YTHDF1’s Regulatory Involvement in Breast Cancer Prognosis, Immunity, and the ceRNA Network
Abstract
1. Introduction
2. Results
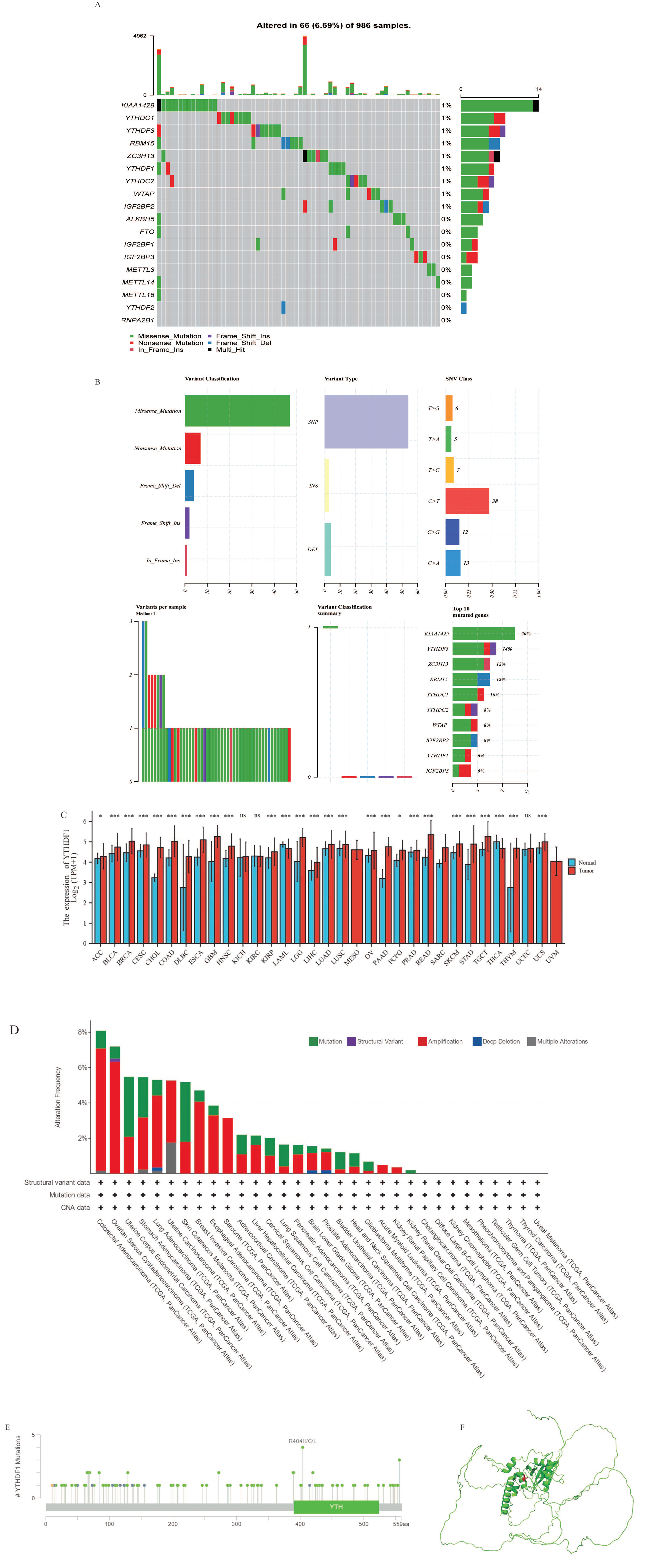
2.1. Transcriptional and Epigenetic Alterations of Major m6A Regulators in Breast Cancer Tissue
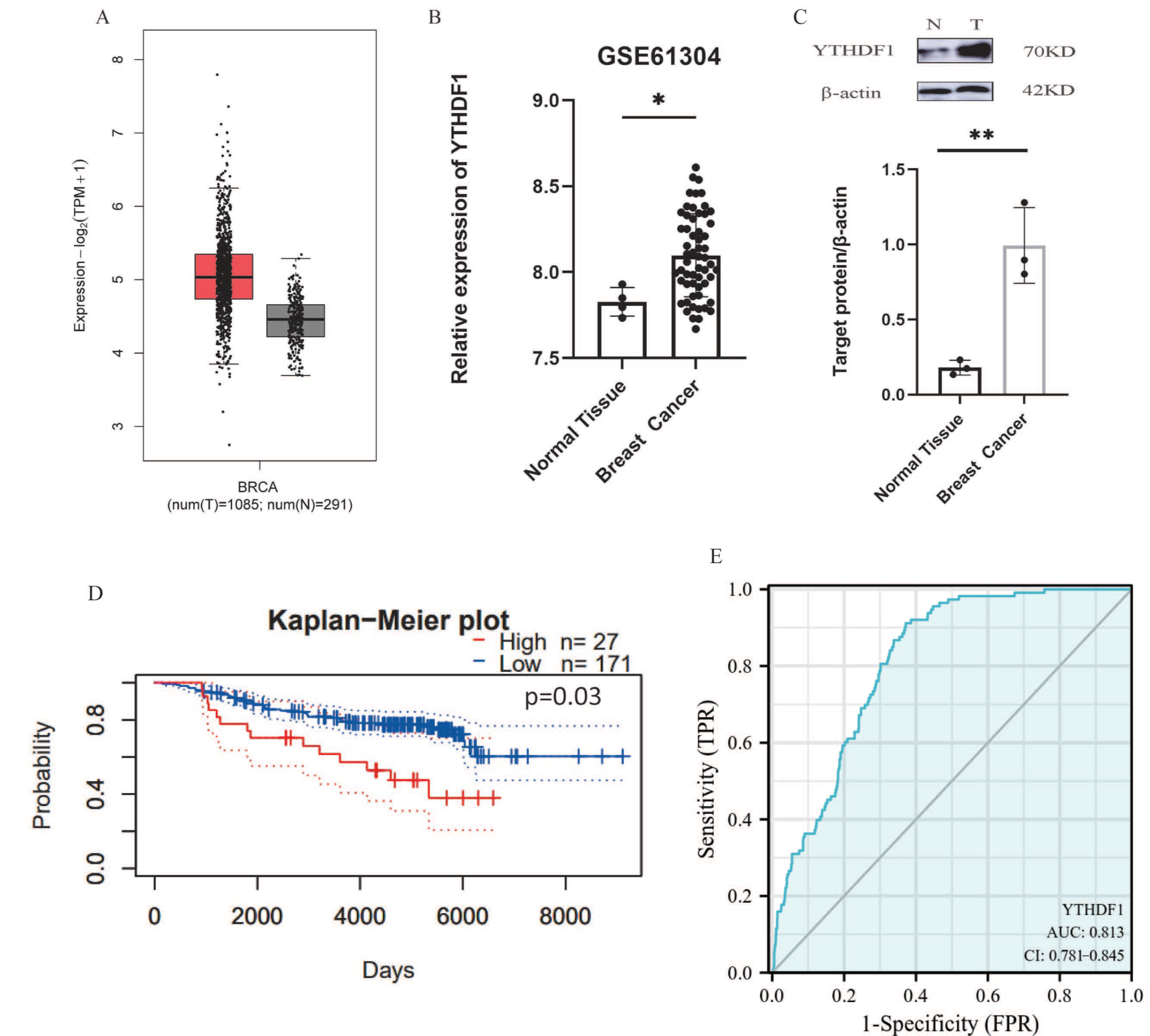
2.2. YTHDF1 Is Up-Regulated in Breast Cancer and Associated with Poor Prognosis
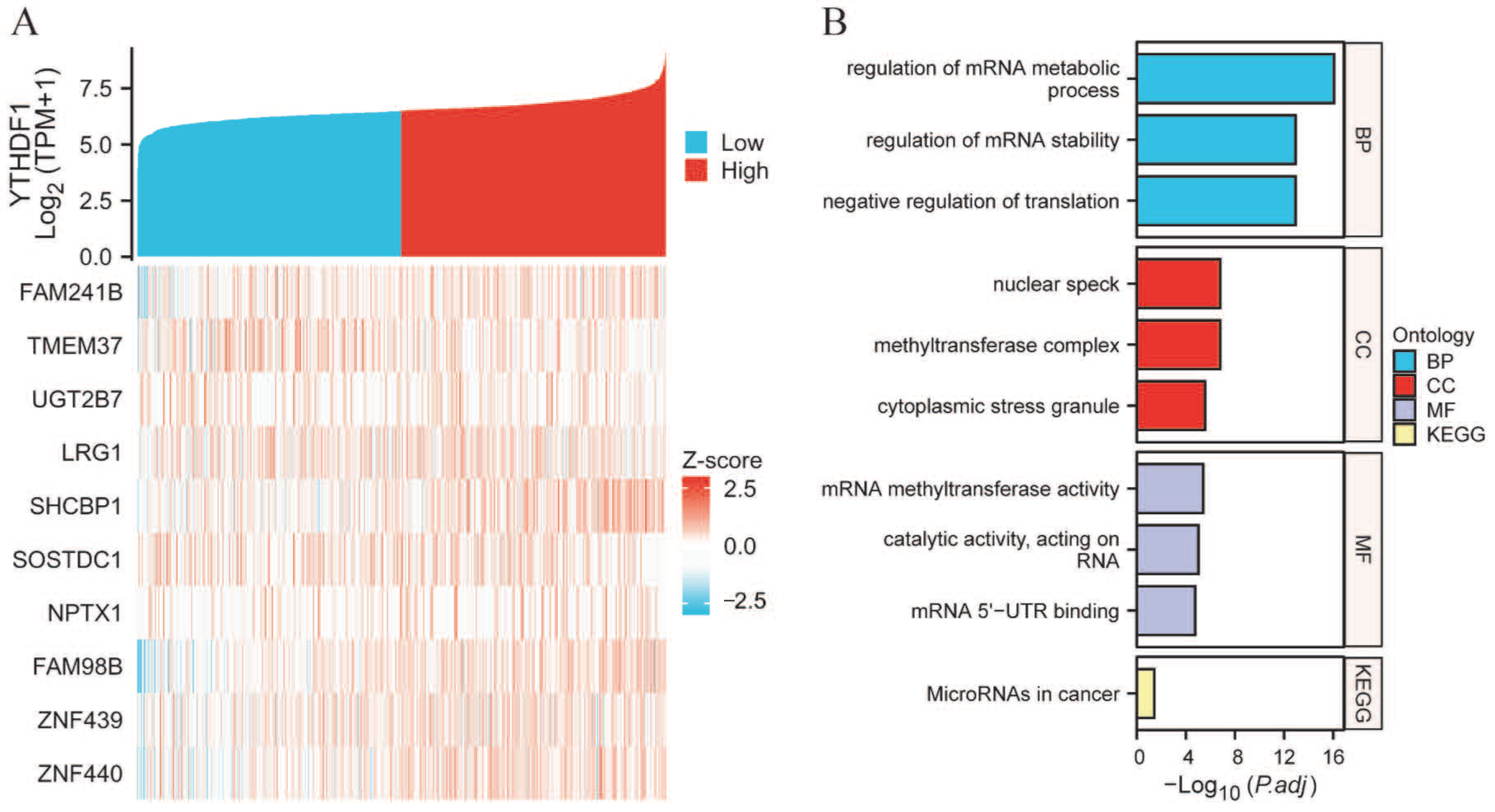
2.3. PPI Network and Functional Annotations
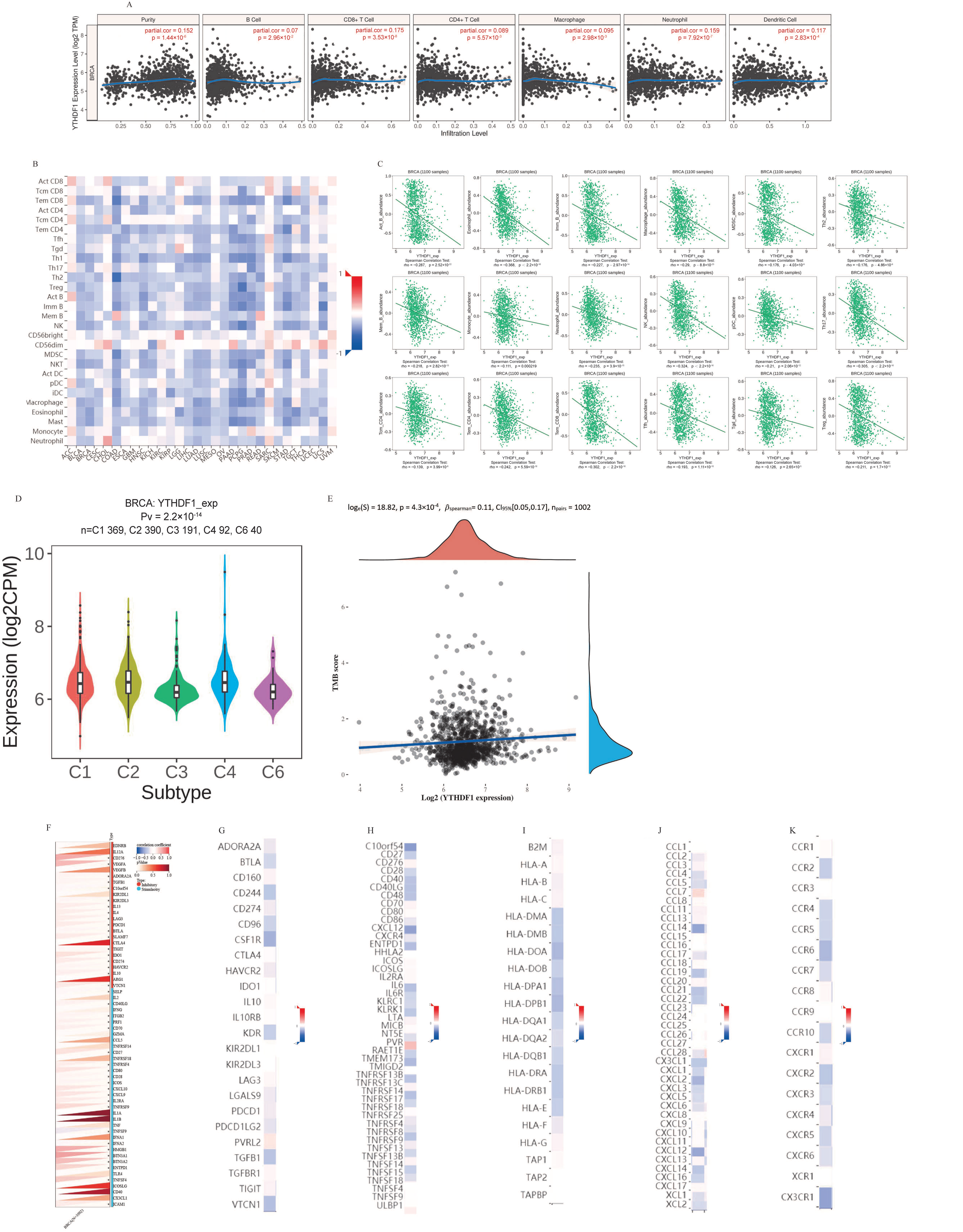
2.4. Analysis of YTHDF1’s Contribution to Breast Cancer Tumor Immunity
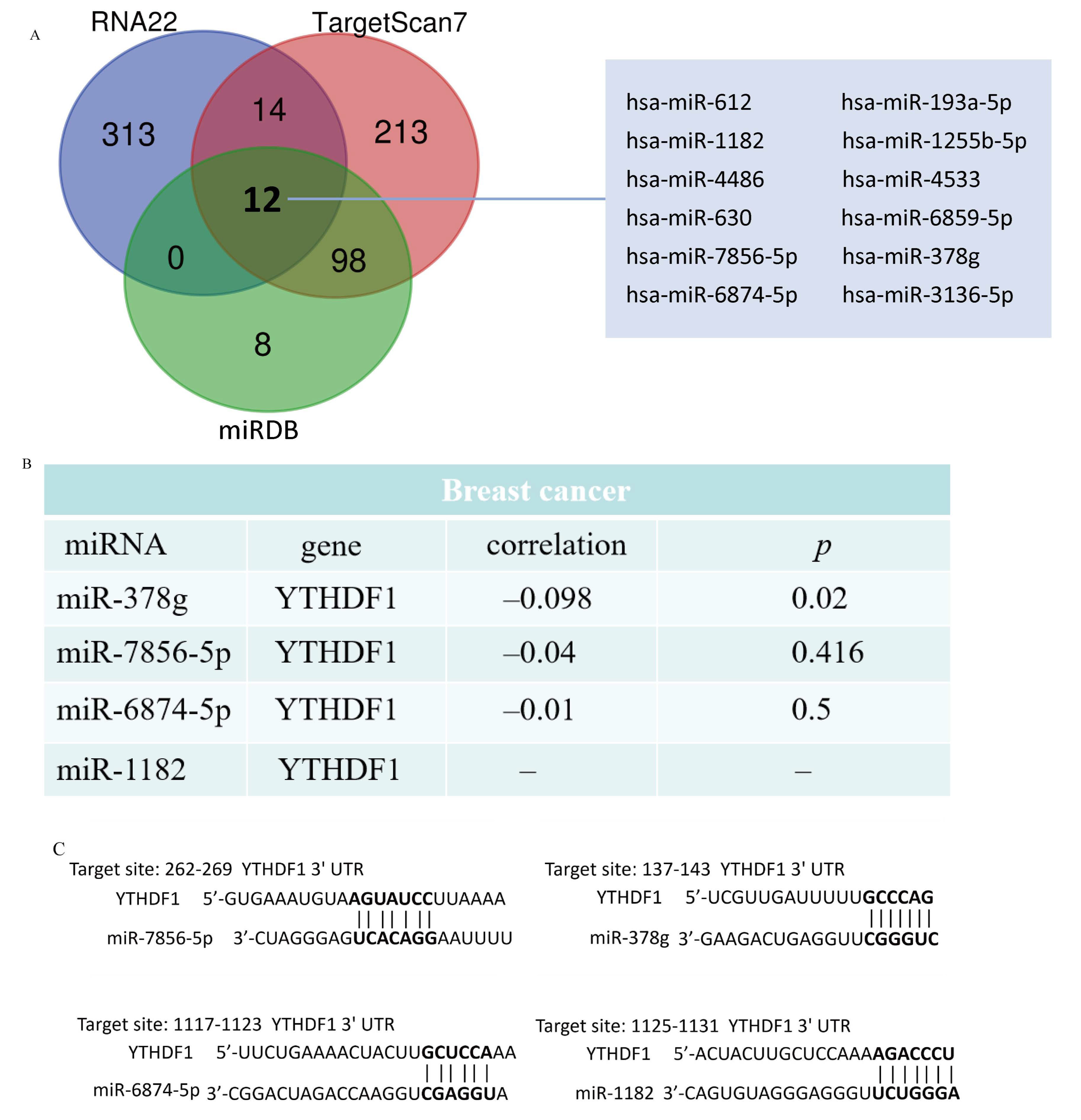
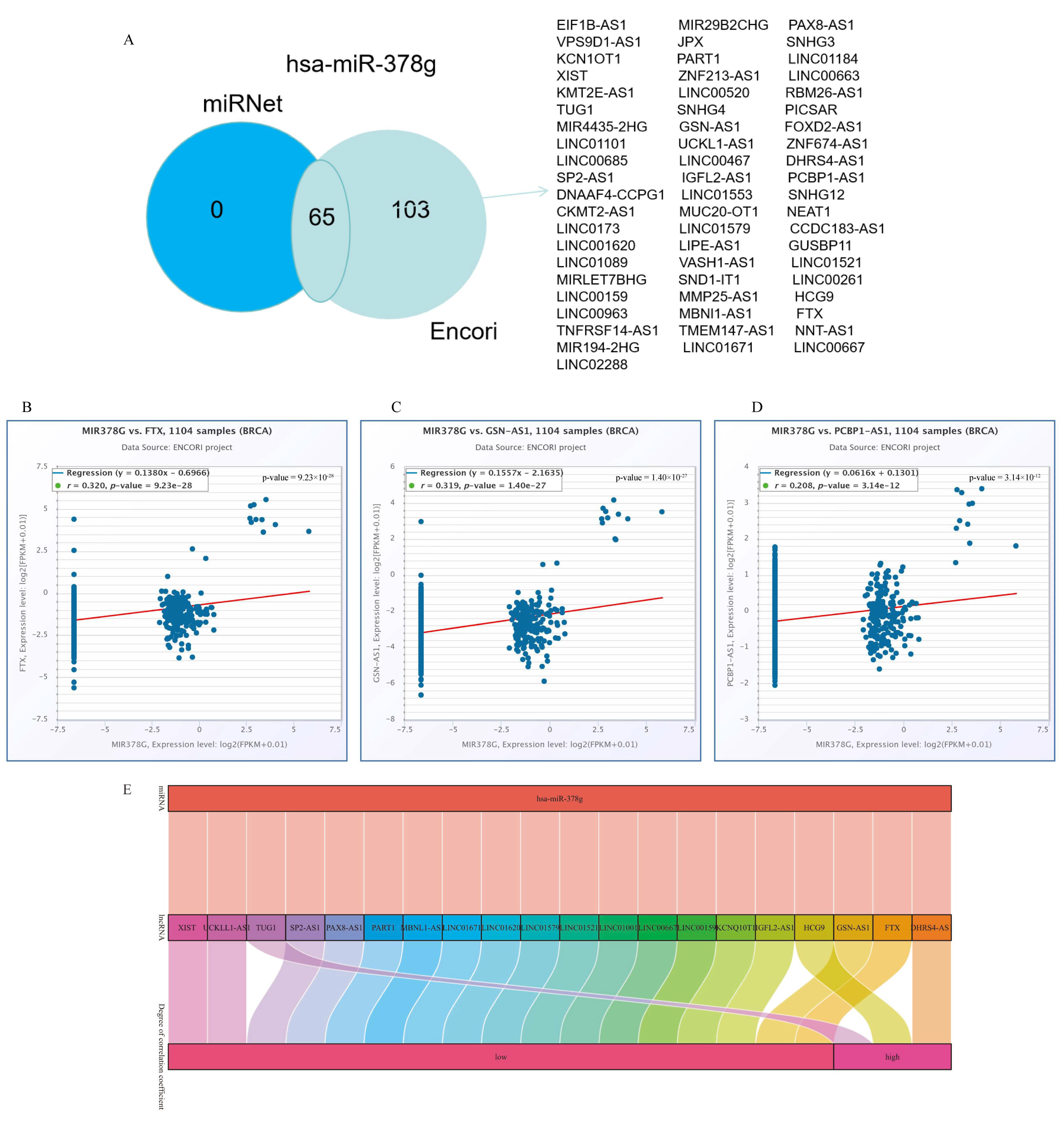
2.5. Analysis of YTHDF1-Associated ceRNA Network in Breast Cancer
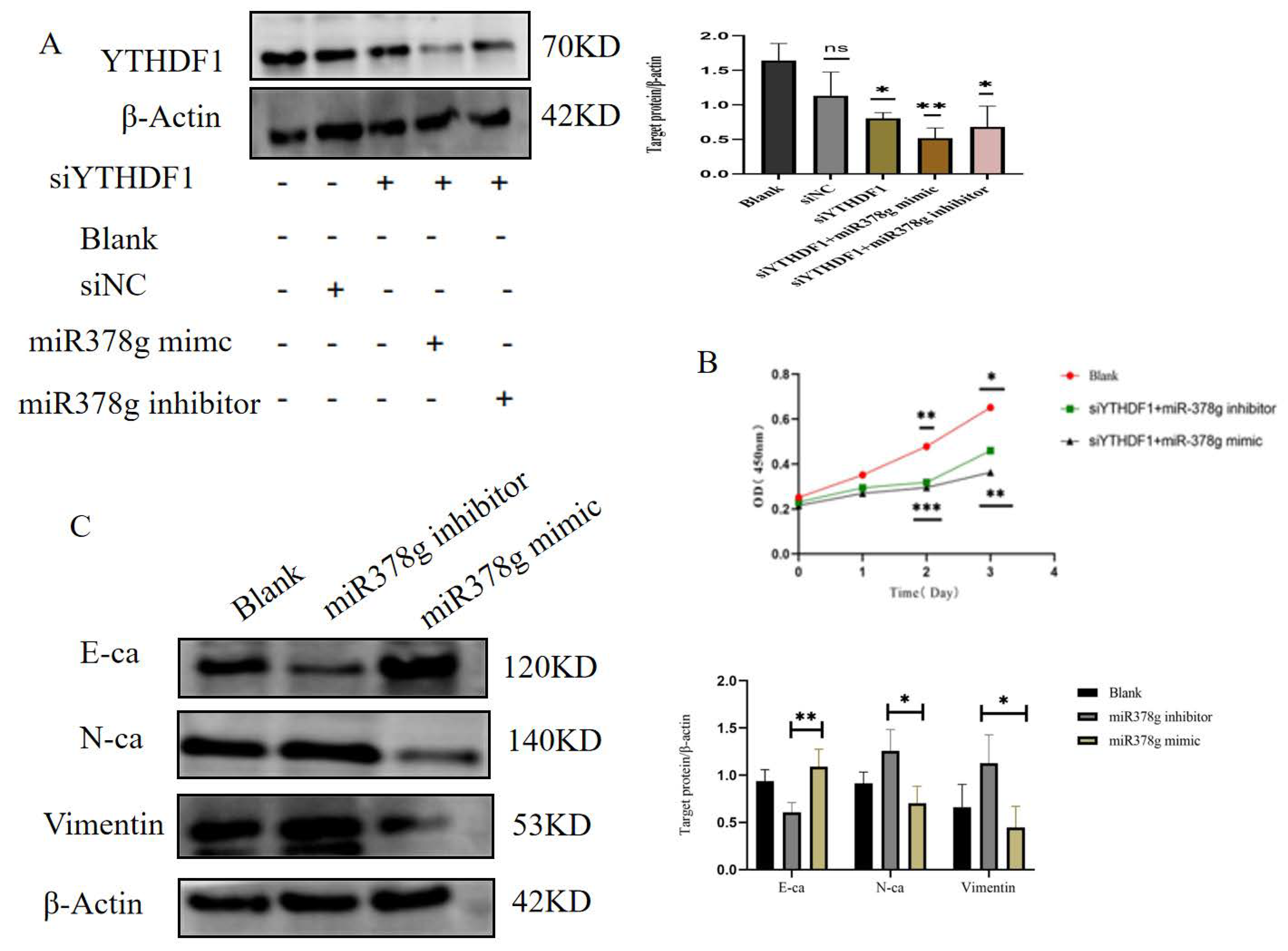
2.6. miR-378g Inhibits YTHDF1 Expression and Proliferation of MDA-MB231 Cells
3. Discussion
4. Materials and Methods
4.1. Tissue Collection
4.2. TCGA Database
4.3. RNA Sequencing Data of YTHDF1 in Breast Cancer
4.4. Protein–Protein Interaction (PPI) Network and Functional Enrichment Analysis
4.5. Tumor Immune Estimation Resource (TIMER) Database
4.6. Tumor Immune System Interaction Database (TISIDB)
4.7. RMVar Database
4.8. PrognoScan Database
4.9. Immune-Checkpoint-Related Database in Breast Cancer
4.10. Tumor Mutational Burden (TMB) Analysis
4.11. Functional Enrichment Analysis
4.12. Construction of ceRNA Network
4.13. Cell Culture
4.14. Cell Transfection
4.15. Cell Proliferation Assay
4.16. Western Blotting
4.17. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Heeke, A.L.; Tan, A.R. Checkpoint inhibitor therapy for metastatic triple-negative breast cancer. Cancer Metastasis Rev. 2021, 40, 537–547. [Google Scholar] [CrossRef]
- Pasculli, B.; Barbano, R.; Parrella, P. Epigenetics of breast cancer: Biology and clinical implication in the era of precision medicine. Semin. Cancer Biol. 2018, 51, 22–35. [Google Scholar] [CrossRef]
