Abstract
Non-small cell lung cancer (NSCLC) represents 80% of all lung cancer cases and is characterized by low survival rates due to chemotherapy and radiation resistance. Novel treatment strategies for NSCLC are urgently needed. Liver kinase B1 (LKB1), a tumor suppressor prevalently mutated in NSCLC, activates AMP-activated protein kinase (AMPK) which in turn inhibits mammalian target of rapamycin complex 1 (mTORC1) and activates unc-51 like autophagy activating kinase 1 (ULK1) to promote autophagy. Sestrin-2 is a stress-induced protein that enhances LKB1-dependent activation of AMPK, functioning as a tumor suppressor in NSCLC. In previous studies, rosemary (Rosmarinus officinalis) extract (RE) activated the AMPK pathway while inhibiting mTORC1 to suppress proliferation, survival, and migration, leading to the apoptosis of NSCLC cells. In the present study, we investigated the anticancer potential of carnosic acid (CA), a bioactive polyphenolic diterpene compound found in RE. The treatment of H1299 and H460 NSCLC cells with CA resulted in concentration and time-dependent inhibition of cell proliferation assessed with crystal violet staining and 3H-thymidine incorporation, and concentration-dependent inhibition of survival, assessed using a colony formation assay. Additionally, CA induced apoptosis of H1299 cells as indicated by decreased B-cell lymphoma 2 (Bcl-2) levels, increased cleaved caspase-3, -7, poly (ADP-ribose) polymerase (PARP), Bcl-2-associated X protein (BAX) levels, and increased nuclear condensation. These antiproliferative and proapoptotic effects coincided with the upregulation of sestrin-2 and the phosphorylation/activation of LKB1 and AMPK. Downstream of AMPK signaling, CA increased levels of autophagy marker light chain 3 (LC3), an established marker of autophagy; inhibiting autophagy with 3-methyladenine (3MA) blocked the antiproliferative effect of CA. Overall, these data indicate that CA can inhibit NSCLC cell viability and that the underlying mechanism of action of CA involves the induction of autophagy through a Sestrin-2/LKB1/AMPK signaling cascade. Future experiments will use siRNA and small molecule inhibitors to better elucidate the role of these signaling molecules in the mechanism of action of CA as well as tumor xenograft models to assess the anticancer properties of CA in vivo.
Keywords:
NSCLC; carnosic acid; autophagy; apoptosis; polyphenols; lung cancer; AMPK; sestrin-2; LKB1 1. Introduction
Cancer is a leading cause of death globally, second only to cardiovascular disease, which made up nearly 10 million deaths in 2020 [1]. Lung cancer is the leading cause of cancer mortality globally, comprising approximately 18% of cancer deaths [2]. Around 80–85% of lung cancer cases are categorized as non-small cell lung cancer (NSCLC) which is further divided into large cell carcinoma, squamous cell carcinoma, and adenocarcinoma accounting for 10–15%, 25%, and 40% of all lung cancer cases, respectively [3]. The treatment for NSCLC typically involves surgery if the primary tumor is resectable, combined with radiation, chemotherapy, immunotherapy, or targeted therapies [4]. Despite treatment options, roughly 72% of NSCLC patients will not survive beyond 5 years underscoring a need for research into novel treatments [5].
A key feature of NSCLC is dysregulated signal transduction cascades that ultimately contribute to uncontrolled cell proliferation, the evasion of apoptosis, and metastasis. Two common pathways implicated in NSCLC are the Ras-Raf-MEK-extracellular signal-regulated kinase (ERK) cascade and the phosphoinositide 3-kinase (PI3K)-Akt-mammalian target of rapamycin (mTOR) cascade [6]. Epidermal growth factor receptor (EGFR) is frequently overexpressed in NSCLC leading to the increased activation of Ras-Raf-MEK-ERK signaling. Additionally, Ras and Raf mutations can further contribute to increased ERK activation and enhanced cell survival and proliferation. Activated Ras also binds and activates PI3K leading to the activation of Akt-mTORC1 signaling resulting in increased protein synthesis and proliferation, and the inhibition of autophagy [6]. PIK3CA mutations that yield a constitutively active form of PI3K are present in some NSCLC cases, which also contributes to enhanced Akt-mTORC1 signaling [6]. Compounds capable of targeting these dysregulated cascades such as the EGFR tyrosine kinase inhibitor gefitinib are often used to treat NSCLC; however, acquired therapy-resistance in NSCLC is a significant problem [7].
Many chemotherapy drugs used to treat NSCLC are derived from plants including etoposide from wild mandrake (Podophyllum peltatum) [8] and paclitaxel from pacific yew (Taxus brevifolia) bark [9]. Rosemary (Rosmarinus officinalis) is a herb with reported antioxidant [10], antimicrobial [11], antidiabetic [12,13], and anticancer properties [14]. Several studies have shown that rosemary extract (RE) and its polyphenolic compounds—carnosol, carnosic acid (CA), and rosmarinic acid—reduce the proliferation and survival of cancer cells and inhibit tumor progression in animal models [14,15]. In recent studies by our group, RE inhibited the proliferation and survival, and induced the apoptosis of lung [16,17], breast [18], and prostate [19] cancer cells. In NSCLC cells, RE significantly increased AMPK signaling, but the active pharmaceutical ingredient responsible and the mechanism leading to AMPK activation has not been explored [17].
AMP-activated protein kinase (AMPK) is an energy sensor whose activation is associated with increased survival in NSCLC patients [20]. Additionally, the anticancer effects of several polyphenols are associated with AMPK activation, including quercetin [21], resveratrol [22], and epigallocatechin-3-gallate (EGCG) [23]. Research has indicated that RE and RE polyphenols trigger apoptosis and cell cycle arrest in a variety of cancer cells as well as increased activation of AMPK [14,15]. Sestrin-2 is a stress-induced protein that leads to the activation of AMPK signaling during conditions of nutrient deficiency, DNA damage, oxidative stress, or hypoxia [24,25]. Liver kinase B1 (LKB1) is a tumor suppressor and upstream kinase of the AMPK pathway that is mutated in 39% of NSCLC cell lines and 34% of human lung adenocarcinoma specimens [26,27,28]. Sestrin-2 has been shown to increase the LKB1-dependent activation of AMPK [29]. The phosphorylation of LKB1 at the Ser428 residue mediates its association with, and the activation of AMPK [30]. When activated, AMPK activates fatty acid catabolism and inhibits fatty acid synthesis. Additionally, activated AMPK inhibits mTOR signaling and protein synthesis, and activates autophagy. Autophagy is a process of cellular self-degradation initiated in response to stressors such as nutrient deprivation that have been reported to have conflicting roles in cancer [31]. While low levels of autophagy are thought to aid cancer progression, excessive levels of autophagy, such as those induced by curcumin [32] or apatinib [33], damage cells and induce apoptosis.
