Peripheral Endocannabinoid Components and Lipid Plasma Levels in Patients with Resistant Migraine and Co-Morbid Personality and Psychological Disorders: A Cross-Sectional Study
Abstract
1. Introduction
2. Results
2.1. Patient Population
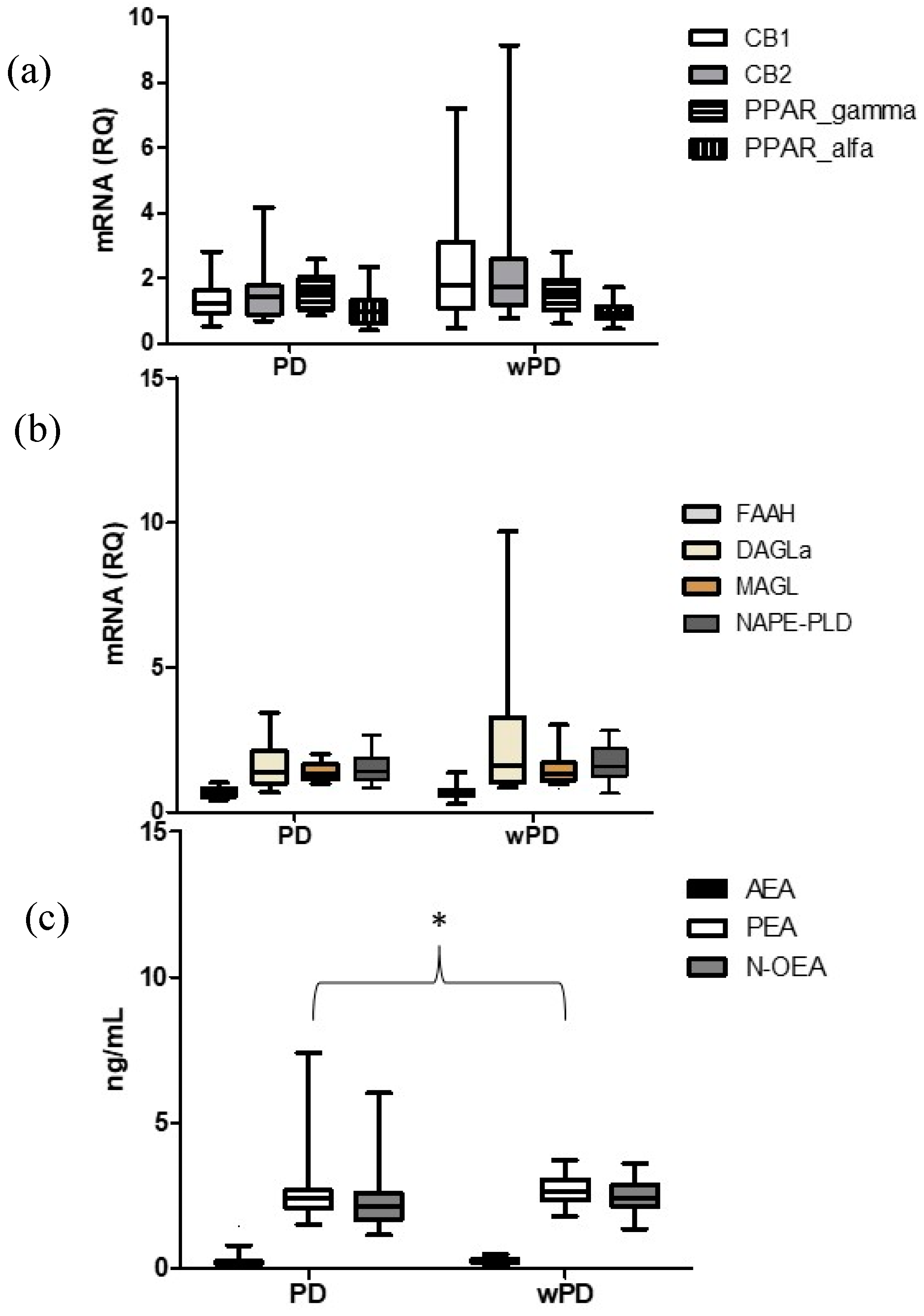
2.2. Comparison between Subjects with PD (PD) and without PD (wPD)
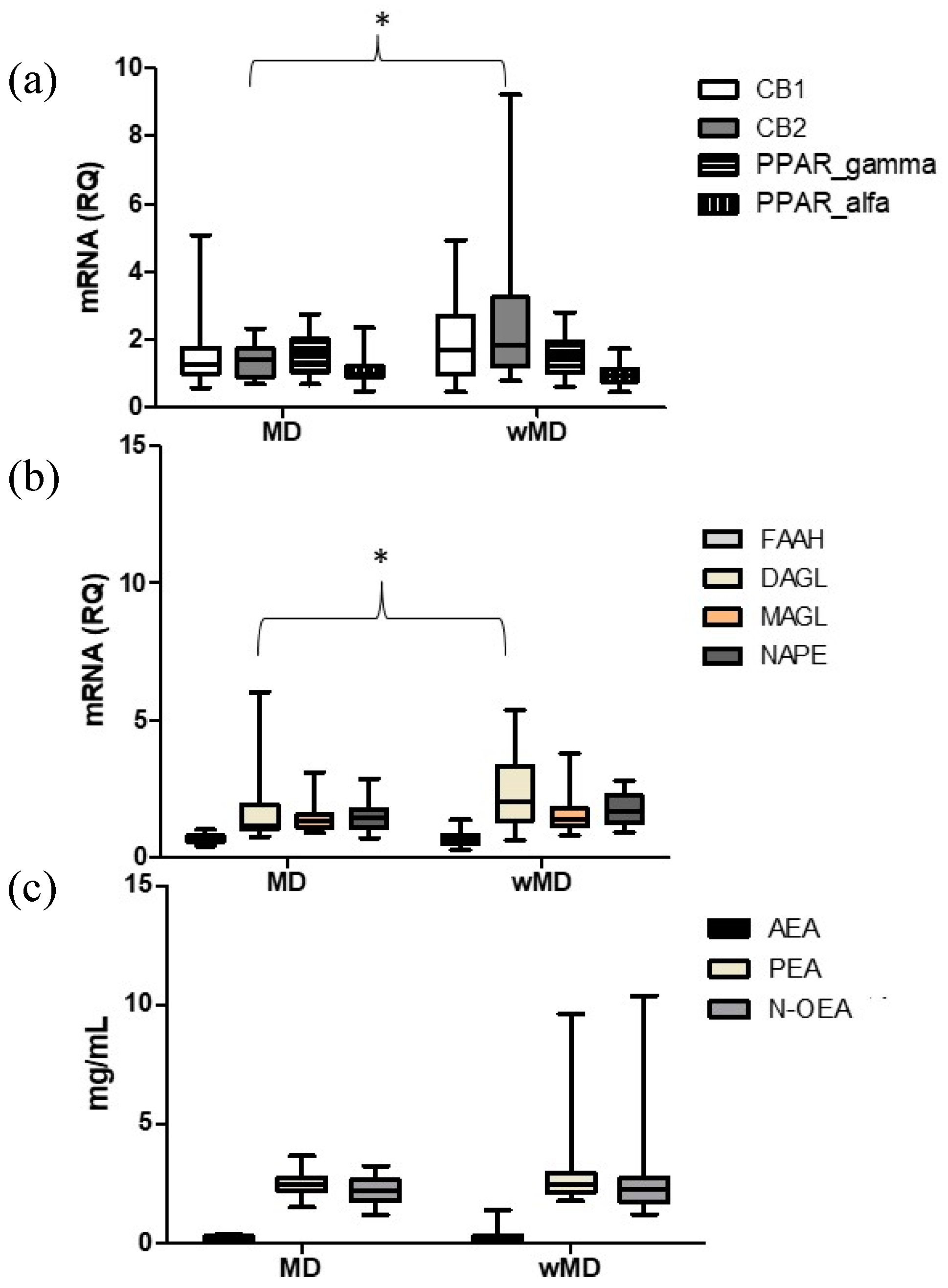
2.3. Comparison between Subjects with MD and Subjects without MD (wMD)
3. Discussion
Limitations of the Study
4. Materials and Methods
4.1. Resistant Migraine Patients
4.2. Procedure
4.3. Psychological Evaluation
4.4. Isolation of Peripheral Blood Mononuclear Cells (PBMCs)
4.5. Gene Expression of ES Components
4.6. Quantitative Profiling of AEA, 2-AG and Related Lipids by LC-MS/MS
4.7. Primary and Secondary Outcome Measures
4.8. Statistical Procedures
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ashina, M.; Terwindt, G.M.; Al-Karagholi, M.A.-M.; de Boer, I.; Lee, M.J.; Hay, D.L.; Schulte, L.H.; Hadjikhani, N.; Sinclair, A.J.; Ashina, H.; et al. Migraine: Disease Characterisation, Biomarkers, and Precision Medicine. Lancet 2021, 397, 1496–1504. [Google Scholar] [CrossRef]
- Schulman, E.A.; Lake, A.E.; Goadsby, P.J.; Peterlin, B.L.; Siegel, S.E.; Markley, H.G.; Lipton, R.B. Defining Refractory Migraine and Refractory Chronic Migraine: Proposed Criteria From the Refractory Headache Special Interest Section of the American Headache Society. Headache J. Head Face Pain 2008, 48, 778–782. [Google Scholar] [CrossRef]
- Sacco, S.; Braschinsky, M.; Ducros, A.; Lampl, C.; Little, P.; van den Brink, A.M.; Pozo-Rosich, P.; Reuter, U.; de la Torre, E.R.; Sanchez Del Rio, M.; et al. European Headache Federation Consensus on the Definition of Resistant and Refractory Migraine. J. Headache Pain 2020, 21, 76. [Google Scholar] [CrossRef] [PubMed]
- Viana, M.; Bottiroli, S.; Sances, G.; Ghiotto, N.; Allena, M.; Guaschino, E.; Nappi, G.; Tassorelli, C. Factors Associated to Chronic Migraine with Medication Overuse: A Cross-Sectional Study. Cephalalgia 2018, 38, 2045–2057. [Google Scholar] [CrossRef]
- Bottiroli, S.; Galli, F.; Viana, M.; De Icco, R.; Bitetto, V.; Allena, M.; Pazzi, S.; Sances, G.; Tassorelli, C. Negative Short-Term Outcome of Detoxification Therapy in Chronic Migraine with Medication Overuse Headache: Role for Early Life Traumatic Experiences and Recent Stressful Events. Front. Neurol. 2019, 10, 173. [Google Scholar] [CrossRef]
- Bottiroli, S.; Viana, M.; Sances, G.; Ghiotto, N.; Guaschino, E.; Galli, F.; Vegni, E.; Pazzi, S.; Nappi, G.; Tassorelli, C. Psychological Factors Associated with Failure of Detoxification Treatment in Chronic Headache Associated with Medication Overuse. Cephalalgia 2016, 36, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Bottiroli, S.; Renzi, A.; Ballante, E.; De Icco, R.; Sances, G.; Tanzilli, A.; Vecchi, T.; Tassorelli, C.; Galli, F. Personality in Chronic Headache: A Systematic Review with Meta-Analysis. Pain Res. Manag. 2023, 2023, 6685372. [Google Scholar] [CrossRef]
- Bottiroli, S.; Allena, M.; Sances, G.; De Icco, R.; Avenali, M.; Fadic, R.; Katsarava, Z.; Lainez, M.J.A.; Goicochea, M.T.; Bendtsen, L.; et al. Psychological, Clinical, and Therapeutic Predictors of the Outcome of Detoxification in a Large Clinical Population of Medication-Overuse Headache: A Six-Month Follow-up of the COMOESTAS Project. Cephalalgia 2019, 39, 135–147. [Google Scholar] [CrossRef]
- Bottiroli, S.; De Icco, R.; Vaghi, G.; Pazzi, S.; Guaschino, E.; Allena, M.; Ghiotto, N.; Martinelli, D.; Tassorelli, C.; Sances, G. Psychological Predictors of Negative Treatment Outcome with Erenumab in Chronic Migraine: Data from an Open Label Long-Term Prospective Study. J. Headache Pain 2021, 22, 114. [Google Scholar] [CrossRef] [PubMed]