- Fang, R.; Ye, L.; Shi, H. Understanding the roles of N6-methyladenosine writers, readers and erasers in breast cancer. Neoplasia 2021, 23, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.A.; Issa, J.-P.J.; Baylin, S. Targeting the cancer epigenome for therapy. Nat. Rev. Genet. 2016, 17, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Begik, O.; Lucas, M.C.; Ramirez, J.M.; Mason, C.E.; Wiener, D.; Schwartz, S.; Mattick, J.S.; Smith, M.A.; Novoa, E.M. Accurate detection of m6A RNA modifications in native RNA sequences. Nat. Commun. 2019, 10, 4079. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wei, Q.; Jin, J.; Luo, Q.; Liu, Y.; Yang, Y.; Cheng, C.; Li, L.; Pi, J.; Si, Y. The m6A reader YTHDF1 promotes ovarian cancer progression via augmenting EIF3C translation. Nucleic Acids Res. 2020, 48, 3816–3831. [Google Scholar] [CrossRef] [PubMed]
- Paramasivam, A.; Priyadharsini, J.V.; Raghunandhakumar, S. Implications of m6A modification in autoimmune disorders. Cell. Mol. Immunol. 2020, 17, 550–551. [Google Scholar] [CrossRef] [PubMed]
- Paramasivam, A.; Vijayashree Priyadharsini, J. Novel insights into m6A modification in circular RNA and implications for immunity. Cell. Mol. Immunol. 2020, 17, 668–669. [Google Scholar] [CrossRef] [PubMed]
- Paramasivam, A.; Vijayashree Priyadharsini, J.; Raghunandhakumar, S. N6-adenosine methylation (m6A): A promising new molecular target in hypertension and cardiovascular diseases. Hypertens. Res. 2020, 43, 153–154. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Kong, S.; Tao, M.; Ju, S. The potential role of RNA N6-methyladenosine in Cancer progression. Mol. Cancer 2020, 19, 88. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.-K.; Ma, C.; Hu, K.; Ma, R.-J.; Zhang, N.; Sun, Z.-G. Roles of N6-Methyladenosine Demethylase FTO in Malignant Tumors Progression. OncoTargets Ther. 2021, 14, 4837. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, J.; Gu, Q.; Ma, Y.; Yang, Y.; Zhu, J.; Zhang, Q.A. The biological function of m6A demethylase ALKBH5 and its role in human disease. Cancer Cell Int. 2020, 20, 347. [Google Scholar] [CrossRef] [PubMed]
