Serological Antibodies against Kidney, Liver, and Spleen Membrane Antigens as Potential Biomarkers in Patients with Immune Disorders
Abstract
1. Introduction
2. Results
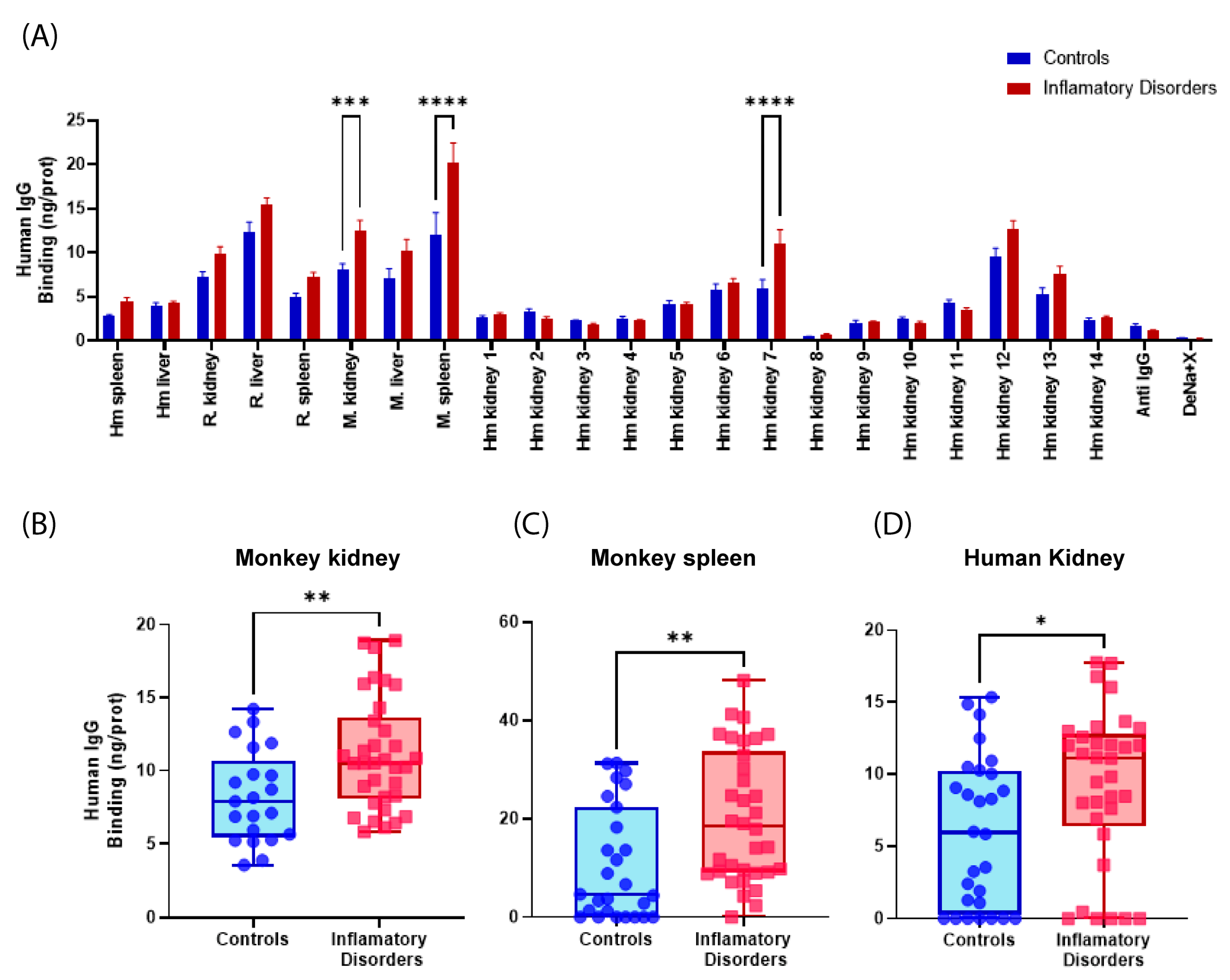
2.1. Detection of Self-Reactive Antibodies in Sera from Patients with LN and Other Autoimmune Disorders
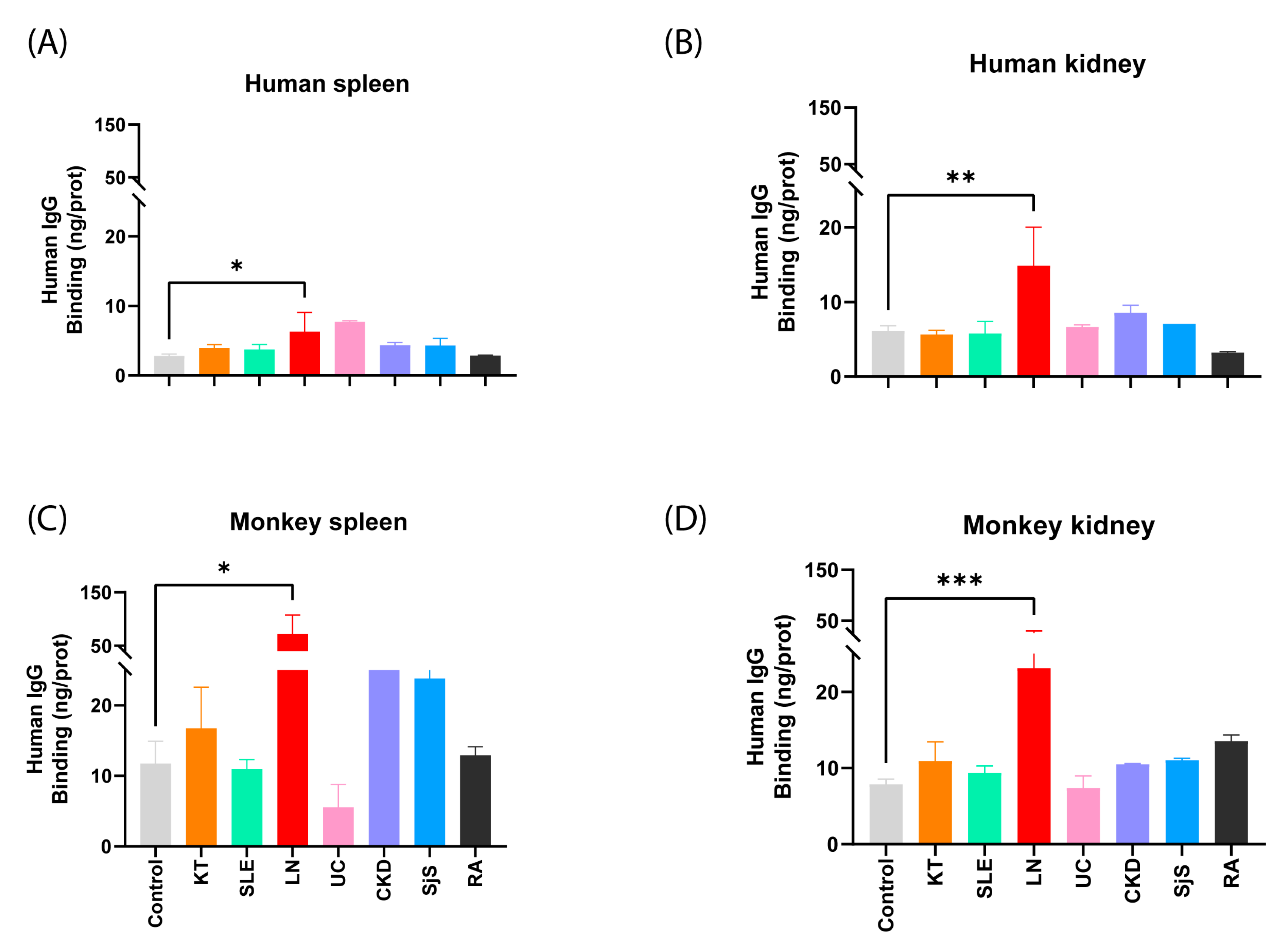
2.1.1. Examination of the Responsiveness of Sera Categorized by Groups
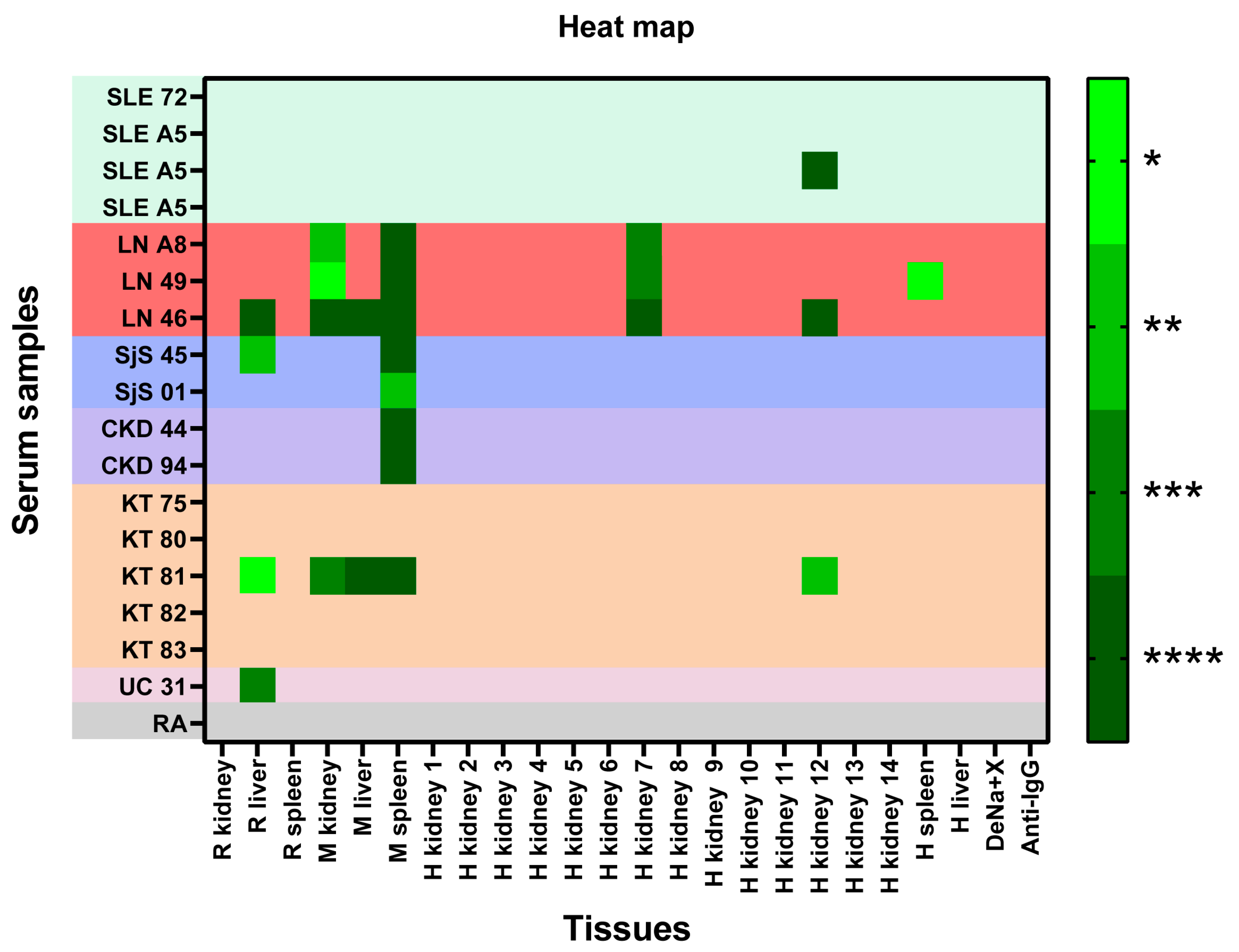
2.1.2. Reactivity of Each Serum to the Panel of Samples Immobilized in CMMAs—Heat Map
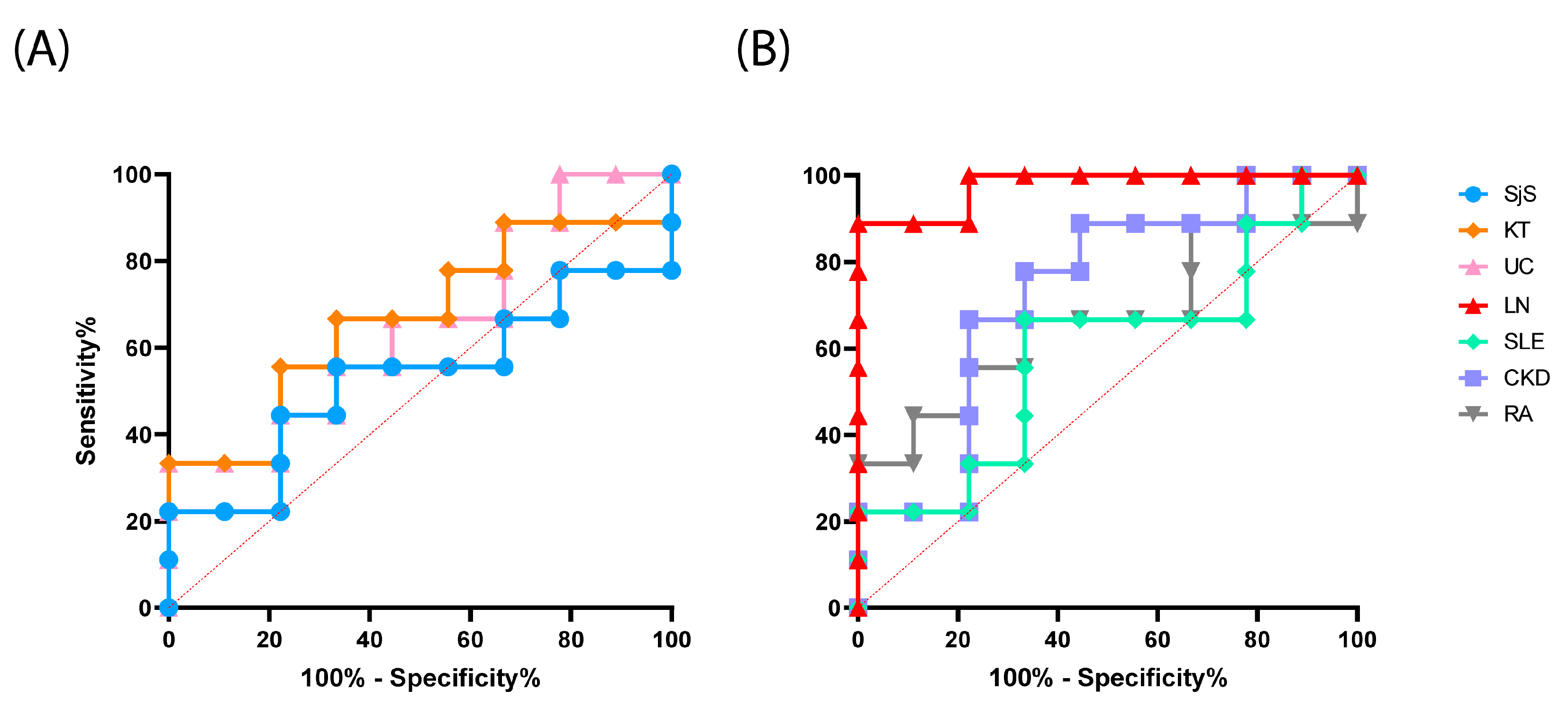
2.1.3. Evaluation of the Test Performance in the Detection of Reactive Antibodies
2.2. Detection of Self-Reactive Antibodies in Sera from Patients with KT
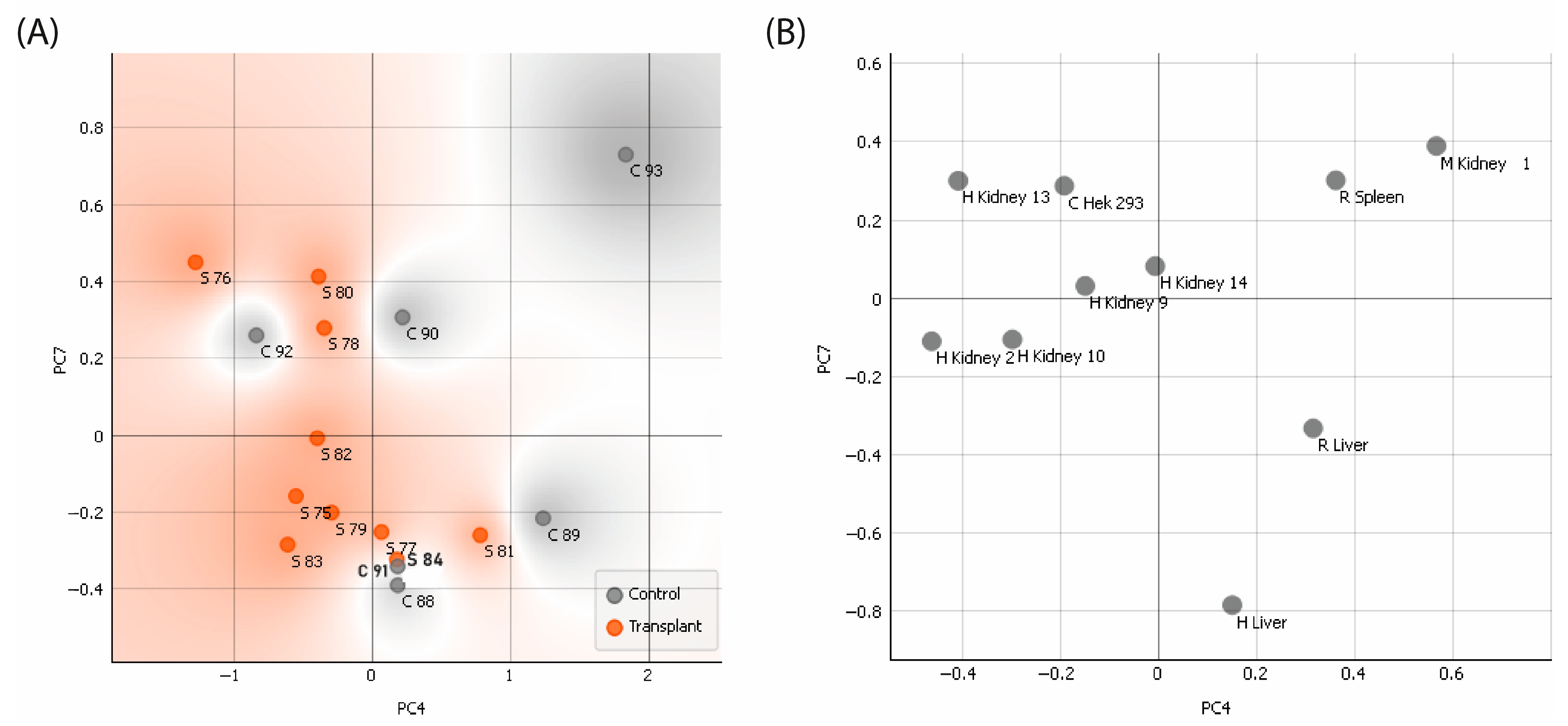
2.2.1. Examination of the Responsiveness of Sera Categorized by Groups—PCA
2.2.2. Reactivity of Renal Transplant Patients’ Sera to the CMMAs Antigenic Panel
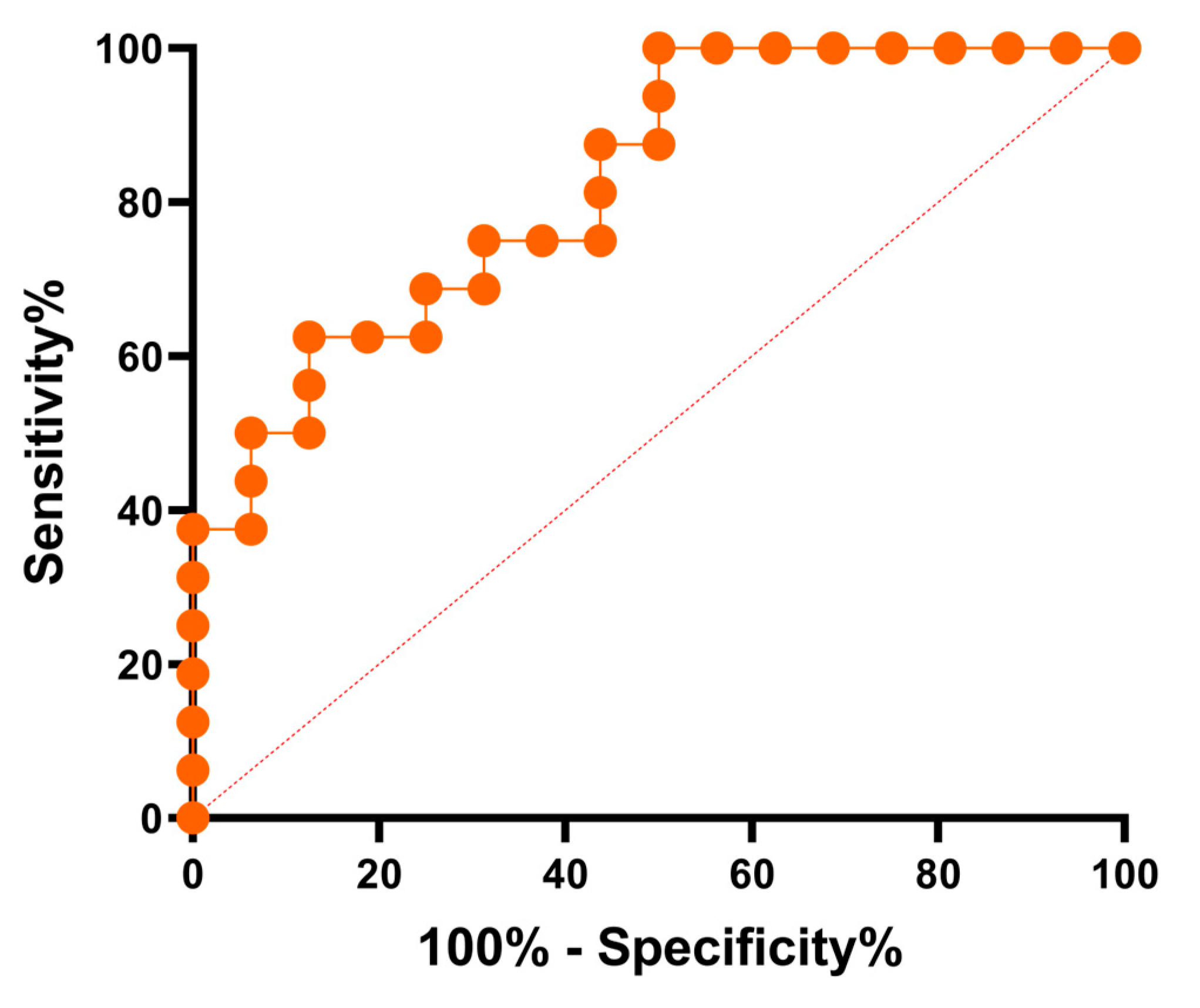
2.2.3. Evaluation of the Test Performance—Receiver Operating Characteristic Curve
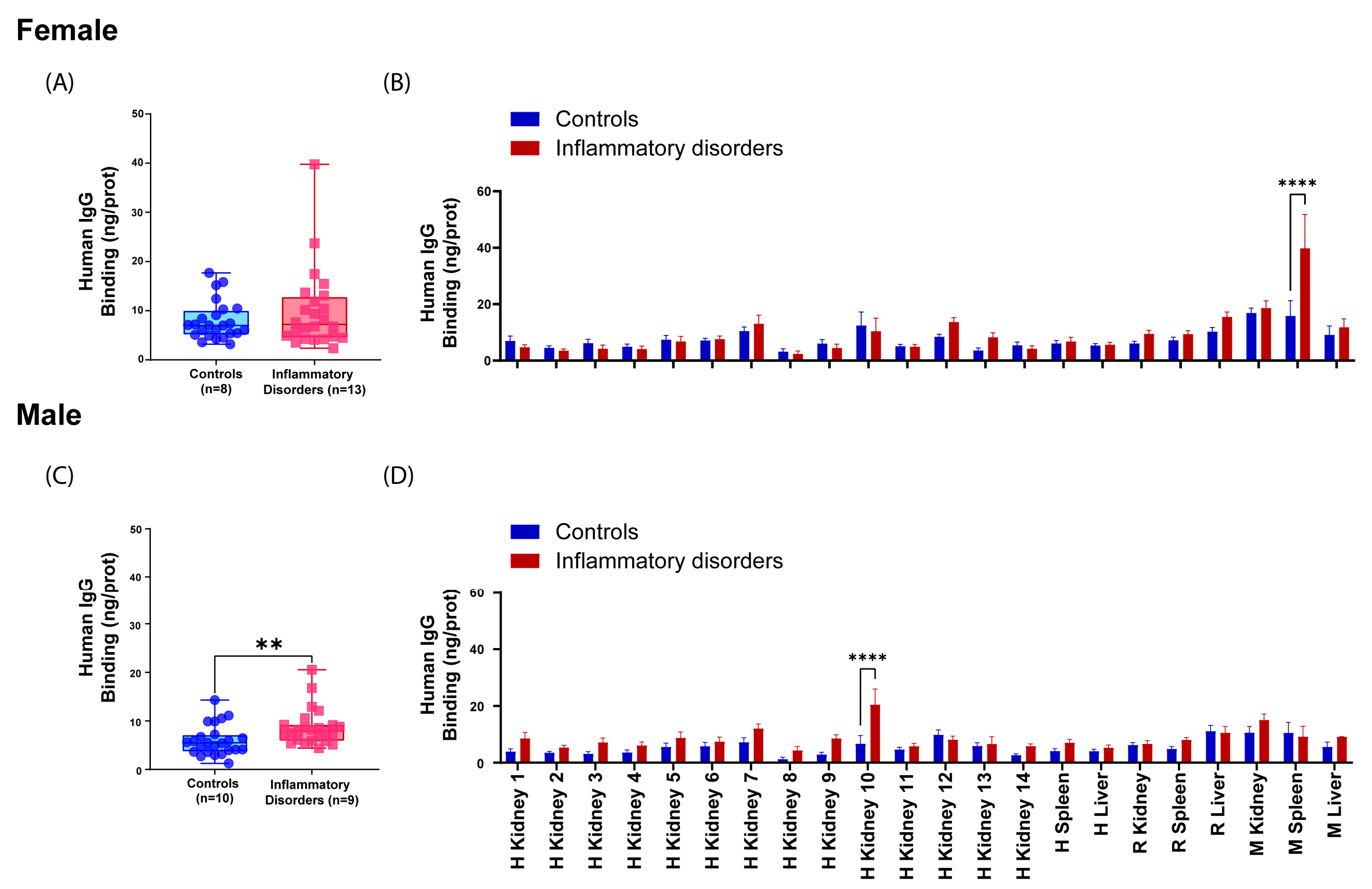
2.3. Examining the Influence of Sex and Age on Reactive Antibody Occurrence
3. Discussion
4. Materials and Methods
4.1. Serum Samples
4.2. Tissue Samples
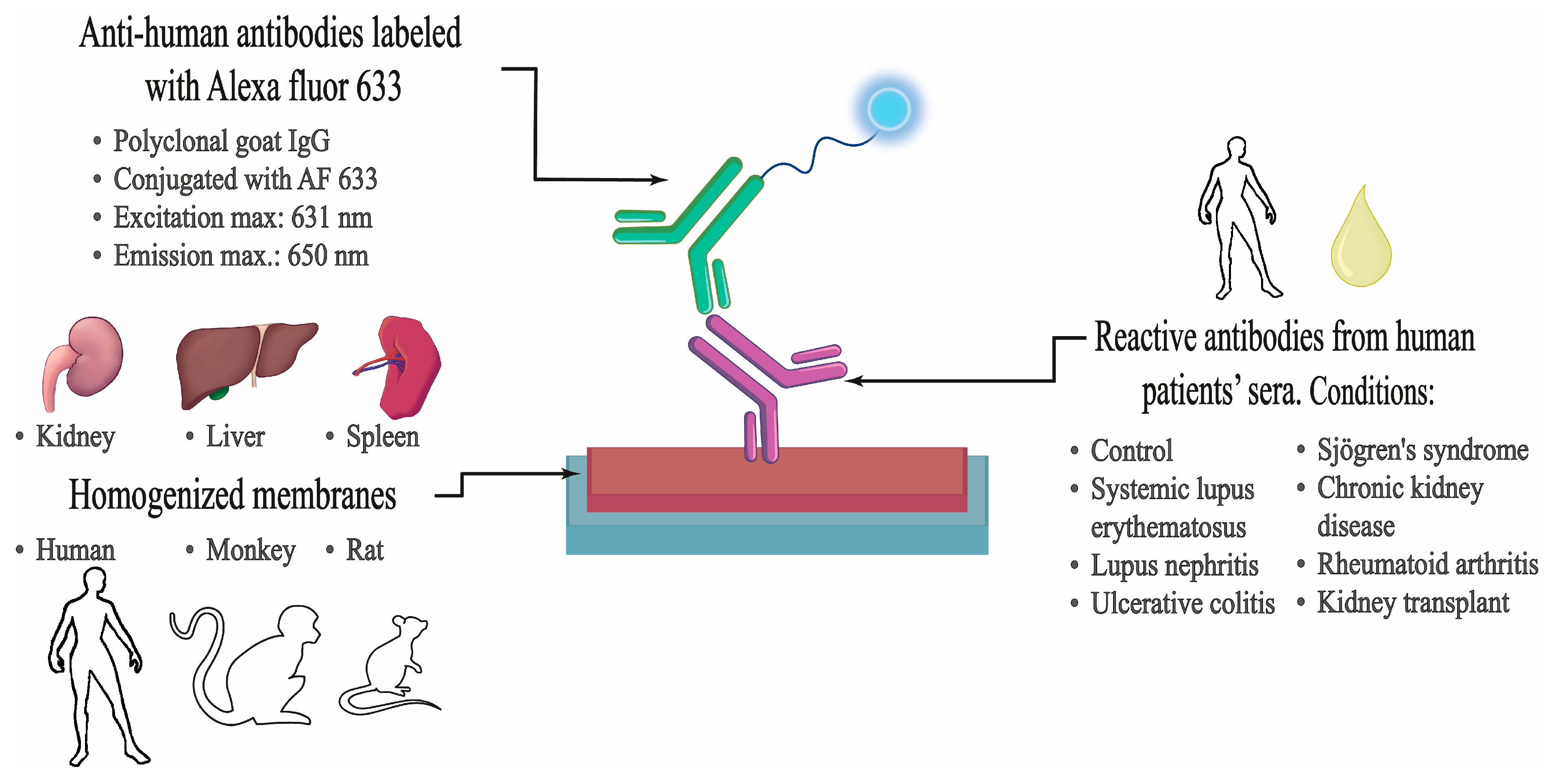
4.3. Design and Methodology of the Study
4.4. Cell Membrane Microarray Fabrication
4.5. Immunoassay Procedure
4.6. Data Processing and Normalization
4.7. Statistical Analyses
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pahwa, R.; Goyal, A.; Jialal, I. Chronic Inflammation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Michels da Silva, D.; Langer, H.; Graf, T. Inflammatory and Molecular Pathways in Heart Failure-Ischemia, HFpEF and Transthyretin Cardiac Amyloidosis. Int. J. Mol. Sci. 2019, 20, 2322. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and Tumor Progression: Signaling Pathways and Targeted Intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Etxebarria, A.; Díez-Martín, E.; Astigarraga, E.; Barreda-Gómez, G. Role of the Immune System in Renal Transplantation, Types of Response, Technical Approaches and Current Challenges. Immuno 2022, 2, 548–570. [Google Scholar] [CrossRef]
- Domínguez-Fernández, C.; Egiguren-Ortiz, J.; Razquin, J.; Gómez-Galán, M.; De Las Heras-García, L.; Paredes-Rodríguez, E.; Astigarraga, E.; Miguélez, C.; Barreda-Gómez, G. Review of Technological Challenges in Personalised Medicine and Early Diagnosis of Neurodegenerative Disorders. Int. J. Mol. Sci. 2023, 24, 3321. [Google Scholar] [CrossRef]
- Hannoodee, S.; Nasuruddin, D.N. Acute Inflammatory Response. In StatPearls; StatPearls Publishing: Treasure, FL, USA, 2023. [Google Scholar]
