Paradigm Shift: A Comprehensive Review of Ovarian Cancer Management in an Era of Advancements
Abstract
1. Introduction
2. Disease Aetiology and Prevention
3. Disease Diagnosis and Prognosis Assessment
4. Current Treatments and Innovations
4.1. Antiangiogenic Agents
4.2. DNA Damage Repair-Based Therapeutics
4.3. Hyperthermic Intraperitoneal Chemotherapy (HIPEC)
4.4. Hormone Receptor Modulators
4.5. FRα-Targeting Drugs
5. Emerging Therapies
5.1. Immunomodulators
5.2. Gene Therapies
5.3. Drug Repurposing
5.4. Small-Molecule Kinase Inhibitors
5.5. Coagulation-Targeting Approaches
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Cabasag, C.J.; Fagan, P.J.; Ferlay, J.; Vignat, J.; Laversanne, M.; Liu, L.; van der Aa, M.A.; Bray, F.; Soerjomataram, I. Ovarian cancer today and tomorrow: A global assessment by world region and Human Development Index using GLOBOCAN 2020. Int. J. Cancer 2022, 151, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Momenimovahed, Z.; Tiznobaik, A.; Taheri, S.; Salehiniya, H. Ovarian cancer in the world: Epidemiology and risk factors. Int. J. Women’s Health 2019, 11, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.; Aggarwal, S.; Rauthan, A.; Warrier, N. Maintenance therapy for newly diagnosed epithelial ovarian cancer—A review. J. Ovarian Res. 2022, 15, 88. [Google Scholar] [CrossRef] [PubMed]
- Jayson, G.C.; Kohn, E.C.; Kitchener, H.C.; Ledermann, J.A. Ovarian cancer. Lancet 2014, 384, 1376–1388. [Google Scholar] [CrossRef]
- DiSilvestro, P.; Secord, A.A. Maintenance treatment of recurrent ovarian cancer: Is it ready for prime time? Cancer Treat. Rev. 2018, 69, 53–65. [Google Scholar] [CrossRef]
- Nik, N.N.; Vang, R.; Shih, I.-M.; Kurman, R.J. Origin and pathogenesis of pelvic (ovarian, tubal, and primary peritoneal) serous carcinoma. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 27–45. [Google Scholar] [CrossRef]
- Helm, C.W. The role of hyperthermic intraperitoneal chemotherapy (HIPEC) in ovarian cancer. Oncologist 2009, 14, 683–694. [Google Scholar] [CrossRef]
- Meinhold-Heerlein, I.; Fotopoulou, C.; Harter, P.; Kurzeder, C.; Mustea, A.; Wimberger, P.; Hauptmann, S.; Sehouli, J. The new WHO classification of ovarian, fallopian tube, and primary peritoneal cancer and its clinical implications. Arch. Gynecol. Obstet. 2016, 293, 695–700. [Google Scholar] [CrossRef]
- De Leo, A.; Santini, D.; Ceccarelli, C.; Santandrea, G.; Palicelli, A.; Acquaviva, G.; Chiarucci, F.; Rosini, F.; Ravegnini, G.; Pession, A. What is new on ovarian carcinoma: Integrated morphologic and molecular analysis following the new 2020 World Health Organization classification of female genital tumors. Diagnostics 2021, 11, 697. [Google Scholar] [CrossRef]
- Moch, H. Female genital tumours. In WHO Classification of Tumours; World Health Organization: Geneva, Switzerland, 2020; Volume 4. [Google Scholar]
- Stewart, C.; Ralyea, C.; Lockwood, S. Ovarian cancer: An integrated review. Semin. Oncol. Nurs. 2019, 35, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Kurman, R.J.; Shih, I.-M. The origin and pathogenesis of epithelial ovarian cancer—A proposed unifying theory. Am. J. Surg. Pathol. 2010, 34, 433. [Google Scholar] [CrossRef] [PubMed]
- Kurman, R.J.; Shih, I.-M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer—Shifting the paradigm. Hum. Pathol. 2011, 42, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Network, C.G.A.R. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609. [Google Scholar] [CrossRef]
- Grandi, G.; Toss, A.; Cortesi, L.; Botticelli, L.; Volpe, A.; Cagnacci, A. The association between endometriomas and ovarian cancer: Preventive effect of inhibiting ovulation and menstruation during reproductive life. BioMed Res. Int. 2015, 2015, 751571. [Google Scholar] [CrossRef] [PubMed]
- Salazar, C.; Campbell, I.G.; Gorringe, K.L. When is “type I” ovarian cancer not “type I”? Indications of an out-dated dichotomy. Front. Oncol. 2018, 8, 654. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, N.; Rassy, E.; Vermorken, J.B.; Assi, T.; Kattan, J.; Boussios, S.; Smith-Gagen, J. The outcome of patients with serous papillary peritoneal cancer, fallopian tube cancer, and epithelial ovarian cancer by treatment eras: 27 years data from the SEER registry. Cancer Epidemiol. 2021, 75, 102045. [Google Scholar] [CrossRef]
- Baslan, T.; Hicks, J. Unravelling biology and shifting paradigms in cancer with single-cell sequencing. Nat. Rev. Cancer 2017, 17, 557–569. [Google Scholar] [CrossRef]
- Cook, D.P.; Vanderhyden, B.C. Ovarian cancer and the evolution of subtype classifications using transcriptional profiling. Biol. Reprod. 2019, 101, 645–658. [Google Scholar] [CrossRef]
- Acs, B.; Rantalainen, M.; Hartman, J. Artificial intelligence as the next step towards precision pathology. J. Intern. Med. 2020, 288, 62–81. [Google Scholar] [CrossRef]
- Zhou, J.; Cao, W.; Wang, L.; Pan, Z.; Fu, Y. Application of artificial intelligence in the diagnosis and prognostic prediction of ovarian cancer. Comput. Biol. Med. 2022, 146, 105608. [Google Scholar] [CrossRef]
- Wentzensen, N.; Poole, E.M.; Trabert, B.; White, E.; Arslan, A.A.; Patel, A.V.; Setiawan, V.W.; Visvanathan, K.; Weiderpass, E.; Adami, H.-O. Ovarian cancer risk factors by histologic subtype: An analysis from the ovarian cancer cohort consortium. J. Clin. Oncol. 2016, 34, 2888. [Google Scholar] [CrossRef]
- González-Martín, A.; Harter, P.; Leary, A.; Lorusso, D.; Miller, R.; Pothuri, B.; Ray-Coquard, I.; Tan, D.; Bellet, E.; Oaknin, A. Newly diagnosed and relapsed epithelial ovarian cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Flaum, N.; Crosbie, E.J.; Edmondson, R.J.; Smith, M.J.; Evans, D.G. Epithelial ovarian cancer risk: A review of the current genetic landscape. Clin. Genet. 2020, 97, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Hunn, J.; Rodriguez, G.C. Ovarian cancer: Etiology, risk factors, and epidemiology. Clin. Obstet. Gynecol. 2012, 55, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Singla, A. Epidemiology and Risk Factors for Ovarian Cancer. In Preventive Oncology for the Gynecologist; Springer: Singapore, 2019; pp. 223–231. [Google Scholar]