- Patil, D.P.; Pickering, B.F.; Jaffrey, S.R. Reading m6A in the transcriptome: m6A-binding proteins. Trends Cell Biol. 2018, 28, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Rauch, S.; He, C.; Dickinson, B.C. Targeted m6A reader proteins to study epitranscriptomic regulation of single RNAs. J. Am. Chem. Soc. 2018, 140, 11974–11981. [Google Scholar] [CrossRef]
- Han, S.H.; Choe, J. Diverse molecular functions of m6A mRNA modification in cancer. Exp. Mol. Med. 2020, 52, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Lan, Q.; Liu, P.Y.; Haase, J.; Bell, J.L.; Hüttelmaier, S.; Liu, T. The Critical Role of RNA m6A Methylation in Cancer. Cancer Res. 2019, 79, 1285–1292. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, B.; Nie, Z.; Duan, L.; Xiong, Q.; Jin, Z.; Yang, C.; Chen, Y. The role of m6A modification in the biological functions and diseases. Signal Transduct. Target. Ther. 2021, 6, 74. [Google Scholar] [CrossRef]
- Kumar, S.; Nagpal, R.; Kumar, A.; Ashraf, M.U.; Bae, Y.-S. Immunotherapeutic potential of m6A-modifiers and MicroRNAs in controlling acute myeloid leukaemia. Biomedicines 2021, 9, 690. [Google Scholar] [CrossRef]
- Wei, M.; Bai, J.-W.; Niu, L.; Zhang, Y.-Q.; Chen, H.-Y.; Zhang, G.-J. The complex roles and therapeutic implications of m6A modifications in breast cancer. Front. Cell Dev. Biol. 2021, 8, 615071. [Google Scholar] [CrossRef]
- Chen, M.; Wong, C.-M. The emerging roles of N6-methyladenosine (m6A) deregulation in liver carcinogenesis. Mol. Cancer 2020, 19, 44. [Google Scholar] [CrossRef]
- Pan, Y.; Ma, P.; Liu, Y.; Li, W.; Shu, Y. Multiple functions of m6A RNA methylation in cancer. J. Hematol. Oncol. 2018, 11, 48. [Google Scholar] [CrossRef]
- Dai, D.; Wang, H.; Zhu, L.; Jin, H.; Wang, X. N6-methyladenosine links RNA metabolism to cancer progression. Cell Death Dis. 2018, 9, 124. [Google Scholar] [CrossRef]