- Pontrelli, P.; Grandaliano, G.; Van Kooten, C. Editorial: Kidney Transplantation and Innate Immunity. Front. Immunol. 2020, 11, 603982. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Varela, M.L.; Mogildea, M.; Moreno, I.; Lopes, A. Acute Inflammation and Metabolism. Inflammation 2018, 41, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, L.B. The Immune System. Essays Biochem. 2016, 60, 275–301. [Google Scholar] [CrossRef]
- Ferrero-Miliani, L.; Nielsen, O.H.; Andersen, P.S.; Girardin, S.E. Chronic Inflammation: Importance of NOD2 and NALP3 in Interleukin-1beta Generation. Clin. Exp. Immunol. 2007, 147, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Siu, J.H.Y.; Surendrakumar, V.; Richards, J.A.; Pettigrew, G.J. T Cell Allorecognition Pathways in Solid Organ Transplantation. Front. Immunol. 2018, 9, 2548. [Google Scholar] [CrossRef]
- Strzelec, M.; Detka, J.; Mieszczak, P.; Sobocińska, M.K.; Majka, M. Immunomodulation—A General Review of the Current State-of-the-Art and New Therapeutic Strategies for Targeting the Immune System. Front. Immunol. 2023, 14, 1127704. [Google Scholar] [CrossRef]
- Tsai, D.-H.; Riediker, M.; Berchet, A.; Paccaud, F.; Waeber, G.; Vollenweider, P.; Bochud, M. Effects of Short- and Long-Term Exposures to Particulate Matter on Inflammatory Marker Levels in the General Population. Environ. Sci. Pollut. Res. Int. 2019, 26, 19697–19704. [Google Scholar] [CrossRef]
- Siebert, S.; Tsoukas, A.; Robertson, J.; McInnes, I. Cytokines as Therapeutic Targets in Rheumatoid Arthritis and Other Inflammatory Diseases. Pharmacol. Rev. 2015, 67, 280–309. [Google Scholar] [CrossRef]
- Richard-Eaglin, A.; Smallheer, B.A. Immunosuppressive/Autoimmune Disorders. Nurs. Clin. N. Am. 2018, 53, 319–334. [Google Scholar] [CrossRef]
- Ilchmann-Diounou, H.; Menard, S. Psychological Stress, Intestinal Barrier Dysfunctions, and Autoimmune Disorders: An Overview. Front. Immunol. 2020, 11, 1823. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Zhang, M.; Jiang, D.; Su, Q.; Shi, J. The Role of Inflammation in Autoimmune Disease: A Therapeutic Target. Front. Immunol. 2023, 14, 1267091. [Google Scholar] [CrossRef] [PubMed]
- Prideaux, L.; De Cruz, P.; Ng, S.C.; Kamm, M.A. Serological Antibodies in Inflammatory Bowel Disease: A Systematic Review. Inflamm. Bowel Dis. 2012, 18, 1340–1355. [Google Scholar] [CrossRef] [PubMed]
- Charles, A.; Janeway, J.; Travers, P.; Walport, M.; Shlomchik, M.J. Autoimmune Responses Are Directed against Self Antigens. In Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Xu, X.; Ng, S.M.; Hassouna, E.; Warrington, A.; Oh, S.-H.; Rodriguez, M. Human-Derived Natural Antibodies: Biomarkers and Potential Therapeutics. Future Neurol. 2015, 10, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.X.; Miller, J.S.; Zheng, S.G. An Updated Advance of Autoantibodies in Autoimmune Diseases. Autoimmun. Rev. 2021, 20, 102743. [Google Scholar] [CrossRef] [PubMed]