In the current study, we focused on CA, a bioactive benzenediol abietane diterpene, examined its effects in lung cancer cells, and investigated the role of upstream and downstream AMPK signaling in its mechanism of action.
2. Results
2.1. Carnosic Acid Inhibits Proliferation and Survival of NSCLC Cells
The accumulation of mutations in cancer cells results in unregulated cell proliferation. The proliferation of H1299 and H460 cells in the presence of CA was assessed using crystal violet and thymidine incorporation assays. CA caused concentration and time-dependent inhibition of H1299 cell proliferation with an IC50 of 47.3 µM and 27.1 µM for 24 h and 48 h, respectively (Figure 1A). Proliferating cells incorporate thymidine as they synthesize new DNA for mitosis. CA inhibited 3H-thymidine incorporation in H1299 cells in a concentration-dependent manner with significant inhibition observed with 10 µM (74.44 ± 3.93% of control, p < 0.05), maximum inhibition with 50 µM (13.11 ± 2.92% of control, p < 0.0001), and an IC50 of 41.09 µM (Figure 1C).
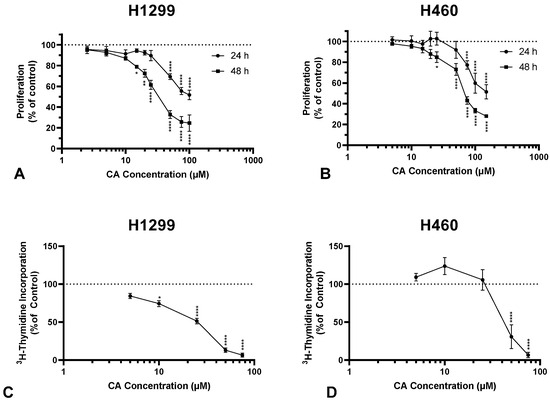
Figure 1.
Carnosic acid (CA) inhibits proliferation of NSCLC cells. Proliferation was assessed using a crystal violet assay (A,B) and a 3H-thymidine incorporation assay (C,D). H1299 and H460 cells were seeded and then treated the following day with CA (A: 2.5–100 µM; B: 5–150 µM) for 24 or 48 h, fixed, stained with crystal violet, crystal violet dye solubilized, and then absorbance measured at 590 nm and expressed as a percentage of control; data are the mean ± SEM for three individual experiments (A). Cells were treated with increasing concentrations of CA (5–75 µM) and exposed to 3H-thymidine for 24 h (B). Cells were lysed and radioactivity measured using liquid scintillation counting. Data are the mean ± SEM for four individual experiments expressed as a percentage of control (B). * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, compared to control (A,B).
In addition, CA treatment dose-dependently inhibited the proliferation of H460 cells, with an IC50 of 89.6 µM and 67 µM for 24 h and 48 h, respectively (Figure 1B). The maximum inhibition of 3H-thymidine incorporation in H460 cells was observed with 50 µM CA (30.77 ± 15.73% of control, p < 0.0001; Figure 1D).
Colony formation is a characteristic of cancer cells grown in vitro and is representative of an individual cell’s ability to survive in isolation to form a new clonal subpopulation. A clonogenic survival assay [18] was used to assess the survival of NSCLC cells under CA treatment. Cells were treated with increasing concentrations of CA for 7 days. Following treatment, the cells were fixed and stained, and colonies greater than 50 cells were counted. CA caused a concentration-dependent inhibition of survival in both H1299 and H460 cells. Significant inhibition of H1299 colony formation was observed with concentrations of 10 µM (62.4 ± 9.8% of control, p < 0.01; Figure 2A) or greater and with IC50 of 11.7 µM. CA concentrations of 25 and 50 µM completely inhibited H1299 cell survival (25 µM: 0.5 ± 0.5% of control, p < 0.001; Figure 2A). Significant inhibition of H460 colony formation was observed with concentrations of 20 µM (62.65 ± 15.98% of control, p < 0.01; Figure 2C) or greater and with an IC50 of 29.7 µM.
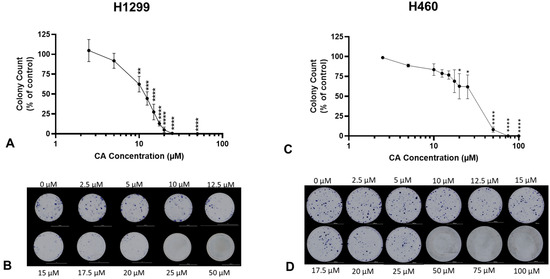
Figure 2.
Carnosic acid (CA) inhibits survival of NSCLC cells. H1299 (A,B) and H460 (C,D) cells were seeded at low density and treated the following day with increasing (A: 2.5–50 µM; C: 2.5–100 µM) concentrations of CA for 7 days to assess colony formation. Whole-well representative images were taken using a BioTek Cytation5 plate reader and BioTek Gen5 software (B,D). Following treatment, colonies greater than 50 cells were counted and expressed as a percentage of control; data are the mean ± SEM for three individual experiments. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, compared to control.
2.2. Carnosic Acid Induces Apoptosis of H1299 NSCLC Cells
The evasion of apoptosis is another characteristic of cancer cells that contributes to increased proliferation and survival. During apoptosis, proapoptotic Bcl-2-associated X protein (BAX) neutralizes antiapoptotic B-cell lymphoma 2 (Bcl-2) leading to the release of apoptogenic factors from mitochondria. These factors lead to activation of caspase-3 and caspase-7 which promote apoptosis through proteolytic cleavage and the inactivation of proteins necessary for survival such as poly (ADP-ribose) polymerase (PARP). The treatment of H1299 cells with 50 µM CA for 12 h or 24 h significantly increased the levels of cleaved caspase-7 (12 h: 214.8 ± 87.3%, p < 0.05; 24 h: 191.7 ± 48.0%, p < 0.05; Figure 3A) and cleaved PARP (12 h: 410.3 ± 163.9%, p < 0.01; 24 h: 447.9 ± 183.5%, p < 0.01; Figure 3B). Additionally, there was a trend toward increased levels of cleaved caspase-3 and BAX, and decreased levels of Bcl-2 (Figure 3A,D,E).