- Dai, X.-Y.; Shi, L.; Li, Z.; Yang, H.-Y.; Wei, J.-F.; Ding, Q. Main N6-methyladenosine readers: YTH family proteins in cancers. Front. Oncol. 2021, 11, 635329. [Google Scholar] [CrossRef]
- Anita, R.; Paramasivam, A.; Priyadharsini, J.V.; Chitra, S. The m6A readers YTHDF1 and YTHDF3 aberrations associated with metastasis and predict poor prognosis in breast cancer patients. Am. J. Cancer Res. 2020, 10, 2546–2554. [Google Scholar]
- Wang, X.; Zhao, B.S.; Roundtree, I.A.; Lu, Z.; Han, D.; Ma, H.; Weng, X.; Chen, K.; Shi, H.; He, C. N6-methyladenosine modulates messenger RNA translation efficiency. Cell 2015, 161, 1388–1399. [Google Scholar] [CrossRef]
- Chen, F.; Chen, Z.; Guan, T.; Zhou, Y.; Ge, L.; Zhang, H.; Wu, Y.; Jiang, G.M.; He, W.; Li, J.; et al. N6-Methyladenosine Regulates mRNA Stability and Translation Efficiency of KRT7 to Promote Breast Cancer Lung Metastasis. Cancer Res. 2021, 81, 2847–2860. [Google Scholar] [CrossRef]
- Jin, D.; Guo, J.; Wu, Y.; Yang, L.; Wang, X.; Du, J.; Dai, J.; Chen, W.; Gong, K.; Miao, S. m6A demethylase ALKBH5 inhibits tumor growth and metastasis by reducing YTHDFs-mediated YAP expression and inhibiting miR-107/LATS2–mediated YAP activity in NSCLC. Mol. Cancer 2020, 19, 40. [Google Scholar] [CrossRef]
- Nishizawa, Y.; Konno, M.; Asai, A.; Koseki, J.; Kawamoto, K.; Miyoshi, N.; Takahashi, H.; Nishida, N.; Haraguchi, N.; Sakai, D. Oncogene c-Myc promotes epitranscriptome m6A reader YTHDF1 expression in colorectal cancer. Oncotarget 2018, 9, 7476. [Google Scholar] [CrossRef]
- Liu, L.; Liu, X.; Dong, Z.; Li, J.; Yu, Y.; Chen, X.; Ren, F.; Cui, G.; Sun, R. N6-methyladenosine-related genomic targets are altered in breast cancer tissue and associated with poor survival. J. Cancer 2019, 10, 5447. [Google Scholar] [CrossRef]
- Han, D.; Liu, J.; Chen, C.; Dong, L.; Liu, Y.; Chang, R.; Huang, X.; Liu, Y.; Wang, J.; Dougherty, U. Anti-tumour immunity controlled through mRNA m6A methylation and YTHDF1 in dendritic cells. Nature 2019, 566, 270–274. [Google Scholar] [CrossRef]
