Structural Characterization and Antidepressant-like Effects of Polygonum sibiricum Polysaccharides on Regulating Microglial Polarization in Chronic Unpredictable Mild Stress-Induced Zebrafish
Abstract
1. Introduction
2. Results
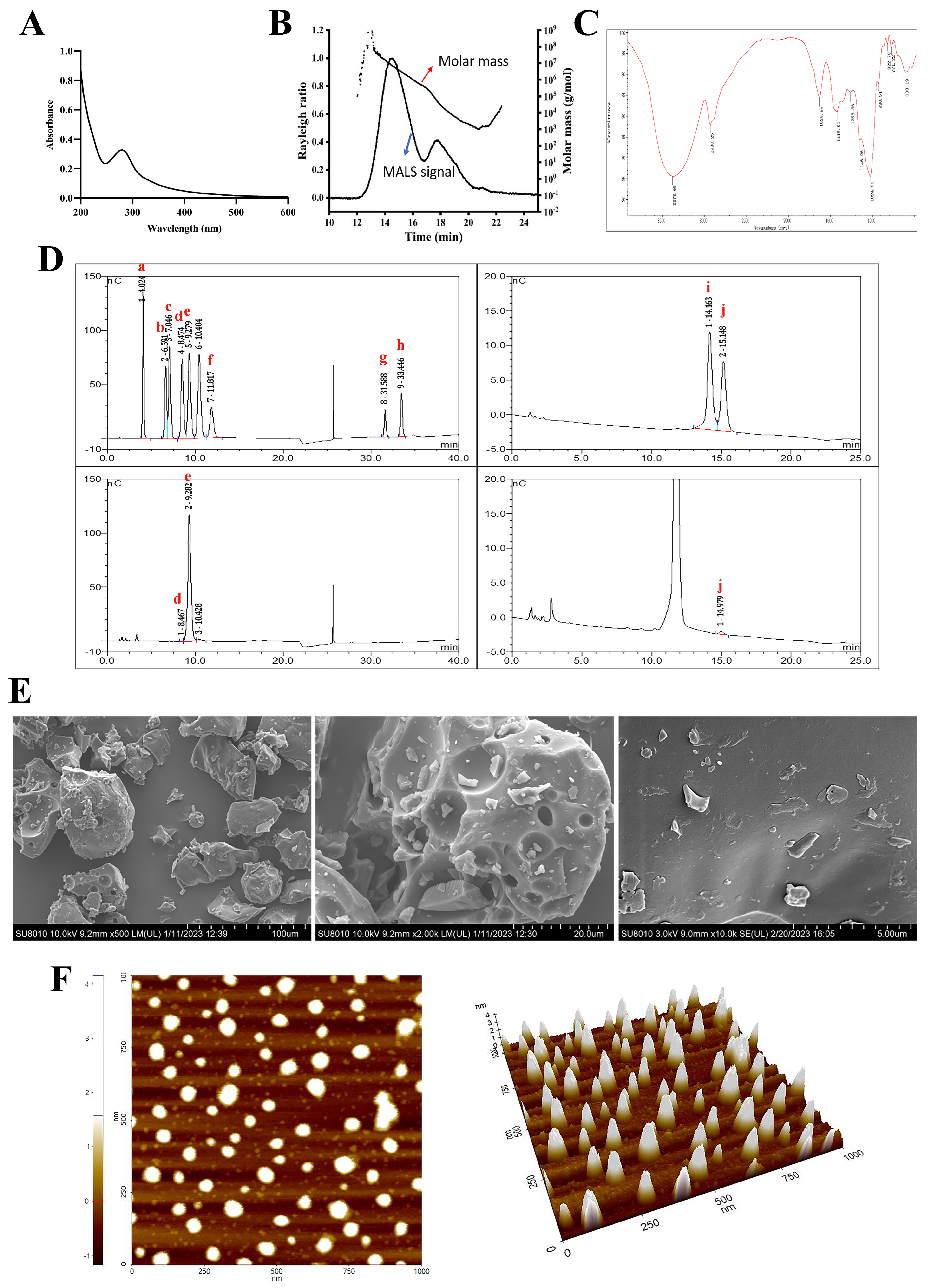
2.1. Structural Characterization of PSP
2.1.1. Chemical, Molecular Weight (Mw), and Monosaccharide Composition of PSP
2.1.2. UV and FT-IR Analysis
2.1.3. Conformational Structure Analysis of PSP
2.2. Effects of PSP on the Body Weight and Depressive-like Behaviors of CUMS-Induced Zebrafish
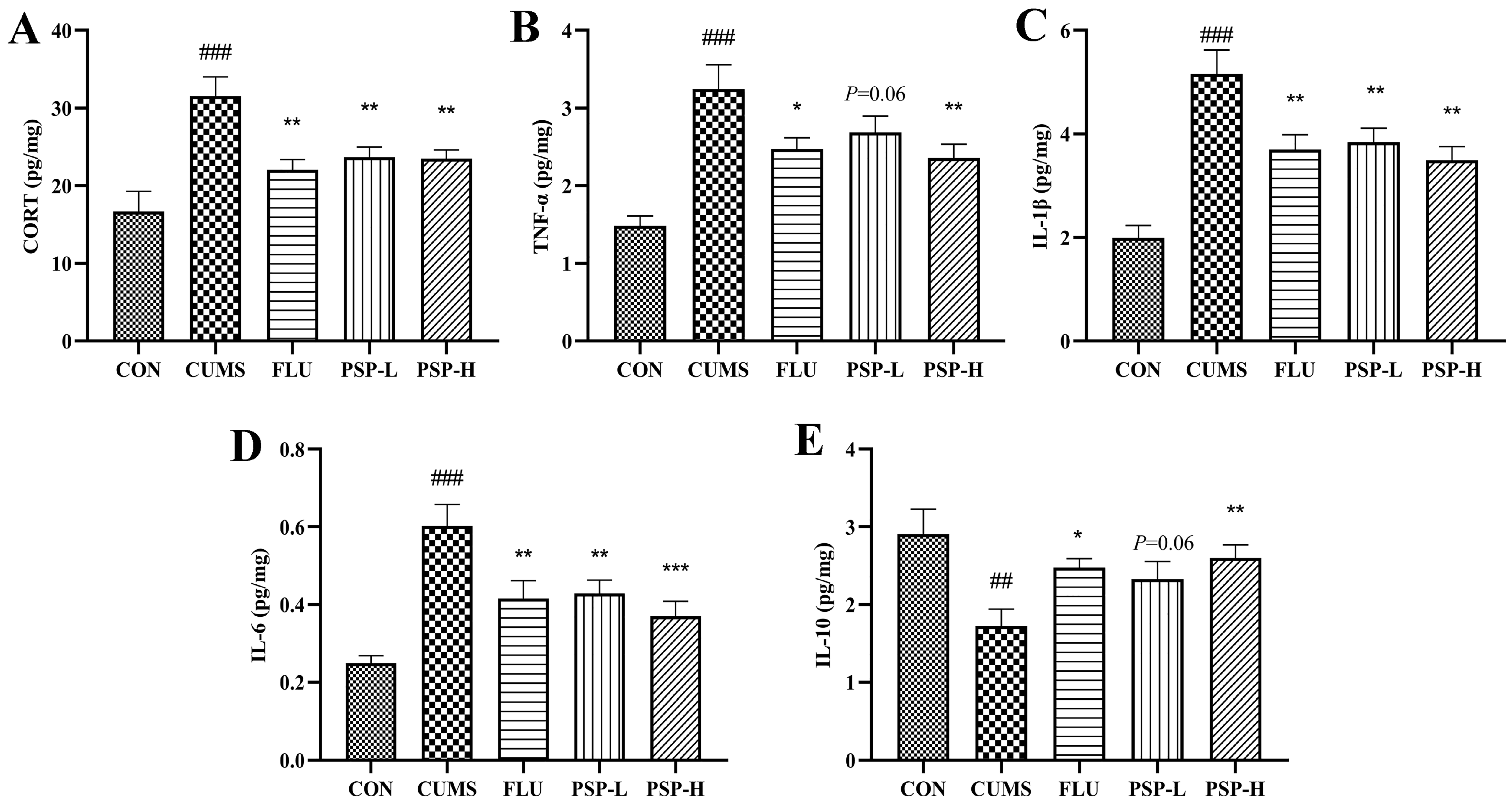
2.3. Effects of PSP on the HPI Function of CUMS-Induced Zebrafish
2.4. Effects of PSP on the Peripheral Inflammation of CUMS-Induced Zebrafish
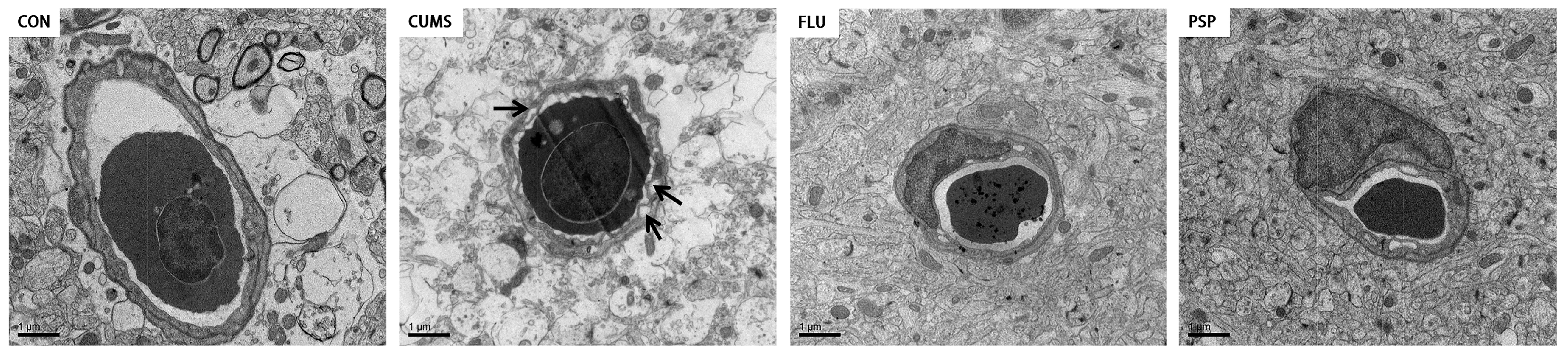
2.5. Effects of PSP on Brain Health in CUMS-Induced Zebrafish
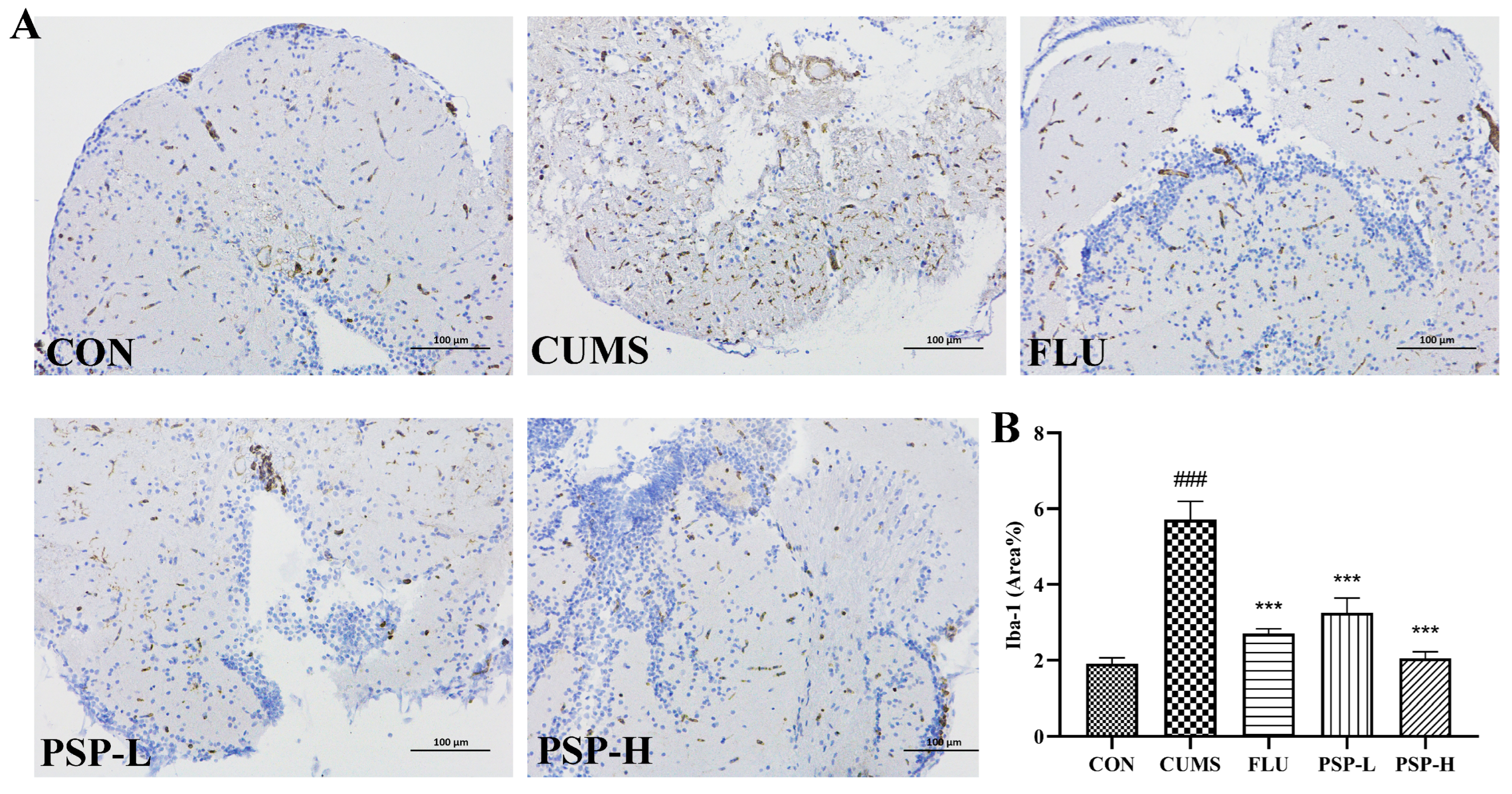
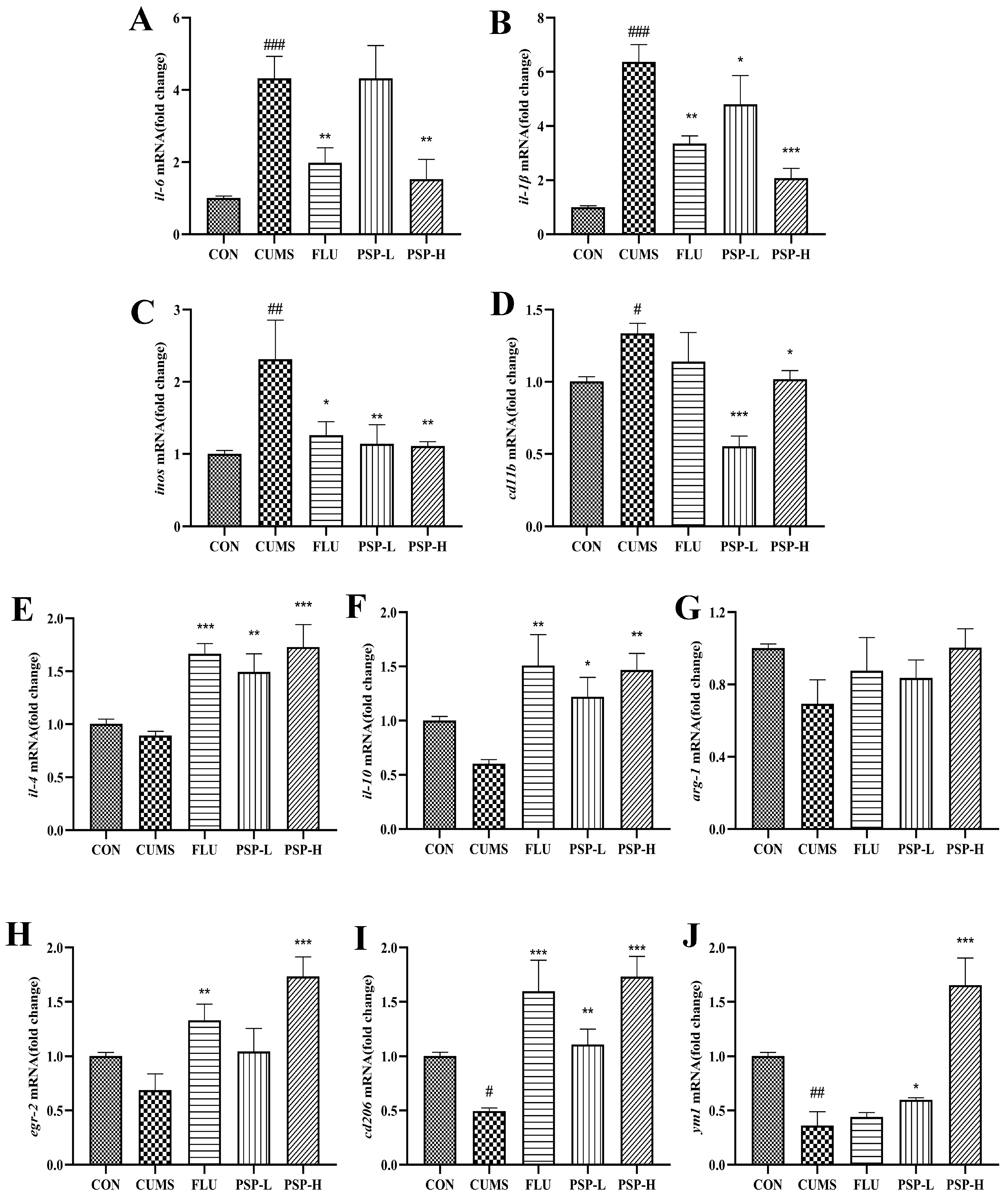
2.6. Effects of PSP on Microglia of CUMS-Induced Zebrafish
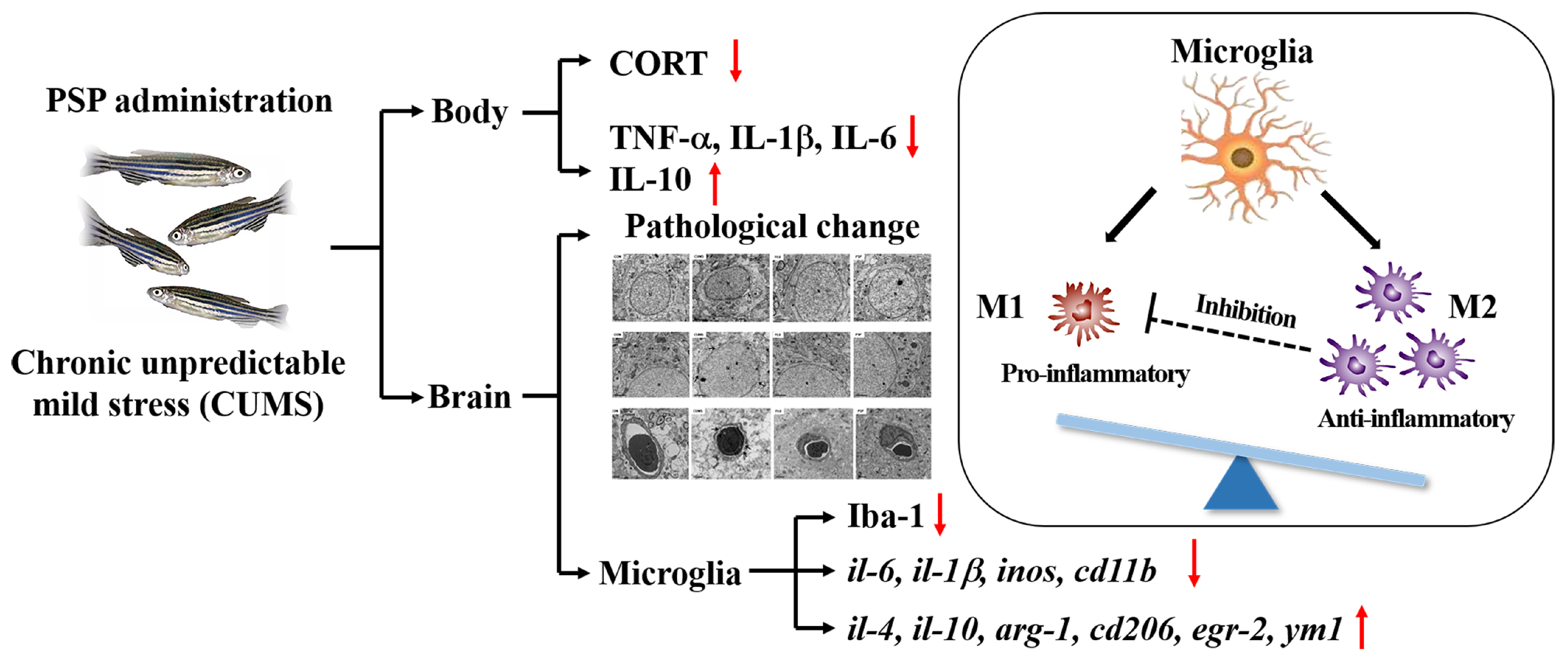
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Structure Characterization of PSP
4.2.1. Determination of Content of Total Carbohydrate, Protein, Phenols, Flavonoids, Crude Fat, Ash and Water
4.2.2. Molecular Weight (Mw) Analysis
4.2.3. Monosaccharide Composition Determination
4.2.4. Ultraviolet (UV) Spectrum Scan and Fourier Transform Infrared Spectroscopy (FT-IR)
4.2.5. Scanning Electron Microscope (SEM) Analysis
4.2.6. Atomic Force Microscope (AFM) Analysis
4.3. Animal Experiment
4.3.1. Animals
4.3.2. Evaluation of PSP after Acute Administration
4.3.3. CUMS Procedure and PSP Administration
4.4. Behavioral Tests
4.4.1. Body Weight
4.4.2. Novel Tank Test (NTT)
4.4.3. Light and Dark Tank Test (LDT)
4.5. Sample Collection
4.6. Nissl Staining
4.7. Transmission Electron Microscope (TEM) Observation
4.8. Immunohistochemistry
4.9. RNA Isolation and Real-Time Quantitative PCR
4.10. Enzyme-Linked Immunosorbent Assay (ELISA)
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Deng, J.W.; Zhou, F.W.; Hou, W.T.; Silver, Z.; Wong, C.Y.; Chang, O.; Huang, E.; Zuo, K.Q. The prevalence of depression, anxiety, and sleep disturbances in COVID-19 patients: A meta-analysis. Ann. N. Y. Acad. Sci. J. 2021, 1486, 90–111. [Google Scholar] [CrossRef]
