Size Dependence of Gold Nanorods for Efficient and Rapid Photothermal Therapy
Abstract
1. Introduction
2. Results
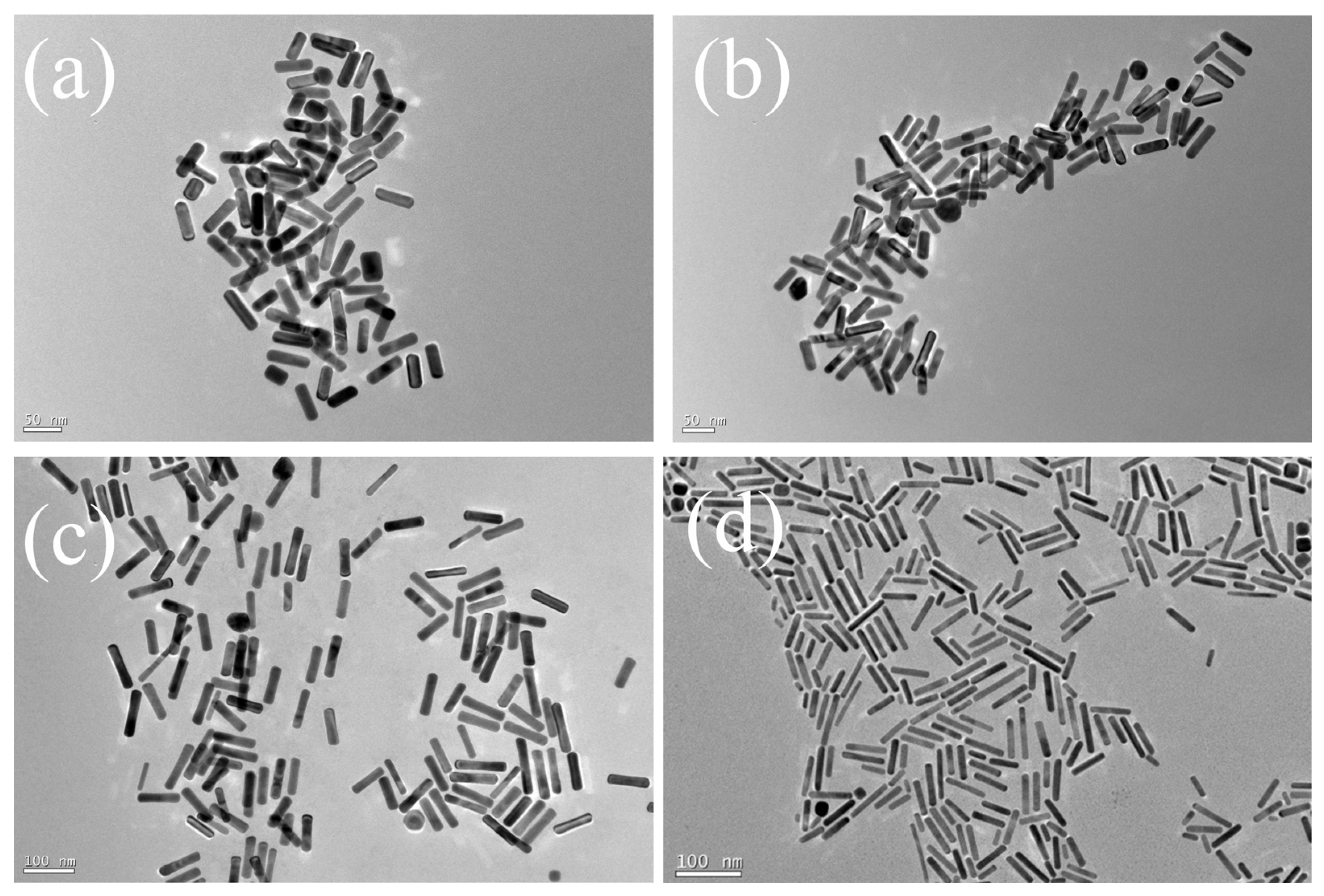
2.1. Characterization of GNRs
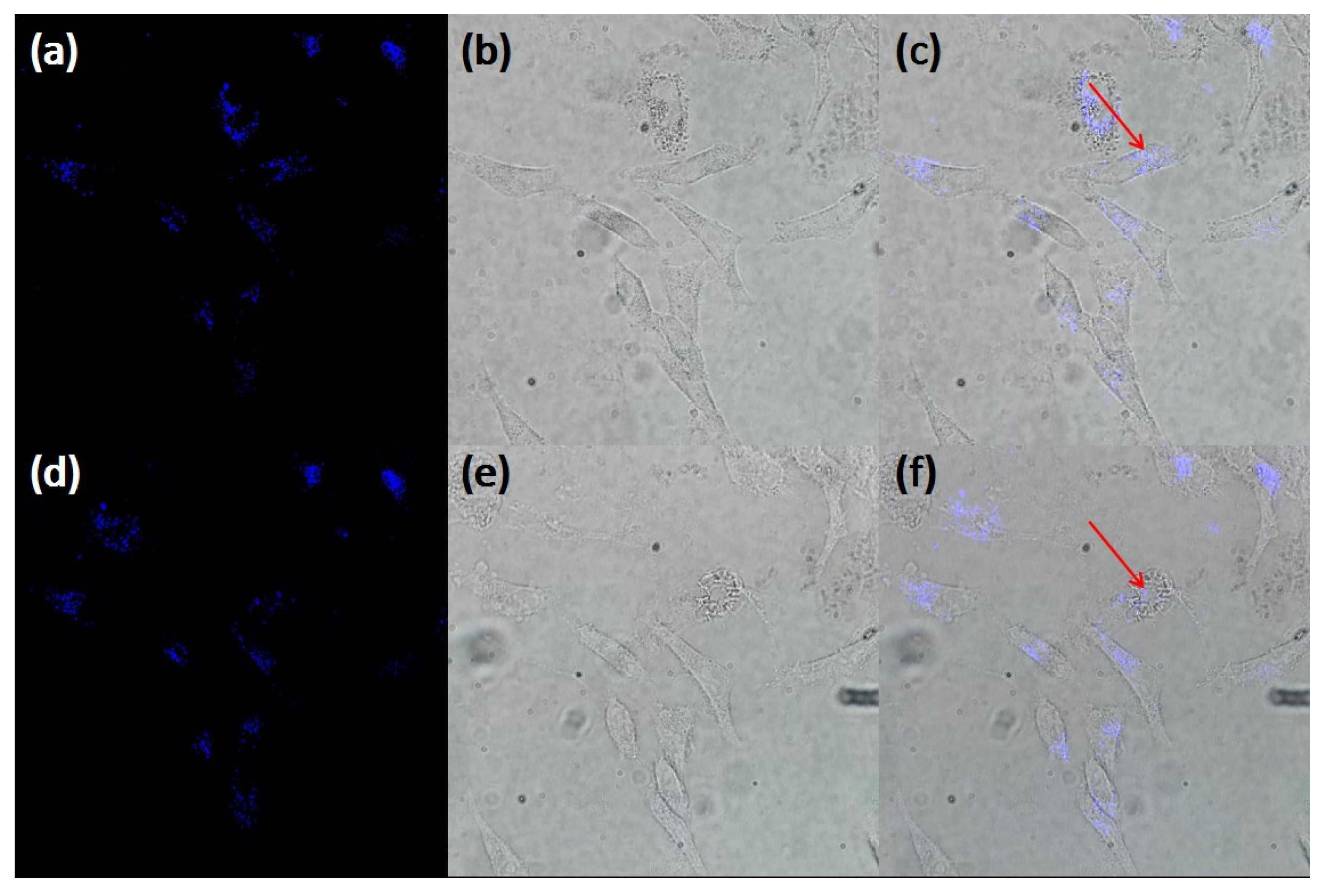
2.2. Cytoxicity Studies and Cellular Uptake
2.3. In Vitro Two-Photon Fluorescence Imaging
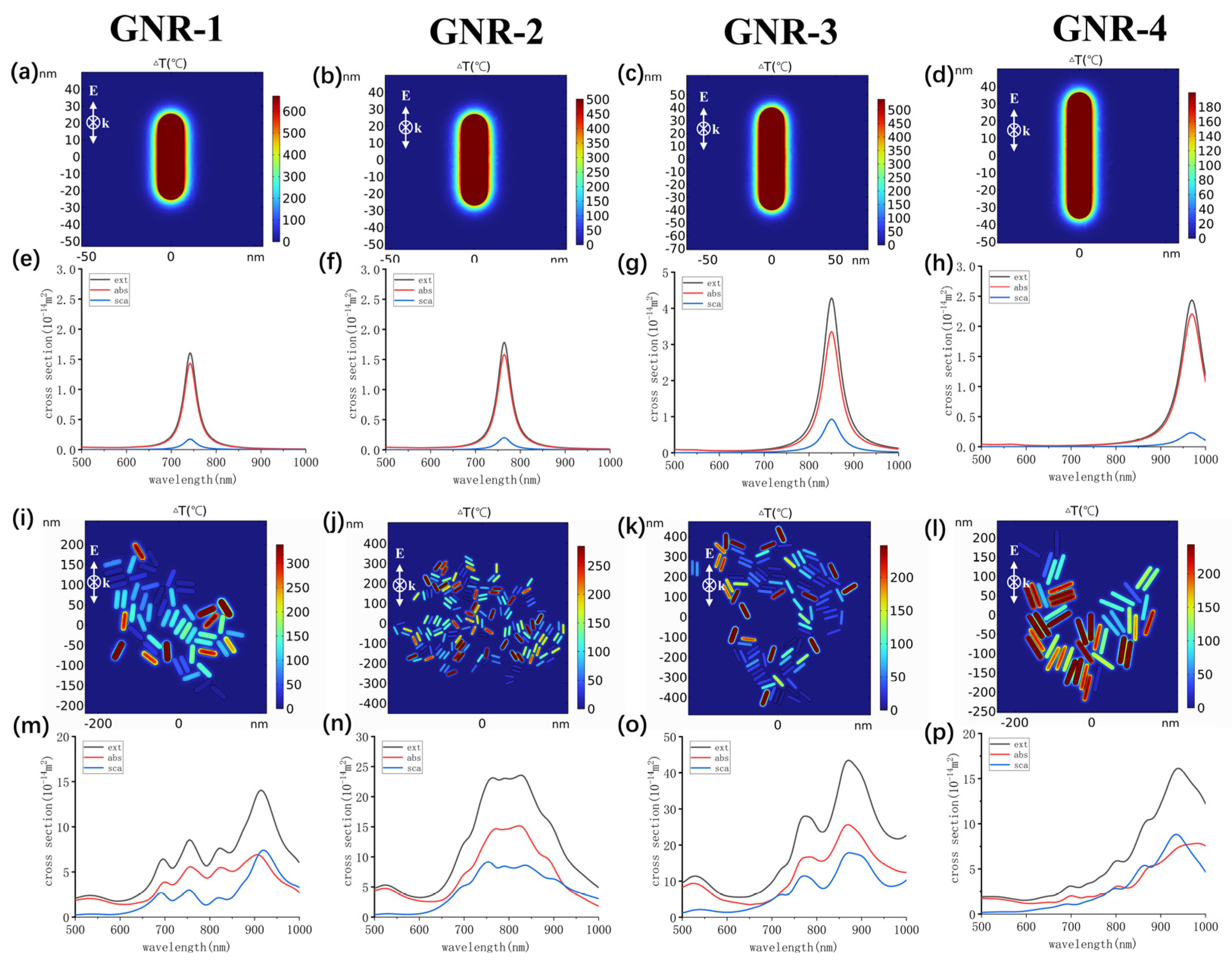
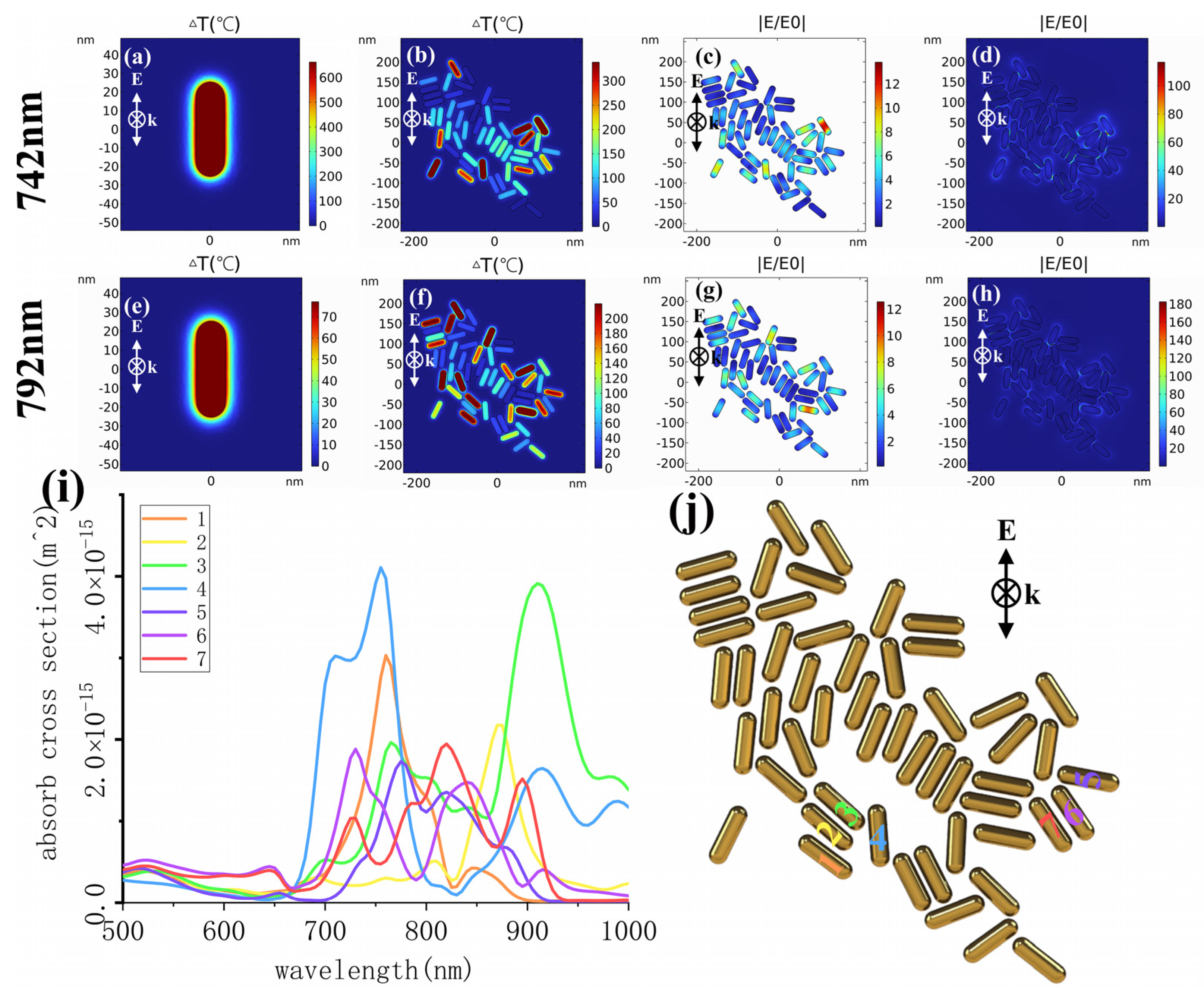
2.4. In Vitro Photothermal Therapy
3. Discussion
4. Materials and Methods
4.1. GNR Synthesis of Different Aspect Ratios
4.2. Cell Culture and Cytotoxicity Experiments
4.3. Photothermal Therapy Experiments
4.4. Cellular Uptake of GNRs
4.5. Simulation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Laser Med. Sci. 2008, 23, 217–228. [Google Scholar] [CrossRef]
- Suarasan, S.; Campu, A.; Vulpoi, A.; Banciu, M.; Astilean, S. Assessing the efficiency of triangular gold nanoparticles as NIR photothermal agents in vitro and melanoma tumor model. Int. J. Mol. Sci. 2022, 23, 13724. [Google Scholar] [CrossRef] [PubMed]
- Arellano-Galindo, L.; Villar-Alvarez, E.; Varela, A.; Figueroa, V.; Fernandez-Vega, J.; Cambón, A.; Prieto, G.; Barbosa, S.; Taboada, P. Hybrid Gold Nanorod-Based Nanoplatform with Chemo and Photothermal Activities for Bimodal Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 13109. [Google Scholar] [CrossRef]
- Shukurov, I.; Mohamed, M.S.; Mizuki, T.; Palaninathan, V.; Ukai, T.; Hanajiri, T.; Maekawa, T. Biological synthesis of bioactive gold nanoparticles from Inonotus obliquus for dual chemo-photothermal effects against human brain cancer cells. Int. J. Mol. Sci. 2022, 23, 2292. [Google Scholar] [CrossRef]
- Gan, R.; Fan, H.; Wei, Z.; Liu, H.; Lan, S.; Dai, Q. Photothermal response of hollow gold nanorods under femtosecond laser irradiation. Nanomaterials 2019, 9, 711. [Google Scholar] [CrossRef]