- Bottiroli, S.; Allena, M.; Sances, G.; De Icco, R.; Avenali, M.; Fadic, R.; Katsarava, Z.; Lainez, M.J.A.; Goicochea, M.T.; Jensen, R.H.; et al. Changes in Anxiety and Depression Symptoms Associated to the Outcome of MOH: A Post-Hoc Analysis of the Comoestas Project. Cephalalgia 2018, 38, 646–654. [Google Scholar] [CrossRef]
- Seng, E.K.; Seng, C.D. Understanding Migraine and Psychiatric Comorbidity. Curr. Opin. Neurol. 2016, 29, 309–313. [Google Scholar] [CrossRef]
- De Icco, R.; Fiamingo, G.; Greco, R.; Bottiroli, S.; Demartini, C.; Zanaboni, A.M.; Allena, M.; Guaschino, E.; Martinelli, D.; Grillo, V.; et al. Neurophysiological and Biomolecular Effects of Erenumab in Chronic Migraine: An Open Label Study. Cephalalgia 2020, 40, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Andrasik, F.; Flor, H.; Turk, D.C. An Expanded View of Psychological Aspects in Head Pain: The Biopsychosocial Model. Neurol. Sci. 2005, 26, s87–s91. [Google Scholar] [CrossRef] [PubMed]
- Rosignoli, C.; Ornello, R.; Onofri, A.; Caponnetto, V.; Grazzi, L.; Raggi, A.; Leonardi, M.; Sacco, S. Applying a Biopsychosocial Model to Migraine: Rationale and Clinical Implications. J. Headache Pain 2022, 23, 100. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Lecue, I.; Pilar-Cuéllar, F.; Muguruza, C.; Florensa-Zanuy, E.; Díaz, Á.; Urigüen, L.; Castro, E.; Pazos, A.; Callado, L.F. The Endocannabinoid System in Mental Disorders: Evidence from Human Brain Studies. Biochem. Pharmacol. 2018, 157, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Marco, E.M.; García-Gutiérrez, M.S.; Bermúdez-Silva, F.-J.; Moreira, F.A.; Guimarães, F.; Manzanares, J.; Viveros, M.-P. Endocannabinoid System and Psychiatry: In Search of a Neurobiological Basis for Detrimental and Potential Therapeutic Effects. Front. Behav. Neurosci. 2011, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Parolaro, D.; Realini, N.; Vigano, D.; Guidali, C.; Rubino, T. The Endocannabinoid System and Psychiatric Disorders. Exp. Neurol. 2010, 224, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Garani, R.; Watts, J.J.; Mizrahi, R. Endocannabinoid System in Psychotic and Mood Disorders, a Review of Human Studies. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 106, 110096. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Gao, M.; Gao, F.; Su, Q.; Wu, J. Brain Cannabinoid Receptor 2: Expression, Function and Modulation. Acta Pharmacol. Sin. 2017, 38, 312–316. [Google Scholar] [CrossRef]
- Juhasz, G.; Csepany, E.; Hullam, G.; Kokonyei, G.; Bagdy, G. Variants in the CNR1 Gene Predispose to Headache with nausea in the presence of life stress. Genes Brain Behav. 2016, 16, 384–393. [Google Scholar] [CrossRef]
- Juhasz, G.; Lazary, J.; Chase, D.; Pegg, E.; Downey, D.; Toth, Z.G.; Stones, K.; Platt, H.; Mekli, K.; Payton, A.; et al. Variations in the Cannabinoid Receptor 1 Gene Predispose to Migraine. Neurosci. Lett. 2009, 461, 116–120. [Google Scholar] [CrossRef]
- Gonda, X.; Rihmer, Z.; Juhasz, G.; Zsombok, T.; Bagdy, G. High Anxiety and Migraine Are Associated with the s Allele of the 5HTTLPR Gene Polymorphism. Psychiatry Res. 2007, 149, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Lazary, J.; Eszlari, N.; Juhasz, G.; Bagdy, G. Genetically Reduced FAAH Activity May Be a Risk for the Development of Anxiety and Depression in Persons with Repetitive Childhood Trauma. Eur. Neuropsychopharmacol. 2016, 26, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Greco, R.; Demartini, C.; Zanaboni, A.M.; Piomelli, D.; Tassorelli, C. Endocannabinoid System and Migraine Pain: An Update. Front. Neurosci. 2018, 12, 172. [Google Scholar] [CrossRef] [PubMed]