- Moret, C.; Isaac, M.; Briley, M. Problems associated with long-term treatment with selective serotonin reuptake inhibitors. J. Psychopharmacol. 2009, 23, 967–974. [Google Scholar] [CrossRef]
- Gao, C.; Xu, J.; Liu, Y.; Yang, Y.X. Nutrition policy and healthy China 2030 building. Eur. J. Clin. Nutr. 2021, 75, 238–246. [Google Scholar] [CrossRef]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; Hage, W.E.; Surget, A.; Belzung, C.; et al. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef]
- Cao, L.H.; Zhao, Y.Y.; Bai, M.; Geliebter, D.; Geliebter, J.; Tiwari, R.; He, H.J.; Wang, Z.Z.; Jia, X.Y.; Li, J.; et al. Mechanistic studies of Gypenosides in microglial state transition and its implications in depression-like behaviors: Role of TLR4/MyD88/NF-kappaB signaling. Front. Pharmacol. 2022, 13, 838261. [Google Scholar] [CrossRef]
- Guo, S.R.; Wang, H.; Yin, Y.F. Microglia polarization from M1 to M2 in neurodegenerative diseases. Front. Aging Neurosci. 2022, 14, 815347. [Google Scholar] [CrossRef]
- Du, L.; Zhang, Y.; Chen, Y.; Zhu, J.; Yang, Y.; Zhang, H.L. Role of microglia in neurological disorders and their potentials as a therapeutic target. Mol. Neurobiol. 2017, 54, 7567–7584. [Google Scholar] [CrossRef]
- Tang, Y.; Le, W. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Darwish, S.F.; Elbadry, A.M.; Elbokhomy, A.S.; Salama, G.A.; Salama, R.M. The dual face of microglia (M1/M2) as a potential target in the protective effect of nutraceuticals against neurodegenerative diseases. Front. Aging 2023, 4, 1231706. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.W.; Wang, S.Y.; Cao, H.; Guo, H.; Li, Y.J.; Xu, F.X.; Zheng, M.M.; Xi, X.Z.; Han, C.C. A Review: The Bioactivities and Pharmacological Applications of Polygonatum sibiricum polysaccharides. Molecules. 2018, 23, 1170. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.M.; Song, Z.J.; Xie, P.; Li, L.; Wang, B.; Peng, D.Y.; Zhu, G.Q. Polygonatum sibiricum polysaccharide prevents depression-like behaviors by reducing oxidative stress, inflammation, and cellular and synaptic damage. J. Ethnopharmacol. 2021, 275, 114164. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.M.; Xie, P.; Li, C.T.; Bian, Z.J.; Wang, X.C.; Peng, D.Y.; Zhu, G.Q. Polysaccharides from Polygonatum cyrtonema Hua reduce depression-like behavior in mice by inhibiting oxidative stress-calpain-1-NLRP3 signaling axis. Oxidative Med. Cell. Longev. 2022, 2022, 2566917. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Sun, Y.; Liu, Y.P.; Liu, J.M.; Sun, J.; Bai, Y.J.; Fan, B.; Lu, C.; Wang, F.Z. Polygonum sibiricum polysaccharides alleviate chronic unpredictable mild stress-induced depressive-like behaviors by regulating the gut microbiota composition and SCFAs levels. J. Funct. Foods 2023, 101, 105411. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Sun, Y.; Liu, Y.P.; Liu, J.M.; Sun, J.; Liu, X.M.; Fan, B.; Lu, C.; Wang, F.Z. Polygonum sibiricum polysaccharides exert the antidepressant-like effects in chronic unpredictable mild stress-induced depressive mice by modulating microbiota-gut-brain axis. Phytother. Res. 2023, 37, 3408–3423. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Liu, B.P.; Zhang, Y.P.; Peng, Z.; Wang, J.; Collier, A.D.; Echevarria, D.J.; Savelieva, K.V.; Lawrence, R.F.; Rex, C.S.; et al. Modeling consequences of prolonged strong unpredictable stress in zebrafish: Complex effects on behavior and physiology. Prog. Neuro-Psychoph. 2018, 81, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Li, X.; Wang, Y.; Yan, L.; Guo, L.; Huang, L.; Gao, W. Characterisation and saccharide mapping of polysaccharides from four common Polygonatum spp. Carbohydr. Polym. 2020, 233, 115836. [Google Scholar] [CrossRef]
- Zhu, R.; Zhang, X.; Wang, Y.; Zhang, L.; Zhao, J.; Chen, G.; Fan, J.; Jia, Y.; Yan, F.; Ning, C. Characterization of polysaccharide fractions from fruit of Actinidia arguta and assessment of their antioxidant and antiglycated activities. Carbohydr. Polym. 2019, 210, 73–84. [Google Scholar] [CrossRef]
- Pei, J.J.; Wang, Z.B.; Ma, H.L.; Yan, J.K. Structural features and antitumor activity of a novel polysaccharide from alkaline extract of Phellinus linteus mycelia. Carbohydr. Polym. 2015, 115, 472–477. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, D.; Wu, Y.; Tao, W.; Luorong, Q.; Luo, J.; Tan, L.; Chen, H.; Cao, W. A new polysaccharide from Hawk tea: Structural characterization and immunomodulatory activity associated with regulating gut microbiota. Food Chem. 2023, 418, 135917. [Google Scholar] [CrossRef]
- Willner, P.; Muscat, R.; Papp, M. Chronic mild stress-induced anhedonia: A realistic animal model of depression. Neurosci. Biobehav. Rev. 1992, 16, 525–534. [Google Scholar] [CrossRef]