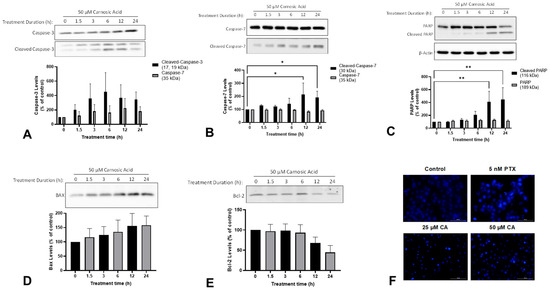
Figure 3.
Carnosic acid (CA) induces apoptosis of H1299 NSCLC cells. Whole cell lysates were prepared from H1299 cells treated without (control) or with 50 µM CA for 1.5–24 h. Cell lysates (20 µg) were resolved by SDS-PAGE and immunoblotted with specific antibodies against caspase-3 (A) caspase-7 (B), PARP (C), BAX (D), Bcl-2 (E), or β-actin (C). Upper panel: A representative immunoblot is shown. Lower panel: The densitometry of the bands, expressed in arbitrary units was measured using ImageJ 1.54g software. H1299 cells were treated for 24 h with 5 nM paclitaxel (PTX), 25 µM CA, or 50 µM CA, and nuclear morphology was assessed qualitatively using Hoechst 33342 and a BioTek cytation 5 plate reader (F). The data are expressed as percent of control and are the mean ± SEM of 3–6 separate experiments. * p< 0.05, ** p < 0.01, compared to control.
Apart from examining the aforementioned apoptosis markers, we also examined morphological changes in the cells. During apoptosis, cells undergo a process of controlled disassembly that includes nuclear condensation and nuclear fragmentation. The treatment of H1299 cells with CA resulted in substantial changes in nuclear morphology consistent with apoptosis (i.e., nuclear fragmentation) when compared to untreated cells. Specifically, CA-treated cells had noticeably smaller nuclei which is consistent with the nuclear condensation that occurs during apoptosis (Figure 3F). The treatment of the cells with 5 nM paclitaxel—a chemotherapy drug used in the treatment of NSCLC—resulted in the same changes in nuclear morphology.
2.3. Carnosic Acid Activates Sestrin-2/LKB1/AMPK Signalling in H1299 NSCLC Cells
Treatment of H1299 NSCLC cells with 50 µM CA caused a time-dependent increase in levels of sestrin-2 (12 h: 297.9 ± 70.0%, p < 0.05; 24 h: 446.1 ± 102.2%, p < 0.001; Figure 4A). Treatment of H1299 NSCLC cells with CA resulted in a significant increase in the level of phosphorylated LKB1 after 1.5 h (175.0 ± 28.3%, p < 0.001). The levels of phosphorylated LKB1 decreased over time, returning to approximately basal levels at the 3 h (112.7 ± 6.1%, p = 0.9028) and 6 h (100.8 ± 10.2%, p > 0.9999) timepoints, and decreasing below basal at the 12 h (51.4 ± 11.7%, p < 0.05) and 24 h (28.9 ± 9.7%, p < 0.001) timepoints (Figure 4B). The treatment of H1299 NSCLC cells with CA caused a time-dependent increase in the levels of phosphorylated AMPK (Figure 4C). A significant increase was observed with treatment times of 6 h and above, with a tenfold increase at 24 h (1051.8 ± 262.7%, p < 0.0001; Figure 4C).
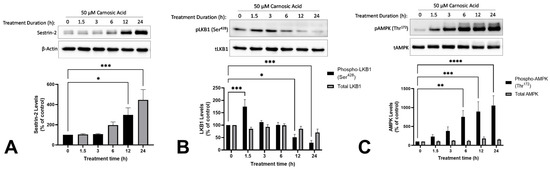
Figure 4.
Carnosic acid (CA) increases Sestrin-2 (A), phospho-LKB1 (B), and phospho-AMPK (C) in H1299 NSCLC cells. Whole cell lysates were prepared from H1299 cells treated without (control) or with 50 µM CA for 1.5–24 h. Cell lysates (20 µg) were resolved by SDS-PAGE and immunoblotted with specific antibodies against sestrin-2 (A), β-actin (A), total and phospho-LKB1 (Ser428; B), or total and phospho-AMPK (Thr172; C). Upper panel: A representative immunoblot is shown. Lower panel: The densitometry of the bands, expressed in arbitrary units was measured using ImageJ 1.54 g software. The data are expressed as percent of control and are the mean ± SEM of six separate experiments. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, compared to control.
2.4. Carnosic Acid Activates Autophagy Signalling in H1299 NSCLC Cells
LC3-I is converted to LC3-II during the later stages of autophagy and is used as a marker of autophagy. The treatment of H1299 cells with CA resulted in a time-dependent increase in levels of LC3-II with significance at 6 h (175.5 ± 35.9%, p < 0.05), 12 h (216.8 ± 46.3%, p < 0.001), and 24 h (331.4 ± 68.0%, p < 0.0001; Figure 5).
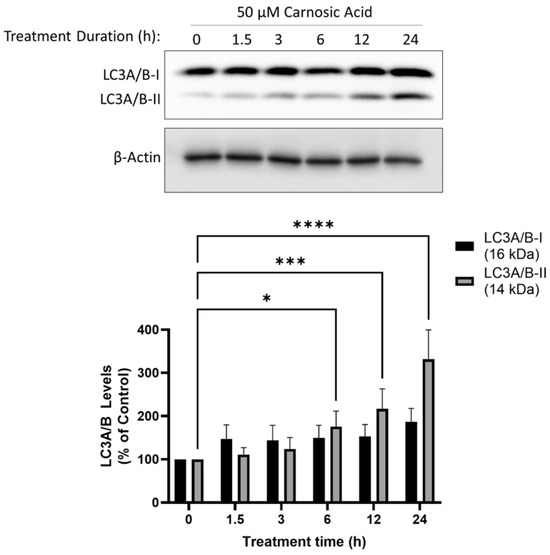
Figure 5.
Carnosic acid (CA) induces autophagy in H1299 NSCLC cells. Whole cell lysates were prepared from H1299 cells treated without (control) or with 50 µM CA for 1.5–24 h. Cell lysates (20 µg) were resolved by SDS-PAGE and immunoblotted with specific antibodies against LC3A/B or β-actin. Upper panel: A representative immunoblot is shown. Lower panel: The densitometry of the bands, expressed in arbitrary units was measured using ImageJ 1.54g software. The data are expressed as percent of control and are the mean ± SEM of six separate experiments. * p< 0.05, *** p < 0.001, **** p < 0.0001, compared to control.