- Roett, M.A.; Evans, P. Ovarian cancer: An overview. Am. Fam. Physician 2009, 80, 609–616. [Google Scholar] [PubMed]
- Bergfeldt, K.; Rydh, B.; Granath, F.; Grönberg, H.; Thalib, L.; Adami, H.-O.; Hall, P. Risk of ovarian cancer in breast-cancer patients with a family history of breast or ovarian cancer: A population-based cohort study. Lancet 2002, 360, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Malander, S.; Rambech, E.; Kristoffersson, U.; Halvarsson, B.; Ridderheim, M.; Borg, Å.; Nilbert, M. The contribution of the hereditary nonpolyposis colorectal cancer syndrome to the development of ovarian cancer. Gynecol. Oncol. 2006, 101, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Tu, C.; Song, X.; Cai, L. Case report: Analysis of BRCA1 and BRCA2 gene mutations in a hereditary ovarian cancer family. J. Assist. Reprod. Genet. 2020, 37, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- Pajenga, E.; Rexha, T.; Çeliku, S.; Bejtja, G.; Pisha, M. Hormonal risk factors for ovarian cancer in the Albanian case-control study. Bosn. J. Basic Med. Sci. 2013, 13, 89. [Google Scholar] [CrossRef][Green Version]
- Budiana, I.N.G.; Angelina, M.; Pemayun, T.G.A. Ovarian cancer: Pathogenesis and current recommendations for prophylactic surgery. J. Turk. Ger. Gynecol. Assoc. 2019, 20, 47. [Google Scholar] [CrossRef]
- Collaborative Group on Epidemiological Studies of Ovarian Cancer; Beral, V.; Gaitskell, K.; Hermon, C.; Moser, K.; Reeves, G.; Peto, R. Menopausal hormone use and ovarian cancer risk: Individual participant meta-analysis of 52 epidemiological studies. Lancet 2015, 385, 1835–1842. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, L.; Yang, X.; Bie, J.; Li, D.; Sun, C.; Zhang, J.; Meng, Y.; Lin, J. Menopausal hormone replacement therapy and the risk of ovarian cancer: A meta-analysis. Front. Endocrinol. 2019, 10, 801. [Google Scholar] [CrossRef]
- Toufakis, V.; Katuwal, S.; Pukkala, E.; Tapanainen, J.S. Impact of parity on the incidence of ovarian cancer subtypes: A population-based case–control study. Acta Oncol. 2021, 60, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Collaborative Group on Epidemiological Studies of Ovarian Cancer. Ovarian cancer and oral contraceptives: Collaborative reanalysis of data from 45 epidemiological studies including 23 257 women with ovarian cancer and 87 303 controls. Lancet 2008, 371, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Gaitskell, K.; Green, J.; Pirie, K.; Barnes, I.; Hermon, C.; Reeves, G.K.; Beral, V.; Collaborators, M.W.S. Histological subtypes of ovarian cancer associated with parity and breastfeeding in the prospective Million Women Study. Int. J. Cancer 2018, 142, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.P.; Chen, H.L.; Shen, M.Y. Breastfeeding and the risk of ovarian cancer: A meta-analysis. J. Midwifery Women’s Health 2014, 59, 428–437. [Google Scholar] [CrossRef]
- Mallen, A.; Soong, T.R.; Townsend, M.K.; Wenham, R.M.; Crum, C.P.; Tworoger, S.S. Surgical prevention strategies in ovarian cancer. Gynecol. Oncol. 2018, 151, 166–175. [Google Scholar] [CrossRef]
- Walker, J.L.; Powell, C.B.; Chen, L.m.; Carter, J.; Bae Jump, V.L.; Parker, L.P.; Borowsky, M.E.; Gibb, R.K. Society of Gynecologic O ncology recommendations for the prevention of ovarian cancer. Cancer 2015, 121, 2108–2120. [Google Scholar] [CrossRef] [PubMed]
- Langdon, S.P.; Herrington, C.S.; Hollis, R.L.; Gourley, C. Estrogen signaling and its potential as a target for therapy in ovarian cancer. Cancers 2020, 12, 1647. [Google Scholar] [CrossRef] [PubMed]
- Lukanova, A.; Kaaks, R. Endogenous hormones and ovarian cancer: Epidemiology and current hypotheses. Cancer Epidemiol. Biomark. Prev. 2005, 14, 98–107. [Google Scholar] [CrossRef]
- Guo, H.; Guo, J.; Xie, W.; Yuan, L.; Sheng, X. The role of vitamin D in ovarian cancer: Epidemiology, molecular mechanism and prevention. J. Ovarian Res. 2018, 11, 71. [Google Scholar] [CrossRef]
- Gaona-Luviano, P.; Medina-Gaona, L.A.; Magaña-Pérez, K. Epidemiology of ovarian cancer. Chin. Clin. Oncol. 2020, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Tanha, K.; Mottaghi, A.; Nojomi, M.; Moradi, M.; Rajabzadeh, R.; Lotfi, S.; Janani, L. Investigation on factors associated with ovarian cancer: An umbrella review of systematic review and meta-analyses. J. Ovarian Res. 2021, 14, 153. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.-L.; Fang, Y.; Zhang, M.; Zhang, Y.-Z. Phytoestrogen intake and risk of ovarian cancer: A meta-analysis of 10 observational studies. Asian Pac. J. Cancer Prev. 2014, 15, 9085–9091. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, P.J.; Gotlieb, W.H. Missed therapeutic and prevention opportunities in women with BRCA-mutated epithelial ovarian cancer and their families due to low referral rates for genetic counseling and BRCA testing: A review of the literature. CA A Cancer J. Clin. 2017, 67, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Berliner, J.L.; Cummings, S.A.; Boldt Burnett, B.; Ricker, C.N. Risk assessment and genetic counseling for hereditary breast and ovarian cancer syndromes—Practice resource of the National Society of Genetic Counselors. J. Genet. Couns. 2021, 30, 342–360. [Google Scholar] [CrossRef] [PubMed]