- Cusick, M.F.; Libbey, J.E.; Fujinami, R.S. Molecular Mimicry as a Mechanism of Autoimmune Disease. Clin. Rev. Allergy Immunol. 2012, 42, 102–111. [Google Scholar] [CrossRef]
- Gao, Z.; Feng, Y.; Xu, J.; Liang, J. T-Cell Exhaustion in Immune-Mediated Inflammatory Diseases: New Implications for Immunotherapy. Front. Immunol. 2022, 13, 977394. [Google Scholar] [CrossRef]
- Calabrò, A.; Accardi, G.; Aiello, A.; Caruso, C.; Candore, G. Sex and Gender Affect Immune Aging. Front. Aging 2023, 4, 1272118. [Google Scholar] [CrossRef] [PubMed]
- Ramírez Sepúlveda, J.I.; Bolin, K.; Mofors, J.; Leonard, D.; Svenungsson, E.; Jönsen, A.; Bengtsson, C.; DISSECT consortium; Nordmark, G.; Rantapää Dahlqvist, S.; et al. Sex Differences in Clinical Presentation of Systemic Lupus Erythematosus. Biol. Sex. Differ. 2019, 10, 60. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex Differences in Immune Responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Bose, M.; Jefferies, C. Sex Bias in Systemic Lupus Erythematosus: A Molecular Insight. Immunometabolism 2022, 4, e00004. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.B.; Aziz, M.; Malepati, D.; Lee-Smith, W.; Clark, J.; Brearley, A.; Nachman, P.H. Evaluating Sex Differences in the Characteristics and Outcomes of Lupus Nephritis: A Systematic Review and Meta-Analysis. Glomerular Dis. 2024, 4, 19–32. [Google Scholar] [CrossRef]
- Mihailovic, J.; Ribi, C.; Chizzolini, C.; Trendelenburg, M.; Von Kempis, J.; Dahdal, S.; Huynh-Do, U. Worse Cardiovascular and Renal Outcome in Male SLE Patients. Sci. Rep. 2023, 13, 18628. [Google Scholar] [CrossRef]
- Amador-Patarroyo, M.J.; Rodriguez-Rodriguez, A.; Montoya-Ortiz, G. How Does Age at Onset Influence the Outcome of Autoimmune Diseases? Autoimmune Dis. 2012, 2012, 251730. [Google Scholar] [CrossRef]
- Castro, C.; Gourley, M. Diagnostic Testing and Interpretation of Tests for Autoimmunity. J. Allergy Clin. Immunol. 2010, 125, S238–S247. [Google Scholar] [CrossRef]
- Ho, J.; Wiebe, C.; Gibson, I.W.; Rush, D.N.; Nickerson, P.W. Immune Monitoring of Kidney Allografts. Am. J. Kidney Dis. 2012, 60, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Joos, T.O.; Schrenk, M.; Höpfl, P.; Kröger, K.; Chowdhury, U.; Stoll, D.; Schörner, D.; Dürr, M.; Herick, K.; Rupp, S.; et al. A Microarray Enzyme-Linked Immunosorbent Assay for Autoimmune Diagnostics. Electrophoresis 2000, 21, 2641–2650. [Google Scholar] [CrossRef] [PubMed]