- Hatef, A.; Fortin-Deschênes, S.; Boulais, E.; Lesage, F.; Meunier, M. Photothermal response of hollow gold nanoshell to laser irradiation: Continuous wave, short and ultrashort pulse. Int. J. Heat Mass Transfer. 2015, 89, 866–871. [Google Scholar] [CrossRef]
- Baffou, G.; Quidant, R. Thermo-plasmonics: Using metallic nanostructures as nano-sources of heat. Laser Photonics Rev. 2013, 7, 171–187. [Google Scholar] [CrossRef]
- Sanchot, A.; Baffou, G.; Marty, R.; Arbouet, A.; Quidant, R.; Girard, C.; Dujardin, E. Plasmonic nanoparticle networks for light and heat concentration. ACS Nano 2012, 6, 3434–3440. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liang, H.; Ma, S.; Wang, D.; Huang, J. Gold nanoparticle based photothermal therapy: Development and application for effective cancer treatment. Sustain. Mater. Technol. 2019, 22, e00109. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, T.; Li, M. Quantitative Analysis of the Shape Effect of Thermoplasmonics in Gold Nanostructures. J. Phys. Chem. Lett. 2023, 14, 3853–3860. [Google Scholar] [CrossRef]
- Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Zhang, H.; Huang, X.Q.; Wan, H.Y.; Li, J.; Fan, X.X.; Luo, K.Q.; Wang, J.H.; Zhu, X.M.; Wang, J.F. Antian-giogenesis-combined photothermal therapy in the second near-infrared window at laser powers below the skin tolerance threshold. Nano-Micro Lett. 2019, 11, 1–20. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, X.; Zhang, S.; Wei, G.; Su, Z. Biomedical and bioactive engineered nanomaterials for targeted tumor photothermal therapy: A review. Mater. Sci. Eng. C 2019, 104, 109891. [Google Scholar] [CrossRef] [PubMed]