- Chen, D.-H.; Zhang, J.-G.; Wu, C.-X.; Li, Q. Non-Coding RNA m6A Modification in Cancer: Mechanisms and Therapeutic Targets. Front. Cell Dev. Biol. 2021, 9, 778582. [Google Scholar] [CrossRef]
- Guo, Y.; Feng, L. N6-methyladenosine-mediated upregulation of LINC00520 accelerates breast cancer progression via regulating miR-577/POSTN axis and downstream ILK/AKT/mTOR signaling pathway. Arch. Biochem. Biophys. 2022, 729, 109381. [Google Scholar] [CrossRef]
- Chen, H.; Yu, Y.; Yang, M.; Huang, H.; Ma, S.; Hu, J.; Xi, Z.; Guo, H.; Yao, G.; Yang, L.; et al. YTHDF1 promotes breast cancer progression by facilitating FOXM1 translation in an m6A-dependent manner. Cell Biosci. 2022, 12, 19. [Google Scholar] [CrossRef]
- Yao, X.; Li, W.; Li, L.; Li, M.; Zhao, Y.; Fang, D.; Zeng, X.; Luo, Z. YTHDF1 upregulation mediates hypoxia-dependent breast cancer growth and metastasis through regulating PKM2 to affect glycolysis. Cell Death Dis. 2022, 13, 258. [Google Scholar] [CrossRef]
- Lv, W.; Tan, Y.; Xiong, M.; Zhao, C.; Wang, Y.; Wu, M.; Wu, Y.; Zhang, Q. Analysis and validation of m6A regulatory network: A novel circBACH2/has-miR-944/HNRNPC axis in breast cancer progression. J. Transl. Med. 2021, 19, 527. [Google Scholar] [CrossRef]
- Zhong, X.; Li, J.; Wu, X.; Wu, X.; Hu, L.; Ding, B.; Qian, L. Identification of N6-Methyladenosine-Related LncRNAs for Predicting Overall Survival and Clustering of a Potentially Novel Molecular Subtype of Breast Cancer. Front. Oncol. 2021, 11, 742944. [Google Scholar] [CrossRef]
- Lan, Q.; Liu, P.Y.; Bell, J.L.; Wang, J.Y.; Hüttelmaier, S.; Zhang, X.D.; Zhang, L.; Liu, T. The Emerging Roles of RNA m6A Methylation and Demethylation as Critical Regulators of Tumorigenesis, Drug Sensitivity, and Resistance. Cancer Res. 2021, 81, 3431–3440. [Google Scholar] [CrossRef]
