Identification and Analysis of PEPC Gene Family Reveals Functional Diversification in Orchidaceae and the Regulation of Bacterial-Type PEPC
Abstract
1. Introduction
2. Results
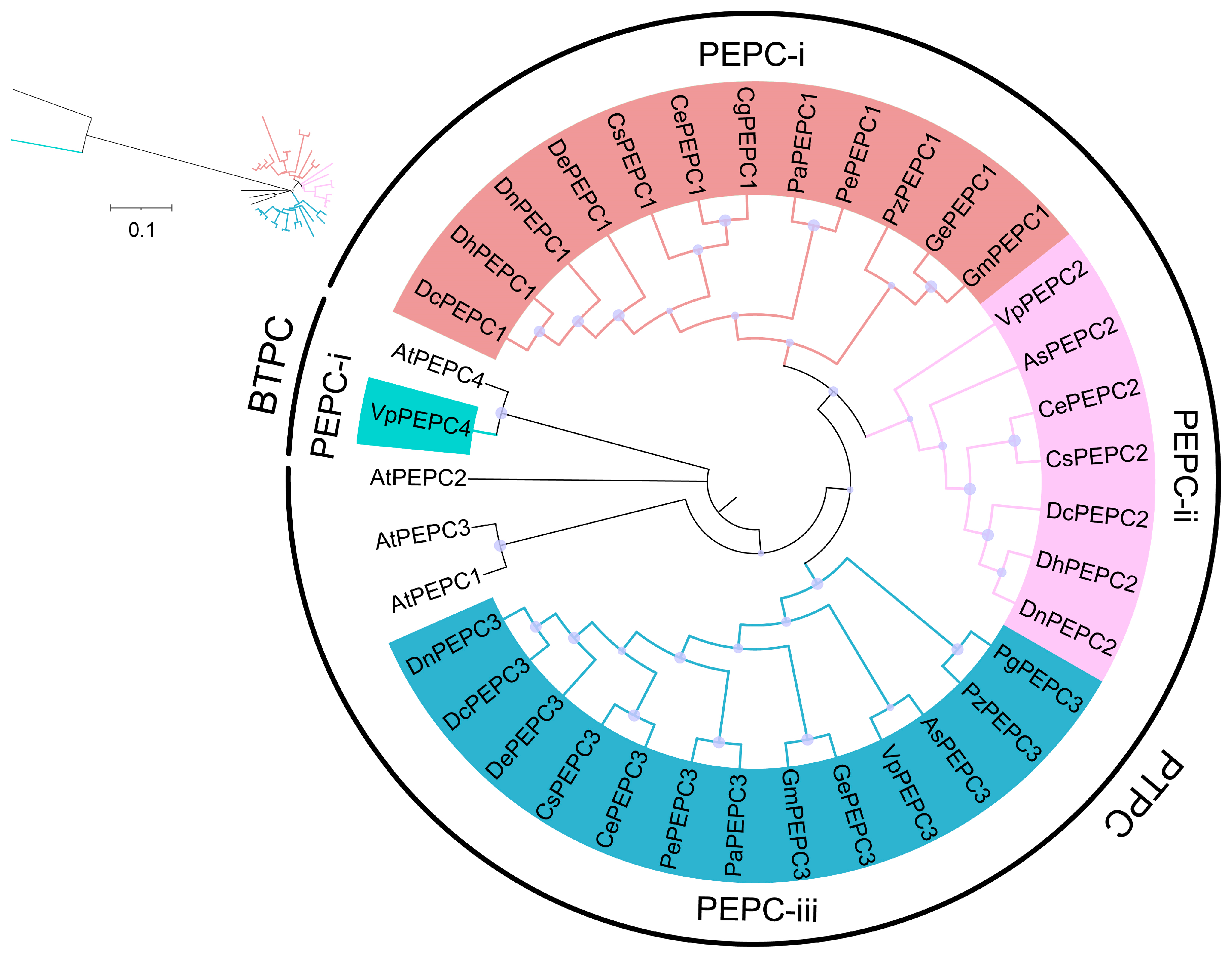
2.1. Identification and Phylogenetic Analysis of PEPC Genes
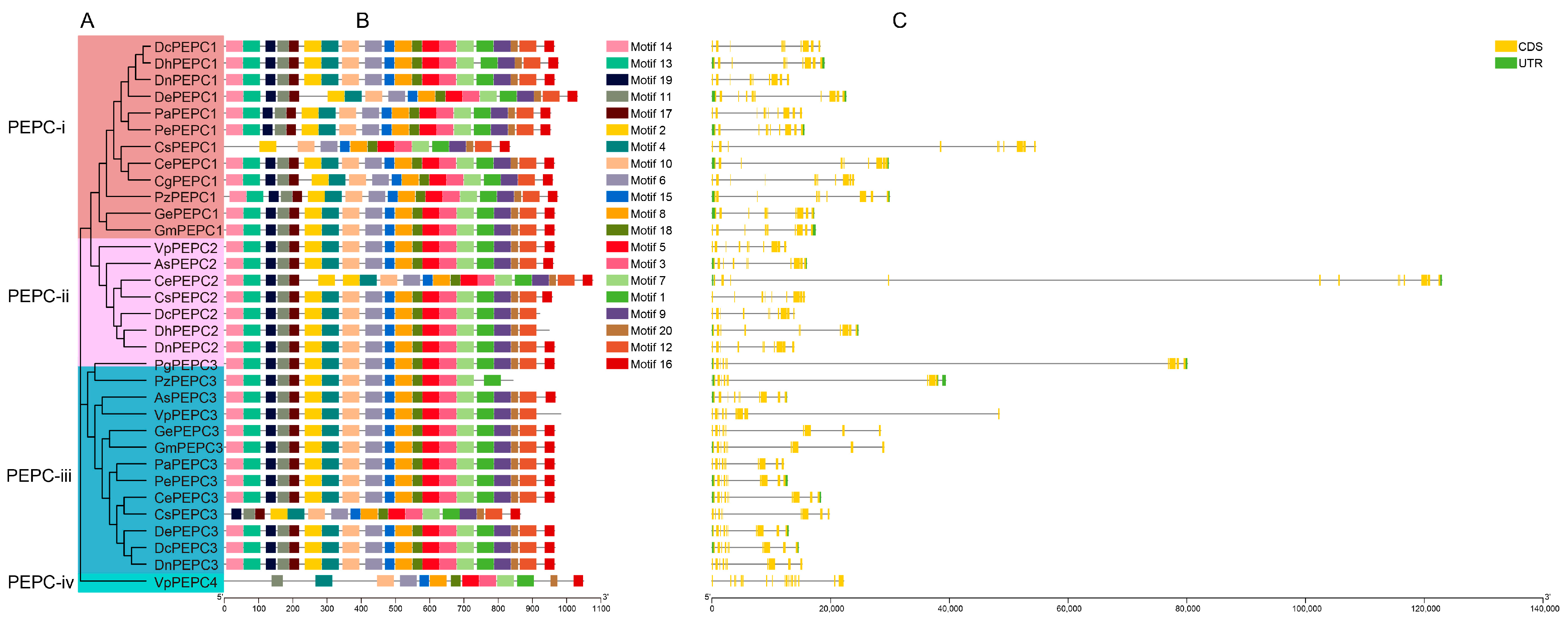
2.2. Motif and Gene Structure Analysis of PEPC Genes
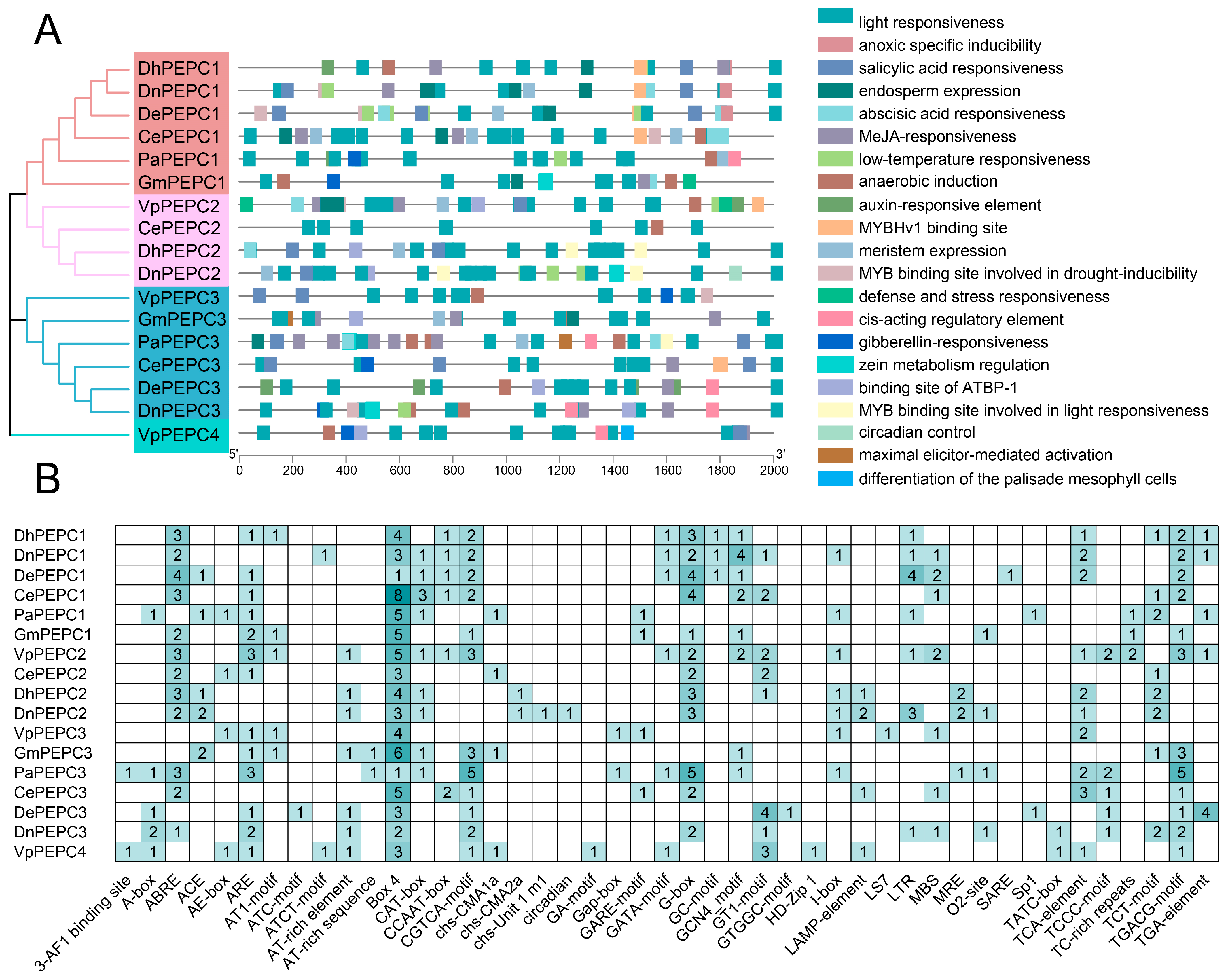
2.3. Cis-Elements in the Promoter Regions of PEPC Genes
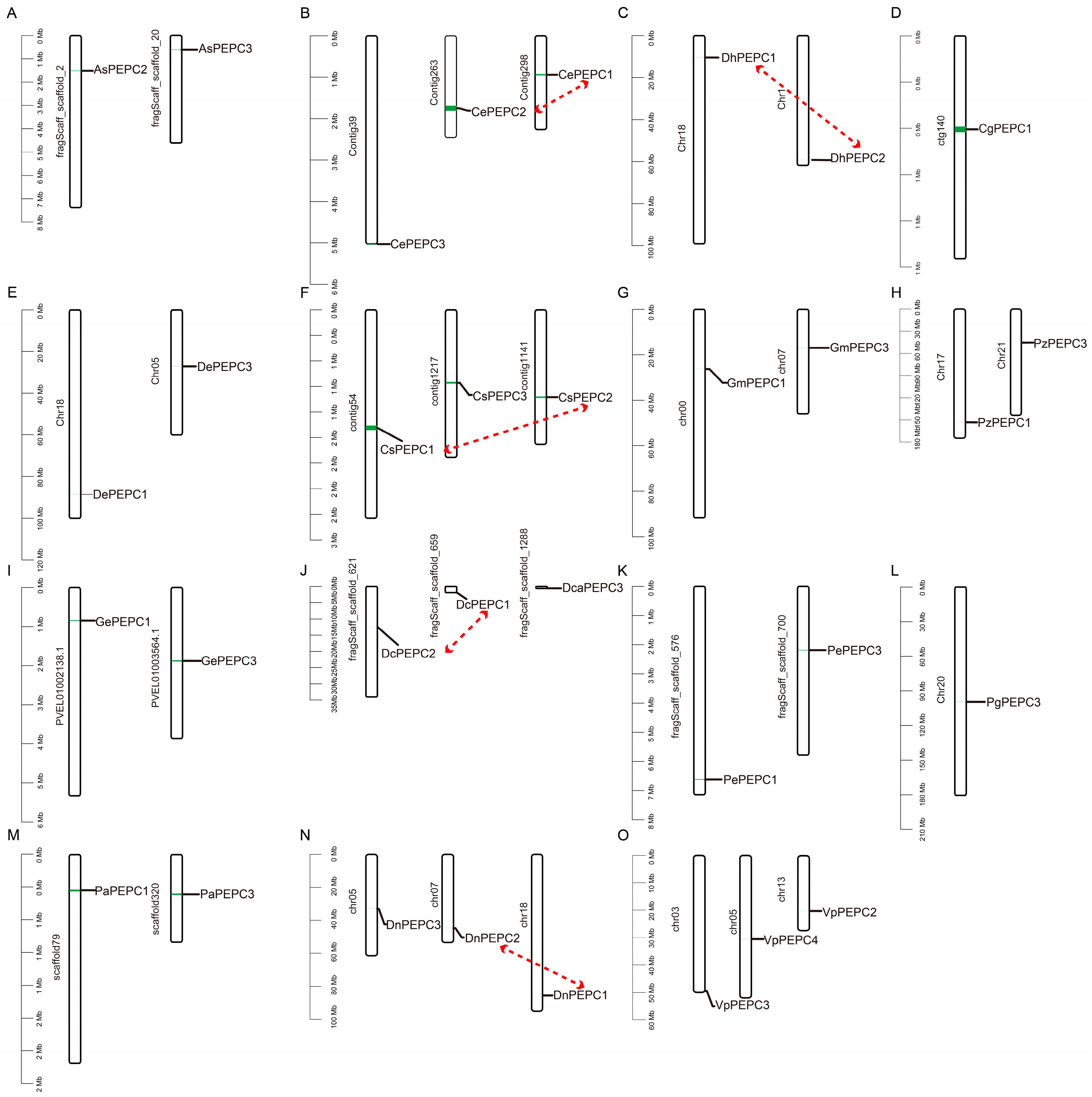
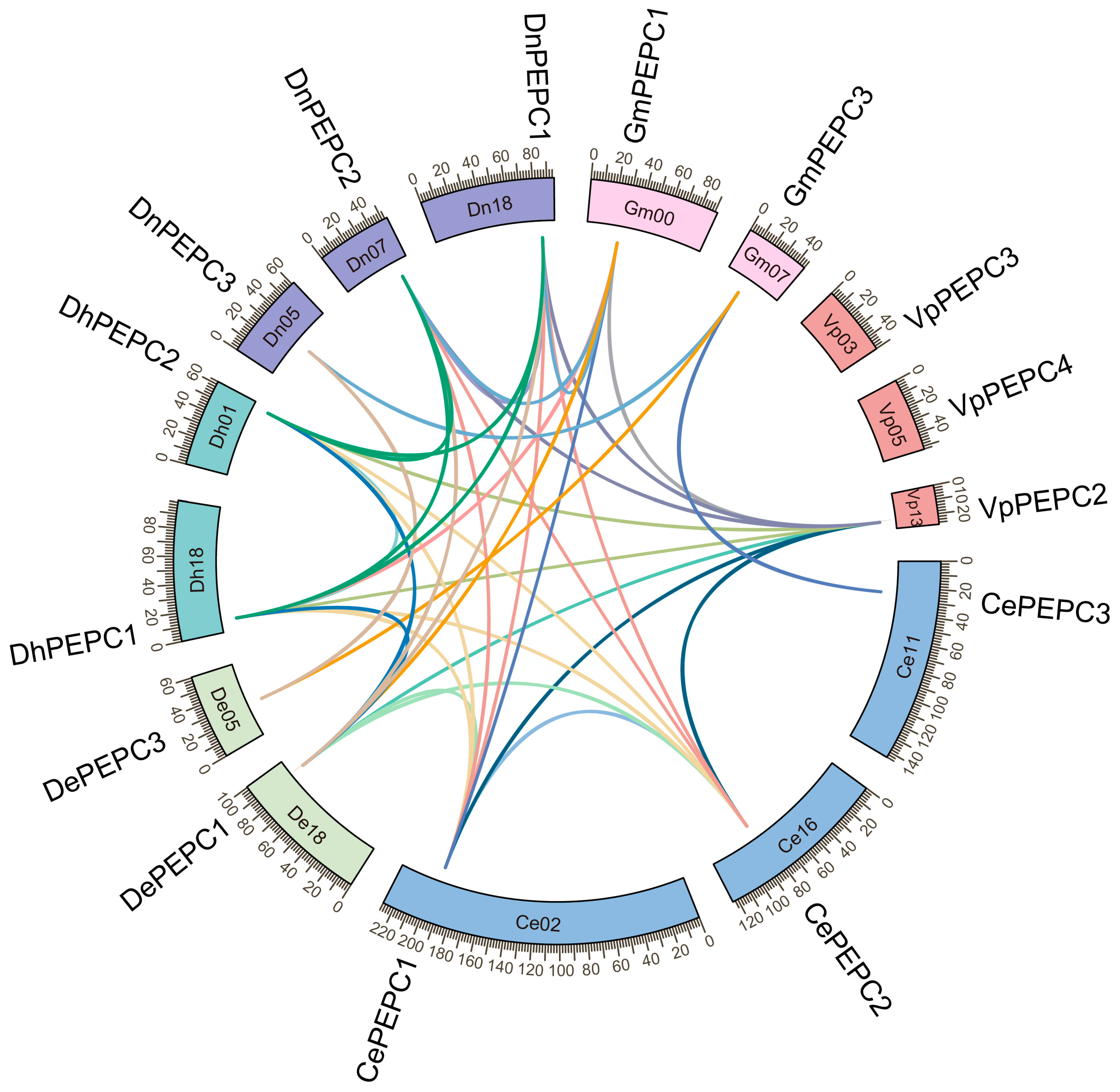
2.4. Chromosomal Localization and Collinearity Analysis of PEPC Genes
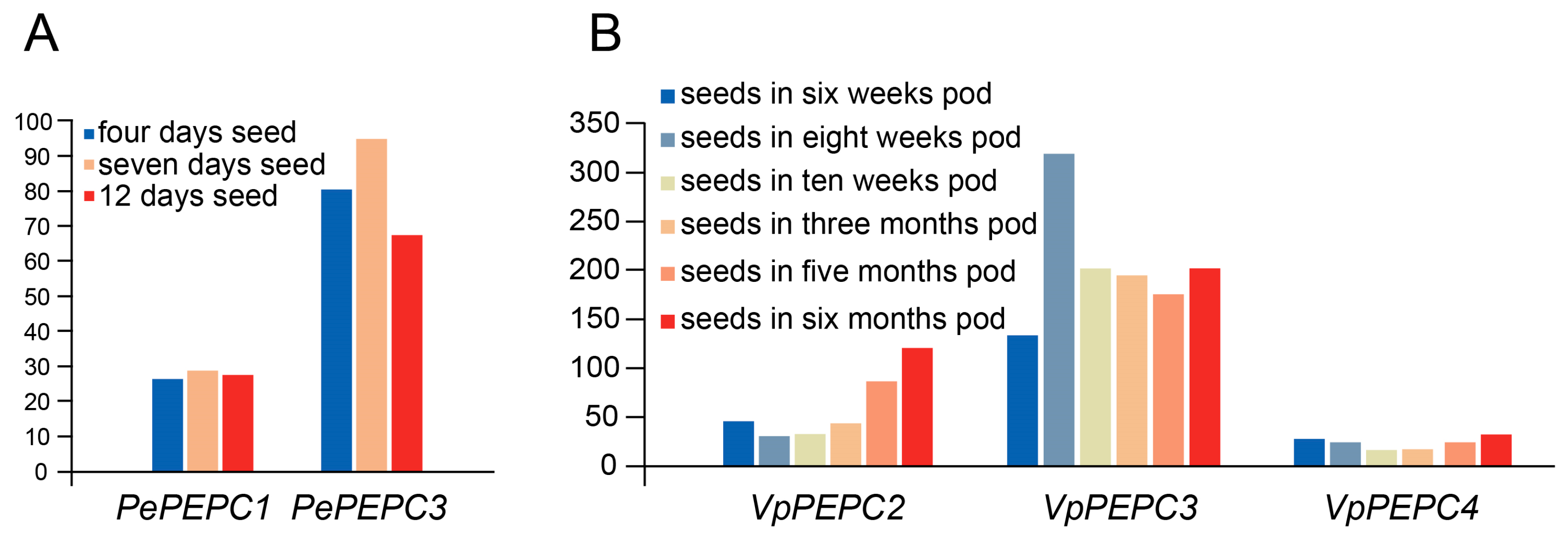
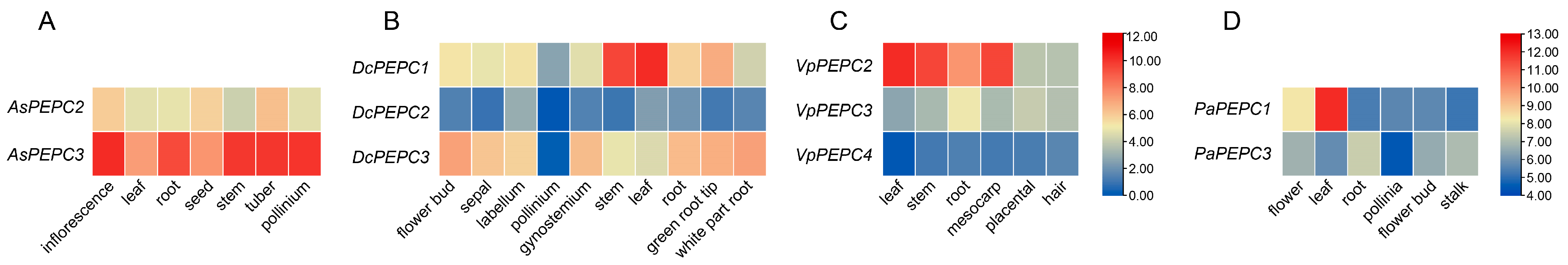
2.5. Expression Analysis of PEPC Genes
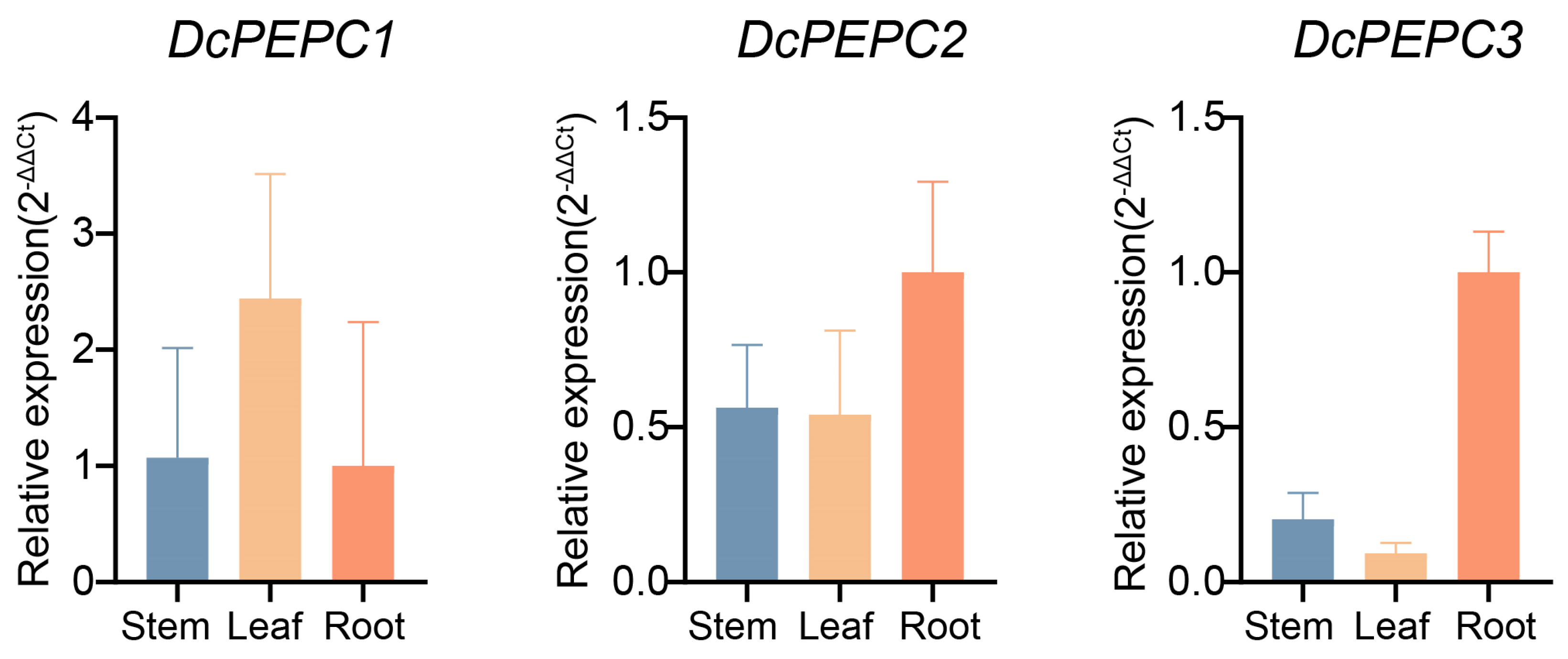
2.6. RT-qPCR of PEPC Genes
3. Discussion
3.1. Identification and Phylogenetic Analysis of PEPC Genes
3.2. Motif and Gene Structure Analysis of PEPC Genes
3.3. Cis-Elements in the Promoter Regions of PEPC Genes
3.4. Chromosomal Localization and Collinearity Analysis of PEPC Genes
3.5. Expression Analysis and RT-qPCR of PEPC Genes
4. Materials and Methods
4.1. Data Sources
4.2. Homolog Gene Identification and Sequence Analysis
4.3. Multiple Sequence Alignment and Phylogenetic Tree Construction
4.4. Prediction of Cis-Acting Elements
4.5. Chromosomal Localization and Collinearity Analysis
4.6. Expression Patterns Analysis
4.7. RT-qPCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chollet, R.; Vidal, J.; O’Leary, M.H. Phosphoenolpyruvate carboxylase: A ubiquitous, highly regulated enzyme in plants. Annu. Rev. Plant Biol. 1996, 47, 273–298. [Google Scholar] [CrossRef] [PubMed]
- Izui, K.; Matsumura, H.; Furumoto, T.; Kai, Y. Phosphoenolpyruvate carboxylase, a new era of structural biology. Annu. Rev. Plant Biol. 2004, 55, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Silvera, K.; Winter, K.; Rodriguez, B.L.; Albion, R.L.; Cushman, J.C. Multiple isoforms of phosphoenolpyruvate carboxylase in the Orchidaceae (subtribe Oncidiinae), implications for the evolution of crassulacean acid metabolism. J. Exp. Bot. 2014, 65, 3623–3636. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, B.; Park, J.; Plaxton, W.C. The remarkable diversity of plant PEPC (phosphoenolpyruvate carboxylase), recent insights into the physiological functions and post-translational controls of non-photosynthetic PEPCs. Biochem. J. 2011, 436, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Gennidakis, S.; Rao, S.; Greenham, K.; Uhrig, R.G.; O’Leary, B.; Snedden, W.A.; Lu, C.F.; Plaxton, W.C. Bacterial- and plant-type phosphoenolpyruvate carboxylase polypeptides interact in the hetero-oligomeric Class-2 PEPC complex of developing castor oil seeds. Plant J. 2007, 52, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Igawa, T.; Fujiwara, M.; Tanaka, I.; Fukao, Y.; Yanagawa, Y. Characterization of bacterial-type phosphoenolpyruvate carboxylase expressed in male gametophyte of higher plants. BMC Plant Biol. 2010, 10, 200. [Google Scholar] [CrossRef]
