Does Chronic Pancreatitis in Growing Pigs Lead to Articular Cartilage Degradation and Alterations in Subchondral Bone?
Abstract
1. Introduction
2. Results
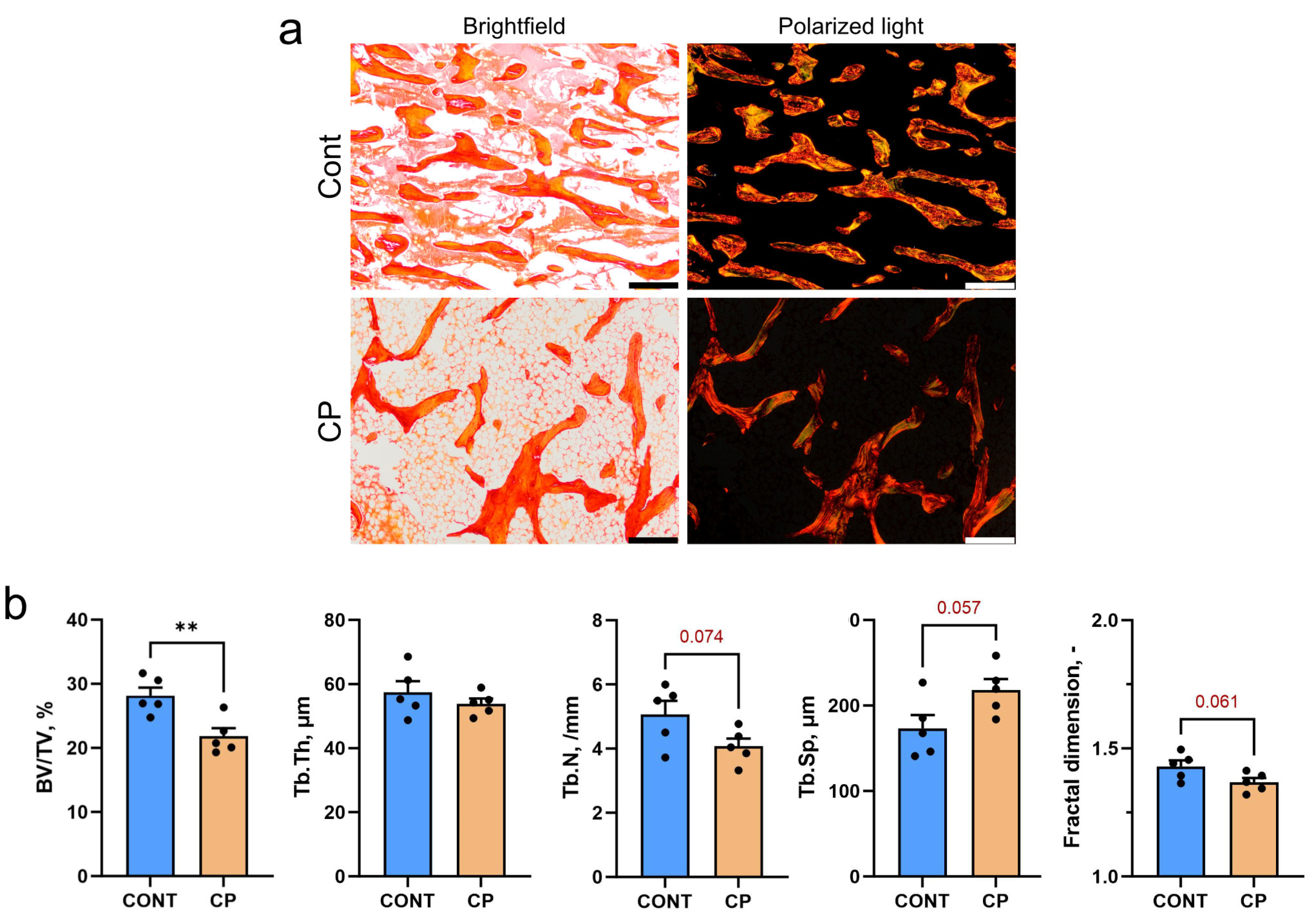
2.1. Subchondral Bone
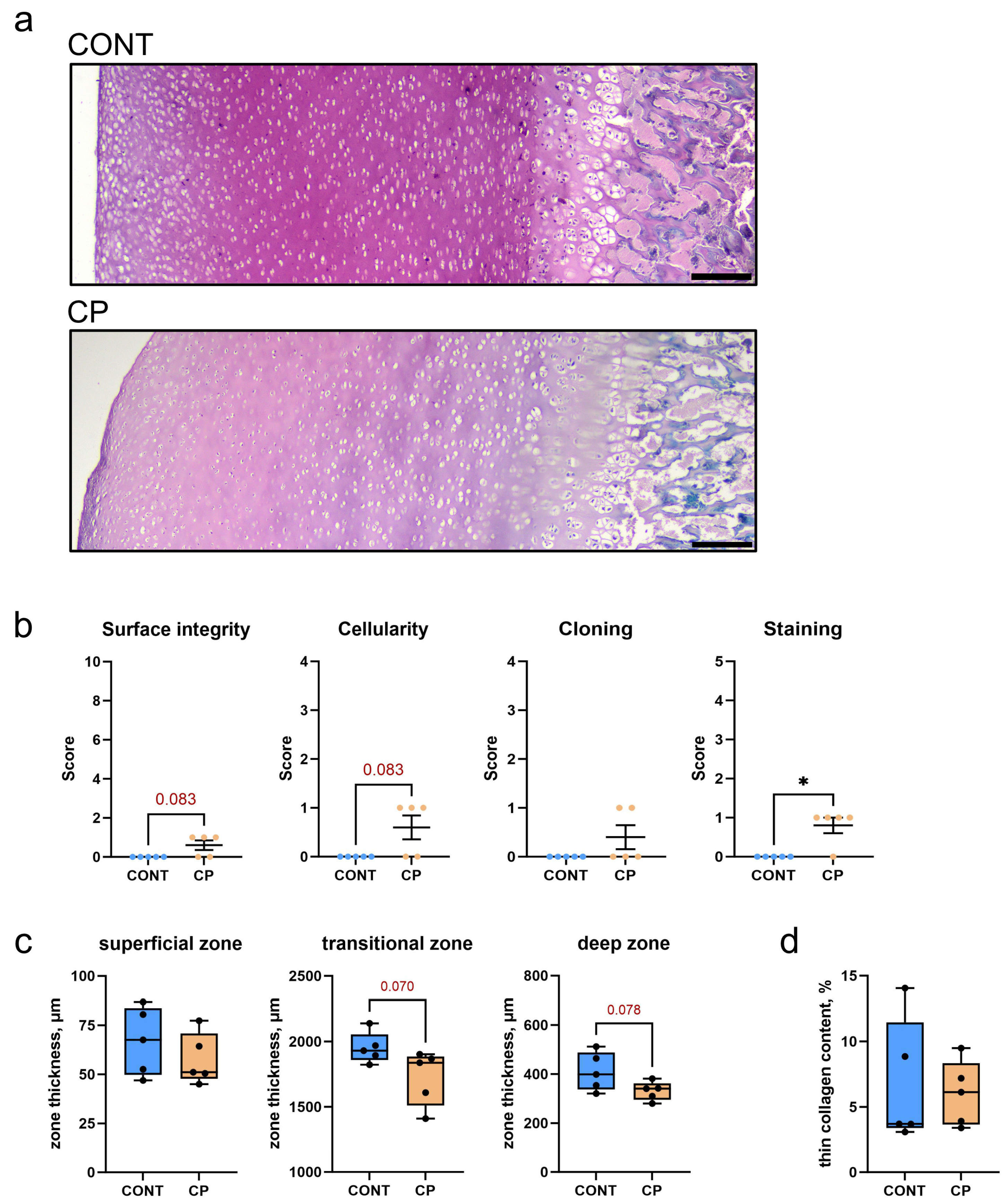
2.2. Articular Cartilage
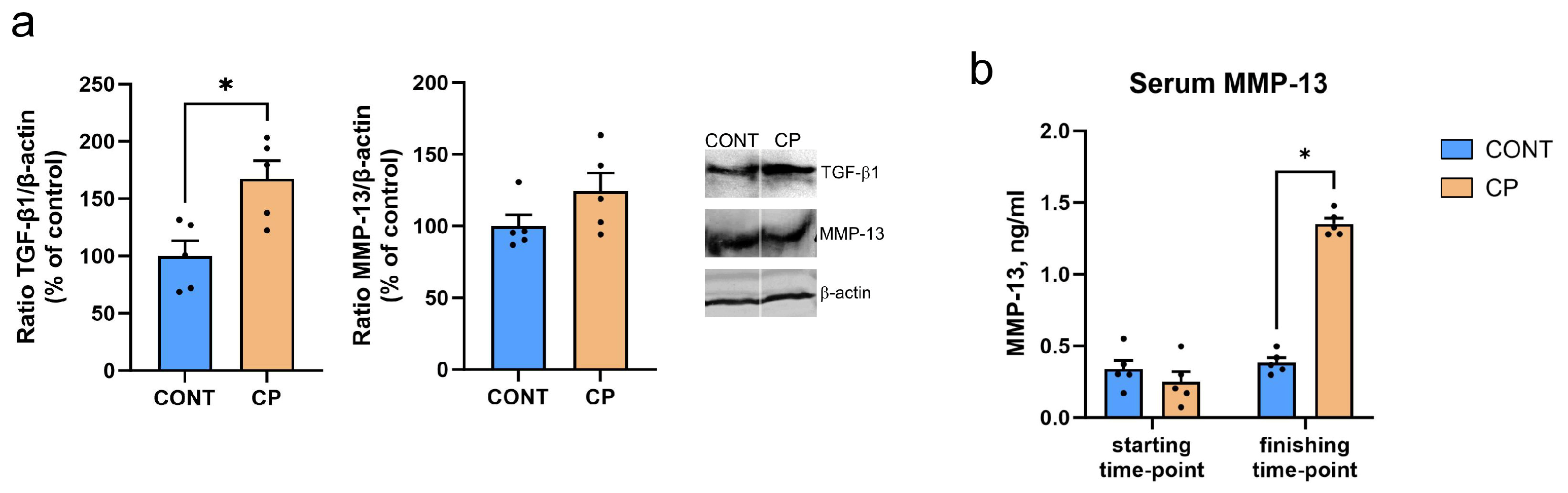
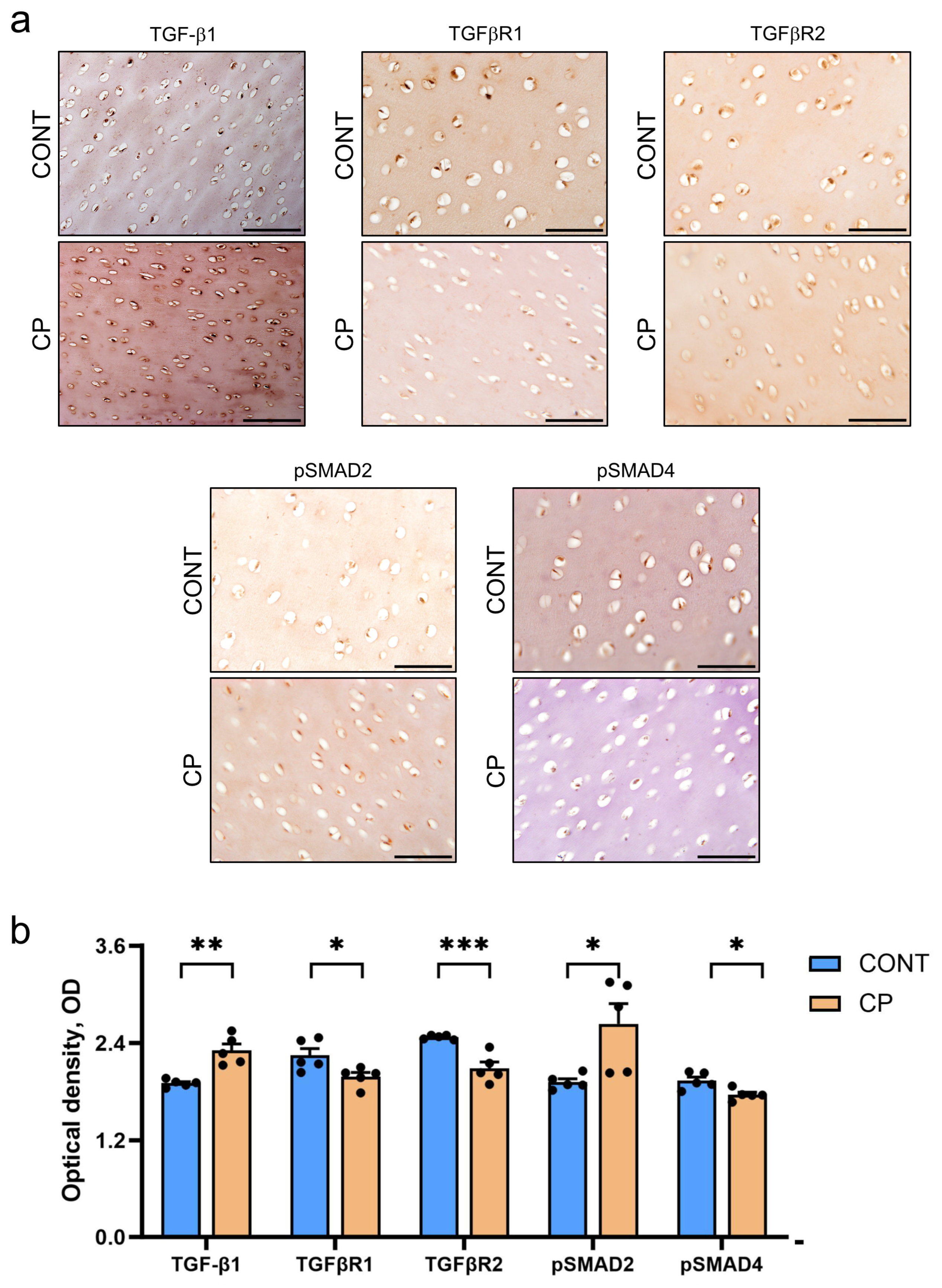
2.3. Immunohistochemistry of Articular Cartilage
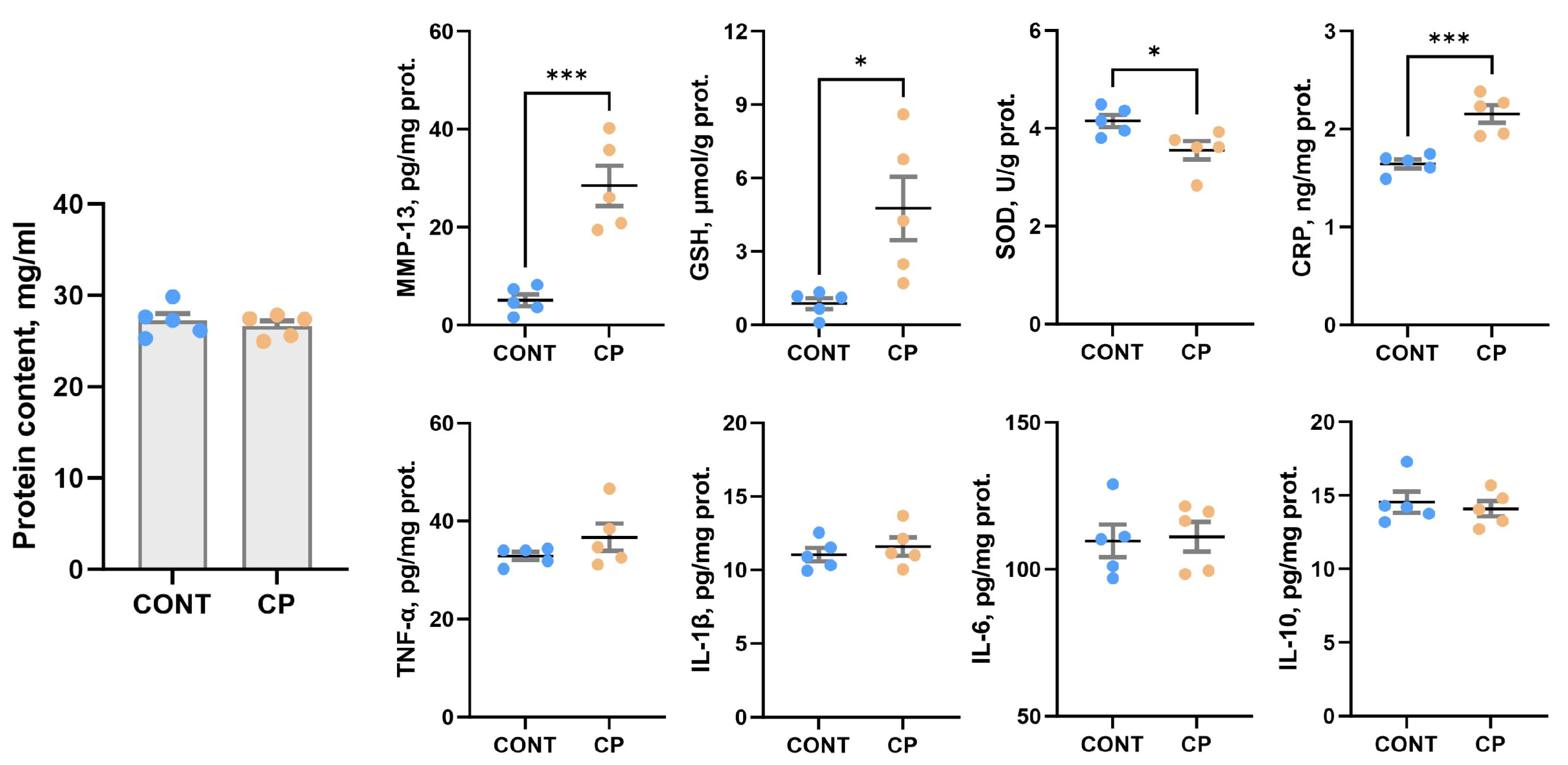
2.4. Synovial Fluid
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Sample Collection
4.3. Biofluids Parameters
4.4. Cartilage Histochemical and Immunohistochemical Analyses
4.5. Western Blot Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gardner, T.B.; Adler, D.G.; Forsmark, C.E.; Sauer, B.G.; Taylor, J.R.; Whitcomb, D.C. ACG Clinical Guideline: Chronic Pancreatitis. Am. J. Gastroenterol. 2020, 115, 322–339. [Google Scholar] [CrossRef]
- Suzuki, M.; Minowa, K.; Isayama, H.; Shimizu, T. Acute recurrent and chronic pancreatitis in children. Pediatr. Int. 2021, 63, 137–149. [Google Scholar] [CrossRef]
- Ellery, K.M.; Uc, A. Recurrent Pancreatitis in Children: Past, Present, and Future. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.D.; Chacko, A.; Ramakrishna, B.S.; Dutta, A.K.; Augustine, J.; Koshy, A.K.; Simon, E.G.; Joseph, A.J. Clinical profile and outcome of chronic pancreatitis in children. Indian Pediatr. 2013, 50, 1016–1019. [Google Scholar] [CrossRef]
- Perito, E.; Gonska, T.; Bellin, M.D.; Schwarzenberg, S.J. Complications of chronic pancreatitis in children. Curr. Opin. Gastroenterol. 2021, 37, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Uc, A.; Cress, G.A.; Wang, F.; Abu-El-Haija, M.; Ellery, K.M.; Fishman, D.S.; Gariepy, C.E.; Gonska, T.; Lin, T.K.; Liu, Q.Y.; et al. Analysis of INSPPIRE-2 Cohort: Risk Factors and Disease Burden in Children with Acute Recurrent or Chronic Pancreatitis. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 643–649. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Świątkiewicz, M.; Muszyński, S.; Donaldson, J.; Ropka-Molik, K.; Arciszewski, M.B.; Murawski, M.; Schwarz, T.; Dobrowolski, P.; Szymańczyk, S.; et al. Repetitive Cerulein-Induced Chronic Pancreatitis in Growing Pigs—A Pilot Study. Int. J. Mol. Sci. 2023, 24, 7715. [Google Scholar] [CrossRef]
- Ahmed, A.; Deep, A.; Kothari, D.J.; Sheth, S.G. Bone disease in chronic pancreatitis. World J. Clin. Cases 2020, 8, 1574–1579. [Google Scholar] [CrossRef]