To determine whether CA-induced autophagy was cytoprotective or antiproliferative, cells were treated with 10 µM CA in the presence of the autophagy inhibitor 3-methyladenine (3MA) or the autophagy activator rapamycin (RAPA). CA alone decreased thymidine incorporation to 82.00 ± 2.08% of control (p < 0.01 vs. control), and pre-treatment with 3MA blocked the effect of CA (99.00 ± 1.16% of control; p < 0.01 vs. CA). Pre-treatment with RAPA did not alter the effect of CA (84.67 ± 2.91% of control, p = 0.7187 vs. control; Figure 6). 3MA or RAPA alone did not affect 3H-thymidine incorporation (Figure S1).
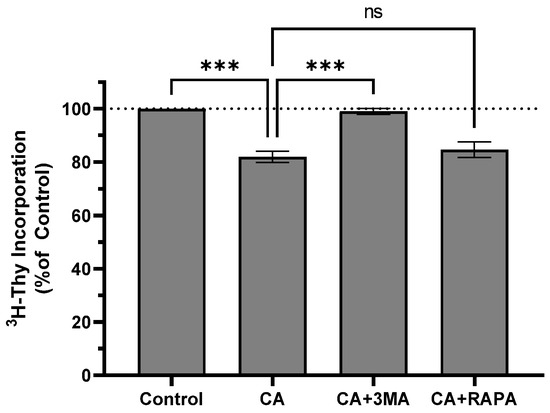
Figure 6.
Autophagy inhibitor 3MA attenuates inhibitory effect of CA on thymidine incorporation. H1299 cells were pretreated for 1 h with autophagy inhibitor 3-methyladenine (3MA; 5 mM) or rapamycin (RAPA; 200 nM) followed by exposure to 10 µM carnosic acid (CA) for an additional 24 h. Cells were exposed to 3H-thymidine for 24 h. Cells were lysed, and radioactivity measured using liquid scintillation counting. Data are the mean ± SEM for three individual experiments expressed as a percentage of control. *** p < 0.001; ns, not significant.
3. Discussion
In the present study, we observed a significant inhibition of NSCLC cell proliferation with CA treatment. While several studies have indicated that CA possesses anticancer properties, its specific impact on NSCLC remains incompletely understood. Zhao et al. [34] examined the effects of CA in A549 NSCLC cells and found a dose-dependent inhibition of proliferation and survival, the induction of apoptosis, and the inhibition of migration and invasion. The effects of CA coincided with decreased levels of matrix metalloproteinase (MMP)-9, and inhibition of PI3K/Akt/mTOR signaling [34]. In the current study, higher concentrations of CA were required to inhibit the proliferation and 3H-thymidine incorporation in H460 cells compared to H1299 cells. This lower sensitivity of H460 cells to CA treatment may be due to their lack of the tumor suppressor LKB1 and is in agreement with the findings of Corveloni et al. [35] showing that similar concentrations of CA are required to inhibit H460 cells.
Although few studies have examined the effects of CA in NSCLC (and none in H1299), there are several studies showing antiproliferative effects in other types of cancer. Yesil-Celiktas et al. [36] assessed the antiproliferative effects of CA across a panel of cancer cell lines (K-562, MCF-7, Hep-3B, PC-3, DU-14, and MDA-MB-231) and found potent antiproliferative effects ranging from 13 to 30% of control with 19 µM CA treatment for 48 h. CA treatment for 24–72 h inhibited the proliferation of AGS and MKN-45 gastric cancer cells with the IC50 ranging from 53 to 72 µM [37]. Similarly, 24 h CA treatment inhibited the proliferation of colorectal cancer cells (caco-2, HT29 and LoVo) with the IC50 ranging from 24 to 96 µM [38]. CA caused a dose-dependent inhibition of CaSki and SiHa cervical cancer cell proliferation and colony formation at concentrations over 10 µM [39]. Our findings in H1299 confirm the antiproliferative effects of CA in the micromolar range (Figure 1) and provide novel information related to CA treatment in a human NSCLC cell line.
Apoptosis is controlled cell death in response to damage that plays an essential role in tissue homeostasis [40]. Apoptosis is triggered when a stress signal such as irradiation or increased reactive oxygen species (ROS) leads to the activation of proapoptotic members of the Bcl-2 family, such as Bax, which neutralize antiapoptotic proteins like Bcl-2 leading to disruption of mitochondrial membrane permeability and release of apoptogenic factors such as cytochrome-c [41]. Cytochrome-c in turn triggers the formation of the apoptosome which recruits the initiator pro-caspase-9 causing its autoactivation. The activation of initiator caspases activates downstream executor caspases-3, -6, and -7 which cleave and inactivate the substrates necessary for proliferation such as the DNA repair protein PARP [42]. In the current study, we observed a time-dependent induction of apoptosis as indicated by significantly increased levels of cleaved caspase-7 and cleaved PARP (Figure 3B,C). We also saw a trend towards increased levels of the apoptotic markers cleaved caspase-3 and Bax, and decreased levels of the antiapoptotic protein Bcl-2. Additionally, we observed nuclear condensation that is consistent with apoptosis (Figure 3F). Our findings are in agreement with microscopic evidence of CA-induced apoptosis observed in A549 NSCLC cells [34]. CA-induced apoptosis has also been observed in gastric [37], cervical [39], and colorectal [38] cancer cells including increased caspase-7 and PARP cleavage, and changes in nuclear morphology.
Some studies suggest that AMPK is a potential target for cancer treatment and prevention. The expression of phosphorylated AMPK in histological samples from lung adenocarcinoma patients was associated with higher survival compared to patients who tested negative for phosphorylated AMPK [20]. In a previous study, we observed AMPK activation in H1299 cells with RE treatment, but the signaling upstream and downstream of AMPK was not investigated extensively [17]. Here, we hypothesized that CA activates AMPK and we wanted to investigate potential upstream signaling molecules: sestrin-2 and LKB1. The induction of sestrin-2 has been shown to have anticancer effects in NSCLC cells [43]. The knockdown of orphan nuclear receptor TR3 in A549, H460, and H1299 cells led to the inhibition of proliferation and the induction of apoptosis which was attributed to the induction of sestrin-2 and the activation of AMPK [43]. LKB1 is an upstream kinase of AMPK and it has been shown that sestrin-2 acts as a scaffold to facilitate the interaction between LKB1 and AMPK [29,44,45]
Under physiological conditions, sestrin-2 expression is induced by DNA damage in a p53-dependent manner or by ROS independent of p53. In the current study, we report a time-dependent increase in sestrin-2 expression (Figure 4A). The treatment of colon cancer cells (HCT116 and HT-29) with quercetin also increases sestrin-2 expression and induces apoptosis [46]. These effects are ROS-induced, p53-independent, as well as sestrin-2 and AMPK-dependent [46]. H1299 cells lack the tumor suppressor p53 and have low basal levels of sestrin-2. It is possible that CA exhibits antitumor effects in H1299 (which lack p53 expression) cells by inducing sestrin-2 expression independent of p53, similar to the effects of quercetin in colon cancer cells.