- Zhang, C.; Fu, J.; Zhou, Y. A review in research progress concerning m6A methylation and immunoregulation. Front. Immunol. 2019, 10, 922. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, X.; Xia, M.; Zhong, J. The roles and mechanisms of the m6A reader protein YTHDF1 in tumor biology and human diseases. Mol. Ther.-Nucleic Acids 2021, 26, 1270–1279. [Google Scholar] [CrossRef]
- Shi, Y.; Fan, S.; Wu, M.; Zuo, Z.; Li, X.; Jiang, L.; Shen, Q.; Xu, P.; Zeng, L.; Zhou, Y.; et al. YTHDF1 links hypoxia adaptation and non-small cell lung cancer progression. Nat. Commun. 2019, 10, 4892. [Google Scholar] [CrossRef]
- Jia, R.; Chai, P.; Wang, S.; Sun, B.; Xu, Y.; Yang, Y.; Ge, S.; Jia, R.; Yang, Y.-G.; Fan, X. m6A modification suppresses ocular melanoma through modulating HINT2 mRNA translation. Mol. Cancer 2019, 18, 161. [Google Scholar] [CrossRef]
- Sun, Y.; Dong, D.; Xia, Y.; Hao, L.; Wang, W.; Zhao, C. YTHDF1 promotes breast cancer cell growth, DNA damage repair and chemoresistance. Cell Death Dis. 2022, 13, 230. [Google Scholar] [CrossRef]
- Lotfinejad, P.; Asghari Jafarabadi, M.; Abdoli Shadbad, M.; Kazemi, T.; Pashazadeh, F.; Sandoghchian Shotorbani, S.; Jadidi Niaragh, F.; Baghbanzadeh, A.; Vahed, N.; Silvestris, N. Prognostic role and clinical significance of tumor-infiltrating lymphocyte (TIL) and programmed death ligand 1 (PD-L1) expression in triple-negative breast cancer (TNBC): A systematic review and meta-analysis study. Diagnostics 2020, 10, 704. [Google Scholar] [CrossRef]
- Hu, Y.; Pan, Q.; Wang, M.; Ai, X.; Yan, Y.; Tian, Y.; Jing, Y.; Tang, P.; Jiang, J. m6A RNA Methylation Regulator YTHDF1 Correlated with Immune Microenvironment Predicts Clinical Outcomes and Therapeutic Efficacy in Breast Cancer. Front. Med. 2021, 8, 667543. [Google Scholar] [CrossRef]
- Li, C.; Zhang, C.; Zhang, G.; Chen, B.; Li, X.; Li, K.; Ren, C.; Wen, L.; Liao, N. YTHDF1 amplification is correlated with worse outcome and lower immune cell infiltrations in breast cancer. Cancer Biomark. 2022, 35, 127–142. [Google Scholar] [CrossRef]