- Parhiala, M.; Ukkonen, M.; Sand, J.; Laukkarinen, J. Osteoporosis and sarcopenia are common and insufficiently diagnosed among chronic pancreatitis patients. BMC Gastroenterol. 2023, 23, 124. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; Oyende, O.; Humes, D.J.; Lobo, D.N. Risk of osteopaenia, osteoporosis and osteoporotic fractures in patients with chronic pancreatitis: A systematic review and meta-analysis. Clin. Nutr. 2023, 42, 1086–1094. [Google Scholar] [CrossRef]
- Barkin, J.A.; Barkin, J.S. Chronic Pancreatitis and Bone Disease. J. Clin. Densitom. 2020, 23, 237–243. [Google Scholar] [CrossRef]
- Li, Y.; Xie, B.; Jiang, Z.; Yuan, B. Relationship between osteoporosis and osteoarthritis based on DNA methylation. Int. J. Clin. Exp. Pathol. 2019, 12, 3399–3407. [Google Scholar]
- Yoshimura, N.; Muraki, S.; Oka, H.; Mabuchi, A.; Kinoshita, H.; Yosihda, M.; Kawaguchi, H.; Nakamura, K.; Akune, T. Epidemiology of lumbar osteoporosis and osteoarthritis and their causal relationship-is osteoarthritis a predictor for osteoporosis or vice versa?: The miyama study. Osteoporos. Int. 2009, 20, 999–1008. [Google Scholar] [CrossRef]
- Im, G.I.I.; Kim, M.K. The relationship between osteoarthritis and osteoporosis. J. Bone Miner. Metab. 2014, 32, 101–109. [Google Scholar] [CrossRef]
- Geusens, P.P.; Van Den Bergh, J.P. Osteoporosis and osteoarthritis: Shared mechanisms and epidemiology. Curr. Opin. Rheumatol. 2016, 28, 97–103. [Google Scholar] [CrossRef]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Wiegertjes, R.; Van De Loo, F.A.J.; Blaney Davidson, E.N. A roadmap to target interleukin-6 in osteoarthritis. Rheumatology 2020, 59, 2681–2694. [Google Scholar] [CrossRef] [PubMed]
- Stannus, O.; Jones, G.; Cicuttini, F.; Parameswaran, V.; Quinn, S.; Burgess, J.; Ding, C. Circulating levels of IL-6 and TNF-α are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthr. Cartil. 2010, 18, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Luo, P.; Yang, M.; Wang, J.; Hou, W.; Xu, P. The role of oxidative stress in the development of knee osteoarthritis: A comprehensive research review. Front. Mol. Biosci. 2022, 9, 1001212. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, Z.; Alexander, P.G.; Ocasio-Nieves, B.D.; Yocum, L.; Lin, H.; Tuan, R.S. Pathogenesis of osteoarthritis: Risk factors, regulatory pathways in chondrocytes, and experimental models. Biology 2020, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Ecker, M. Overview of MMP-13 as a promising target for the treatment of osteoarthritis. Int. J. Mol. Sci. 2021, 22, 1742. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, X.; Wang, S.; Jing, Y.; Su, J. Subchondral bone microenvironment in osteoarthritis and pain. Bone Res. 2021, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Lites, T.D.; Foster, A.L.; Boring, M.A.; Fallon, E.A.; Odom, E.L.; Seth, P. Arthritis Among Children and Adolescents Aged < 18 Years—United States, 2017–2021. Morb. Mortal. Wkly. Rep. 2023, 72, 788–792. [Google Scholar]