- Rienda, B.; Elexpe, A.; Tolentino-Cortez, T.; Gulak, M.; Bruzos-Cidón, C.; Torrecilla, M.; Astigarraga, E.; Barreda-Gómez, G. Analysis of Acetylcholinesterase Activity in Cell Membrane Microarrays of Brain Areas as a Screening Tool to Identify Tissue Specific Inhibitors. Analytica 2021, 2, 25–36. [Google Scholar] [CrossRef]
- Fernández, R.; Garate, J.; Tolentino-Cortez, T.; Herraiz, A.; Lombardero, L.; Ducrocq, F.; Rodríguez-Puertas, R.; Trifilieff, P.; Astigarraga, E.; Barreda-Gómez, G.; et al. Microarray and Mass Spectrometry-Based Methodology for Lipid Profiling of Tissues and Cell Cultures. Anal. Chem. 2019, 91, 15967–15973. [Google Scholar] [CrossRef]
- de la Fuente, M.; Rodríguez-Agirretxe, I.; Vecino, E.; Astigarraga, E.; Acera, A.; Barreda-Gómez, G. Elevation of Tear MMP-9 Concentration as a Biomarker of Inflammation in Ocular Pathology by Antibody Microarray Immunodetection Assays. Int. J. Mol. Sci. 2022, 23, 5639. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, T. An Introduction to ROC Analysis. Pattern Recognit. Lett. 2006, 27, 861–874. [Google Scholar] [CrossRef]
- Hajian-Tilaki, K. Receiver Operating Characteristic (ROC) Curve Analysis for Medical Diagnostic Test Evaluation. Casp. J. Intern. Med. 2013, 4, 627–635. [Google Scholar]
- Pisetsky, D.S. Pathogenesis of Autoimmune Disease. Nat. Rev. Nephrol. 2023, 19, 509–524. [Google Scholar] [CrossRef]
- Lewis, S.M.; Williams, A.; Eisenbarth, S.C. Structure-Function of the Immune System in the Spleen. Sci. Immunol. 2019, 4, eaau6085. [Google Scholar] [CrossRef]
- Rahman, A.; Manson, J.J.; Isenberg, D.A. Autoantibodies and Lupus Nephritis. In Lupus Nephritis; Lewis, E.J., Schwartz, M.M., Korbet, S.M., Chan, D.T.M., Eds.; Oxford University Press: Oxford, UK, 2010; pp. 35–58. ISBN 978-0-19-956805-5. [Google Scholar]
- Ungaro, R.; Colombel, J.-F.; Lissoos, T.; Peyrin-Biroulet, L. A Treat-to-Target Update in Ulcerative Colitis: A Systematic Review. Am. J. Gastroenterol. 2019, 114, 874–883. [Google Scholar] [CrossRef]
- Mitsuyama, K.; Niwa, M.; Takedatsu, H.; Yamasaki, H.; Kuwaki, K.; Yoshioka, S.; Yamauchi, R.; Fukunaga, S.; Torimura, T. Antibody Markers in the Diagnosis of Inflammatory Bowel Disease. World J. Gastroenterol. 2016, 22, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.-J.; Saxena, R.; Zhao, M.; Parodis, I.; Salmon, J.E.; Mohan, C. Lupus Nephritis. Nat. Rev. Dis. Primers 2020, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Schaub, J.A.; Kretzler, M. Chapter 33—Systems Biology in Diagnosis and Treatment of Kidney Disease. In Regenerative Nephrology, 2nd ed.; Goligorsky, M.S., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 465–479. ISBN 978-0-12-823318-4. [Google Scholar]
- Borchers, A.T.; Leibushor, N.; Naguwa, S.M.; Cheema, G.S.; Shoenfeld, Y.; Gershwin, M.E. Lupus Nephritis: A Critical Review. Autoimmun. Rev. 2012, 12, 174–194. [Google Scholar] [CrossRef] [PubMed]