- Younis, M.R.; An, R.B.; Yin, Y.C.; Wang, S.; Ye, D.; Xia, X.H. Plasmonic nanohybrid with high photothermal conversion efficiency for simultaneously effective antibacterial/anticancer photothermal therapy. ACS Appl. Bio Mater. 2019, 2, 3942–3953. [Google Scholar] [CrossRef] [PubMed]
- Vigderman, L.; Manna, P.; Zubarev, E.R. Quantitative replacement of cetyl trimethylammonium bromide by cationic thiol ligands on the surface of gold nanorods and their extremely large uptake by cancer cells. Angew. Chem. 2012, 124, 660–665. [Google Scholar] [CrossRef]
- de Melo-Diogo, D.; Pais-Silva, C.; Dias, D.R.; Moreira, A.F.; Correia, I.J. Strategies to improve cancer photothermal therapy mediated by nanomaterials. Adv. Healthc. Mater. 2017, 6, 1700073. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, L.; Wang, J.; Jiang, X.; Li, X.; Hu, Z.; Chen, C. Mesoporous silica-coated gold nanorods as a light-mediated multifunctional theranostic platform for cancer treatment. Adv. Mater. 2012, 24, 1418–1423. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, L.; Zhang, L.; Wu, X.; Nie, G.; Chen, C.; Wang, Y. Metabolic characteristics of 16HBE and A549 cells exposed to different surface modified gold nanorods. Adv. Healthc. Mater. 2016, 5, 2363–2375. [Google Scholar] [CrossRef]
- Huang, X.; Qian, W.; El-Sayed, I.H.; El-Sayed, M.A. The potential use of the enhanced nonlinear properties of gold nanospheres in photothermal cancer therapy. Lasers Surg. Med. 2007, 39, 747–753. [Google Scholar] [CrossRef]
- Wei, X.; Chen, H.; Tham, H.P.; Zhang, N.; Xing, P.; Zhang, G.; Zhao, Y. Combined photodynamic and photothermal therapy using cross-linked polyphosphazene nanospheres decorated with gold nanoparticles. ACS Appl. Nano Mater. 2018, 1, 3663–3672. [Google Scholar] [CrossRef]