- Daly, M.B.; Pal, T.; Berry, M.P.; Buys, S.S.; Dickson, P.; Domchek, S.M.; Elkhanany, A.; Friedman, S.; Goggins, M.; Hutton, M.L. Genetic/familial high-risk assessment: Breast, ovarian, and pancreatic, version 2.2021, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 77–102. [Google Scholar] [CrossRef] [PubMed]
- Baandrup, L.; Kjær, S.K.; Olsen, J.; Dehlendorff, C.; Friis, S. Low-dose aspirin use and the risk of ovarian cancer in Denmark. Ann. Oncol. 2015, 26, 787–792. [Google Scholar] [CrossRef]
- Zhang, D.; Bai, B.; Xi, Y.; Wang, T.; Zhao, Y. Is aspirin use associated with a decreased risk of ovarian cancer? A systematic review and meta-analysis of observational studies with dose-response analysis. Gynecol. Oncol. 2016, 142, 368–377. [Google Scholar] [CrossRef]
- Baandrup, L.; Faber, M.T.; Christensen, J.; Jensen, A.; Andersen, K.K.; Friis, S.; Kjær, S.K. Nonsteroidal anti-inflammatory drugs and risk of ovarian cancer: Systematic review and meta-analysis of observational studies. Acta Obstet. Et Gynecol. Scand. 2013, 92, 245–255. [Google Scholar] [CrossRef]
- Raffle, A.E.; Gray, J.M. Screening: Evidence and Practice; Oxford University Press: New York, NY, USA, 2019. [Google Scholar]
- Temming, L.A.; Macones, G.A. What is prenatal screening and why to do it? Semin. Perinatol. 2016, 40, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Mathis, J.; Jellouli, M.A.; Sabiani, L.; Fest, J.; Blache, G.; Mathevet, P. Ovarian cancer screening in the general population. Horm. Mol. Biol. Clin. Investig. 2019, 41, 20190038. [Google Scholar] [CrossRef] [PubMed]
- Patni, R. Screening for ovarian cancer: An update. J. Mid-Life Health 2019, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Grossman, D.C.; Curry, S.J.; Owens, D.K.; Barry, M.J.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W.; Kemper, A.R.; Krist, A.H.; Kurth, A.E. Screening for ovarian cancer: US preventive services task force recommendation statement. Jama 2018, 319, 588–594. [Google Scholar]
- Stirling, D.; Evans, D.G.R.; Pichert, G.; Shenton, A.; Kirk, E.N.; Rimmer, S.; Steel, C.M.; Lawson, S.; Busby-Earle, R.C.; Walker, J. Screening for familial ovarian cancer: Failure of current protocols to detect ovarian cancer at an early stage according to the international Federation of gynecology and obstetrics system. J. Clin. Oncol. 2005, 23, 5588–5596. [Google Scholar] [CrossRef]
- Bodurka, D.C.; Deavers, M.T.; Tian, C.; Sun, C.C.; Malpica, A.; Coleman, R.L.; Lu, K.H.; Sood, A.K.; Birrer, M.J.; Ozols, R. Reclassification of serous ovarian carcinoma by a 2-tier system: A gynecologic oncology group study. Cancer 2012, 118, 3087–3094. [Google Scholar] [CrossRef] [PubMed]
- Jacob, F.; Meier, M.; Caduff, R.; Goldstein, D.; Pochechueva, T.; Hacker, N.; Fink, D.; Heinzelmann-Schwarz, V. No benefit from combining HE4 and CA125 as ovarian tumor markers in a clinical setting. Gynecol. Oncol. 2011, 121, 487–491. [Google Scholar] [CrossRef]
- Dochez, V.; Caillon, H.; Vaucel, E.; Dimet, J.; Winer, N.; Ducarme, G. Biomarkers and algorithms for diagnosis of ovarian cancer: CA125, HE4, RMI and ROMA, a review. J. Ovarian Res. 2019, 12, 28. [Google Scholar] [CrossRef]
- Buamah, P. Benign conditions associated with raised serum CA-125 concentration. J. Surg. Oncol. 2000, 75, 264–265. [Google Scholar] [CrossRef]
- Anastasi, E.; Manganaro, L.; Granato, T.; Benedetti Panici, P.; Frati, L.; Porpora, M.G. Is CA72-4 a useful biomarker in differential diagnosis between ovarian endometrioma and epithelial ovarian cancer? Dis. Markers 2013, 35, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yu, J.; Song, X.; Mi, H. Serum CA125, CA199 and CEA combined detection for epithelial ovarian cancer diagnosis: A meta-analysis. Open Med. 2017, 12, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Kurosaki, A.; Hasegawa, K.; Kato, T.; Abe, K.; Hanaoka, T.; Miyara, A.; O’Shannessy, D.J.; Somers, E.B.; Yasuda, M.; Sekino, T. Serum folate receptor alpha as a biomarker for ovarian cancer: Implications for diagnosis, prognosis and predicting its local tumor expression. Int. J. Cancer 2016, 138, 1994–2002. [Google Scholar] [CrossRef] [PubMed]
- Nebgen, D.R.; Lu, K.H.; Bast, R.C. Novel approaches to ovarian cancer screening. Curr. Oncol. Rep. 2019, 21, 75. [Google Scholar] [CrossRef] [PubMed]
- Biskup, E.; Wils, R.S.; Hogdall, C.; Hogdall, E. Prospects of improving early ovarian cancer diagnosis using cervical cell swabs. Anticancer Res. 2022, 42, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cragun, J.M. Screening for ovarian cancer. Cancer Control 2011, 18, 16–21. [Google Scholar] [CrossRef]
- Jacobs, I.; Oram, D.; Fairbanks, J.; Turner, J.; Frost, C.; Grudzinskas, J. A risk of malignancy index incorporating CA 125, ultrasound and menopausal status for the accurate preoperative diagnosis of ovarian cancer. BJOG Int. J. Obstet. Gynaecol. 1990, 97, 922–929. [Google Scholar] [CrossRef]
- Bristow, R.E.; Smith, A.; Zhang, Z.; Chan, D.W.; Crutcher, G.; Fung, E.T.; Munroe, D.G. Ovarian malignancy risk stratification of the adnexal mass using a multivariate index assay. Gynecol. Oncol. 2013, 128, 252–259. [Google Scholar] [CrossRef]
- Moore, R.G.; McMeekin, D.S.; Brown, A.K.; DiSilvestro, P.; Miller, M.C.; Allard, W.J.; Gajewski, W.; Kurman, R.; Bast, R.C., Jr.; Skates, S.J. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol. Oncol. 2009, 112, 40–46. [Google Scholar] [CrossRef]
- Zhang, R.; Siu, M.K.; Ngan, H.Y.; Chan, K.K. Molecular biomarkers for the early detection of ovarian cancer. Int. J. Mol. Sci. 2022, 23, 12041. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.A.; Andrews, K.S.; Brooks, D.; Fedewa, S.A.; Manassaram-Baptiste, D.; Saslow, D.; Wender, R.C. Cancer screening in the United States, 2019: A review of current American Cancer Society guidelines and current issues in cancer screening. CA A Cancer J. Clin. 2019, 69, 184–210. [Google Scholar] [CrossRef] [PubMed]