- O’Leary, B.; Fedosejevs, E.T.; Hill, A.T.; Bettridge, J.; Park, J.; Rao, S.K.; Leach, C.A.; Plaxton, W.C. Tissue-specific expression and post-translational modifications of plant-and bacterial-type phosphoenolpyruvate carboxylase isozymes of the castor oil plant, Ricinus communis L. J. Exp. Bot. 2011, 62, 5485–5495. [Google Scholar] [CrossRef]
- Sánchez, R.; Cejudo, F.J. Identification and expression analysis of a gene encoding a bacterial-type phosphoenolpyruvate carboxylase from Arabidopsis and rice. Plant Physiol. 2003, 132, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Borland, A.M.; Griffiths, H.; Hartwell, J.; Smith, J.A.C. Exploiting the potential of plants with crassulacean acid metabolism for bioenergy production on marginal lands. J. Exp. Bot. 2009, 60, 2879–2896. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, Y.; Zhao, Z.; Lu, S.; Lu, Q.; Cui, C.; Parry, M.A.J.; Hu, Y.G. Genome-wide identification and comparative analyses of key genes involved in C4 photosynthesis in five main gramineous crops. Front. Plant Sci. 2023, 14, 1134170. [Google Scholar] [CrossRef] [PubMed]
- Cousins, A.B.; Baroli, I.; Badger, M.R.; Ivakov, A.; Lea, P.J.; Leegood, R.C.; von Caemmerer, S. The role of phosphoenolpyruvate carboxylase during C4 photosynthetic isotope exchange and stomatal conductance. Plant Physiol. 2007, 145, 1006–1017. [Google Scholar] [CrossRef]
- Ruiz-Ballesta, I.; Baena, G.; Gandullo, J.; Wang, L.Q.; She, Y.M.; Plaxton, W.C.; Echevarría, C. New insights into the post-translational modification of multiple phosphoenolpyruvate carboxylase isoenzymes by phosphorylation and monoubiquitination during sorghum seed development and germination. J. Exp. Bot. 2016, 67, 3523–3536. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Reddy, G.M.; Agarwal, A.; Chandrasekhar, K.; Sopory, S.K.; Reddy, M.K.; Kaul, T. Molecular and structural analysis of C4-specific PEPC isoform from Pennisetum glaucum plays a role in stress adaptation. Gene 2012, 500, 224–231. [Google Scholar] [CrossRef]
- Besnard, G.; Pincon, G.; D’Hont, A.; Hoarau, J.Y.; Cadet, F.; Offmann, B. Characterisation of the phosphoenolpyruvate carboxylase gene family in sugarcane (Saccharum spp.). Theor. Appl. Genet. 2003, 107, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Boxall, S.F.; Kadu, N.; Dever, L.V.; Kneřová, J.; Waller, J.L.; Gould, P.J.; Hartwell, J. Kalanchoë PPC1 is essential for crassulacean acid metabolism and the regulation of core circadian clock and guard cell signaling genes. Plant Cell 2020, 32, 1136–1160. [Google Scholar] [CrossRef]