- Tassorelli, C.; Greco, R.; Silberstein, S.D. The Endocannabinoid System in Migraine: From Bench to Pharmacy and Back. Curr. Opin. Neurol. 2019, 32, 405–412. [Google Scholar] [CrossRef]
- Perrotta, A.; Arce-Leal, N.; Tassorelli, C.; Gasperi, V.; Sances, G.; Blandini, F.; Serrao, M.; Bolla, M.; Pierelli, F.; Nappi, G.; et al. Acute Reduction of Anandamide-Hydrolase (FAAH) Activity Is Coupled With a Reduction of Nociceptive Pathways Facilitation in Medication-Overuse Headache Subjects After Withdrawal Treatment. Headache J. Head Face Pain 2012, 52, 1350–1361. [Google Scholar] [CrossRef]
- Greco, R.; Demartini, C.; Zanaboni, A.M.; Tumelero, E.; De Icco, R.; Sances, G.; Allena, M.; Tassorelli, C. Peripheral Changes of Endocannabinoid System Components in Episodic and Chronic Migraine Patients: A Pilot Study. Cephalalgia 2021, 41, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Sacco, S.; Lampl, C.; Maassen van den Brink, A.; Caponnetto, V.; Braschinsky, M.; Ducros, A.; Little, P.; Pozo-Rosich, P.; Reuter, U.; Ruiz de la Torre, E.; et al. Burden and Attitude to Resistant and Refractory Migraine: A Survey from the European Headache Federation with the Endorsement of the European Migraine & Headache Alliance. J. Headache Pain 2021, 22, 39. [Google Scholar] [CrossRef]
- Navarrete, F.; García-Gutiérrez, M.S.; Jurado-Barba, R.; Rubio, G.; Gasparyan, A.; Austrich-Olivares, A.; Manzanares, J. Endocannabinoid System Components as Potential Biomarkers in Psychiatry. Front. Psychiatry 2020, 11, 315. [Google Scholar] [CrossRef]
- Kolla, N.J.; Boileau, I.; Bagby, R.M. Higher Trait Neuroticism Is Associated with Greater Fatty Acid Amide Hydrolase Binding in Borderline and Antisocial Personality Disorders. Sci. Rep. 2022, 12, 1126. [Google Scholar] [CrossRef]
- Burke, N.N.; Finn, D.P.; Mcguire, B.E.; Roche, M. Review Psychological Stress in Early Life as a Predisposing Factor for the Development of Chronic Pain: Clinical and Preclinical Evidence and Neurobiological Mechanisms. J. Neurosci. Res. 2016, 95, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Cupini, L.M.; Costa, C.; Sarchielli, P.; Bari, M.; Battista, N.; Eusebi, P.; Calabresi, P.; Maccarrone, M. Degradation of Endocannabinoids in Chronic Migraine and Medication Overuse Headache. Neurobiol. Dis. 2008, 30, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Sarchielli, P.; Pini, L.A.; Coppola, F.; Rossi, C.; Baldi, A.; Mancini, M.L.; Calabresi, P. Endocannabinoids in Chronic Migraine: CSF Findings Suggest a System Failure. Neuropsychopharmacology 2007, 32, 1384–1390. [Google Scholar] [CrossRef] [PubMed]
- Alger, B.E.; Kim, J. Supply and Demand for Endocannabinoids. Trends Neurosci. 2011, 34, 304–315. [Google Scholar] [CrossRef]
- Taber, K.H.; Hurley, R.A. Endocannabinoids: Stress, Anxiety, and Fear. J. Neuropsychiatry Clin. Neurosci. 2009, 21, iv-113. [Google Scholar] [CrossRef] [PubMed]
- Angstman, K.B.; Rasmussen, N.H. Personality Disorders: Review and Clinical Application in Daily Practice. Am. Fam. Physician 2011, 84, 1253–1260. [Google Scholar]
- Yang, F.; Alvarenga, I.; Rodrigo, S.; Gomez, S.; Kummer, A. Personality Disorders Are Associated with More Severe Forms of Migraine. Acta Neurol. Belg. 2019, 119, 201–205. [Google Scholar] [CrossRef]
- Kayhan, F.; Ilik, F. Prevalence of Personality Disorders in Patients with Chronic Migraine. Compr. Psychiatry 2016, 68, 60–64. [Google Scholar] [CrossRef]
- Wang, W.; Larstone, R.; Health, N.; Jang, K.L. Disordered personality traits in primary headaches. Soc. Behav. Personal. Int. J. 2019, 33, 495–502. [Google Scholar] [CrossRef]