- Mejia-Vilet, J.M.; Rovin, B.H. 59—Epidemiology and Management of Lupus Nephritis. In Dubois’ Lupus Erythematosus and Related Syndromes, 9th ed.; Wallace, D.J., Hahn, B.H., Eds.; Elsevier: London, UK, 2019; pp. 727–744. ISBN 978-0-323-47927-1. [Google Scholar]
- Musa, R.; Brent, L.H.; Qurie, A. Lupus Nephritis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Stinton, L.; Barr, S.; Tibbles, L.A.; Yilmaz, S.; Sar, A.; Benediktsson, H.; Fritzler, M. Autoantibodies in Lupus Nephritis Patients Requiring Renal Transplantation. Lupus 2007, 16, 394–400. [Google Scholar] [CrossRef]
- Elsayed, S.A.; Mohafez, O.M.M. Autoantibodies Spectrum in Lupus Nephritis in a Cohort of Egyptian Patients: Relation to Disease Activity and Prognostic Value. Egypt. Rheumatol. Rehabil. 2020, 47, 39. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Y.-Y.; Zhao, M.-H.; Zhang, Y.-K.; Wang, H.-Y. Autoantibodies against Glomerular Mesangial Cells and Their Target Antigens in Lupus Nephritis. Ren. Fail. 2005, 27, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.J.; Schwartz, M.M. Pathology of Lupus Nephritis. Lupus 2005, 14, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Riquelme, M.E. The heterogeneity of systemic lupus erythematosus: Looking for a molecular answer. Rev. Colomb. Reumatol. 2021, 28, 31–38. [Google Scholar] [CrossRef]
- Carsons, S.E.; Patel, B.C. Sjogren Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Aiyegbusi, O.; McGregor, L.; McGeoch, L.; Kipgen, D.; Geddes, C.C.; Stevens, K.I. Renal Disease in Primary Sjögren’s Syndrome. Rheumatol. Ther. 2021, 8, 63–80. [Google Scholar] [CrossRef]
- Icardi, A.; Araghi, P.; Ciabattoni, M.; Romano, U.; Lazzarini, P.; Bianchi, G. Kidney involvement in rheumatoid arthritis. Reumatismo 2003, 55, 76–85. [Google Scholar] [CrossRef]
- Santambrogio, L.; Marrack, P. The Broad Spectrum of Pathogenic Autoreactivity. Nat. Rev. Immunol. 2023, 23, 69–70. [Google Scholar] [CrossRef]
- Trier, N.H.; Houen, G. Antibody Cross-Reactivity in Auto-Immune Diseases. Int. J. Mol. Sci. 2023, 24, 13609. [Google Scholar] [CrossRef]
- Tipton, C.M.; Fucile, C.F.; Darce, J.; Chida, A.; Ichikawa, T.; Gregoretti, I.; Schieferl, S.; Hom, J.; Jenks, S.; Feldman, R.J.; et al. Diversity, Cellular Origin and Autoreactivity of Antibody-Secreting Cell Expansions in Acute Systemic Lupus Erythematosus. Nat. Immunol. 2015, 16, 755–765. [Google Scholar] [CrossRef]