- Wang, N.; Zhong, X.; Cong, Y.; Wang, T.; Yang, S.; Li, Y.; Gai, J. Genome-wide analysis of phosphoenolpyruvate carboxylase gene family and their response to abiotic stresses in soybean. Sci. Rep. 2016, 6, 38448. [Google Scholar] [CrossRef]
- Besnard, G.; Muasya, A.M.; Russier, F.; Roalson, E.H.; Salamin, N.; Christin, P.A. Phylogenomics of C4 photosynthesis in sedges (Cyperaceae), multiple appearances and genetic convergence. Mol. Biol. Evol. 2009, 26, 1909–1919. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Zhang, L.S.; Zhang, G.Q.; Zheng, B.Q.; Liu, Z.J.; Wang, Y. Evolutionary history of PEPC genes in green plants, implications for the evolution of CAM in orchids. Mol. Phylogenet. Evol. 2016, 94, 559–564. [Google Scholar] [CrossRef]
- Alonso-Cantabrana, H.; Cousins, A.B.; Danila, F.; Ryan, T.; Sharwood, R.E.; von Caemmerer, S.; Furbank, R.T. Diffusion of CO2 across the mesophyll-bundle sheath cell interface in a C4 plant with genetically reduced PEP carboxylase activity. Plant Physiol. 2018, 178, 72–81. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Lu, W.; Wei, X.; Zhang, Q.; Lv, C.; Song, N. Enhanced photorespiration in transgenic rice over-expressing maize C4 phosphoenolpyruvate carboxylase gene contributes to alleviating low nitrogen stress. Plant Physiol. Biochem. 2018, 130, 577–588. [Google Scholar] [CrossRef]
- Liu, D.; Hu, R.; Zhang, J.; Guo, H.B.; Cheng, H.; Li, L.; Borland, A.M.; Qin, H.; Chen, J.-G.; Muchero, W.; et al. Overexpression of an agave phosphoenolpyruvate carboxylase improves plant growth and stress tolerance. Cells 2021, 10, 582. [Google Scholar] [CrossRef]
- Behera, D.; Swain, A.; Karmakar, S.; Dash, M.; Swain, P.; Baig, M.J.; Molla, K.A. Overexpression of Setaria italica phosphoenolpyruvate carboxylase gene in rice positively impacts photosynthesis and agronomic traits. Plant Physiol. Biochem. 2023, 194, 169–181. [Google Scholar] [CrossRef]
- Sunagawa, H.; Cushman, J.; Agarie, S. Crassulacean acid metabolism may alleviate production of reactive oxygen species in a facultative CAM plant; the common ice plant Mesembryanthemum crystallinum L. Plant Prod. Sci. 2010, 13, 256–260. [Google Scholar] [CrossRef]
- Ming, R.; VanBuren, R.; Wai, C.M.; Tang, H.; Schatz, M.C.; Bowers, J.E.; Lyons, E.; Wang, M.L.; Chen, J.; Biggers, E.; et al. The pineapple genome and the evolution of CAM photosynthesis. Nat. Genet. 2015, 47, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; He, Z.S.; Zhe, M.; Feng, J.Q.; Zhang, L.; Huang, Y.W.; Liu, F.; Huang, J.L.; Ya, J.D.; Zhang, S.B.; et al. High-quality Cymbidium mannii genome and multifaceted regulation of crassulacean acid metabolism in epiphytes. Plant Commun. 2023, 4, 100564. [Google Scholar] [CrossRef]