- Westen, D.; Shedler, J. Revising and Assessing Axis II, Part II: Toward an Empirically Based and Clinically Useful Classification of Personality Disorders. Am. J. Psychiatry 1999, 156, 273–285. [Google Scholar] [CrossRef]
- Westen, D.; Shedler, J. Revising and Assessing Axis II, Part I: Developing a Clinically and Empirically Valid Assessment Method. Am. J. Psychiatry 1999, 156, 258–272. [Google Scholar] [CrossRef]
- Fava, M.; Farabaugh, A.H.; Sickinger, A.H.; Wright, E.; Alpert, J.E.; Sonawalla, S.; Nierenberg, A.A.; Worthington, J.J., III. Personality Disorders and Depression. Psychol. Med. 2002, 32, 1049–1057. [Google Scholar] [CrossRef]
- Shea, M.T.; Pilkonis, P.A.; Beckham, E.; Collins, J.F.; Elkin, I.; Sotsky, S.M.; Docherty, J.P. Personality Disorders and Treatment Outcome in the NIMH Treatment of Depression Collaborative Research Program. Am. J. Psychiatry 1990, 147, 711–718. [Google Scholar] [CrossRef]
- Pozza, A.; Domenichetti, S.; Mazzoni, G.P.; Dèttore, D. The Comorbidity of Cluster C Personality Disorders in Obsessive Compulsive Disorder as a Marker of Anxiety and Depression Severity. Eur. Psychiatry 2016, 33, S202–S203. [Google Scholar] [CrossRef]
- Skodol, A.E.; Stout, R.L.; McGlashan, T.H.; Grilo, C.M.; Gunderson, J.G.; Shea, M.T.; Morey, L.C.; Zanarini, M.C.; Dyck, I.R.; Oldham, J.M. Co-Occurrence of Mood and Personality Disorders: A Report from the Collaborative Longitudinal Personality Disorders Study (CLPS). Depress. Anxiety 1999, 10, 175–182. [Google Scholar] [CrossRef]
- Hillard, C.J.; Weinlander, K.M.; Stuhr, K.L. Contributions of Endocannabinoid Signaling to Psychiatric Disorders in Humans: Genetic and Biochemical Evidence. Neuroscience 2012, 204, 207–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.-W.; Ishiguro, H.; Ohtsuki, T.; Hess, J.; Carillo, F.; Walther, D.; Onaivi, E.S.; Arinami, T.; Uhl, G.R. Human Cannabinoid Receptor 1: 5′ Exons, Candidate Regulatory Regions, Polymorphisms, Haplotypes and Association with Polysubstance Abuse. Mol. Psychiatry 2004, 9, 916–931. [Google Scholar] [CrossRef] [PubMed]
- Lazary, J.; Lazary, A.; Gonda, X.; Benko, A.; Molnar, E.; Hunyady, L.; Juhasz, G.; Bagdy, G. Promoter Variants of the Cannabinoid Receptor 1 Gene (CNR1) in Interaction with 5-HTTLPR Affect the Anxious Phenotype. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2009, 150B, 1118–1127. [Google Scholar] [CrossRef]
- Domschke, K.; Dannlowski, U.; Ohrmann, P.; Lawford, B.; Bauer, J.; Kugel, H.; Heindel, W.; Young, R.; Morris, P.; Arolt, V.; et al. Cannabinoid Receptor 1 (CNR1) Gene: Impact on Antidepressant Treatment Response and Emotion Processing in Major Depression. Eur. Neuropsychopharmacol. 2008, 18, 751–759. [Google Scholar] [CrossRef]
- Rubino, T.; Zamberletti, E.; Parolaro, D. Endocannabinoids and Mental Disorders. In Endocannabinoids; Springer: Berlin/Heidelberg, Germany, 2015; pp. 261–283. [Google Scholar]
- Centonze, D.; Battistini, L.; Maccarrone, M. The Endocannabinoid System in Peripheral Lymphocytes as a Mirror of Neuroinflammatory Diseases. Curr. Pharm. Des. 2008, 14, 2370–2382. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, D.; D’Agata, A.; Starkweather, A.R.; Young, E.E. Contribution of Endocannabinoid Gene Expression and Genotype on Low Back Pain Susceptibility and Chronicity. Clin. J. Pain 2018, 34, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Leweke, F.M.; Giuffrida, A.; Wurster, U.; Emrich, H.M.; Piomelli, D. Elevated Endogenous Cannabinoids in Schizophrenia. Neuroreport 1999, 10, 1665–1669. [Google Scholar] [CrossRef]