- D’Aquila, P.S.; Brain, P.; Willner, P. Effects of chronic mild stress on performance in behavioural tests relevant to anxiety and depression. Physiol. Behav. 1994, 56, 861–867. [Google Scholar] [CrossRef]
- Dang, R.Z.; Wang, M.Y.; Li, X.H.; Wang, H.Y.; Liu, L.X.; Wu, Q.Y.; Zhao, J.T.; Ji, P.; Zhong, L.M.; Licinio, J.; et al. Edaravone ameliorates depressive and anxiety-like behaviors via Sirt1/Nrf2/HO-1/Gpx4 pathway. J. Neuroinflamm. 2022, 19, 41. [Google Scholar] [CrossRef]
- Liu, E.Y.; Yang, C.L.; Tsai, J.C.; Cheng, H.Y.; Peng, W.H. Antidepressive mechanisms of rhynchophylline in mice with chronic unpredictable stress-induced depression. J. Ethnopharmacol. 2023, 309, 116302. [Google Scholar] [CrossRef]
- Fontana, B.D.; Alnassar, N.; Parker, M.O. The zebrafish (Danio rerio) anxiety test battery: Comparison of behavioral responses in the novel tank diving and light-dark tasks following exposure to anxiogenic and anxiolytic compounds. Psychopharmacology 2022, 239, 287–296. [Google Scholar] [CrossRef]
- Bensi, N.; Bertuzzi, M.; Armario, A.; Gauna, H.F. Chronic immobilization stress reduces sodium intake and renal excretion in rats. Physiol. Behav. 1997, 62, 1391–1396. [Google Scholar] [CrossRef]
- Zeng, J.; Ji, Y.; Luan, F.; Hu, J.; Rui, Y.; Liu, Y.; Rao, Z.; Liu, R.; Zeng, N. Xiaoyaosan ethyl acetate fraction alleviates depression-like behaviors in CUMS mice by promoting hippocampal neurogenesis via modulating the IGF-1Rbeta/PI3K/Akt signaling pathway. J. Ethnopharmacol. 2022, 288, 115005. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Chen, H.Y.; Wang, T.; Su, H.; Li, J.; He, Y.J.; Su, S.Y. Electroacupuncture promotes synaptic plasticity in rats with chronic inflammatory pain-related depression by upregulating BDNF/TrkB/CREB signaling pathway. Brain Behav. 2023, 13, e3310. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Z.; Huang, H.; Li, L.; Yuan, Y.N.; Xiang, L.; Wu, X.W.; Ni, C.F.; Yu, W.F. Mol Med Rep. Intracarotid cold saline infusion contributes to neuroprotection in MCAO-induced ischemic stroke in rats via serum and glucocorticoid-regulated kinase 1. Mol. Med. Rep. 2021, 20, 3942–3950. [Google Scholar] [CrossRef]
- Dion-Albert, L.; Cadoret, A.; Doney, E.; Kaufmann, F.N.; Dudek, K.A.; Daigle, B.; Parise, L.F.; Cathomas, F.; Samba, N.; Hudson, N.; et al. Vascular and blood-brain barrier-related changes underlie stress responses and resilience in female mice and depression in human tissue. Nat. Commun. 2022, 13, 164. [Google Scholar] [CrossRef] [PubMed]