- Yang, X.; Hu, R.; Yin, H.; Jenkins, J.; Shu, S.; Tang, H.; Liu, D.G.; Weighill, D.A.; Yim, W.C.; Ha, J.; et al. The Kalanchoë genome provides insights into convergent evolution and building blocks of crassulacean acid metabolism. Nat. Commun. 2017, 8, 1899. [Google Scholar] [CrossRef] [PubMed]
- Wickell, D.; Kuo, L.Y.; Yang, H.P.; Ashok, A.D.; Irisarri, I.; Dadras, A.; de Vries, S.; de Vries, J.; Huang, Y.M.; Li, Z.; et al. Underwater CAM photosynthesis elucidated by Isoetes genome. Nat. Commun. 2021, 12, 6348. [Google Scholar] [CrossRef] [PubMed]
- Silvera, K.; Neubig, K.M.; Whitten, W.; Williams, N.H.; Winter, K.; Cushman, J.C. Evolution along the crassulacean acid metabolism continuum. Funct. Plant Biol. 2010, 37, 995–1010. [Google Scholar] [CrossRef]
- Gamisch, A.; Winter, K.; Fischer, G.A.; Comes, H.P. Evolution of crassulacean acid metabolism (CAM) as an escape from ecological niche conservatism in Malagasy Bulbophyllum (Orchidaceae). New Phytol. 2021, 231, 1236–1248. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.C.; Winter, K. Crassulacean Acid Metabolism: Biochemistry, Ecophysiology and Evolution; Springer: Berlin/Heidelberg, Germany, 1996; pp. 427–436. [Google Scholar]
- Silvera, K.; Santiago, L.S.; Cushman, J.C.; Winter, K. Crassulacean acid metabolism and epiphytism linked to adaptive radiations in the Orchidaceae. Plant Physiol. 2009, 149, 1838–1847. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Liu, K.W.; Li, Z.; Lohaus, R.; Hsiao, Y.Y.; Niu, S.C.; Wang, J.Y.; Lin, Y.C.; Xu, Q.; Chen, L.J.; et al. The Apostasia genome and the evolution of orchids. Nature 2017, 549, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Li, Z.; Sun, W.-H.; Chen, J.; Zhang, D.; Ma, L.; Zhang, Q.-H.; Chen, M.-K.; Zheng, Q.-D.; Liu, J.-F.; et al. The Cymbidium genome reveals the evolution of unique morphological traits. Hortic. Res. 2021, 8, 255. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Gao, J.; Wei, Y.; Ren, R.; Zhang, G.; Lu, C.; Jin, J.; Ai, Y.; Wang, Y.; Chen, L.; et al. The genome of Cymbidium sinense revealed the evolution of orchid traits. Plant Biotechnol. J. 2021, 19, 2501–2516. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, G.-Z.; Huang, J.; Liu, D.-K.; Xue, F.; Chen, X.-L.; Chen, S.-Q.; Liu, C.-G.; Liu, H.; Ma, H.; et al. The Cymbidium goeringii genome provides insight into organ development and adaptive evolution in orchids. Ornam. Plant Res. 2021, 1, 10. [Google Scholar]
- Zhang, G.Q.; Xu, Q.; Bian, C.; Tsai, W.C.; Yeh, C.M.; Liu, K.W.; Yoshida, K.; Zhang, L.S.; Chang, S.B.; Chen, F.; et al. The Dendrobium catenatum Lindl. genome sequence provides insights into polysaccharide synthase; floral development and adaptive evolution. Sci. Rep. 2016, 6, 19029. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, G.Q.; Zhang, D.; Liu, X.D.; Xu, X.Y.; Sun, W.H.; Yu, X.; Zhu, X.; Wang, Z.W.; Zhao, X.; et al. Chromosome-scale assembly of the Dendrobium chrysotoxum genome enhances the understanding of orchid evolution. Hortic. Res. 2021, 8, 183. [Google Scholar] [CrossRef]