- McLaughlin, D.; Keir, M.; Foster, H.; Jandial, S. Chronic arthritis in children and young people. Medicine 2022, 50, 149–158. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Il-6 in inflammation, Immunity, And disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Newman, A.B. Inflammatory markers in population studies of aging. Ageing Res. Rev. 2011, 10, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M. From C-Reactive Protein to Interleukin-6 to Interleukin-1: Moving Upstream to Identify Novel Targets for Atheroprotection. Circ. Res. 2016, 118, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Liang, L.; Guo, X. Serum interleukin-10 level in patients with inflammatory bowel disease: A meta-analysis. Eur. J. Inflamm. 2019, 17, 1–7. [Google Scholar] [CrossRef]
- Ariane, A.R.; Morais, N.D.S.; Priore, S.E.; Franceschini, S.D.C.C. Inflammatory Biomarkers and Components of Metabolic Syndrome in Adolescents: A Systematic Review. Inflammation 2022, 45, 14–30. [Google Scholar]
- Ooi, P.H.; Thompson-Hodgetts, S.; Pritchard-Wiart, L.; Gilmour, S.M.; Mager, D.R. Pediatric Sarcopenia: A Paradigm in the Overall Definition of Malnutrition in Children? J. Parenter. Enter. Nutr. 2020, 44, 407–418. [Google Scholar] [CrossRef]
- Wang, L.; You, X.; Zhang, L.; Zhang, C.; Zou, W. Mechanical regulation of bone remodeling. Bone Res. 2022, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Vauhkonen, M.; Peltonen, J.; Karaharju, E.; Aalto, K.; Alitalo, I. Collagen synthesis and mineralization in the early phase of distraction bone healing. Bone Miner. 1990, 10, 171–181. [Google Scholar] [CrossRef]
- Khatri, M.; Naughton, R.J.; Clifford, T.; Harper, L.D.; Corr, L. The effects of collagen peptide supplementation on body composition, collagen synthesis, and recovery from joint injury and exercise: A systematic review. Amino Acids 2021, 53, 1493–1506. [Google Scholar] [CrossRef]
- Zwerina, J.; Hayer, S.; Tohidast-Akrad, M.; Bergmeister, H.; Redlich, K.; Feige, U.; Dunstan, C.; Kollias, G.; Steiner, G.; Smolen, J.; et al. Single and Combined Inhibition of Tumor Necrosis Factor, Interleukin-1, and RANKL Pathways in Tumor Necrosis Factor-Induced Arthritis: Effects on Synovial Inflammation, Bone Erosion, and Cartilage Destruction. Arthritis Rheum. 2004, 50, 277–290. [Google Scholar] [CrossRef]