- De Marchi, N.; De Petrocellis, L.; Orlando, P.; Daniele, F.; Fezza, F.; Di Marzo, V. Endocannabinoid Signalling in the Blood of Patients with Schizophrenia. Lipids Health Dis. 2003, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Ferretjans, R.; de Campos, S.M.; Ribeiro-Santos, R.; Guimarães, F.C.; de Oliveira, K.; Cardoso, A.C.A.; Araújo, M.S.; Teixeira-Carvalho, A.; Martins-Filho, O.A.; Teixeira, A.L.; et al. Cognitive Performance and Peripheral Endocannabinoid System Receptor Expression in Schizophrenia. Schizophr. Res. 2014, 156, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Im, D.-S. GPR119 and GPR55 as Receptors for Fatty Acid Ethanolamides, Oleoylethanolamide and Palmitoylethanolamide. Int. J. Mol. Sci. 2021, 22, 1034. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.N.; Miller, G.E.; Carrier, E.J.; Gorzalka, B.B.; Hillard, C.J. Circulating Endocannabinoids and N-Acyl Ethanolamines Are Differentially Regulated in Major Depression and Following Exposure to Social Stress. Psychoneuroendocrinology 2009, 34, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, D.; Manchia, M.; Carpiniello, B.; Valtorta, F.; Nobile, M.; Gobbi, G.; Comai, S. Role of Palmitoylethanolamide (PEA) in Depression: Translational Evidence: Special Section on “Translational and Neuroscience Studies in Affective Disorders”. Section Editor, Maria Nobile MD, PhD. This Section of JAD Focuses on the Relevance of Translatio. J. Affect. Disord. 2019, 255, 195–200. [Google Scholar] [CrossRef]
- Lama, A.; Pirozzi, C.; Annunziata, C.; Morgese, M.G.; Senzacqua, M.; Severi, I.; Calignano, A.; Trabace, L.; Giordano, A.; Meli, R.; et al. Palmitoylethanolamide Counteracts Brain Fog Improving Depressive-like Behaviour in Obese Mice: Possible Role of Synaptic Plasticity and Neurogenesis. Br. J. Pharmacol. 2021, 178, 845–859. [Google Scholar] [CrossRef]
- Hill, M.N.; Bierer, L.M.; Makotkine, I.; Golier, J.A.; Galea, S.; McEwen, B.S.; Hillard, C.J.; Yehuda, R. Reductions in Circulating Endocannabinoid Levels in Individuals with Post-Traumatic Stress Disorder Following Exposure to the World Trade Center Attacks. Psychoneuroendocrinology 2013, 38, 2952–2961. [Google Scholar] [CrossRef]
- Mariotti, A. The Effects of Chronic Stress on Health: New Insights into the Molecular Mechanisms of Brain–Body Communication. Future Sci. OA 2015, 1. [Google Scholar] [CrossRef]
- Shonesy, B.C.; Bluett, R.J.; Ramikie, T.S.; Báldi, R.; Hermanson, D.J.; Kingsley, P.J.; Marnett, L.J.; Winder, D.G.; Colbran, R.J.; Patel, S. Genetic Disruption of 2-Arachidonoylglycerol Synthesis Reveals a Key Role for Endocannabinoid Signaling in Anxiety Modulation. Cell Rep. 2014, 9, 1644–1653. [Google Scholar] [CrossRef] [PubMed]
- Galiègue, S.; Mary, S.; Marchand, J.; Dussossoy, D.; Carrière, D.; Carayon, P.; Bouaboula, M.; Shire, D.; Le Fur, G.; Casellas, P. Expression of Central and Peripheral Cannabinoid Receptors in Human Immune Tissues and Leukocyte Subpopulations. Eur. J. Biochem. 1995, 232, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Cabral, G.A.; Griffin-Thomas, L. Emerging Role of the Cannabinoid Receptor CB2 in Immune Regulation: Therapeutic Prospects for Neuroinflammation. Expert Rev. Mol. Med. 2009, 11, e3. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.-P.; Onaivi, E.S.; Ishiguro, H.; Liu, Q.-R.; Tagliaferro, P.A.; Brusco, A.; Uhl, G.R. Cannabinoid CB2 Receptors: Immunohistochemical Localization in Rat Brain. Brain Res. 2006, 1071, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Onaivi, E.S. Neuropsychobiological Evidence for the Functional Presence and Expression of Cannabinoid CB2 Receptors in the Brain. Neuropsychobiology 2006, 54, 231–246. [Google Scholar] [CrossRef]