- Han, B.; Jing, Y.; Dai, J.; Zheng, T.; Gu, F.; Zhao, Q.; Zhu, F.; Song, X.; Deng, H.; Wei, P.; et al. A chromosome-level genome assembly of Dendrobium huoshanense using long reads and Hi-C data. Genome Biol. Evol. 2020, 12, 2486–2490. [Google Scholar] [CrossRef]
- Xu, Q.; Niu, S.-C.; Li, K.-L.; Zheng, P.-J.; Zhang, X.-J.; Jia, Y.; Liu, Y.; Niu, Y.-X.; Yu, L.-H.; Chen, D.-F.; et al. Chromosome-scale assembly of the Dendrobium nobile genome provides insights into the molecular mechanism of the biosynthesis of the medicinal active ingredient of Dendrobium. Front. Genet. 2022, 13, 844622. [Google Scholar] [CrossRef]
- Yuan, Y.; Jin, X.; Liu, J.; Zhao, X.; Zhou, J.; Wang, X.; Wang, D.; Lai, C.; Xu, W.; Huang, J. The Gastrodia elata genome provides insights into plant adaptation to heterotrophy. Nat. Commun. 2018, 9, 1615. [Google Scholar] [CrossRef]
- Jiang, Y.; Hu, X.D.; Yuan, Y.; Guo, X.L.; Chase, M.W.; Ge, S.; Li, J.W.; Fu, J.L.; Li, K.; Hao, M.; et al. The Gastrodia menghaiensis (Orchidaceae) genome provides new insights of orchid mycorrhizal interactions. BMC Plant Biol. 2022, 22, 179. [Google Scholar] [CrossRef]
- Chao, Y.T.; Chen, W.C.; Chen, C.Y.; Ho, H.Y.; Yeh, C.H.; Kuo, Y.T.; Su, C.L.; Yen, S.H.; Hsueh, H.Y.; Yeh, J.H.; et al. Chromosome-level assembly; genetic and physical mapping of Phalaenopsis aphrodite genome provides new insights into species adaptation and resources for orchid breeding. Plant Biotechnol. 2018, 16, 2027–2041. [Google Scholar] [CrossRef]
- Cai, J.; Liu, X.; Vanneste, K.; Tsai, W.C.; Liu, K.W.; Chen, L.J.; He, Y.; Xu, Q.; Bian, C.; Zheng, Z.; et al. The genome sequence of the orchid Phalaenopsis equestris. Nat. Genet. 2015, 47, 65–72. [Google Scholar] [CrossRef]
- Li, M.H.; Liu, K.W.; Li, Z.; Lu, H.C.; Ye, Q.L.; Zhang, D.; Wang, J.Y.; Li, Y.F.; Zhong, Z.M.; Liu, X.; et al. Genomes of leafy and leafless Platanthera orchids illuminate the evolution of mycoheterotrophy. Nat. Plants 2022, 8, 373–388. [Google Scholar] [CrossRef]
- Hasing, T.; Tang, H.; Brym, M.; Khazi, F.; Huang, T.; Chambers, A.H. A phased Vanilla planifolia genome enables genetic improvement of flavour and production. Nat. Food 2020, 1, 811–819. [Google Scholar] [CrossRef]