- Medina-Rodriguez, E.M.; Beurel, E. Blood brain barrier and inflammation in depression. Neurobiol. Dis. 2022, 175, 105926. [Google Scholar] [CrossRef] [PubMed]
- Kandola, A.; Ashdown-Franks, G.; Hendrikse, J.; Sabiston, C.M.; Stubbs, B. Physical activity and depression: Towards understanding the antidepressant mechanisms of physical activity. Neurosci. Biobehav. Rev. 2019, 107, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.K.; Lin, W.; Zha, Q.J.; Guo, H.H.; Zhang, D.D.; Yang, L.P.; Li, L.; Li, D.P.; Tang, R. Persistent exposure to environmental levels of microcystin-lr disturbs cortisol production via hypothalamic-pituitary-interrenal (HPI) axis and subsequently liver glucose metabolism in adult male zebrafish (Danio rerio). Toxins 2020, 12, 282. [Google Scholar] [CrossRef] [PubMed]
- Bury, N.R.; Sturm, A. Evolution of the corticosteroid receptor signalling pathway in fish. Gen. Comp. Endocrinol. 2007, 153, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, M.J.; Chatzopoulou, A.; Spaink, H.P. The zebrafish as a model system for glucocorticoid receptor research. Comp. Biochem. Physiol. Part A 2009, 153, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Engelsma, M.Y.; Huising, M.O.; van Muiswinkel, W.B.; Flik, G.; Kwang, J.; Savelkoul, H.F.; Verburg-van Kemenade, B.M. Neuroendocrine-immune interactions in fish: A role for interleukin-1. Vet. Immunol. Immunopathol. 2002, 87, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Rutz, S.; Crellin, N.K.; Valdez, P.A.; Hymowitz, S.G. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu. Rev. Immunol. 2011, 29, 71–109. [Google Scholar] [CrossRef] [PubMed]
- Porro, C.; Cianciulli, A.; Panaro, M.A. The regulatory role of IL-10 in neurodegenerative diseases. Biomolecules 2020, 10, 1017. [Google Scholar] [CrossRef]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef]
- Calcia, M.A.; Bonsall, D.R.; Bloomfield, P.S.; Selvaraj, S.; Barichello, T.; Howes, O.D. Stress and neuroinflammation: A systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology 2016, 233, 1637–1650. [Google Scholar] [CrossRef]
- Wang, H.X.; He, Y.; Sun, Z.L.; Ren, S.Y.; Liu, M.X.; Wang, G.; Yang, J. Microglia in depression: An overview of microglia in the pathogenesis and treatment of depression. J. Neuroinflamm. 2022, 19, 132. [Google Scholar] [CrossRef]