- Tamer, T.M. Hyaluronan and synovial joint: Function, distribution and healing. Interdiscip. Toxicol. 2013, 6, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Bolduc, J.A.; Collins, J.A.; Loeser, R.F. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic. Biol. Med. 2019, 132, 73–82. [Google Scholar] [CrossRef]
- Setti, T.; Arab, M.G.L.; Santos, G.S.; Alkass, N.; Andrade, M.A.P.; Lana, J.F.S.D. The protective role of glutathione in osteoarthritis. J. Clin. Orthop. Trauma 2021, 15, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Li, X.D.; Sun, G.F.; Zhu, W.B.; Wang, Y.H. Effects of high intensity exhaustive exercise on SOD, MDA, and NO levels in rats with knee osteoarthritis. Genet. Mol. Res. 2015, 14, 12367–12376. [Google Scholar] [CrossRef]
- Xiao, Z.; Yu, X.; Zhang, S.; Liang, A. The Expression Levels and Significance of GSH, MDA, SOD, and 8-OHdG in Osteochondral Defects of Rabbit Knee Joints. Biomed Res. Int. 2022, 2022, 6916179. [Google Scholar] [CrossRef]
- Tetreault, M.W.; Wetters, N.G.; Moric, M.; Gross, C.E.; Della Valle, C.J. Is Synovial C-reactive Protein a Useful Marker for Periprosthetic Joint Infection? Clin. Orthop. Relat. Res. 2014, 472, 3997–4003. [Google Scholar] [CrossRef]
- Delpuech, P.; Desch, G.; Magnan, F.; Arlaud, J.; Lam-My, S. La proteine C-reactive dans les affections inflammatoires articulaires: Comparaison des dosages sanguin et synovial. Clin. Biochem. 1989, 22, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Q.; Li, R.; Duan, J.Y.; Wang, C. Bin Synovial fluid C-reactive protein as a diagnostic marker for periprosthetic joint infection: A systematic review and meta-analysis. Chin. Med. J. 2016, 129, 1987–1993. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Deng, Z.; Chen, K.; Jian, S.; Zhou, F.; Yang, Y.; Fu, Z.; Xie, H.; Xiong, J.; Zhu, W. Cartilage tissue engineering: From proinflammatory and anti-inflammatory cytokines to osteoarthritis treatments (Review). Mol. Med. Rep. 2022, 25, 99. [Google Scholar] [CrossRef] [PubMed]
- Saklatvala, J. Tumour necrosis factor α stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature 1986, 322, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Mazur, C.M.; Woo, J.J.; Yee, C.S.; Fields, A.J.; Acevedo, C.; Bailey, K.N.; Kaya, S.; Fowler, T.W.; Lotz, J.C.; Dang, A.; et al. Osteocyte dysfunction promotes osteoarthritis through MMP13-dependent suppression of subchondral bone homeostasis. Bone Res. 2019, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Burton, D.W.; Foster, M.; Johnson, K.A.; Hiramoto, M.; Deftos, L.J.; Terkeltaub, R. Chondrocyte calcium-sensing receptor expression is up-regulated in early guinea pig knee osteoarthritis and modulates PTHrP, MMP-13, and TIMP-3 expression. Osteoarthr. Cartil. 2005, 13, 395–404. [Google Scholar] [CrossRef]