- Berek, J.S.; Kehoe, S.T.; Kumar, L.; Friedlander, M. Cancer of the ovary, fallopian tube, and peritoneum. Int. J. Gynecol. Obstet. 2018, 143, 59–78. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Sessa, C.; du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I. ESMO–ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef]
- Lataifeh, I.; Marsden, D.E.; Robertson, G.; Gebski, V.; Hacker, N.F. Presenting symptoms of epithelial ovarian cancer. Aust. N. Z. J. Obstet. Gynaecol. 2005, 45, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Mustafin, C.; Vesnin, S.; Turnbull, A.; Dixon, M.; Goltsov, A.; Goryanin, I. Diagnostics of Ovarian Tumors in Postmenopausal Patients. Diagnostics 2022, 12, 2619. [Google Scholar] [CrossRef] [PubMed]
- Arora, T.; Mullangi, S.; Lekkala, M.R. Ovarian Cancer; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Kemppainen, J.; Hynninen, J.; Virtanen, J.; Seppänen, M. PET/CT for evaluation of ovarian cancer. Semin. Nucl. Med. 2019, 49, 484–492. [Google Scholar] [CrossRef]
- Javadi, S.; Ganeshan, D.M.; Qayyum, A.; Iyer, R.B.; Bhosale, P. Ovarian Cancer, the Revised FIGO Staging System, and the Role of Imaging. Am. J. Roentgenol. 2016, 206, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Berek, J.S.; Renz, M.; Kehoe, S.; Kumar, L.; Friedlander, M. Cancer of the ovary, fallopian tube, and peritoneum: 2021 update. Int. J. Gynecol. Obstet. 2021, 155, 61–85. [Google Scholar] [CrossRef]
- Cooper, B.C.; Sood, A.K.; Davis, C.S.; Ritchie, J.M.; Sorosky, J.I.; Anderson, B.; Buller, R.E. Preoperative CA 125 levels: An independent prognostic factor for epithelial ovarian cancer. Obstet. Gynecol. 2002, 100, 59–64. [Google Scholar] [CrossRef]
- Meinhold-Heerlein, I.; Hauptmann, S. The heterogeneity of ovarian cancer. Arch. Gynecol. Obstet. 2014, 289, 237–239. [Google Scholar] [CrossRef]
- Matz, M.; Coleman, M.P.; Sant, M.; Chirlaque, M.D.; Visser, O.; Gore, M.; Allemani, C.; Bouzbid, S.; Hamdi-Chérif, M.; Zaidi, Z.; et al. The histology of ovarian cancer: Worldwide distribution and implications for international survival comparisons (CONCORD-2). Gynecol. Oncol. 2017, 144, 405–413. [Google Scholar] [CrossRef]
- Heintz, A.; Odicino, F.; Maisonneuve, P.; Quinn, M.; Benedet, J.; Creasman, W.; Ngan, H.; Pecorelli, S.; Beller, U. Carcinoma of the ovary. Int. J. Gynecol. Obstet. 2006, 95, S161–S192. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Sood, A.K.; Fallowfield, L.; Howitt, B.E.; Sehouli, J.; Karlan, B.Y. Ovarian cancer. Nat. Rev. Dis. Primers 2016, 2, 16061. [Google Scholar] [CrossRef]
- Filis, P.; Mauri, D.; Markozannes, G.; Tolia, M.; Filis, N.; Tsilidis, K. Hyperthermic intraperitoneal chemotherapy (HIPEC) for the management of primary advanced and recurrent ovarian cancer: A systematic review and meta-analysis of randomized trials. ESMO Open 2022, 7, 100586. [Google Scholar] [CrossRef]
- Opławski, M.; Średnicka, A.; Niewiadomska, E.; Boroń, D.; Januszyk, P.; Grabarek, B.O. Clinical and molecular evaluation of patients with ovarian cancer in the context of drug resistance to chemotherapy. Front. Oncol. 2022, 12, 954008. [Google Scholar] [CrossRef]
- Millstein, J.; Budden, T.; Goode, E.L.; Anglesio, M.S.; Talhouk, A.; Intermaggio, M.P.; Leong, H.; Chen, S.; Elatre, W.; Gilks, B. Prognostic gene expression signature for high-grade serous ovarian cancer. Ann. Oncol. 2020, 31, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Wilczyński, J.; Paradowska, E.; Wilczyński, M. Personalization of Therapy in High-Grade Serous Tubo-Ovarian Cancer—The Possibility or the Necessity? J. Pers. Med. 2023, 14, 49. [Google Scholar] [CrossRef]
- Bowtell, D.D.; Böhm, S.; Ahmed, A.A.; Aspuria, P.-J.; Bast, R.C., Jr.; Beral, V.; Berek, J.S.; Birrer, M.J.; Blagden, S.; Bookman, M.A. Rethinking ovarian cancer II: Reducing mortality from high-grade serous ovarian cancer. Nat. Rev. Cancer 2015, 15, 668–679. [Google Scholar] [CrossRef]
- Tothill, R.W.; Tinker, A.V.; George, J.; Brown, R.; Fox, S.B.; Lade, S.; Johnson, D.S.; Trivett, M.K.; Etemadmoghadam, D.; Locandro, B. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin. Cancer Res. 2008, 14, 5198–5208. [Google Scholar] [CrossRef] [PubMed]
- Verhaak, R.G.; Tamayo, P.; Yang, J.-Y.; Hubbard, D.; Zhang, H.; Creighton, C.J.; Fereday, S.; Lawrence, M.; Carter, S.L.; Mermel, C.H. Prognostically relevant gene signatures of high-grade serous ovarian carcinoma. J. Clin. Investig. 2012, 123, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Konecny, G.E.; Wang, C.; Hamidi, H.; Winterhoff, B.; Kalli, K.R.; Dering, J.; Ginther, C.; Chen, H.-W.; Dowdy, S.; Cliby, W. Prognostic and therapeutic relevance of molecular subtypes in high-grade serous ovarian cancer. J. Natl. Cancer Inst. 2014, 106, dju249. [Google Scholar] [CrossRef]
- Dion, L.; Carton, I.; Jaillard, S.; Nyangoh Timoh, K.; Henno, S.; Sardain, H.; Foucher, F.; Leveque, J.; de la Motte Rouge, T.; Brousse, S. The landscape and therapeutic implications of molecular profiles in epithelial ovarian cancer. J. Clin. Med. 2020, 9, 2239. [Google Scholar] [CrossRef]