- Wang, L.; Li, M.; Zhu, C.P.; Qin, A.P.; Wang, J.C.; Wei, X.N. The protective effect of Palmatine on depressive like behavior by modulating microglia polarization in LPS-induced mice. Neurochem. Res. 2022, 47, 3178–3191. [Google Scholar] [CrossRef]
- Wu, S.T.; Nguyen, L.T.M.; Pan, H.R.; Hassan, S.; Dai, Y.M.; Xu, J.; Wen, Z.L. Two phenotypically and functionally distinct microglial populations in adult zebrafish. Sci. Adv. 2020, 6, 1160. [Google Scholar] [CrossRef]
- Mojzesz, M.; Widziolek, M.; Adamek, M.; Orzechowska, U.; Podlasz, P.; Prajsnar, T.K.; Pooranachandran, N.; Pecio, A.; Michalik, A.; Surachetpong, W.; et al. Tilapia lake virus-induced neuroinflammation in zebrafish: Microglia activation and sickness behavior. Front. Immunol. 2021, 12, 760882. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.P.; Zhang, B.P.; Huang, S.B.; Zhou, Z.L.; Jia, X.B.; Qiao, C.M.; Wang, F.; Sun, M.F.; Shi, Y.; Yao, L.; et al. Insulin-like growth factor-1 enhances motoneuron survival and inhibits neuroinflammation after spinal cord transection in zebrafish. Cell. Mol. Neurobiol. 2022, 42, 1373–1384. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yang, L.E.; Wang, J.; Hu, G.; Liu, Z.Y.; Yan, D.; Serikuly, N.; Alpyshov, E.T.; Demin, K.A.; Galstyan, D.S.; et al. Behavioral and physiological effects of acute and chronic kava exposure in adult zebrafish. Neurotoxicology Teratol. 2020, 79, 106881. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Guo, A.; Huang, Z.; Guan, K.; Zhu, Y.; Chan, C.; Gui, J.; Song, C.; Li, X. The exploration of neuroinflammatory mechanism by which CRHR2 deficiency induced anxiety disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2024, 128, 110844. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Zhou, X.; Chen, H.G. Structure-activity relationship of plant polysaccharides. Zhongguo Zhong Yao Za Zhi 2017, 42, 4104–4109. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.X.; Chen, X.F.; Cong, P.; Li, Z.X.; Wu, Y.P.; Zhang, J.; Wang, J.T.; Yao, W.B.; Yang, W.J.; Chen, F.X. Advances in the mechanisms of polysaccharides in alleviating depression and its complications. Phytomedicine 2023, 109, 154566. [Google Scholar] [CrossRef] [PubMed]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Walker, J.M. The bicinchoninic acid (BCA) assay for protein quantitation. Methods Mol. Biology. 1994, 32, 5–8. [Google Scholar] [CrossRef]