- Masumoto, C.; Miyazawa, S.I.; Ohkawa, H.; Fukudaa, T.; Taniguchia, Y.; Murayamaa, S.; Kusanob, M.; Saitob, K.; Fukayamaa, H.; Miyao, M. Phosphoenolpyruvate carboxylase intrinsically located in the chloroplast of rice plays a crucial role in ammonium assimilation. Proc. Natl. Acad. Sci. USA 2010, 107, 5226–5231. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, L.; Columbus, J.T.; Hu, Y.; Zhao, Y.; Tang, L.; Guo, Z.H.; Chen, W.L.; McKain, M.; Bartlett, M.; et al. A well-supported nuclear phylogeny of Poaceae and implications for the evolution of C4 photosynthesis. Mol. Plant 2022, 15, 755–777. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, F.; Zhang, G.Q.; Zhang, Y.Q.; Niu, S.; Xiong, J.-S.; Lin, Z.; Cheng, Z.M.; Liu, Z.J. Origin and mechanism of crassulacean acid metabolism in orchids as implied by comparative transcriptomics and genomics of the carbon fixation pathway. Plant J. 2016, 86, 175–185. [Google Scholar] [CrossRef]
- Motomura, H.; Yukawa, T.; Ueno, O.; Kagawa, A. The occurrence of crassulacean acid metabolism in Cymbidium (Orchidaceae) and its ecological and evolutionary implications. J. Plant Res. 2008, 121, 163–177. [Google Scholar] [CrossRef]
- Li, M.H.; Liu, D.K.; Zhang, G.Q.; Deng, H.; Tu, X.D.; Wang, Y.; Lan, S.R.; Liu, Z.J. A perspective on crassulacean acid metabolism photosynthesis evolution of orchids on different continents: Dendrobium as a case study. J. Exp. Bot. 2019, 70, 6611–6619. [Google Scholar] [CrossRef]
- Torres-Morales, G.; Lasso, E.; Silvera, K.; Turner, B.L.; Winter, K. Occurrence of crassulacean acid metabolism in Colombian orchids determined by leaf carbon isotope ratios. Bot. J. Linn. Soc. 2020, 193, 431–477. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, A.; Wang, Y.; Hua, J. Evolution of PEPC gene family in Gossypium reveals functional diversification and GhPEPC genes responding to abiotic stresses. Gene 2019, 698, 61–71. [Google Scholar] [CrossRef]
- Ting, M.K.Y.; She, Y.M.; Plaxton, W.C. Transcript profiling indicates a widespread role for bacterial-type phosphoenolpyruvate carboxylase in malate-accumulating sink tissues. J. Exp. Bot. 2017, 68, 5857–5869. [Google Scholar] [CrossRef]
- Zhang, D.; Lan, S.; Yin, W.L.; Liu, Z.J. Genome-wide identification and expression pattern analysis of KNOX gene family in Orchidaceae. Front. Plant Sci. 2022, 13, 901089. [Google Scholar] [CrossRef]
- Jo, B.S.; Choi, S.S. Introns, the functional benefits of introns in genomes. Genomics inform. 2015, 13, 112. [Google Scholar] [CrossRef]
- Hernandez-Garcia, C.M.; Finer, J.J. Identification and validation of promoters and cis-acting regulatory elements. Plant Sci. 2014, 217, 109–119. [Google Scholar] [CrossRef]
- Flagel, L.E.; Wendel, J.F. Gene duplication and evolutionary novelty in plants. New Phytol. 2009, 183, 557–564. [Google Scholar] [CrossRef]
- Fay, J.C.; Wu, C.I. Sequence divergence; functional constraint; and selection in protein evolution. Annu. Rev. Genomics Hum. Genet. 2003, 4, 213–235. [Google Scholar] [CrossRef]
- Cvijović, I.; Good, B.H.; Desai, M.M. The effect of strong purifying selection on genetic diversity. Genetics 2018, 209, 1235–1278. [Google Scholar] [CrossRef]