- Macintyre, G.; Goranova, T.E.; De Silva, D.; Ennis, D.; Piskorz, A.M.; Eldridge, M.; Sie, D.; Lewsley, L.-A.; Hanif, A.; Wilson, C. Copy number signatures and mutational processes in ovarian carcinoma. Nat. Genet. 2018, 50, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Harris, F.R.; Zhang, P.; Yang, L.; Hou, X.; Leventakos, K.; Weroha, S.J.; Vasmatzis, G.; Kovtun, I.V. Targeting HER 2 in patient-derived xenograft ovarian cancer models sensitizes tumors to chemotherapy. Mol. Oncol. 2019, 13, 132–152. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Y.; Kim, H.J.; Park, S.A.; Lee, S.H.; Kim, L.K.; Lee, J.Y.; Kim, S.; Kim, Y.T.; Kim, S.W.; Nam, E.J. Genetic profiles associated with chemoresistance in patient-derived xenograft models of ovarian cancer. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2019, 51, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.E.; Salinas, E.A.; Devor, E.J.; Newtson, A.M.; Thiel, K.W.; Goodheart, M.J.; Bender, D.P.; Smith, B.J.; Leslie, K.K.; Gonzalez-Bosquet, J. Molecular characterization of non-responders to chemotherapy in serous ovarian cancer. Int. J. Mol. Sci. 2019, 20, 1175. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Li, J.; Zhang, Q.; Xu, F.; Xie, B.; Lu, H.; Wu, X.; Zhou, X. Single-cell transcriptomes reveal heterogeneity of high-grade serous ovarian carcinoma. Clin. Transl. Med. 2021, 11, e500. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Wang, F.; Gao, C.; Cao, Y.; Wang, J. Identification of specific cell subpopulations and marker genes in ovarian cancer using single-cell RNA sequencing. BioMed Res. Int. 2021, 2021, 1005793. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.K.; Alvarez, R.D.; Bakkum-Gamez, J.N.; Barroilhet, L.; Behbakht, K.; Berchuck, A.; Chen, L.-M.; Cristea, M.; DeRosa, M.; Eisenhauer, E.L.; et al. Ovarian Cancer, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 191–226. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Pius, C.; Nabeel, M.; Nair, M.; Vishwanatha, J.K.; Ahmad, S.; Basha, R. Ovarian cancer: Current status and strategies for improving therapeutic outcomes. Cancer Med. 2019, 8, 7018–7031. [Google Scholar] [CrossRef]
- McMullen, M.; Madariaga, A.; Lheureux, S. New approaches for targeting platinum-resistant ovarian cancer. Semin. Cancer Biol. 2020, 77, 167–181. [Google Scholar] [CrossRef]
- Guo, T.; Dong, X.; Xie, S.; Zhang, L.; Zeng, P.; Zhang, L. Cellular mechanism of gene mutations and potential therapeutic targets in ovarian cancer. Cancer Manag. Res. 2021, 13, 3081–3100. [Google Scholar] [CrossRef]
- Basta, A.; Bidziński, M.; Bieńkiewicz, A.; Blecharz, P.; Bodnar, L.; Jach, R.; Knapp, P.; Kojs, Z.; Kotarski, J.; Markowska, J. Recommendation of the Polish Society of Oncological Gynaecology on the diagnosis and treatment of epithelial ovarian cancer. Oncol. Clin. Pract. 2015, 11, 233–243. [Google Scholar]
- Macchia, G.; Titone, F.; Restaino, S.; Arcieri, M.; Pellecchia, G.; Andreetta, C.; Driul, L.; Vizzielli, G.; Donato, P. Is It Time to Reassess the Role of Radiotherapy Treatment in Ovarian Cancer? Healthcare 2023, 11, 2413. [Google Scholar] [CrossRef]
- Durno, K.; Powell, M.E. The role of radiotherapy in ovarian cancer. Int. J. Gynecol. Cancer 2022, 32, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Herrera, F.G.; Irving, M.; Kandalaft, L.E.; Coukos, G. Rational combinations of immunotherapy with radiotherapy in ovarian cancer. Lancet Oncol. 2019, 20, e417–e433. [Google Scholar] [CrossRef]
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of ovarian cancer: A review. Cancer Biol. Med. 2017, 14, 9. [Google Scholar]
- Kim, S.; Han, Y.; Kim, S.I.; Kim, H.-S.; Kim, S.J.; Song, Y.S. Tumor evolution and chemoresistance in ovarian cancer. npj Precis. Oncol. 2018, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Foley, O.W.; Rauh-Hain, J.A.; Del Carmen, M.G. Recurrent epithelial ovarian cancer: An update on treatment. Oncology 2013, 27, 288. [Google Scholar] [PubMed]
- Cortez, A.J.; Tudrej, P.; Kujawa, K.A.; Lisowska, K.M. Advances in ovarian cancer therapy. Cancer Chemother. Pharmacol. 2018, 81, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Markman, M.; Rothman, R.; Hakes, T.; Reichman, B.; Hoskins, W.; Rubin, S.; Jones, W.; Almadrones, L.; Lewis, J., Jr. Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J. Clin. Oncol. 1991, 9, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Kaye, S.B. Ovarian cancer: Strategies for overcoming resistance to chemotherapy. Nat. Rev. Cancer 2003, 3, 502–516. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, K.; Gibbs, E.; Åvall-Lundqvist, E.; dePont Christensen, R.; Woie, K.; Kalling, M.; Auranen, A.; Grenman, S.; Hoegberg, T.; Rosenberg, P. Chemotherapy vs tamoxifen in platinum-resistant ovarian cancer: A phase III, randomised, multicentre trial (Ovaresist). Br. J. Cancer 2017, 116, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Lakusta, C.M.; Atkinson, M.J.; Robinson, J.W.; Nation, J.; Taenzer, P.A.; Campo, M.G. Quality of life in ovarian cancer patients receiving chemotherapy. Gynecol. Oncol. 2001, 81, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Stiggelbout, A.M.; De Haes, J. Patient preference for cancer therapy: An overview of measurement approaches. J. Clin. Oncol. 2001, 19, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Ahmed-Lecheheb, D.; Joly, F. Ovarian cancer survivors’ quality of life: A systematic review. J. Cancer Surviv. 2016, 10, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Wang, X.; Jin, H.; Mi, Y.; Liu, L.; Dong, M.; Chen, Y.; Zou, Z. Cisplatin-induced ototoxicity: Updates on molecular mechanisms and otoprotective strategies. Eur. J. Pharm. Biopharm. 2021, 163, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Martin, V. Overview of paclitaxel (Taxol®). Semin. Oncol. Nurs. 1993, 2, 2–5. [Google Scholar] [CrossRef]
- Nero, C.; Ciccarone, F.; Pietragalla, A.; Duranti, S.; Daniele, G.; Salutari, V.; Carbone, M.V.; Scambia, G.; Lorusso, D. Ovarian cancer treatments strategy: Focus on PARP inhibitors and immune check point inhibitors. Cancers 2021, 13, 1298. [Google Scholar] [CrossRef]
- Bamberger, E.; Perrett, C. Angiogenesis in epithelian ovarian cancer. Mol. Pathol. 2002, 55, 348. [Google Scholar] [CrossRef]
- Burger, R.A.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Huang, H.; Mannel, R.S.; Homesley, H.D.; Fowler, J.; Greer, B.E. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 2011, 365, 2473–2483. [Google Scholar] [CrossRef]