Here, we saw an increase in Ser428 phosphorylation of LKB1 with acute LKB1 treatment (Figure 4B). LKB1 is implicated as an upstream kinase required for the anticancer effects of several natural products. The polyphenol honokiol increased LKB1 expression in breast cancer cells (MCF7 and MDA-MB-231) and LKB1 knockdown blocked the anticancer effects [47]. Similarly, the anticancer effects of the polyphenol resveratrol in leukemic cells (HL-60) were dependent on autophagy activated via LKB1-AMPK signaling [48]. Curcumin exhibited anticancer effects in colon cancer cells (Caco-2) by increasing Ser428 phosphorylation of LKB1 and the subsequent activation of AMPK [49]. In a xenograft model of breast cancer, mango (Mangifera indica L.) polyphenols decreased tumor weight and volume, and this was associated with increased sestrin-2, LKB1, and AMPK levels [50]. Taken together, our data indicate that sestrin-2/LKB1/AMPK signaling may be a possible mechanism contributing to the antiproliferative and proapoptotic effects of CA, similar to other polyphenols, and this could explain why CA was less effective in the LKB1-null H460 cell line.
In the current study, CA treatment resulted in a time-dependent increase in LC3-II levels consistent with induction of autophagy (Figure 5). CA-induced autophagy could be a cytoprotective mechanism in response to cellular stress caused by CA. This was the case with the naturally sourced anticancer compounds polyphyllin II and pyoluteorin whose anticancer effects were enhanced by autophagy inhibition with 3MA [51,52]. Alternatively, CA-induced autophagy could be contributing to cell death, as was the case with quercetin. Quercetin treatment of H1299 resulted in the inhibition of proliferation, AMPK activation, and the induction of autophagy and autophagy inhibition with 3MA-inhibited quercetin-induced apoptosis [21]. To determine whether autophagy was contributing to cell death or cell survival, we treated H1299 cells with a sub-maximal dose (10 µM) of CA in the presence of the autophagy inhibitor 3MA or the autophagy activator RAPA. We hypothesized that if the CA-induced autophagy was cytoprotective, then 3MA should enhance the antiproliferative effect of CA, and RAPA would attenuate the antiproliferative effect of CA. We found that 3MA blocked the effect of CA while RAPA had no effect. This suggests that in our model, autophagy is contributing to the CA-induced inhibition of proliferation.
Other studies also suggest that sestrin-2-mediated autophagy has antiproliferative effects in some cancer cells. ChlA-F, a derivative of cheliensisin A [53], and isorhapontigenin, a derivative of stilbene [54], inhibit the anchorage-independent growth of bladder cancer cells by inducing sestrin-2-dependent autophagy. In colorectal cancer cells, the combination of oxaliplatin and docosahexaenoic acid caused autophagic cell death mediated by endoplasmic reticulum stress and upregulation of sestrin-2 [55]. In hepatocellular carcinoma cells (HepG2 and PLC/PRF/5), fangchinoline inhibited cell proliferation survival, and induced Sestirn-2/AMPK mediated autophagic cell death [56]. The effects of tanshinone IIA, a diterpenoid naphthoquinone against osteosarcoma cells, also involved sestrin-2-mediated autophagy [57]. CA may act through similar mechanisms to induce autophagy and cell death in lung cancer cells.
4. Materials and Methods
4.1. Materials
Human H1299 cells were obtained from the American Type Culture Collection (ATCC). Cell culture (RPMI) media, fetal bovine serum (FBS), trypsin, antibiotic, and Hoechst 33342 were from Thermo Fisher Scientific (Burlington, ON, Canada). Antibodies against caspase-3 (cat. No. 9662), caspase-7 (cat. No. 9494), PARP (cat. No. 9542), BAX (cat. No. 2772), Bcl-2 (cat. No. 2872), β-actin (cat. No. 4967), sestrin-2 (cat. No. 8487), pLKB1, LKB1 (cat. No. 3047), pAMPK (cat. No. 2535), AMPK (cat. No. 5831), and LC3 (cat. No. 12741) were purchased from Cell Signaling Technology via New England Biolabs (Mississauga, ON, Canada). Bovine serum albumin (BSA), 10% formalin, dimethyl sulfoxide (DMSO), crystal violet stain, and paclitaxel were purchased from Millipore Sigma (Oakville, ON, Canada). Carnosic acid (purity 98.17%) and 3-methyladenine were purchased from Med Chem Express (Monmouth Junction, NJ, USA).
4.2. Cell Culture
H1299 and H460 cells were cultured in RPMI media supplemented with 10% (v/v) FBS and 1% (v/v) antibiotic-antimycotic in a humidified incubator at 37 °C with 5% CO2. A stock solution of 100 mM CA was prepared in DMSO and diluted to a working concentration of 1 mM with cell culture media. The final concentration and time of exposure are indicated in each figure.
4.3. Crystal Violet Proliferation Assay
The crystal violet cell proliferation assay was performed as described previously [17]. H1299 or H460 cells were seeded (1000 cells/well) in sextuplicate in 96-well plates and treated with the indicated concentration of CA for 24 or 48 h. Following treatment, cells were fixed with 10% formalin and stained with 0.5% (w/v) crystal violet. Crystal violet dye was solubilized, and absorbance was read at 570 nm using a KC4 plate reader (Bio-Tek, Winooski, VT, USA). The data are expressed as a percentage of the control.
4.4. [3H]-Thymidine Incorporation Assay
The [3H]-thymidine incorporation assay was performed as described previously [58]. Subconfluent H1299 was serum-deprived for 24 h and then treated with the indicated concentrations of CA followed by the addition of 10 μM [3H]-thymidine for 24 h. Following treatment, the media was removed, and the cells were rinsed three times with ice-cold HEPES-buffered saline (HBS). Unincorporated [3H]-thymidine was solubilized with 10% trichloroacetic acid (TCA) for 10 min at 4 °C. The TCA was aspirated, and the cells were rinsed twice with ice-cold HBS, followed by lysis with 0.05 N NaOH. The radioactivity of lysates was determined using liquid scintillation counting and data were expressed as a percentage of control.