- Xin, X.; Tan, Q.; Li, F.; Chen, Z.; Zhang, K.; Li, F.; Yang, B.; Xing, Z.; Zhou, F.; Tian, Y.; et al. Potential Value of Matrix Metalloproteinase-13 as a Biomarker for Osteoarthritis. Front. Surg. 2021, 8, 750047. [Google Scholar] [CrossRef]
- Yang, Q.; Wu, S.; Mao, X.; Wang, W.; Tai, H. Inhibition effect of curcumin on TNF-α and MMP-13 expression induced by advanced glycation end products in chondrocytes. Pharmacology 2013, 91, 77–85. [Google Scholar] [CrossRef]
- Speziali, A.; Delcogliano, M.; Tei, M.; Placella, G.; Chillemi, M.; Tiribuzi, R.; Cerulli, G. Chondropenia: Current concept review. Musculoskelet. Surg. 2015, 99, 189–200. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Martel-Pelletier, J.; Lascau-Coman, V.; Moldovan, F.; Jovanovic, D.; Raynauld, J.P.; Pelletier, J.P. Collagenase-1 and collagenase-3 synthesis in normal and early experimental osteoarthritic canine cartilage: An immunohistochemical study. J. Rheumatol. 1998, 25, 1585–1594. [Google Scholar]
- Varzaneh, M.B.; Zhao, Y.; Rozynek, J.; Han, M.; Reed, D.A. Disrupting mechanical homeostasis promotes matrix metalloproteinase-13 mediated processing of neuron glial antigen 2 in mandibular condylar cartilage. Eur. Cells Mater. 2023, 45, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; He, Z.; Qu, X.; Liu, X.; Zhang, W.; Zhang, S.; Han, X.; Yan, M.; Xu, Q.; Zhang, S.; et al. Different subchondral trabecular bone microstructure and biomechanical properties between developmental dysplasia of the hip and primary osteoarthritis. J. Orthop. Transl. 2020, 22, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Scanzello, C.R.; Goldring, S.R. The role of synovitis in osteoarthritis pathogenesis. Bone 2012, 51, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Hui, A.Y.; McCarty, W.J.; Masuda, K.; Firestein, G.S.; Sah, R.L. A systems biology approach to synovial joint lubrication in health, injury, and disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2012, 4, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.; Pesesse, L.; Lambert, C. Targeting the synovial angiogenesis as a novel treatment approach to osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2014, 6, 20–34. [Google Scholar] [CrossRef]