- Dufresne, A.; Lesluyes, T.; Ménétrier-Caux, C.; Brahmi, M.; Darbo, E.; Toulmonde, M.; Italiano, A.; Mir, O.; Le Cesne, A.; Le Guellec, S. Specific immune landscapes and immune checkpoint expressions in histotypes and molecular subtypes of sarcoma. Oncoimmunology 2020, 9, 1792036. [Google Scholar] [CrossRef]
- Hu, J.; Qiu, D.; Yu, A.; Hu, J.; Deng, H.; Li, H.; Yi, Z.; Chen, J.; Zu, X. YTHDF1 is a potential pan-cancer biomarker for prognosis and immunotherapy. Front. Oncol. 2021, 11, 607224. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, J. Functions of immune checkpoint molecules beyond immune evasion. Regul. Cancer Immune Checkp. 2020, 1248, 201–226. [Google Scholar]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Ge, J.; Xiang, B.; Wu, X.; Ma, J.; Zhou, M.; Li, X. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol. Cancer 2019, 18, 10. [Google Scholar] [CrossRef]
- Xu, D.; Xu, Z.; Bi, X.; Cai, J.; Cao, M.; Zheng, D.; Chen, L.; Li, P.; Wang, H.; Wu, D.; et al. Identification and functional analysis of N6-methyladenine (m(6) A)-related lncRNA across 33 cancer types. Cancer Med. 2023, 12, 2104–2116. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, K.; Zou, X.; Hua, Z.; Wang, H.; Bian, W.; Wang, H.; Chen, F.; Dai, T. LncRNA DHRS4-AS1 ameliorates hepatocellular carcinoma by suppressing proliferation and promoting apoptosis via miR-522-3p/SOCS5 axis. Bioengineered 2021, 12, 10862–10877. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.; Qi, L.; Ling, L. Long Noncoding RNA HCG9 Promotes Osteosarcoma Progression through RAD51 by Acting as a ceRNA of miR-34b-3p. Mediat. Inflamm. 2021, 2021, 9978882. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cheng, M.; Zheng, X.; Zheng, L.; Liu, H.; Lu, J.; Liu, Y.; Chen, W. Interactions between lncRNA TUG1 and miR-9-5p Modulate the Resistance of Breast Cancer Cells to Doxorubicin by Regulating eIF5A2. Oncotargets Ther. 2020, 13, 13159–13170. [Google Scholar] [CrossRef]
- Mizuno, H.; Kitada, K.; Nakai, K.; Sarai, A. PrognoScan: A new database for meta-analysis of the prognostic value of genes. BMC Med. Genom. 2009, 2, 18. [Google Scholar] [CrossRef]