- Tsai, M.F.; Chang, S.H.G.; Cheng, F.Y.; Shanmugam, V.; Cheng, Y.S.; Su, C.H.; Yeh, C.S. Au nanorod design as light-absorber in the first and second biological near-infrared windows for in vivo photothermal therapy. ACS Nano 2013, 7, 5330–5342. [Google Scholar] [CrossRef]
- Li, Z.; Huang, H.; Tang, S.; Li, Y.; Yu, X.F.; Wang, H.; Li, P.; Sun, Z.; Zhang, H.; Liu, C.; et al. Small gold nanorods laden macrophages for enhanced tumor coverage in photothermal therapy. Biomaterials 2016, 74, 144–154. [Google Scholar] [CrossRef]
- Deng, X.; Liang, S.; Cai, X.; Huang, S.; Cheng, Z.; Shi, Y.; Pang, M.; Ma, P.; Lin, J. Yolk–shell structured Au nanostar@ metal–organic framework for synergistic chemo-photothermal therapy in the second near-infrared window. Nano Lett. 2019, 19, 6772–6780. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, N.; Elbadri, K.; Correia, A.; Santos, H.A. Polyethylene Glycol-Stabilized Gold Nanostars-Loaded Microneedles for Photothermal Therapy of Melanoma. Adv. Mater. Technol. 2023, 8, 2301159. [Google Scholar] [CrossRef]
- Chen, J.; Wiley, B.; Li, Z.Y.; Campbell, D.; Saeki, H.; Cang, H.; Au, L.; Lee, J.; Li, X.; Xia, Y. Gold nanocages: Engineering their structure for biomedical applications. Adv. Mater. 2005, 17, 2255–2261. [Google Scholar] [CrossRef]
- Xia, Y.; Li, W.; Cobley, C.M.; Chen, J.; Xia, X.; Zhang, Q.; Yang, M.; Cho, E.C.; Brown, P.K. Gold nanocages: From synthesis to theranostic applications. Acc. Chem. Res. 2011, 44, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, S.; Zhang, M.; Ma, Y.; Liu, Y.; Gao, W.; Zhang, J.; Gu, Y. Laser-triggered small interfering RNA releasing gold nanoshells against heat shock protein for sensitized photothermal therapy. Adv. Sci. 2017, 4, 1600327. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Bian, Y.; Liu, Y.; Zhang, X.; Wang, M.; Xing, S.; Li, L.; Gao, D. Combined near infrared photothermal therapy and chemotherapy using gold nanoshells coated liposomes to enhance antitumor effect. Small 2016, 12, 4103–4112. [Google Scholar] [CrossRef]