- Aref-Eshghi, E.; Liu, M.; Harper, P.E.; Doré, J.; Martin, G.; Furey, A.; Green, R.; Rahman, P.; Zhai, G. Overexpression of MMP13 in human osteoarthritic cartilage is associated with the SMAD-independent TGF-β signalling pathway. Arthritis Res. Ther. 2015, 17, 264. [Google Scholar] [CrossRef]
- Saiganesh, S.; Saathvika, R.; Udhaya, V.; Arumugam, B.; Vishal, M.; Selvamurugan, N. Matrix metalloproteinase-13: A special focus on its regulation by signaling cascades and microRNAs in bone. Int. J. Biol. Macromol. 2018, 109, 338–349. [Google Scholar]
- Wang, W.; Rigueur, D.; Lyons, K.M. TGFβ signaling in cartilage development and maintenance. Birth Defects Res. Part C—Embryo Today Rev. 2014, 102, 37–51. [Google Scholar] [CrossRef]
- Shen, J.; Li, S.; Chen, D. TGF-β signaling and the development of osteoarthritis. Bone Res. 2014, 2, 14002. [Google Scholar] [CrossRef]
- Liu, N.; Qi, D.; Jiang, J.; Zhang, J.; Yu, C. Expression pattern of p–Smad2/Smad4 as a predictor of survival in invasive breast ductal carcinoma. Oncol. Lett. 2020, 19, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- van Caam, A.; Madej, W.; Garcia de Vinuesa, A.; Goumans, M.J.; ten Dijke, P.; Blaney Davidson, E.; van der Kraan, P. TGFβ1-induced SMAD2/3 and SMAD1/5 phosphorylation are both ALK5-kinase-dependent in primary chondrocytes and mediated by TAK1 kinase activity. Arthritis Res. Ther. 2017, 19, 112. [Google Scholar] [CrossRef]
- Finnson, K.W.; Chi, Y.; Bou-Gharios, G.; Leask, A.; Philip, A. TGF-b signaling in cartilage homeostasis and osteoarthritis. Front. Biosci. 2012, 4, 251–268. [Google Scholar] [CrossRef]
- Wu, J.; Liu, W.; Bemis, A.; Wang, E.; Qiu, Y.; Morris, E.A.; Flannery, C.R.; Yang, Z. Comparative proteomic characterization of articular cartilage tissue from normal donors and patients with osteoarthritis. Arthritis Rheum. 2007, 56, 3675–3684. [Google Scholar] [CrossRef]
- Verdier, M.P.; Seité, S.; Guntzer, K.; Pujol, J.P.; Boumédiène, K. Immunohistochemical analysis of transforming growth factor beta isoforms and their receptors in human cartilage from normal and osteoarthritic femoral heads. Rheumatol. Int. 2005, 25, 118–124. [Google Scholar] [CrossRef]
- Xiao, J.; Li, T.; Wu, Z.; Shi, Z.; Chen, J.; Lam, S.K.L.; Zhao, Z.; Yang, L.; Qiu, G. REST corepressor (CoREST) repression induces phenotypic gene regulation in advanced osteoarthritic chondrocytes. J. Orthop. Res. 2010, 28, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Blaney Davidson, E.N.; Remst, D.F.G.; Vitters, E.L.; van Beuningen, H.M.; Blom, A.B.; Goumans, M.-J.; van den Berg, W.B.; van der Kraan, P.M. Increase in ALK1/ALK5 Ratio as a Cause for Elevated MMP-13 Expression in Osteoarthritis in Humans and Mice. J. Immunol. 2009, 182, 7937–7945. [Google Scholar] [CrossRef] [PubMed]