- Gaynor, N.; Crown, J.; Collins, D.M. Immune checkpoint inhibitors: Key trials and an emerging role in breast cancer. Semin. Cancer Biol. 2022, 79, 44–57. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Zou, X.; Shi, Y.; Liu, Q.; Huyan, T.; Su, J.; Wang, Q.; Zhang, F.; Li, X. FOXO1 inhibition prevents renal ischemia–reperfusion injury via cAMP-response element binding protein/PPAR-γ coactivator-1α-mediated mitochondrial biogenesis. Br. J. Pharmacol. 2020, 177, 432–448. [Google Scholar] [CrossRef]
- Ravaioli, S.; Limarzi, F.; Tumedei, M.M.; Palleschi, M.; Maltoni, R.; Bravaccini, S. Are we ready to use TMB in breast cancer clinical practice? Cancer Immunol. Immunother. 2020, 69, 1943–1945. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, W.; Zhou, Y.; Wang, J.; Wang, K.; Lin, Q.; Li, Y.; Xie, Y.; Li, M.; Wang, J.; Xiong, L. YTHDF1’s Regulatory Involvement in Breast Cancer Prognosis, Immunity, and the ceRNA Network. Int. J. Mol. Sci. 2024, 25, 1879. https://doi.org/10.3390/ijms25031879
Luo W, Zhou Y, Wang J, Wang K, Lin Q, Li Y, Xie Y, Li M, Wang J, Xiong L. YTHDF1’s Regulatory Involvement in Breast Cancer Prognosis, Immunity, and the ceRNA Network. International Journal of Molecular Sciences. 2024; 25(3):1879. https://doi.org/10.3390/ijms25031879
Chicago/Turabian StyleLuo, Wenting, Youjia Zhou, Jiayang Wang, Keqin Wang, Qing Lin, Yuqiu Li, Yujie Xie, Miao Li, Jie Wang, and Lixia Xiong. 2024. "YTHDF1’s Regulatory Involvement in Breast Cancer Prognosis, Immunity, and the ceRNA Network" International Journal of Molecular Sciences 25, no. 3: 1879. https://doi.org/10.3390/ijms25031879
APA StyleLuo, W., Zhou, Y., Wang, J., Wang, K., Lin, Q., Li, Y., Xie, Y., Li, M., Wang, J., & Xiong, L. (2024). YTHDF1’s Regulatory Involvement in Breast Cancer Prognosis, Immunity, and the ceRNA Network. International Journal of Molecular Sciences, 25(3), 1879. https://doi.org/10.3390/ijms25031879