- Bao, Y.F.; Li, J.Y.; Zheng, L.F.; Li, H.Y. Antioxidant activities of cold-nature Tibetan herbs are signifcantly greater than hot-nature ones and are associated with their levels of total phenolic components. Chin. J. Nat. Med. 2015, 13, 609–617. [Google Scholar] [CrossRef]
- Vegh, R.; Csoka, M.; Stefanovits-Banyai, E.; Juhasz, R.; Sipos, L. Biscuits enriched with monofloral bee pollens: Nutritional properties, techno-functional parameters, sensory profile, and consumer preference. Foods 2022, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Jheng, Y.H.; Lee, L.H.; Ting, C.; Pan, H.; Hui, C.F.; Chen, J.Y. Zebrafish fed on recombinant Artemia expressing epinecidin-1 exhibit increased survival and altered expression of immunomodulatory genes upon Vibrio vulnificus infection. Fish Shellfish. Immunol. 2015, 42, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Elkhayat, S.I.; Bartels, B.K.; Tien, A.K.; Tien, D.H.; et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain Res. 2009, 205, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Cachat, J.; Kyzar, E.J.; Collins, C.; Gaikwad, S.; Green, J.; Roth, A.; El-Ounsi, M.; Davis, A.; Pham, M.; Landsman, S.; et al. Unique and potent effects of acute ibogaine on zebrafish: The developing utility of novel aquatic models for hallucinogenic drug research. Behav. Brain Res. 2013, 236, 258–269. [Google Scholar] [CrossRef]
- Chandrasekar, G.; Arner, A.; Kitambi, S.S.; Dahlman-Wright, K.; Lendahl, M.A. Developmental toxicity of the environmental pollutant 4-nonylphenol in zebrafish. Neurotoxicology Teratol. 2011, 33, 752–764. [Google Scholar] [CrossRef]
- Togao, M.; Nakayama, S.M.M.; Ikenaka, Y.; Mizukawa, H.; Makino, Y.; Kubota, A.; Matsukawa, T.; Yokoyama, K.; Hirata, T.; Ishizuka, M. Bioimaging of Pb and STIM1 in mice liver, kidney and brain using laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) and immunohistochemistry. Chemosphere 2020, 238, 124581. [Google Scholar] [CrossRef]



| Parameters | Units |
|---|---|
| Molar mass moments (g/mol) | |
| Weight-average molecular weight (Mw) | 2.048 × 104 ± 0.03 |
| Number-average molecular weight (Mn) | 2.180 × 103 ± 0.05 |
| Peak-position molecular weight (Mp) | 1.557 × 103 ± 0.04 |
| Z-average molecular weight (Mz) | 7.340 × 106 ± 0.08 |
| Polydispersity | |
| Mw/Mn | 9.394 ± 0.08 |
| Chemical composition (%, w/w) | |