- Li, T.F.; Darowish, M.; Zuscik, M.J.; Chen, D.; Schwarz, E.M.; Rosier, R.N.; Drissi, H.; O’Keefe, R.J. Smad3-deficient chondrocytes have enhanced BMP signaling and accelerated differentiation. J. Bone Miner. Res. 2006, 21, 4–16. [Google Scholar] [CrossRef]
- Hills, R.L.; Belanger, L.M.; Morris, E.A. Bone morphogenetic protein 9 is a potent anabolic factor for juvenile bovine cartilage, but not adult cartilage. J. Orthop. Res. 2005, 23, 611–617. [Google Scholar] [CrossRef]
- Thielen, N.G.M.; Van der Kraan, P.M.; Van Caam, A.P.M. TGFβ/BMP signaling pathway in cartilage homeostasis. Cells 2019, 8, 969. [Google Scholar] [CrossRef]
- Posey, K.L.; Coustry, F.; Hecht, J.T. Cartilage oligomeric matrix protein: COMPopathies and beyond. Matrix Biol. 2018, 71–72, 161–173. [Google Scholar] [CrossRef]
- Koelling, S.; Clauditz, T.S.; Kaste, M.; Miosge, N. Cartilage oligomeric matrix protein is involved in human limb development and in the pathogenesis of osteoarthritis. Arthritis Res. Ther. 2006, 8, R56. [Google Scholar] [CrossRef] [PubMed]
- Obafemi, T.F.; Yu, P.; Li, J.; Davis, J.M.; Liu, K.; Cheng, B.; Zhao, X.; Shen, Q.; Younes, M.; Ko, T.C.; et al. Comparable Responses in Male and Female Mice to Cerulein-Induced Chronic Pancreatic Injury and Recovery. J. Pancreas 2018, 19, 236–243. [Google Scholar]
- National Research Council. Nutrient Requirements of Swine: Eleventh Revised Edition (2012), 12th ed.; The National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Grela, E.R.; Skomial, J. Feeding Guidelines for Pigs, 3rd ed.; The Kielanowski Institute of Animal Physiology and Nutrition: Jabłonna, Poland, 2020. [Google Scholar]
- Kahveci, Z.; Minday, F.Z.; Cavusoglu, I. Safranin O staining using a microwave oven. Biotech. Histochem. 2000, 75, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, L.C.U.; Bignolas, G.; Brentani, R.R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 1979, 11, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Moody, H.R.; Heard, B.J.; Frank, C.B.; Shrive, N.G.; Oloyede, A.O. Investigating the potential value of individual parameters of histological grading systems in a sheep model of cartilage damage: The Modified Mankin method. J. Anat. 2012, 221, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Doube, M.; Klosowski, M.M.; Arganda-Carreras, I.; Cordelières, F.P.; Dougherty, R.P.; Jackson, J.S.; Schmid, B.; Hutchinson, J.R.; Shefelbine, S.J. BoneJ: Free and extensible bone image analysis in ImageJ. Bone 2010, 47, 1076–1079. [Google Scholar] [CrossRef] [PubMed]