4.5. Clonogenic Survival Assay
Clonogenic survival assays were performed as described previously [17]. H1299 or H460 cells were seeded in duplicate in 12-well plates (500 cells/well), allowed to adhere overnight, and exposed to media containing the indicated concentrations of CA for 7 days. Colonies were fixed with 10% formalin and stained with 0.5% (w/v) crystal violet; representative images were taken using a BioTek Cytation5 plate reader. Colonies (>50 cells) were counted, and the data were expressed as the surviving fraction compared to the control.
4.6. SDS-PAGE and Western Blotting
SDS-PAGE and Western blotting were performed as described previously [17]. Following treatment, cells were washed with ice-cold PBS and lysed with ice-cold RIPA buffer containing phosphatase and protease inhibitor. Lysate was combined with Laemmli sample buffer and boiled for 5 min. Proteins (20 µg) were separated using SDS-PAGE, transferred to a PVDF membrane, and incubated with the indicated antibodies. Low molecular weight targets (<40 kDa), mid-range targets (40–100 kDa), and high molecular weight targets (>100 kDa) were electrophoresed using hand-cast 12.5%, 10%, or 7.5% polyacrylamide gels, respectively. β-actin was used as a loading control. Blots were visualized using a BioRad chemidoc imager. Densitometric analysis was performed using Image J 1.54g software. The data are expressed as the mean ± SEM relative to the control.
4.7. Statistical Analysis
All data are expressed as the mean ± SEM for the indicated number of individual experiments. Analysis of variance (ANOVA) was performed using GraphPad Prism 8 software. All p-values ≤ 0.05 were considered significant. Any significant ANOVA result was followed with Dunnett’s post hoc test.
5. Conclusions
Previously, we have shown that rosemary (Rosmarinus officinalis) extract (RE) inhibits the proliferation, survival, and migration of H1299 NSCLC cells via a mechanism involving the activation of AMPK and the induction of apoptosis [17]. In the current study, we found that the treatment of H1299 and H460 NSCLC cells with CA (a RE polyphenolic diterpene) resulted in the inhibition of proliferation and survival. In H1299 cells, CA induced apoptosis and autophagy, and these effects correlated with increased levels of sestrin-2, phospho-LKB1, and phospho-AMPK. Inhibiting autophagy with 3MA blocked the antiproliferative effect of CA (see graphical abstract). Taken together, these results indicate that CA can induce the apoptosis of H1299 NSCLC cells through a mechanism involving sestrin-2/LKB1/AMPK signaling and the induction of autophagy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms25041950/s1.
Author Contributions
Conceptualization, E.J.O. and E.T.; methodology, E.J.O. and E.T.; resources, E.T.; data curation, E.J.O.; writing—original draft preparation, E.J.O.; writing—review and editing, E.J.O., E.T., N.S.K.S. and R.E.K.M.; visualization, E.J.O.; supervision, E.T. All authors have read and agreed to the published version of the manuscript.
Funding
E.J.O. was supported by a NSERC Canada Graduate Scholarship.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and supplementary information.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, A.G.; Tsao, M.S.; Beasley, M.B.; Borczuk, A.C.; Brambilla, E.; Cooper, W.A.; Dacic, S.; Jain, D.; Kerr, K.M.; Lantuejoul, S.; et al. The 2021 WHO Classification of Lung Tumors: Impact of Advances since 2015. J. Thorac. Oncol. 2022, 17, 362–387. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, D.S.; Wood, D.E.; Aggarwal, C.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 1.2020. J. Natl. Compr. Cancer Netw. JNCCN 2019, 17, 1464–1472. [Google Scholar] [CrossRef]
- National Cancer Institute. SEER*Explorer: An Interactive Website for SEER Cancer Statistics. Available online: https://seer.cancer.gov/statistics-network/explorer/ (accessed on 26 September 2023).
- Brambilla, E.; Gazdar, A. Pathogenesis of Lung Cancer Signaling Pathways: Roadmap for Therapies. Eur. Respir. J. Off. J. Eur. Soc. Clin. Respir. Physiol. 2009, 33, 1485–1497. [Google Scholar] [CrossRef]
- Westover, D.; Zugazagoitia, J.; Cho, B.C.; Lovly, C.M.; Paz-Ares, L. Mechanisms of Acquired Resistance to First- and Second-Generation EGFR Tyrosine Kinase Inhibitors. Ann. Oncol. 2018, 29, i10–i19. [Google Scholar] [CrossRef]
- Stephenson, J. Potent Anti-Cancer Drug from Witches’ Umbrella. Lancet Oncol. 2000, 1, 8. [Google Scholar] [CrossRef]
- Renneberg, R. Biotech History: Yew Trees, Paclitaxel Synthesis and Fungi. Biotechnol. J. 2007, 2, 1207–1209. [Google Scholar] [CrossRef]
- Moreno, S.; Scheyer, T.; Romano, C.S.; Vojnov, A.A. Antioxidant and Antimicrobial Activities of Rosemary Extracts Linked to Their Polyphenol Composition. Free Radic. Res. 2006, 40, 223–231. [Google Scholar] [CrossRef]
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and Antimicrobial Properties of Rosemary (Rosmarinus officinalis, L.): A Review. Medicines 2018, 5, 98. [Google Scholar] [CrossRef] [PubMed]
- Naimi, M.; Vlavcheski, F.; Shamshoum, H.; Tsiani, E. Rosemary Extract as a Potential Anti-Hyperglycemic Agent: Current Evidence and Future Perspectives. Nutrients 2017, 9, 968. [Google Scholar] [CrossRef]
- Vlavcheski, F.; Tsiani, E. Attenuation of Free Fatty Acid-Induced Muscle Insulin Resistance by Rosemary Extract. Nutrients 2018, 10, 1623. [Google Scholar] [CrossRef]