- Maji, S.K.; Yu, S.; Chung, K.; Sekkarapatti Ramasamy, M.; Lim, J.W.; Wang, J.; Lee, H.; Kim, D.H. Synergistic nanozymetic activity of hybrid gold bipyramid–molybdenum disulfide core@ shell nanostructures for two-photon imaging and anticancer therapy. ACS Appl. Mater. Interfaces 2018, 10, 42068–42076. [Google Scholar] [CrossRef]
- Li, C.; Mei, E.; Chen, C.; Li, Y.; Nugasur, B.; Hou, L.; Ding, X.; Hu, M.; Zhang, Y.; Su, Z.; et al. Gold-nanobipyramid-based nanotheranostics for dual-modality imaging-guided phototherapy. ACS Appl. Mater. Interfaces 2020, 12, 12541–12548. [Google Scholar] [CrossRef]
- Jiang, K.; Smith, D.A.; Pinchuk, A. Size-dependent photothermal conversion efficiencies of plasmonically heated gold nanoparticles. J. Phys. Chem. C 2013, 117, 27073–27080. [Google Scholar] [CrossRef]
- Chen, Y.S.; Hung, Y.C.; Liau, I.; Huang, G.S. Assessment of the in vivo toxicity of gold nanoparticles. Nanoscale Res. Lett. 2009, 4, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Mackey, M.A.; Ali, M.R.K.; Austin, L.A.; Near, R.D.; EI-Sayed, M.A. The most effective gold nanorod size for plasmonic photothermal therapy: Theory and in vitro experiments. J. Phys. Chem. B 2014, 118, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, W.; Wang, T.; Miao, S.; Lan, S.; Wei, Z.; Meng, Z.; Dai, Q.; Fan, H. Highly localized, efficient, and rapid photothermal therapy using gold nanobipyramids for liver cancer cells triggered by femtosecond laser. Sci. Rep. 2023, 13, 3372. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, J.Y.; Brech, A.; Fang, C.; Wang, J.; Helm, P.J.; Peng, Q. The use of femto-second lasers to trigger powerful explosions of gold nanorods to destroy cancer cells. Biomaterials 2013, 34, 6157–6162. [Google Scholar] [CrossRef]