- Ehrenstein, M.R.; Katz, D.R.; Griffiths, M.H.; Papadaki, L.; Winkler, T.H.; Kalden, J.R.; Isenberg, D.A. Human IgG Anti-DNA Antibodies Deposit in Kidneys and Induce Proteinuria in SCID Mice. Kidney Int. 1995, 48, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Leadbetter, E.A.; Rifkin, I.R.; Hohlbaum, A.M.; Beaudette, B.C.; Shlomchik, M.J.; Marshak-Rothstein, A. Chromatin-IgG Complexes Activate B Cells by Dual Engagement of IgM and Toll-like Receptors. Nature 2002, 416, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Hakkim, A.; Fürnrohr, B.G.; Amann, K.; Laube, B.; Abed, U.A.; Brinkmann, V.; Herrmann, M.; Voll, R.E.; Zychlinsky, A. Impairment of Neutrophil Extracellular Trap Degradation Is Associated with Lupus Nephritis. Proc. Natl. Acad. Sci. USA 2010, 107, 9813–9818. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Boone, M.; Meuris, L.; Lemmens, I.; Van Roy, N.; Soete, A.; Reumers, J.; Moisse, M.; Plaisance, S.; Drmanac, R.; et al. Genome Dynamics of the Human Embryonic Kidney 293 Lineage in Response to Cell Biology Manipulations. Nat. Commun. 2014, 5, 4767. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.-H.; Baek, I.-C.; Hong, C.-H.; Park, K.H.; Lee, H.; Oh, E.-J.; Kim, T.-G. Establishment of HLA Class I and MICA/B Null HEK-293T Panel Expressing Single MICA Alleles to Detect Anti-MICA Antibodies. Sci. Rep. 2021, 11, 15716. [Google Scholar] [CrossRef] [PubMed]
- Shaw, G.; Morse, S.; Ararat, M.; Graham, F.L. Preferential Transformation of Human Neuronal Cells by Human Adenoviruses and the Origin of HEK 293 Cells. FASEB J. 2002, 16, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Oka, Y.; Nakajima, K.; Nagao, K.; Miura, K.; Ishii, N.; Kobayashi, H. 293FT Cells Transduced with Four Transcription Factors (OCT4, SOX2, NANOG, and LIN28) Generate Aberrant ES-like Cells. J. Stem Cells Regen. Med. 2010, 6, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.W.; Cho, E.-Y.; Park, M.S.; Lee, K.-B.; Kim, H.; Kim, U. Polycystin-1 Expression in Fetal, Adult and Autosomal Dominant Polycystic Kidney. J. Korean Med. Sci. 2006, 21, 425–429. [Google Scholar] [CrossRef]
- Larrea, A.; Elexpe, A.; Díez-Martín, E.; Torrecilla, M.; Astigarraga, E.; Barreda-Gómez, G. Neuroinflammation in the Evolution of Motor Function in Stroke and Trauma Patients: Treatment and Potential Biomarkers. Curr. Issues Mol. Biol. 2023, 45, 8552–8585. [Google Scholar] [CrossRef]
- Mishra, M.N.; Baliga, K.V. Significance of Panel Reactive Antibodies in Patients Requiring Kidney Transplantation. Saudi J. Kidney Dis. Transpl. 2013, 24, 495–499. [Google Scholar] [CrossRef]
- Friedewald, J.J.; Kurian, S.M.; Heilman, R.L.; Whisenant, T.C.; Poggio, E.D.; Marsh, C.; Baliga, P.; Odim, J.; Brown, M.M.; Ikle, D.N.; et al. Development and Clinical Validity of a Novel Blood-Based Molecular Biomarker for Subclinical Acute Rejection Following Kidney Transplant. Am. J. Transplant. 2019, 19, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Naesens, M.; Khatri, P.; Li, L.; Sigdel, T.K.; Vitalone, M.J.; Chen, R.; Butte, A.J.; Salvatierra, O.; Sarwal, M.M. Progressive Histological Damage in Renal Allografts Is Associated with Expression of Innate and Adaptive Immunity Genes. Kidney Int. 2011, 80, 1364–1376. [Google Scholar] [CrossRef] [PubMed]