- Onaivi, E.S.; Ishiguro, H.; Gong, J.-P.; Patel, S.; Meozzi, P.A.; Myers, L.; Perchuk, A.; Mora, Z.; Tagliaferro, P.A.; Gardner, E.; et al. Functional Expression of Brain Neuronal CB2 Cannabinoid Receptors Are Involved in the Effects of Drugs of Abuse and in Depression. Ann. N. Y. Acad. Sci. 2008, 1139, 434–449. [Google Scholar] [CrossRef]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A Meta-Analysis of Cytokines in Major Depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef]
- Ménard, C.; Pfau, M.L.; Hodes, G.E.; Russo, S.J. Immune and Neuroendocrine Mechanisms of Stress Vulnerability and Resilience. Neuropsychopharmacology 2017, 42, 62–80. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.E.; Teixeira, A.L. Inflammation in Psychiatric Disorders: What Comes First? Ann. N. Y. Acad. Sci. 2019, 1437, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Chase, K.A.; Feiner, B.; Rosen, C.; Gavin, D.P.; Sharma, R.P. Characterization of Peripheral Cannabinoid Receptor Expression and Clinical Correlates in Schizophrenia. Psychiatry Res. 2016, 245, 346–353. [Google Scholar] [CrossRef] [PubMed]
- de Campos-Carli, S.M.; Araújo, M.S.; de Oliveira Silveira, A.C.; de Rezende, V.B.; Rocha, N.P.; Ferretjans, R.; Ribeiro-Santos, R.; Teixeira-Carvalho, A.; Martins-Filho, O.A.; Berk, M.; et al. Cannabinoid Receptors on Peripheral Leukocytes from Patients with Schizophrenia: Evidence for Defective Immunomodulatory Mechanisms. J. Psychiatr. Res. 2017, 87, 44–52. [Google Scholar] [CrossRef]
- Atmaca, M.; Tezcan, E.; Kuloglu, M.; Kirtas, O.; Ustundag, B. Serum Folate and Homocysteine Levels in Patients with Obsessive-Compulsive Disorder. Psychiatry Clin. Neurosci. 2005, 59, 616–620. [Google Scholar] [CrossRef]
- Balandeh, E.; Karimian, M.; Behjati, M.; Mohammadi, A.H. Serum Vitamins and Homocysteine Levels in Obsessive-Compulsive Disorder: A Systematic Review and Meta-Analysis. Neuropsychobiology 2021, 80, 502–515. [Google Scholar] [CrossRef]
- Levine, J.; Stahl, Z.; Sela, B.A.; Gavendo, S.; Ruderman, V.; Belmaker, R.H. Elevated Homocysteine Levels in Young Male Patients With Schizophrenia. Am. J. Psychiatry 2002, 159, 1790–1792. [Google Scholar] [CrossRef] [PubMed]
- Jendričko, T.; Vidović, A.; Grubišić-Ilić, M.; Romić, Ž.; Kovačić, Z.; Kozarić-Kovačić, D. Homocysteine and Serum Lipids Concentration in Male War Veterans with Posttraumatic Stress Disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 134–140. [Google Scholar] [CrossRef] [PubMed]
- László, A.; Lénárt, L.; Illésy, L.; Fekete, A.; Nemcsik, J. The Role of Neurotrophins in Psychopathology and Cardiovascular Diseases: Psychosomatic Connections. J. Neural Transm. 2019, 126, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Galli, F.; Caputi, M.; Sances, G.; Vegni, E.; Bottiroli, S.; Nappi, G.; Tassorelli, C. Alexithymia in Chronic and Episodic Migraine: A Comparative Study. J. Ment. Health 2017, 26, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.C.; Ski, C.F.; Murphy, B.M.; Fernandez, E.; Alvarenga, M.E.; Le Grande, M.R.; Thompson, D.R. What Role Does Personality Play in Cardiovascular Disease? Br. J. Card. Nurs. 2018, 13, 330–337. [Google Scholar] [CrossRef]
- Toda, N.; Nakanishi-Toda, M. How Mental Stress Affects Endothelial Function. Pflügers Arch.-Eur. J. Physiol. 2011, 462, 779–794. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.C.; Bland, R.C. Life Events and the 1-Year Prevalence of Major Depressive Episode, Generalized Anxiety Disorder, and Panic Disorder in a Community Sample. Compr. Psychiatry 1994, 35, 76–82. [Google Scholar] [CrossRef]
- Horesh, N.; Amir, M.; Kedem, P.; Goldberger, Y.; Kotler, M. Life Events in Childhood, Adolescence and Adulthood and the Relationship to Panic Disorder. Acta Psychiatr. Scand. 1997, 96, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Hirschfeld, R.M.A. Personality Disorders and Depression: Comorbidity. Depress. Anxiety 1999, 10, 142–146. [Google Scholar] [CrossRef]