- Tong, L.; Zhao, Y.; Huff, T.B.; Hansen, M.N.; Wei, A.; Cheng, J.X. Gold nanorods mediate tumor cell death by compromising membrane integrity. Adv. Mater. 2007, 19, 3136–3141. [Google Scholar] [CrossRef]
- Zhang, W.; Ji, Y.; Wu, X.; Xu, H. Trafficking of gold nanorods in breast cancer cells: Uptake, lysosome maturation, and elimination. ACS Appl. Mater. Interfaces 2013, 5, 9856–9865. [Google Scholar] [CrossRef]
- Gole, A.; Murphy, C.J. Seed-mediated synthesis of gold nanorods: Role of the size and nature of the seed. Chem. Mater. 2004, 16, 3633–3640. [Google Scholar] [CrossRef]
- Ye, X.; Jin, L.; Caglayan, H.; Chen, J.; Xing, G.; Zheng, C.; Doan-Nguyen, V.; Kang, Y.; Engheta, N.; Kagan, C.R.; et al. Improved size-tunable synthesis of monodisperse gold nanorods through the use of aromatic additives. ACS Nano 2012, 6, 2804–2817. [Google Scholar] [CrossRef]
- Pillai, P.P.; Kowalczyk, B.; Kandere-Grzybowska, K.; Borkowska, M.; Grzybowski, B.A. Engineering gram selectivity of mixed-charge gold nanoparticles by tuning the balance of surface charges. Angew. Chem. Int. Ed. 2016, 55, 8610–8614. [Google Scholar] [CrossRef] [PubMed]
- Leonov, A.P.; Zheng, J.; Clogston, J.D.; Stern, S.T.; Patri, A.K.; Wei, A. Detoxification of gold nanorods by treatment with polystyrenesulfonate. ACS Nano 2008, 2, 2481–2488. [Google Scholar] [CrossRef]