- Perren, T.J.; Swart, A.M.; Pfisterer, J.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; Kurzeder, C. A phase 3 trial of bevacizumab in ovarian cancer. N. Engl. J. Med. 2011, 365, 2484–2496. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.L.; Eisenhauer, E.L.; Herzog, T.J. Emerging therapies: Angiogenesis inhibitors for ovarian cancer. Expert Opin. Emerg. Drugs 2015, 20, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.T.; Ross, L.; Holash, J.; Nakanishi, M.; Hu, L.; Hofmann, J.I.; Yancopoulos, G.D.; Jaffe, R.B. Vascular endothelial growth factor-trap decreases tumor burden, inhibits ascites, and causes dramatic vascular remodeling in an ovarian cancer model. Clin. Cancer Res. 2003, 9, 5721–5728. [Google Scholar]
- Jain, R.K. Antiangiogenic therapy for cancer: Current and emerging concepts. Oncology 2005, 19, 7–16. [Google Scholar] [PubMed]
- Mao, C.-L.; Seow, K.-M.; Chen, K.-H. The Utilization of Bevacizumab in Patients with Advanced Ovarian Cancer: A Systematic Review of the Mechanisms and Effects. Int. J. Mol. Sci. 2022, 23, 6911. [Google Scholar] [CrossRef] [PubMed]
- Nakai, H.; Matsumura, N. The roles and limitations of bevacizumab in the treatment of ovarian cancer. Int. J. Clin. Oncol. 2022, 27, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, S.; Nakai, H.; Yamaguchi, K.; Hamanishi, J.; Mandai, M.; Matsumura, N. Time-dependent changes in risk of progression during use of bevacizumab for ovarian cancer. JAMA Netw. Open 2023, 6, e2326834. [Google Scholar] [CrossRef]
- Garcia, A.; Singh, H. Bevacizumab and ovarian cancer. Ther. Adv. Med. Oncol. 2013, 5, 133–141. [Google Scholar] [CrossRef]
- Burger, R.A.; Sill, M.W.; Monk, B.J.; Greer, B.E.; Sorosky, J.I. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: A Gynecologic Oncology Group Study. J. Clin. Oncol. 2007, 25, 5165–5171. [Google Scholar] [CrossRef]
- Kommoss, S.; Winterhoff, B.; Oberg, A.L.; Konecny, G.E.; Wang, C.; Riska, S.M.; Fan, J.-B.; Maurer, M.J.; April, C.; Shridhar, V. Bevacizumab may differentially improve ovarian cancer outcome in patients with proliferative and mesenchymal molecular subtypes. Clin. Cancer Res. 2017, 23, 3794–3801. [Google Scholar] [CrossRef]
- Arora, S.; Balasubramaniam, S.; Zhang, H.; Berman, T.; Narayan, P.; Suzman, D.; Bloomquist, E.; Tang, S.; Gong, Y.; Sridhara, R.; et al. FDA Approval Summary: Olaparib Monotherapy or in Combination with Bevacizumab for the Maintenance Treatment of Patients with Advanced Ovarian Cancer. Oncologist 2020, 26, e164–e172. [Google Scholar] [CrossRef]
- Mirza, M.; Coleman, R.; González-Martín, A.; Moore, K.; Colombo, N.; Ray-Coquard, I.; Pignata, S. The forefront of ovarian cancer therapy: Update on PARP inhibitors. Ann. Oncol. 2020, 31, 1148–1159. [Google Scholar] [CrossRef]
- Revythis, A.; Limbu, A.; Mikropoulos, C.; Ghose, A.; Sanchez, E.; Sheriff, M.; Boussios, S. Recent insights into PARP and immuno-checkpoint inhibitors in epithelial ovarian cancer. Int. J. Environ. Res. Public Health 2022, 19, 8577. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Q.; Xu, Y.; Cui, M.; Han, L. Advances in the treatment of ovarian cancer using PARP inhibitors and the underlying mechanism of resistance. Curr. Drug Targets 2020, 21, 167–178. [Google Scholar] [CrossRef]
- Xie, T.; Dickson, K.-A.; Yee, C.; Ma, Y.; Ford, C.E.; Bowden, N.A.; Marsh, D.J. Targeting Homologous Recombination Deficiency in Ovarian Cancer with PARP Inhibitors: Synthetic Lethal Strategies That Impact Overall Survival. Cancers 2022, 14, 4621. [Google Scholar] [CrossRef]
- Cook, S.A.; Tinker, A.V. PARP inhibitors and the evolving landscape of ovarian cancer management: A review. BioDrugs 2019, 33, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.R.; Monk, B.J.; Herrstedt, J.; Oza, A.M.; Mahner, S.; Redondo, A.; Fabbro, M.; Ledermann, J.A.; Lorusso, D.; Vergote, I. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N. Engl. J. Med. 2016, 375, 2154–2164. [Google Scholar] [CrossRef] [PubMed]
- Pujade-Lauraine, E.; Ledermann, J.A.; Selle, F.; Gebski, V.; Penson, R.T.; Oza, A.M.; Korach, J.; Huzarski, T.; Poveda, A.; Pignata, S. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Oza, A.M.; Lorusso, D.; Aghajanian, C.; Oaknin, A.; Dean, A.; Colombo, N.; Weberpals, J.I.; Clamp, A.; Scambia, G. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 1949–1961. [Google Scholar] [CrossRef] [PubMed]
- Pennington, K.P.; Walsh, T.; Harrell, M.I.; Lee, M.K.; Pennil, C.C.; Rendi, M.H.; Thornton, A.; Norquist, B.M.; Casadei, S.; Nord, A.S. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin. Cancer Res. 2014, 20, 764–775. [Google Scholar] [CrossRef]
- Franzese, E.; Centonze, S.; Diana, A.; Carlino, F.; Guerrera, L.P.; Di Napoli, M.; De Vita, F.; Pignata, S.; Ciardiello, F.; Orditura, M. PARP inhibitors in ovarian cancer. Cancer Treat. Rev. 2019, 73, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.J.; Ashworth, A. PARP inhibitors: Synthetic lethality in the clinic. Science 2017, 355, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Arisa, O.; Peer, C.J.; Figg, W.D.; Fojo, A. PARP inhibitors: A review of the pharmacology, pharmacokinetics, and pharmacogenetics. Semin. Oncol. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Curtin, N.J. Targeting the DNA damage response for cancer therapy. Biochem. Soc. Trans. 2023, 51, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Huang, T.-T.; Horibata, S.; Lee, J.-M. Cell cycle checkpoints and beyond: Exploiting the ATR/CHK1/WEE1 pathway for the treatment of PARP inhibitor–resistant cancer. Pharmacol. Res. 2022, 178, 106162. [Google Scholar] [CrossRef] [PubMed]