| Total carbohydrate | 72.12 ± 0.90 |
| Protein | 14.40 ± 0.004 |
| Ash | 5.68 ± 0.51 |
| Water | 3.21 ± 0.08 |
| Flavonoids | 0.28 ± 0.06 |
| Phenols | 0.11 ± 0.003 |
| Crude fat | 0.03 ± 0.008 |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| β-actin | ACCACGGCCGAAAGAGAAAT | ATGTCCACGTCGCACTTCAT |
| inos | CCTCCTCATGTACCTGAATCTCG | GCTCCTTGCTTTAGTATGTCGC |
| cd11b | TCCTCGGATTCCAGAAACAC | AGCAGCACAAGTCCTCCAAT |
| ym1 | GCAAGAGGAAGTCCACCTGAAGAC | ATACAGCAGCGGTCAGCATAAGC |
| arg-1 | TCCGTTCTCCAAAGGACAGC | GACTCGTCGTTGGGAAGGTT |
| cd206 | ACGCTTTCGATGGGTTTCCT | CCCTCCGTAGTACATTCCGC |
| egr-2 | TCTGGATGCGGAGAGGTCTATCAAG | AGTAGGATGGCGGAGGATATGAGATG |
| il-4 | TTGGTCCCCGTTTCTGAGTC | CCAGTCCCGGTATATGCTGC |
| il-10 | AAGCACTCCACAACCCCAAT | TGCATTTCACCATATCCCGCT |
| il-6 | AGACCGCTGCCTGTCTAAAA | TTTGATGTCGTTCACCAGGA |
| il-1β | TTCCCCAAGTGCTGCTTATT | AAGTTAAAACCGCTGTGGTCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, D.; Liu, J.; Bai, Y.; Fan, B.; Lu, C.; Wang, F. Structural Characterization and Antidepressant-like Effects of Polygonum sibiricum Polysaccharides on Regulating Microglial Polarization in Chronic Unpredictable Mild Stress-Induced Zebrafish. Int. J. Mol. Sci. 2024, 25, 2005. https://doi.org/10.3390/ijms25042005
Zhang Y, Wang D, Liu J, Bai Y, Fan B, Lu C, Wang F. Structural Characterization and Antidepressant-like Effects of Polygonum sibiricum Polysaccharides on Regulating Microglial Polarization in Chronic Unpredictable Mild Stress-Induced Zebrafish. International Journal of Molecular Sciences. 2024; 25(4):2005. https://doi.org/10.3390/ijms25042005
Chicago/Turabian StyleZhang, Yingyu, Danyang Wang, Jiameng Liu, Yajuan Bai, Bei Fan, Cong Lu, and Fengzhong Wang. 2024. "Structural Characterization and Antidepressant-like Effects of Polygonum sibiricum Polysaccharides on Regulating Microglial Polarization in Chronic Unpredictable Mild Stress-Induced Zebrafish" International Journal of Molecular Sciences 25, no. 4: 2005. https://doi.org/10.3390/ijms25042005
APA StyleZhang, Y., Wang, D., Liu, J., Bai, Y., Fan, B., Lu, C., & Wang, F. (2024). Structural Characterization and Antidepressant-like Effects of Polygonum sibiricum Polysaccharides on Regulating Microglial Polarization in Chronic Unpredictable Mild Stress-Induced Zebrafish. International Journal of Molecular Sciences, 25(4), 2005. https://doi.org/10.3390/ijms25042005