- Moore, J.; Yousef, M.; Tsiani, E. Anticancer Effects of Rosemary (Rosmarinus officinalis L.) Extract and Rosemary Extract Polyphenols. Nutrients 2016, 8, 731. [Google Scholar] [CrossRef]
- O’Neill, E.J.; Den Hartogh, D.J.; Azizi, K.; Tsiani, E. Anticancer Properties of Carnosol: A Summary of In Vitro and In Vivo Evidence. Antioxidants 2020, 9, 961. [Google Scholar] [CrossRef]
- Moore, J.; Megaly, M.; MacNeil, A.J.; Klentrou, P.; Tsiani, E. Rosemary Extract Reduces Akt/MTOR/P70S6K Activation and Inhibits Proliferation and Survival of A549 Human Lung Cancer Cells. Biomed. Pharmacother. 2016, 83, 725–732. [Google Scholar] [CrossRef]
- O’Neill, E.J.; Moore, J.; Song, J.; Tsiani, E. Inhibition of Non-Small Cell Lung Cancer Proliferation and Survival by Rosemary Extract Is Associated with Activation of ERK and AMPK. Life 2021, 12, 52. [Google Scholar] [CrossRef] [PubMed]
- Jaglanian, A.; Tsiani, E. Rosemary Extract Inhibits Proliferation, Survival, Akt, and MTOR Signaling in Triple-Negative Breast Cancer Cells. Int. J. Mol. Sci. 2020, 21, 810. [Google Scholar] [CrossRef] [PubMed]
- Jaglanian, A.; Termini, D.; Tsiani, E. Rosemary (Rosmarinus officinalis L.) Extract Inhibits Prostate Cancer Cell Proliferation and Survival by Targeting Akt and MTOR. Biomed. Pharmacother. 2020, 131, 110717. [Google Scholar] [CrossRef] [PubMed]
- William, W.N.; Kim, J.-S.; Liu, D.D.; Solis, L.; Behrens, C.; Lee, J.J.; Lippman, S.M.; Kim, E.S.; Hong, W.K.; Wistuba, I.I.; et al. The Impact of Phosphorylated AMP-Activated Protein Kinase Expression on Lung Cancer Survival. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2012, 23, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ding, H.; Tang, X.; Liang, M.; Li, S.; Zhang, J.; Cao, J. Quercetin Induces Pro-Apoptotic Autophagy via SIRT1/AMPK Signaling Pathway in Human Lung Cancer Cell Lines A549 and H1299 in Vitro. Thorac. Cancer 2021, 12, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fan, Y.; Zhang, Y.; Liu, Y.; Yu, Y.; Ma, M. Resveratrol Induces Autophagy and Apoptosis in Non-Small-Cell Lung Cancer Cells by Activating the NGFR-AMPK-MTOR Pathway. Nutrients 2022, 14, 2413. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-H.; Hsieh, C.-H.; Tsai, S.-Y.; Wang, C.-Y.; Wang, C.-C. Anticancer Effects of Epigallocatechin-3-Gallate Nanoemulsion on Lung Cancer Cells through the Activation of AMP-Activated Protein Kinase Signaling Pathway. Sci. Rep. 2020, 10, 5163. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Budanov, A.V.; Talukdar, S.; Park, E.J.; Park, H.L.; Park, H.-W.; Bandyopadhyay, G.; Li, N.; Aghajan, M.; Jang, I.; et al. Maintenance of Metabolic Homeostasis by Sestrin2 and Sestrin3. Cell Metab. 2012, 16, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Budanov, A.V.; Karin, M. The P53-Regulated Sestrin Gene Products Inhibit MTOR Signaling. Cell 2008, 134, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Shackelford, D.B.; Shaw, R.J. The LKB1–AMPK Pathway: Metabolism and Growth Control in Tumour Suppression. Nat. Rev. Cancer 2009, 9, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Iwakawa, R.; Takahashi, K.; Kohno, T.; Nakanishi, Y.; Matsuno, Y.; Suzuki, K.; Nakamoto, M.; Shimizu, E.; Minna, J.D.; et al. Prevalence and Specificity of LKB1 Genetic Alterations in Lung Cancers. Oncogene 2007, 26, 5911–5918. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Ramsey, M.R.; Hayes, D.N.; Fan, C.; McNamara, K.; Kozlowski, P.; Torrice, C.; Wu, M.C.; Shimamura, T.; Perera, S.A.; et al. LKB1 Modulates Lung Cancer Differentiation and Metastasis. Nature 2007, 448, 807–810. [Google Scholar] [CrossRef]
- Morrison, A.; Chen, L.; Wang, J.; Zhang, M.; Yang, H.; Ma, Y.; Budanov, A.; Lee, J.H.; Karin, M.; Li, J. Sestrin2 Promotes LKB1-Mediated AMPK Activation in the Ischemic Heart. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2015, 29, 408–417. [Google Scholar] [CrossRef]
- Kullmann, L.; Krahn, M.P. Controlling the Master—Upstream Regulation of the Tumor Suppressor LKB1. Oncogene 2018, 37, 3045–3057. [Google Scholar] [CrossRef]
- Levy, J.M.M.; Towers, C.G.; Thorburn, A. Targeting Autophagy in Cancer. Nat. Rev. Cancer 2017, 17, 528–542. [Google Scholar] [CrossRef]
- Tamaddoni, A.; Mohammadi, E.; Sedaghat, F.; Qujeq, D.; As’Habi, A. The Anticancer Effects of Curcumin via Targeting the Mammalian Target of Rapamycin Complex 1 (MTORC1) Signaling Pathway. Pharmacol. Res. 2020, 156, 104798. [Google Scholar] [CrossRef]
- Lu, W.; Ke, H.; Qianshan, D.; Zhen, W.; Guoan, X.; Honggang, Y. Apatinib Has Anti-Tumor Effects and Induces Autophagy in Colon Cancer Cells. Iran. J. Basic Med. Sci. 2017, 20, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, J.; Fan, Y.; Li, Y. Antiproliferative Activity of Carnosic Acid Is Mediated via Inhibition of Cell Migration and Invasion, and Suppression of Phosphatidylinositol 3-Kinases (PI3K)/AKT/Mammalian Target of Rapamycin (MTOR) Signaling Pathway. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 7864–7871. [Google Scholar] [CrossRef]
- Corveloni, A.C.; Semprebon, S.C.; Baranoski, A.; Biazi, B.I.; Zanetti, T.A.; Mantovani, M.S. Carnosic Acid Exhibits Antiproliferative and Proapoptotic Effects in Tumoral NCI-H460 and Nontumoral IMR-90 Lung Cells. J. Toxicol. Environ. Health A 2020, 83, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Yesil-Celiktas, O.; Sevimli, C.; Bedir, E.; Vardar-Sukan, F. Inhibitory Effects of Rosemary Extracts, Carnosic Acid and Rosmarinic Acid on the Growth of Various Human Cancer Cell Lines. Plant Foods Hum. Nutr. 2010, 65, 158–163. [Google Scholar] [CrossRef] [PubMed]