- Wendel, J.F.; Lisch, D.; Hu, G.; Mason, A.S. The long and short of doubling down, polyploidy; epigenetics; and the temporal dynamics of genome fractionation. Curr. Opin. Genet. Dev. 2018, 49, 1–7. [Google Scholar] [CrossRef]
- Hsiao, Y.Y.; Fu, C.H.; Ho, S.Y.; Li, C.I.; Chen, Y.Y.; Wu, W.L.; Wang, J.S.; Zhang, D.Y.; Hu, W.Q.; Yu, X.; et al. OrchidBase 4.0: A database for orchid genomics and molecular biology. BMC Plant Biol. 2021, 21, 371. [Google Scholar] [CrossRef]
- Chao, Y.-T.; Yen, S.-H.; Yeh, J.-H.; Chen, W.-C.; Shih, M.-C. Orchidstra 2.0—A Transcriptomics Resource for the Orchid Family. Plant Cell Physiol. 2017, 58, e9. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools, an integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.E.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Chou, K.C.; Shen, H.B. Plant-mPLoc, a top-down strategy to augment the power for predicting plant protein subcellular localization. PLoS ONE 2010, 5, e11335. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (ITOL): An online tool for phylogenetic tree display and annotation. Bioinformatics 2007, 23, 127–128. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Hurst, L.D. The Ka/Ks ratio: Diagnosing the form of sequence evolution. Trends Genet. 2002, 18, 486–487. [Google Scholar] [CrossRef]
| Gene Name a | Gene ID | Intron | Amino Acid Number (aa) | Molecular Weight (kDa) | Theoretical Isoelectric Point | Grand Average of Hydrophobicity | Subcellular Localization |
|---|---|---|---|---|---|---|---|
| AsPEPC3 | Ash006877 | 9 | 968 | 110.68 | 5.71 | −0.405 | Cytoplasm |
| AsPEPC2 | Ash003090 | 9 | 961 | 109.32 | 6.28 | −0.375 | Cytoplasm |
| CePEPC3 | JL001583 | 9 | 965 | 110.36 | 5.78 | −0.379 | Cytoplasm |
| CePEPC1 | JL008709 | 9 | 964 | 109.90 | 6.04 | −0.374 | Cytoplasm |
| CePEPC2 | JL007930 | 11 | 1076 | 122.43 | 6.33 | −0.349 | Cytoplasm |
| CgPEPC1 | GL01041 | 10 | 960 | 109.57 | 6.30 | −0.399 | Cytoplasm |
| CsPEPC2 | Mol008966 | 9 | 958 | 108.95 | 6.10 | −0.336 | Cytoplasm |
| CsPEPC3 | Mol012516 | 8 | 865 | 99.01 | 6.17 | −0.381 | Cytoplasm |
| CsPEPC1 | Mol013879 | 8 | 834 | 95.42 | 6.46 | −0.354 | Cytoplasm |
| DcPEPC3 | Dca019608 | 9 | 965 | 110.14 | 5.81 | −0.402 | Cytoplasm |
| DcPEPC1 | Dca010878 | 9 | 964 | 109.85 | 6.00 | −0.390 | Cytoplasm |
| DcPEPC2 | Dca000344 | 9 | 920 | 105.12 | 5.85 | −0.361 | Cytoplasm |
| DePEPC3 | Maker113806 | 9 | 965 | 110.21 | 5.76 | −0.411 | Cytoplasm |
| DePEPC1 | Maker105367 | 10 | 1032 | 117.69 | 6.64 | −0.328 | Cytoplasm |
| DhPEPC1 | Dhu000014644 | 9 | 976 | 111.33 | 5.98 | −0.374 | Cytoplasm |
| DhPEPC2 | Dhu000025281 | 9 | 949 | 108.38 | 5.91 | −0.368 | Cytoplasm |
| DnPEPC3 | cds-KAI0522578.1 | 9 | 965 | 110.22 | 5.75 | −0.392 | Cytoplasm |