- Varghese, F.; Bukhari, A.B.; Malhotra, R.; De, A. IHC profiler: An open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS ONE 2014, 9, e96801. [Google Scholar] [CrossRef]
- Seyed Jafari, S.M.; Hunger, R.E. IHC optical density score: A new practical method for quantitative immunohistochemistry image analysis. Appl. Immunohistochem. Mol. Morphol. 2017, 25, e12–e13. [Google Scholar] [CrossRef]
- Ohgane, K.; Yoshioka, H. Quantification of Gel Bands by an Image J Macro, Band/Peak Quantification Tool. Protocols.io. 2019. Available online: https://www.protocols.io/view/quantification-of-gel-bands-by-an-image-j-macro-ba-bp2l6n4bkgqe/v1 (accessed on 17 December 2023).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomaszewska, E.; Hułas-Stasiak, M.; Dobrowolski, P.; Świątkiewicz, M.; Muszyński, S.; Tomczyk-Warunek, A.; Blicharski, T.; Donaldson, J.; Arciszewski, M.B.; Świetlicki, M.; et al. Does Chronic Pancreatitis in Growing Pigs Lead to Articular Cartilage Degradation and Alterations in Subchondral Bone? Int. J. Mol. Sci. 2024, 25, 1989. https://doi.org/10.3390/ijms25041989
Tomaszewska E, Hułas-Stasiak M, Dobrowolski P, Świątkiewicz M, Muszyński S, Tomczyk-Warunek A, Blicharski T, Donaldson J, Arciszewski MB, Świetlicki M, et al. Does Chronic Pancreatitis in Growing Pigs Lead to Articular Cartilage Degradation and Alterations in Subchondral Bone? International Journal of Molecular Sciences. 2024; 25(4):1989. https://doi.org/10.3390/ijms25041989
Chicago/Turabian StyleTomaszewska, Ewa, Monika Hułas-Stasiak, Piotr Dobrowolski, Małgorzata Świątkiewicz, Siemowit Muszyński, Agnieszka Tomczyk-Warunek, Tomasz Blicharski, Janine Donaldson, Marcin B. Arciszewski, Michał Świetlicki, and et al. 2024. "Does Chronic Pancreatitis in Growing Pigs Lead to Articular Cartilage Degradation and Alterations in Subchondral Bone?" International Journal of Molecular Sciences 25, no. 4: 1989. https://doi.org/10.3390/ijms25041989
APA StyleTomaszewska, E., Hułas-Stasiak, M., Dobrowolski, P., Świątkiewicz, M., Muszyński, S., Tomczyk-Warunek, A., Blicharski, T., Donaldson, J., Arciszewski, M. B., Świetlicki, M., Puzio, I., & Bonior, J. (2024). Does Chronic Pancreatitis in Growing Pigs Lead to Articular Cartilage Degradation and Alterations in Subchondral Bone? International Journal of Molecular Sciences, 25(4), 1989. https://doi.org/10.3390/ijms25041989