- Bouberhan, S.; Bar-Peled, L.; Matoba, Y.; Mazina, V.; Philp, L.; Rueda, B.R. The evolving role of DNA damage response in overcoming therapeutic resistance in ovarian cancer. Cancer Drug Resist. 2023, 6, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Van Driel, W.J.; Koole, S.N.; Sikorska, K.; Schagen van Leeuwen, J.H.; Schreuder, H.W.; Hermans, R.H.; De Hingh, I.H.; Van Der Velden, J.; Arts, H.J.; Massuger, L.F. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef]
- Issels, R.D. Hyperthermia adds to chemotherapy. Eur. J. Cancer 2008, 44, 2546–2554. [Google Scholar] [CrossRef]
- Bakrin, N.; Classe, J.; Pomel, C.; Gouy, S.; Chene, G.; Glehen, O. Hyperthermic intraperitoneal chemotherapy (HIPEC) in ovarian cancer. J. Visc. Surg. 2014, 151, 347–353. [Google Scholar] [CrossRef]
- Armstrong, D.K.; Alvarez, R.D.; Bakkum-Gamez, J.N.; Barroilhet, L.; Behbakht, K.; Berchuck, A.; Berek, J.S.; Chen, L.-M.; Cristea, M.; DeRosa, M. NCCN guidelines insights: Ovarian cancer, version 1.2019: Featured updates to the NCCN guidelines. J. Natl. Compr. Cancer Netw. 2019, 17, 896–909. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, T.H.; Han, E.S. State of the Science: The role of HIPEC in the treatment of ovarian cancer. Gynecol. Oncol. 2021, 160, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.C.; Chang, S.-J.; Yoo, H.J.; Nam, B.-H.; Bristow, R.; Park, S.-Y. Randomized trial of hyperthermic intraperitoneal chemotherapy (HIPEC) in women with primary advanced peritoneal, ovarian, and tubal cancer. J. Clin. Oncol. 2017, 35, 5520. [Google Scholar] [CrossRef]
- González Gil, A.; Cerezuela Fernández-de Palencia, Á.; Gómez Ruiz, Á.J.; Gil Gómez, E.; López Hernández, F.; Nieto Ruiz, A.; Martínez, J.; Marhuenda, I.; Cascales Campos, P.A. HIPEC in Ovarian Cancer Is the Future… and Always Will Be? Results from a Spanish Multicentric Survey. Cancers 2023, 15, 3481. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Singh, N.; Ghatage, P. Past, present, and future of hyperthermic intraperitoneal chemotherapy (HIPEC) in ovarian cancer. Cureus 2021, 13, e15563. [Google Scholar] [CrossRef]
- Simpkins, F.; Garcia-Soto, A.; Slingerland, J. New insights on the role of hormonal therapy in ovarian cancer. Steroids 2013, 78, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Langdon, S.; Hawkes, M.; Lawrie, S.; Hawkins, R.; Tesdale, A.; Crew, A.; Miller, W.; Smyth, J. Oestrogen receptor expression and the effects of oestrogen and tamoxifen on the growth of human ovarian carcinoma cell lines. Br. J. Cancer 1990, 62, 213–216. [Google Scholar] [CrossRef]
- Langdon, S.P.; Ritchie, A.; Young, K.; Crew, A.J.; Smyth, J.F.; Miller, W.R.; Sweeting, V.; Bramley, T.; Hillier, S.; Hawkins, R.A. Contrasting effects of 17 β-estradiol on the growth of human ovarian carcinoma cells in vitro and in vivo. Int. J. Cancer 1993, 55, 459–464. [Google Scholar] [CrossRef]
- Langdon, S.; Hirst, G.; Miller, E.; Hawkins, R.; Tesdale, A.; Smyth, J.; Miller, W. The regulation of growth and protein expression by estrogen in vitro: A study of 8 human ovarian carcinoma cell lines. J. Steroid Biochem. Mol. Biol. 1994, 50, 131–135. [Google Scholar] [CrossRef]
- O’Donnell, A.J.; Macleod, K.G.; Burns, D.J.; Smyth, J.F.; Langdon, S.P. Estrogen receptor-α mediates gene expression changes and growth response in ovarian cancer cells exposed to estrogen. Endocr. -Relat. Cancer 2005, 12, 851–866. [Google Scholar] [CrossRef][Green Version]
- Park, S.-H.; Cheung, L.W.; Wong, A.S.; Leung, P.C. Estrogen regulates Snail and Slug in the down-regulation of E-cadherin and induces metastatic potential of ovarian cancer cells through estrogen receptor α. Mol. Endocrinol. 2008, 22, 2085–2098. [Google Scholar] [CrossRef]
- Lazennec, G. Estrogen receptor beta, a possible tumor suppressor involved in ovarian carcinogenesis. Cancer Lett. 2006, 231, 151–157. [Google Scholar] [CrossRef]
- Bossard, C.; Busson, M.; Vindrieux, D.; Gaudin, F.; Machelon, V.; Brigitte, M.; Jacquard, C.; Pillon, A.; Balaguer, P.; Balabanian, K. Potential role of estrogen receptor beta as a tumor suppressor of epithelial ovarian cancer. PLoS ONE 2012, 7, e44787. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, T.; Brown, W.D.; Sapi, E.; Aschkenazi, S.; Muñoz, A.; Mor, G. Absence of estrogen receptor-β expression in metastatic ovarian cancer. Obstet. Gynecol. 2000, 96, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidis, I.; Papaioannidou, P. Estrogen receptor beta and ovarian cancer: A key to pathogenesis and response to therapy. Arch. Gynecol. Obstet. 2016, 293, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Ignatov, T.; Modl, S.; Thulig, M.; Weißenborn, C.; Treeck, O.; Ortmann, O.; Zenclussen, A.; Costa, S.D.; Kalinski, T.; Ignatov, A. GPER-1 acts as a tumor suppressor in ovarian cancer. J. Ovarian Res. 2013, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Schüler-Toprak, S.; Skrzypczak, M.; Ignatov, T.; Ignatov, A.; Ortmann, O.; Treeck, O. G protein-coupled estrogen receptor 1 (GPER-1) and agonist G-1 inhibit growth of ovarian cancer cells by activation of anti-tumoral transcriptome responses: Impact of GPER-1 mRNA on survival. J. Cancer Res. Clin. Oncol. 2020, 146, 3175–3188. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.O.; Arias-Pulido, H.; Kuo, D.Y.; Howard, T.; Qualls, C.R.; Lee, S.-J.; Verschraegen, C.F.; Hathaway, H.J.; Joste, N.E.; Prossnitz, E.R. GPR30 predicts poor survival for ovarian cancer. Gynecol. Oncol. 2009, 114, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.-X.; Xiong, W.; Wang, M.-L.; Yang, J.; Shi, H.-J.; Chen, H.-Q.; Niu, G. Nuclear G protein-coupled oestrogen receptor (GPR30) predicts poor survival in patients with ovarian cancer. J. Int. Med. Res. 2018, 46, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Jiang, X.; Zhao, Y.; Wen, H.; Liu, G. Role of GPER on proliferation, migration and invasion in ligand-independent manner in human ovarian cancer cell line SKOV3. Cell Biochem. Funct. 2015, 33, 552–559. [Google Scholar] [CrossRef]