- Wongpakaran, N.; Boonyanaruthee, V.; Pinyopornpanish, M.; Intaprasert, S.; Wongpakaran, T. Comorbid Personality Disorders among Patients with Depression. Neuropsychiatr. Dis. Treat. 2015, 11, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, TX, USA, 2013. [Google Scholar]
- First, M.; Williams, J.; Karg, R.; Spitzer, R. Structured Clinical Interview for DSM-5 Disorders, Clinician Version (SCID-5-CV); American Psychiatric Association: Arlington, VA, USA, 2015. [Google Scholar]
- Ferrari, A.; Cicero, A.F.G.; Bertolini, A.; Leone, S.; Pasciullo, G.; Sternieri, E. Need for Analgesics/Drugs of Abuse: A Comparison between Headache Patients and Addicts by the Leeds Dependence Questionnaire (LDQ). Cephalalgia 2006, 26, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Bottiroli, S.; Galli, F.; Ballante, E.; Pazzi, S.; Sances, G.; Guaschino, E.; Allena, M.; Tassorelli, C. Validity of the Severity of Dependence Scale for Detecting Dependence Behaviours in Chronic Migraine with Medication Overuse. Cephalalgia 2022, 42, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Grassi, M.C.; Enea, D.; Ferketich, A.K.; Culasso, F.; Nencini, P. Validity of the Italian Version of the Severity of Dependence Scale (SDS) for Nicotine Dependence in Smokers Intending to Quit. Psychol. Rep. 2014, 114, 1–13. [Google Scholar] [CrossRef]
- Iani, L.; Lauriola, M.; Costantini, M. A Confirmatory Bifactor Analysis of the Hospital Anxiety and Depression Scale in an Italian Community Sample. Health Qual. Life Outcomes 2014, 12, 84. [Google Scholar] [CrossRef]
- Apolone, G.; Mosconi, P. The Italian SF-36 Health Survey. J. Clin. Epidemiol. 1998, 51, 1025–1036. [Google Scholar] [CrossRef]
- Fanelli, F.; Di Lallo, V.D.; Belluomo, I.; De Iasio, R.; Baccini, M.; Casadio, E.; Gasparini, D.I.; Colavita, M.; Gambineri, A.; Grossi, G.; et al. Estimation of Reference Intervals of Five Endocannabinoids and Endocannabinoid Related Compounds in Human Plasma by Two Dimensional-LC/MS/MS. J. Lipid Res. 2012, 53, 481–493. [Google Scholar] [CrossRef]
| Total n = 51 | |
|---|---|
| Age | 46.0 ± 11.7 |
| Gender, female | 45 (88%) |
| CM | 38 (75%) |
| Ongoing antidepressants treatment | 13 (25%) |
| Migraine days per month | 20.2 ± 7.3 |
| Days of acute drug intake per month | 16.5 ± 6.9 |
| Doses of acute drug intake per month | 24.1 ± 15.7 |
| MIDAS | 79.2 ± 38.1 |
| HIT-6 | 67.6 ± 4.3 |
| LDQ | 7.3 ± 4.4 |
| SDS | 6.9 ± 3.1 |
| HADS anxiety subscale | 5.7 ± 3.8 |
| HADS depression subscale | 5.9 ± 4.2 |
| SF-36 physical subscale | 35.4 ± 7.6 |
| SF-36 mental subscale | 39.6 ± 10.9 |
| Personality disorders | 31 (61%) |
| Cluster A | 3 (6%) |
| Paranoid | 3 (6%) |
| Schizoid | 0 (0%) |
| Schizotypal | 0 (0%) |
| Cluster B | 7 (14%) |
| Histrionic | 3 (6%) |
| Narcissistic | 5 (10%) |
| Antisocial | 0 (0%) |
| Borderline | 2 (4%) |
| Cluster C | 31 (61%) |
| Avoidant | 3 (6%) |
| Dependent | 1 (2%) |
| Obsessive compulsive | 31 (61%) |
| Anxiety disorders | 42 (82%) |
| Mood disorders | 31 (61%) |
| Comorbid personality and mood disorders | 22 (43%) |
| PD n = 31 | wPD n = 20 | p | |
|---|---|---|---|
| Age | 46.4 ± 11.8 | 45.5 ± 11.8 | 0.80 |
| Gender, female | 28 (90%) | 17 (85%) | 0.44 |
| CM | 21 (68%) | 17 (85%) | 0.20 |
| Ongoing antidepressants treatment | 11 (36%) | 2 (15%) | 0.04 |
| Migraine days per month | 20.5 ± 7.5 | 20.0 ± 7.2 | 0.90 |
| Days of acute drug intake per month | 15.6 ± 6.1 | 17.8 ± 7.9 | 0.44 |
| Doses of acute drug intake per month | 23.0 ± 16.0 | 25.7 ± 15.6 | 0.30 |
| MIDAS | 86.3 ± 43.5 | 68.2 ± 24.9 | 0.12 |