- El-Huneidi, W.; Bajbouj, K.; Muhammad, J.S.; Vinod, A.; Shafarin, J.; Khoder, G.; Saleh, M.A.; Taneera, J.; Abu-Gharbieh, E. Carnosic Acid Induces Apoptosis and Inhibits Akt/MTOR Signaling in Human Gastric Cancer Cell Lines. Pharmaceuticals 2021, 14, 230. [Google Scholar] [CrossRef]
- Barni, M.V.; Carlini, M.J.; Cafferata, E.G.; Puricelli, L.; Moreno, S. Carnosic Acid Inhibits the Proliferation and Migration Capacity of Human Colorectal Cancer Cells. Oncol. Rep. 2012, 27, 1041–1048. [Google Scholar] [CrossRef]
- Su, K.; Wang, C.; Zhang, Y.; Cai, Y.; Zhang, Y.; Zhao, Q. The Inhibitory Effects of Carnosic Acid on Cervical Cancer Cells Growth by Promoting Apoptosis via ROS-Regulated Signaling Pathway. Biomed. Pharmacother. 2016, 82, 180–191. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as Anticancer Mechanism: Function and Dysfunction of Its Modulators and Targeted Therapeutic Strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef]
- Danial, N.N.; Korsmeyer, S.J. Cell Death: Critical Control Points. Cell 2004, 116, 205–219. [Google Scholar] [CrossRef]
- Degterev, A.; Boyce, M.; Yuan, J. A Decade of Caspases. Oncogene 2003, 22, 8543–8567. [Google Scholar] [CrossRef]
- Lee, S.-O.; Andey, T.; Jin, U.-H.; Kim, K.; Sachdeva, M.; Safe, S. The Nuclear Receptor TR3 Regulates MTORC1 Signaling in Lung Cancer Cells Expressing Wild-Type P53. Oncogene 2012, 31, 3265–3276. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Shao, H.; Liu, S.; Niu, Y.; Fu, L. Globular Adiponectin Ameliorates Insulin Resistance in Skeletal Muscle by Enhancing the LKB1-Mediated AMPK Activation via SESN2. Sports Med. Health Sci. 2022, 5, 34–41. [Google Scholar] [CrossRef]
- Quan, N.; Sun, W.; Wang, L.; Chen, X.; Bogan, J.S.; Zhou, X.; Cates, C.; Liu, Q.; Zheng, Y.; Li, J. Sestrin2 Prevents Age-Related Intolerance to Ischemia and Reperfusion Injury by Modulating Substrate Metabolism. FASEB J. 2017, 31, 4153–4167. [Google Scholar] [CrossRef]
- Kim, G.T.; Lee, S.H.; Kim, J.I.; Kim, Y.M. Quercetin Regulates the Sestrin 2-AMPK-P38 MAPK Signaling Pathway and Induces Apoptosis by Increasing the Generation of Intracellular ROS in a P53-Independent Manner. Int. J. Mol. Med. 2014, 33, 863. [Google Scholar] [CrossRef]
- Nagalingam, A.; Arbiser, J.L.; Bonner, M.Y.; Saxena, N.K.; Sharma, D. Honokiol Activates AMP-Activated Protein Kinase in Breast Cancer Cells via an LKB1-Dependent Pathway and Inhibits Breast Carcinogenesis. Breast Cancer Res. BCR 2012, 14, R35. [Google Scholar] [CrossRef]
- Fan, Y.; Chiu, J.-F.; Liu, J.; Deng, Y.; Xu, C.; Zhang, J.; Li, G. Resveratrol Induces Autophagy-Dependent Apoptosis in HL-60 Cells. BMC Cancer 2018, 18, 581. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Moreau, R. Curcumin Represses MTORC1 Signaling in Caco-2 Cells by a Two-Sided Mechanism Involving the Loss of IRS-1 and Activation of AMPK. Cell. Signal. 2021, 78, 109842. [Google Scholar] [CrossRef] [PubMed]
- Nemec, M.J.; Kim, H.; Marciante, A.B.; Barnes, R.C.; Hendrick, E.D.; Bisson, W.H.; Talcott, S.T.; Mertens-Talcott, S.U. Polyphenolics from Mango (Mangifera indica L.) Suppress Breast Cancer Ductal Carcinoma in Situ Proliferation through Activation of AMPK Pathway and Suppression of MTOR in Athymic Nude Mice. J. Nutr. Biochem. 2017, 41, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Yu, S.; Yang, Y.; Qu, S. Pyoluteorin Induces Apoptosis and Autophagy in NSCLC Cells. Biol. Pharm. Bull. 2021, 44, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Xin, M.; Xu, J.; Xiang, X.; Li, X.; Jiang, J.; Jia, X. Polyphyllin II Induced Apoptosis of NSCLC Cells by Inhibiting Autophagy through the MTOR Pathway. Pharm. Biol. 2022, 60, 1781–1789. [Google Scholar] [CrossRef]
- Hua, X.; Xu, J.; Deng, X.; Xu, J.; Li, J.; Zhu, D.Q.; Zhu, J.; Jin, H.; Tian, Z.; Huang, H. New Compound ChlA-F Induces Autophagy-Dependent Anti-Cancer Effect via Upregulating Sestrin-2 in Human Bladder Cancer. Cancer Lett. 2018, 436, 38–51. [Google Scholar] [CrossRef]
- Liang, Y.; Zhu, J.; Huang, H.; Xiang, D.; Li, Y.; Zhang, D.; Li, J.; Wang, Y.; Jin, H.; Jiang, G.; et al. SESN2/Sestrin 2 Induction-Mediated Autophagy and Inhibitory Effect of Isorhapontigenin (ISO) on Human Bladder Cancers. Autophagy 2016, 12, 1229–1239. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, D.Y.; Kang, S.H.; Yun, H.K.; Kim, J.L.; Kim, B.R.; Park, S.H.; Na, Y.J.; Jo, M.J.; Jeong, Y.A. Docosahexaenoic Acid Enhances Oxaliplatin-Induced Autophagic Cell Death via the ER Stress/Sesn2 Pathway in Colorectal Cancer. Cancers 2019, 11, 982. [Google Scholar] [CrossRef]
- Wang, N.; Pan, W.; Zhu, M.; Zhang, M.; Hao, X.; Liang, G.; Feng, Y. Fangchinoline Induces Autophagic Cell Death via P53/Sestrin2/AMPK Signalling in Human Hepatocellular Carcinoma Cells. Br. J. Pharmacol. 2011, 164, 731–742. [Google Scholar] [CrossRef]
- Yen, J.-H.; Huang, S.-T.; Huang, H.-S.; Fong, Y.-C.; Wu, Y.-Y.; Chiang, J.-H.; Su, Y.-C. HGK-Sestrin 2 Signaling-Mediated Autophagy Contributes to Antitumor Efficacy of Tanshinone IIA in Human Osteosarcoma Cells. Cell Death Dis. 2018, 9, 1003. [Google Scholar] [CrossRef]
- Barron, C.C.; Moore, J.; Tsakiridis, T.; Pickering, G.; Tsiani, E. Inhibition of Human Lung Cancer Cell Proliferation and Survival by Wine. Cancer Cell Int. 2014, 14, 6. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).