- Gibney, E.M.; Cagle, L.R.; Freed, B.; Warnell, S.E.; Chan, L.; Wiseman, A.C. Detection of Donor-Specific Antibodies Using HLA-Coated Microspheres: Another Tool for Kidney Transplant Risk Stratification. Nephrol. Dial. Transplant. 2006, 21, 2625–2629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Reed, E.F. The Importance of Non-HLA Antibodies in Transplantation. Nat. Rev. Nephrol. 2016, 12, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Josephson, M.A. Monitoring and Managing Graft Health in the Kidney Transplant Recipient. Clin. J. Am. Soc. Nephrol. 2011, 6, 1774–1780. [Google Scholar] [CrossRef] [PubMed]
- Han, H.S.; Lubetzky, M.L. Immune Monitoring of Allograft Status in Kidney Transplant Recipients. Front. Nephrol. 2023, 3, 1293907. [Google Scholar] [CrossRef]
- Guerra, G.; Srinivas, T.R.; Meier-Kriesche, H.-U. Calcineurin Inhibitor-Free Immunosuppression in Kidney Transplantation. Transplant Int. 2007, 20, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, S.; Putalun, W.; Vimolmangkang, S.; Phoolcharoen, W.; Shoyama, Y.; Tanaka, H.; Morimoto, S. Enzyme-Linked Immunosorbent Assay for the Quantitative/Qualitative Analysis of Plant Secondary Metabolites. J. Nat. Med. 2018, 72, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Elexpe, A.; Nieto, N.; Fernández-Cuétara, C.; Domínguez-Fernández, C.; Morera-Herreras, T.; Torrecilla, M.; Miguélez, C.; Laso, A.; Ochoa, E.; Bailen, M.; et al. Study of Tissue-Specific Reactive Oxygen Species Formation by Cell Membrane Microarrays for the Characterization of Bioactive Compounds. Membranes 2021, 11, 943. [Google Scholar] [CrossRef]
- Sánchez-Magraner, L.; de la Fuente, M.; Evans, C.; Miles, J.; Elexpe, A.; Rodriguez-Astigarraga, M.; Astigarraga, E.; Barreda-Gómez, G. Quantification of PD-1/PD-L1 Interaction between Membranes from PBMCs and Melanoma Samples Using Cell Membrane Microarray and Time-Resolved Förster Resonance Energy Transfer. Analytica 2021, 2, 156–170. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Puertas, R.R.; Gómez, G.B.; González, J.A.F.; Arribas, E.A.; García, A.A.; Rodríguez, A.M.; Plaza, D.G. Method for the Surface Treatment of Solid Substrates. EP 2 048 534 A1, 15 April 2009. [Google Scholar]
- Hebert-Chatelain, E.; Desprez, T.; Serrat, R.; Bellocchio, L.; Soria-Gomez, E.; Busquets-Garcia, A.; Pagano Zottola, A.C.; Delamarre, A.; Cannich, A.; Vincent, P.; et al. A Cannabinoid Link between Mitochondria and Memory. Nature 2016, 539, 555–559. [Google Scholar] [CrossRef]