| HIT | 67.5 ± 5.2 | 67.6 ± 2.4 | 0.67 |
| LDQ | 7.4 ± 4.4 | 7.2 ± 4.5 | 0.76 |
| SDS | 6.4 ± 2.7 | 7.7 ± 3.4 | 0.29 |
| HADS anxiety subscale | 5.7 ± 3.8 | 5.6 ± 3.9 | 0.88 |
| HADS depression subscale | 5.6 ± 3.8 | 6.5 ± 4.9 | 0.54 |
| SF-36 physical subscale | 35.5 ± 7.9 | 35.3 ± 7.4 | 0.65 |
| SF-36 mental subscale | 38.8 ± 10.6 | 40.9 ± 11.4 | 0.47 |
| Anxiety disorders | 26 (84%) | 16 (80%) | 0.72 |
| Mood disorders | 21 (68%) | 10 (50%) | 0.25 |
| MD n = 31 | wMD n = 20 | p | |
|---|---|---|---|
| Age | 45.8 ± 11.3 | 46.4 ± 12.6 | 0.88 |
| Gender, female | 29 (94%) | 16 (80%) | 0.15 |
| CM | 23 (74%) | 15 (75%) | 0.61 |
| Ongoing antidepressants treatment | 11 (36%) | 2 (15%) | 0.04 |
| Migraine days per month | 20.3 ± 6.9 | 20.2 ± 7.9 | 0.91 |
| Days of acute drug intake per month | 16.1 ± 6.4 | 17.1 ± 7.7 | 0.96 |
| Doses of acute drug intake per month | 23.3 ± 13.5 | 25.3 ± 18.9 | 0.74 |
| MIDAS | 84.3 ± 39.4 | 71.4 ± 35.5 | 0.21 |
| HIT | 68.1 ± 4.8 | 66.7 ± 3.4 | 0.07 |
| LDQ | 8.2 ± 4.5 | 6.1 ± 4.1 | 0.07 |
| SDS | 7.3 ± 3.3 | 6.4 ± 2.7 | 0.29 |
| HADS anxiety subscale | 6.6 ± 3.9 | 4.2 ± 3.3 | 0.03 |
| HADS depression subscale | 7.3 ± 4.6 | 3.9 ± 2.6 | 0.006 |
| SF-36 physical subscale | 34.5 ± 6.7 | 36.8 ± 8.9 | 0.15 |
| SF-36 mental subscale | 36.2 ± 10.4 | 45.0 ± 9.6 | 0.004 |
| Anxiety disorders | 29 (94%) | 13 (65%) | 0.02 |
| Personality disorders | 21 (68%) | 10 (50%) | 0.25 |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Ubiquitin C | AGAGGCTGATCTTTGCTGGA | GGGTGGACTCTTTCTGGATG |
| CB1 | CCTTTTGCTGCCTAAATCCAC | CCACTGCTCAAACATCTGAC |
| CB2 | CATGGAGGAAGGAATGTGCTGGGTGAC | GAGGAAGGCGATGAACAGGAG |
| PPARγ | GGCTTCATGACAAGGGAGTTTC | AACTCAAACTTGGGCTCCATAAAG |
| PPARα | ATGGTGGACACGGAAAGCC | CGATGGATTGCGAAATCTCTTGG |
| FAAH | GAGGACATGTTCCGCTTGGA | TGTTGTCTTGGCAAGAAGGGA |
| NAPE-PLD | TTGTGAATCCGTGGCCAACATGG | TACTGCGATGGTGAAGCACG |
| MAGL | CAAGGCCCTCATCTTTGTGT | ACGTGGAAGTCAGACACTAC |
| DAGLα | AGAATGTCACCCTCGGAATGG | GTGGCTCTCAGCTTCGACAAAGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bottiroli, S.; Greco, R.; Franco, V.; Zanaboni, A.; Palmisani, M.; Vaghi, G.; Sances, G.; De Icco, R.; Tassorelli, C. Peripheral Endocannabinoid Components and Lipid Plasma Levels in Patients with Resistant Migraine and Co-Morbid Personality and Psychological Disorders: A Cross-Sectional Study. Int. J. Mol. Sci. 2024, 25, 1893. https://doi.org/10.3390/ijms25031893
Bottiroli S, Greco R, Franco V, Zanaboni A, Palmisani M, Vaghi G, Sances G, De Icco R, Tassorelli C. Peripheral Endocannabinoid Components and Lipid Plasma Levels in Patients with Resistant Migraine and Co-Morbid Personality and Psychological Disorders: A Cross-Sectional Study. International Journal of Molecular Sciences. 2024; 25(3):1893. https://doi.org/10.3390/ijms25031893
Chicago/Turabian StyleBottiroli, Sara, Rosaria Greco, Valentina Franco, Annamaria Zanaboni, Michela Palmisani, Gloria Vaghi, Grazia Sances, Roberto De Icco, and Cristina Tassorelli. 2024. "Peripheral Endocannabinoid Components and Lipid Plasma Levels in Patients with Resistant Migraine and Co-Morbid Personality and Psychological Disorders: A Cross-Sectional Study" International Journal of Molecular Sciences 25, no. 3: 1893. https://doi.org/10.3390/ijms25031893
APA StyleBottiroli, S., Greco, R., Franco, V., Zanaboni, A., Palmisani, M., Vaghi, G., Sances, G., De Icco, R., & Tassorelli, C. (2024). Peripheral Endocannabinoid Components and Lipid Plasma Levels in Patients with Resistant Migraine and Co-Morbid Personality and Psychological Disorders: A Cross-Sectional Study. International Journal of Molecular Sciences, 25(3), 1893. https://doi.org/10.3390/ijms25031893