| DnPEPC2 | cds-KAI0516232.1 | 9 | 965 | 110.06 | 5.93 | −0.356 | Cytoplasm |
| DnPEPC1 | cds-KAI0492914.1 | 9 | 965 | 110.04 | 6.16 | −0.399 | Cytoplasm |
| GePEPC1 | Gel007954 | 9 | 965 | 109.94 | 6.04 | −0.375 | Cytoplasm |
| GePEPC3 | Gel011478 | 9 | 965 | 110.23 | 6.05 | −0.385 | Cytoplasm |
| GmPEPC3 | Gme.evm.model.scaffold_67.219 | 9 | 965 | 110.34 | 6.12 | −0.396 | Cytoplasm |
| GmPEPC1 | Gme.evm.model.scaffold_80.68 | 9 | 965 | 109.89 | 6.02 | −0.361 | Cytoplasm |
| PaPEPC3 | PAXXG242960-mRNA1 | 9 | 965 | 109.91 | 5.82 | −0.376 | Cytoplasm |
| PaPEPC1 | PAXXG121890-mRNA1 | 9 | 953 | 108.39 | 5.97 | −0.371 | Cytoplasm |
| PePEPC3 | Peq006904 | 9 | 965 | 110.03 | 5.82 | −0.377 | Cytoplasm |
| PePEPC1 | Peq005747 | 9 | 953 | 108.35 | 6.00 | −0.356 | Cytoplasm |
| PgPEPC3 | PGU005496 | 9 | 965 | 109.90 | 5.71 | −0.361 | Cytoplasm |
| PzPEPC1 | PZI019529 | 9 | 974 | 111.01 | 5.91 | −0.348 | Cytoplasm |
| PzPEPC3 | PZI023524 | 8 | 842 | 95.59 | 5.95 | −0.359 | Cytoplasm |
| VpPEPC2 | Vpla_KAG0455466.1 | 9 | 965 | 109.76 | 6.02 | −0.358 | Cytoplasm |
| VpPEPC3 | Vpla_KAG0489408.1 | 10 | 982 | 112.66 | 6.25 | −0.433 | Cytoplasm |
| VpPEPC4 | Vpla_KAG0480734.1 | 19 | 1049 | 118.09 | 6.23 | −0.350 | Cytoplasm |
| AtPEPC1 | AT1G53310.1 | 9 | 967 | 110.29 | 5.72 | −0.391 | Cytoplasm |
| AtPEPC2 | AT2G42600.1 | 9 | 963 | 109.75 | 5.57 | −0.387 | Cytoplasm |
| AtPEPC3 | AT3G14940.1 | 9 | 968 | 110.16 | 5.73 | −0.389 | Cytoplasm |
| AtPEPC4 | AT1G68750.1 | 19 | 1032 | 116.59 | 6.68 | −0.419 | Cytoplasm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Gao, X.; Wu, Y.; Wei, C.; Li, M.-H.; Liu, D.-K.; Liu, Z.-J. Identification and Analysis of PEPC Gene Family Reveals Functional Diversification in Orchidaceae and the Regulation of Bacterial-Type PEPC. Int. J. Mol. Sci. 2024, 25, 2055. https://doi.org/10.3390/ijms25042055
Li R, Gao X, Wu Y, Wei C, Li M-H, Liu D-K, Liu Z-J. Identification and Analysis of PEPC Gene Family Reveals Functional Diversification in Orchidaceae and the Regulation of Bacterial-Type PEPC. International Journal of Molecular Sciences. 2024; 25(4):2055. https://doi.org/10.3390/ijms25042055
Chicago/Turabian StyleLi, Ruyi, Xuyong Gao, Yuwei Wu, Chunyi Wei, Ming-He Li, Ding-Kun Liu, and Zhong-Jian Liu. 2024. "Identification and Analysis of PEPC Gene Family Reveals Functional Diversification in Orchidaceae and the Regulation of Bacterial-Type PEPC" International Journal of Molecular Sciences 25, no. 4: 2055. https://doi.org/10.3390/ijms25042055
APA StyleLi, R., Gao, X., Wu, Y., Wei, C., Li, M.-H., Liu, D.-K., & Liu, Z.-J. (2024). Identification and Analysis of PEPC Gene Family Reveals Functional Diversification in Orchidaceae and the Regulation of Bacterial-Type PEPC. International Journal of Molecular Sciences, 25(4), 2055. https://doi.org/10.3390/ijms25042055