- Rayavarapu, R.G.; Petersen, W.; Hartsuiker, L.; Chin, P.; Janssen, H.; Van Leeuwen, F.W.; Otto, C.; Manohar, S.; Van Leeuwen, T.G. In vitro toxicity studies of polymer-coated gold nanorods. Nanotechnology 2010, 21, 145101. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Na, S. Metal thin film ablation with femtosecond pulsed laser. Opt. Laser Technol. 2007, 39, 1443–1448. [Google Scholar] [CrossRef]
- Boulais, É.; Lachaine, R.; Meunier, M. Plasma mediated off-resonance plasmonic enhanced ultrafast laser-induced nanocavitation. Nano Lett. 2012, 12, 4763–4769. [Google Scholar] [CrossRef] [PubMed]
- Hatef, A.; Darvish, B.; Burke, A.; Dagallier, A.; Meunier, M. Computational characterization of plasma effects in ultrafast laser irradiation of spherical gold nanostructures for photothermal therapy. J. Phys. D Appl. Phys. 2016, 49, 105401. [Google Scholar] [CrossRef]
- Hao, J.; Zhou, L.; Qiu, M. Nearly total absorption of light and heat generation by plasmonic metamaterials. Phys. Rev. B 2011, 83, 165107. [Google Scholar] [CrossRef]
- Baffou, G.; Quidant, R.; Girard, C. Heat generation in plasmonic nanostructures: Influence of morphology. Appl. Phys. Lett. 2009, 94, 153109. [Google Scholar] [CrossRef]
- Baffou, G.; Girard, C.; Quidant, R. Mapping heat origin in plasmonic structures. Phys. Rev. Lett. 2010, 104, 136805. [Google Scholar] [CrossRef] [PubMed]
- Link, S.; El-Sayed, M.A. Shape and size dependence of radiative, non-radiative and photothermal properties of gold nanocrystals. Int. Rev. Phys. Chem. 2000, 19, 409–453. [Google Scholar] [CrossRef]
- Hashimoto, S.; Werner, D.; Uwada, T. Studies on the interaction of pulsed lasers with plasmonic gold nanoparticles toward light manipulation, heat management, and nanofabrication. J. Photochem. Photobiol. C 2012, 13, 28–54. [Google Scholar] [CrossRef]