- Manuel, I.; Barreda-Gómez, G.; González de San Román, E.; Veloso, A.; Fernández, J.A.; Giralt, M.T.; Rodríguez-Puertas, R. Neurotransmitter Receptor Localization: From Autoradiography to Imaging Mass Spectrometry. ACS Chem. Neurosci. 2015, 6, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Demšar, J.; Curk, T.; Erjavec, A.; Gorup, Č.; Hočevar, T.; Milutinovič, M.; Možina, M.; Polajnar, M.; Toplak, M.; Starič, A.; et al. Orange: Data Mining Toolbox in Python. J. Mach. Learn. Res. 2013, 14, 2349–2353. [Google Scholar]


| Condition | Sex | n | Range Age |
|---|---|---|---|
| Lupus Nephritis | Female | 3 | 32–45 |
| Systematic Lupus Erythematosus | Female | 2 | 51–55 |
| Sjögren Syndrome | Female | 2 | 54–55 |
| Chronic Kidney Disease | Female | 2 | 54 |
| Rheumatoid Arthritis | Male | 1 | 72 |
| Ulcerative Colitis | Male | 1 | 60 |
| Kidney Transplant | Female | 3 | 54–64 |
| Male | 7 | 25–70 | |
| Control | Female | 8 | 20–83 |
| Male | 10 | 19–67 |
| Anatomical Site | Sex | n | Range Age |
|---|---|---|---|
| Liver | Male | 1 | 46 |
| Spleen | Male | 1 | 48 |
| Kidney | Male | 8 | 38–64 |
| Female | 6 | 46–70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez-Suarez, L.; Diez-Martin, E.; Egiguren-Ortiz, J.; Fernandez, R.; Etxebarria, A.; Astigarraga, E.; Miguelez, C.; Ramirez-Garcia, A.; Barreda-Gómez, G. Serological Antibodies against Kidney, Liver, and Spleen Membrane Antigens as Potential Biomarkers in Patients with Immune Disorders. Int. J. Mol. Sci. 2024, 25, 2025. https://doi.org/10.3390/ijms25042025
Hernandez-Suarez L, Diez-Martin E, Egiguren-Ortiz J, Fernandez R, Etxebarria A, Astigarraga E, Miguelez C, Ramirez-Garcia A, Barreda-Gómez G. Serological Antibodies against Kidney, Liver, and Spleen Membrane Antigens as Potential Biomarkers in Patients with Immune Disorders. International Journal of Molecular Sciences. 2024; 25(4):2025. https://doi.org/10.3390/ijms25042025
Chicago/Turabian StyleHernandez-Suarez, Leidi, Eguzkiñe Diez-Martin, June Egiguren-Ortiz, Roberto Fernandez, Aitor Etxebarria, Egoitz Astigarraga, Cristina Miguelez, Andoni Ramirez-Garcia, and Gabriel Barreda-Gómez. 2024. "Serological Antibodies against Kidney, Liver, and Spleen Membrane Antigens as Potential Biomarkers in Patients with Immune Disorders" International Journal of Molecular Sciences 25, no. 4: 2025. https://doi.org/10.3390/ijms25042025
APA StyleHernandez-Suarez, L., Diez-Martin, E., Egiguren-Ortiz, J., Fernandez, R., Etxebarria, A., Astigarraga, E., Miguelez, C., Ramirez-Garcia, A., & Barreda-Gómez, G. (2024). Serological Antibodies against Kidney, Liver, and Spleen Membrane Antigens as Potential Biomarkers in Patients with Immune Disorders. International Journal of Molecular Sciences, 25(4), 2025. https://doi.org/10.3390/ijms25042025