- Ekici, O.; Harrison, R.K.; Durr, N.J.; Eversole, D.S.; Lee, M.; Ben-Yakar, A. Thermal analysis of gold nanorods heated with femtosecond laser pulses. J. Phys. D Appl. Phys. 2008, 41, 185501. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of cell viability by the MTT assay. Cold Spring Harb. Protoc. 2018, pdb-prot095505. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.B.; Christy, R.W. Optical Constants of the Noble Metals. Phys. Rev. B 1972, 6, 4370–4379. [Google Scholar] [CrossRef]




| Energy | 28 pJ | 35 pJ | 52 pJ | 69 pJ | 104 pJ | 138 pJ | ||
|---|---|---|---|---|---|---|---|---|
| Aspect Ratio | Death Time | |||||||
| GNR-1 | 90 min | 60 min | 30 min | 0 min | ||||
| GNR-2 | 120 min | 90 min | 30 min | 0 min | ||||
| GNR-3 | 90 min | 30 min | 0 min | |||||
| GNR-4 | 120 min | 90 min | 30 min | 0 min | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Yao, Y.; Qin, H.; Xing, X.; Li, Z.; Ouyang, M.; Fan, H. Size Dependence of Gold Nanorods for Efficient and Rapid Photothermal Therapy. Int. J. Mol. Sci. 2024, 25, 2018. https://doi.org/10.3390/ijms25042018
Zhou W, Yao Y, Qin H, Xing X, Li Z, Ouyang M, Fan H. Size Dependence of Gold Nanorods for Efficient and Rapid Photothermal Therapy. International Journal of Molecular Sciences. 2024; 25(4):2018. https://doi.org/10.3390/ijms25042018
Chicago/Turabian StyleZhou, Wei, Yanhua Yao, Hailing Qin, Xiaobo Xing, Zongbao Li, Min Ouyang, and Haihua Fan. 2024. "Size Dependence of Gold Nanorods for Efficient and Rapid Photothermal Therapy" International Journal of Molecular Sciences 25, no. 4: 2018. https://doi.org/10.3390/ijms25042018
APA StyleZhou, W., Yao, Y., Qin, H., Xing, X., Li, Z., Ouyang, M., & Fan, H. (2024). Size Dependence of Gold Nanorods for Efficient and Rapid Photothermal Therapy. International Journal of Molecular Sciences, 25(4), 2018. https://doi.org/10.3390/ijms25042018