- George, A.; McLachlan, J.; Tunariu, N.; Della Pepa, C.; Migali, C.; Gore, M.; Kaye, S.; Banerjee, S. The role of hormonal therapy in patients with relapsed high-grade ovarian carcinoma: A retrospective series of tamoxifen and letrozole. BMC Cancer 2017, 17, 456. [Google Scholar] [CrossRef]
- Sieh, W.; Köbel, M.; Longacre, T.A.; Bowtell, D.D.; DeFazio, A.; Goodman, M.T.; Høgdall, E.; Deen, S.; Wentzensen, N.; Moysich, K.B. Hormone-receptor expression and ovarian cancer survival: An Ovarian Tumor Tissue Analysis consortium study. Lancet Oncol. 2013, 14, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.R.; Dowsett, M. Aromatase inhibitors for breast cancer: Lessons from the laboratory. Nat. Rev. Cancer 2003, 3, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Saeaib, N.; Peeyananjarassri, K.; Liabsuetrakul, T.; Buhachat, R.; Myriokefalitaki, E. Hormone replacement therapy after surgery for epithelial ovarian cancer. Cochrane Database Syst. Rev. 2020, 1, CD012559. [Google Scholar] [CrossRef] [PubMed]
- Ledermann, J.; Canevari, S.; Thigpen, T. Targeting the folate receptor: Diagnostic and therapeutic approaches to personalize cancer treatments. Ann. Oncol. 2015, 26, 2034–2043. [Google Scholar] [CrossRef] [PubMed]
- Elnakat, H. Distribution, functionality and gene regulation of folate receptor isoforms: Implications in targeted therapy. Adv. Drug Deliv. Rev. 2004, 56, 1067–1084. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.; Bax, H.J.; Josephs, D.H.; Ilieva, K.M.; Pellizzari, G.; Opzoomer, J.; Bloomfield, J.; Fittall, M.; Grigoriadis, A.; Figini, M. Targeting folate receptor alpha for cancer treatment. Oncotarget 2016, 7, 52553. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.B.; Marth, C.; Coleman, R.L. Role of the folate receptor in ovarian cancer treatment: Evidence, mechanism, and clinical implications. Cancer Metastasis Rev. 2015, 34, 41–52. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, D.M.; Matulonis, U.A.; Birrer, M.J.; Castro, C.M.; Gilbert, L.; Vergote, I.; Martin, L.P.; Mantia-Smaldone, G.M.; Martin, A.G.; Bratos, R. Phase Ib study of mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in combination with bevacizumab in patients with platinum-resistant ovarian cancer. Gynecol. Oncol. 2020, 157, 379–385. [Google Scholar] [CrossRef]
- Kalli, K.R.; Oberg, A.L.; Keeney, G.L.; Christianson, T.J.; Low, P.S.; Knutson, K.L.; Hartmann, L.C. Folate receptor alpha as a tumor target in epithelial ovarian cancer. Gynecol. Oncol. 2008, 108, 619–626. [Google Scholar] [CrossRef]
- Bax, H.J.; Chauhan, J.; Stavraka, C.; Santaolalla, A.; Osborn, G.; Khiabany, A.; Grandits, M.; López-Abente, J.; Palhares, L.C.; Chan Wah Hak, C. Folate receptor alpha in ovarian cancer tissue and patient serum is associated with disease burden and treatment outcomes. Br. J. Cancer 2023, 128, 342–353. [Google Scholar] [CrossRef]
- Moore, K.N.; Martin, L.P.; O’Malley, D.M.; Matulonis, U.A.; Konner, J.A.; Vergote, I.; Ponte, J.F.; Birrer, M.J. A review of mirvetuximab soravtansine in the treatment of platinum-resistant ovarian cancer. Future Oncol. 2018, 14, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, C.; Palaia, I.; Giorgini, M.; De Medici, C.; Iadarola, R.; Vertechy, L.; Domenici, L.; Di Donato, V.; Tomao, F.; Muzii, L. Targeted drug delivery via folate receptors in recurrent ovarian cancer: A review. OncoTargets Ther. 2014, 7, 1223–1236. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.-A. Mirvetuximab soravtansine: First approval. Drugs 2023, 83, 265–273. [Google Scholar] [CrossRef]
- Armstrong, D.K.; White, A.J.; Weil, S.C.; Phillips, M.; Coleman, R.L. Farletuzumab (a monoclonal antibody against folate receptor alpha) in relapsed platinum-sensitive ovarian cancer. Gynecol. Oncol. 2013, 129, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Spannuth, W.A.; Sood, A.K.; Coleman, R.L. Farletuzumab in epithelial ovarian carcinoma. Expert Opin. Biol. Ther. 2010, 10, 431–437. [Google Scholar] [CrossRef]
- Vergote, I.; Armstrong, D.; Scambia, G.; Teneriello, M.; Sehouli, J.; Schweizer, C.; Weil, S.C.; Bamias, A.; Fujiwara, K.; Ochiai, K. A randomized, double-blind, placebo-controlled, phase III study to assess efficacy and safety of weekly farletuzumab in combination with carboplatin and taxane in patients with ovarian cancer in first platinum-sensitive relapse. J. Clin. Oncol. 2016, 34, 2271–2278. [Google Scholar] [CrossRef]
- Herzog, T.J.; Pignata, S.; Ghamande, S.A.; Rubio, M.-J.; Fujiwara, K.; Vulsteke, C.; Armstrong, D.K.; Sehouli, J.; Coleman, R.L.; Gabra, H. Randomized phase II trial of farletuzumab plus chemotherapy versus placebo plus chemotherapy in low CA-125 platinum-sensitive ovarian cancer. Gynecol. Oncol. 2023, 170, 300–308. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, W.; Huang, J.; Li, F.; Sheng, J.; Song, H.; Chen, Y. Development of a dendritic cell/tumor cell fusion cell membrane nano-vaccine for the treatment of ovarian cancer. Front. Immunol. 2022, 13, 828263. [Google Scholar] [CrossRef]
- Odunsi, K. Immunotherapy in ovarian cancer. Ann. Oncol. 2017, 28, viii1–viii7. [Google Scholar] [CrossRef]
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 18538–18543. [Google Scholar] [CrossRef]
- Zhang, L.; Conejo-Garcia, J.R.; Katsaros, D.; Gimotty, P.A.; Massobrio, M.; Regnani, G.; Makrigiannakis, A.; Gray, H.; Schlienger, K.; Liebman, M.N. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 2003, 348, 203–213. [Google Scholar] [CrossRef]
- Hamanishi, J.; Mandai, M.; Iwasaki, M.; Okazaki, T.; Tanaka, Y.; Yamaguchi, K.; Higuchi, T.; Yagi, H.; Takakura, K.; Minato, N. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc. Natl. Acad. Sci. USA 2007, 104, 3360–3365. [Google Scholar] [CrossRef]
- Hwang, W.-T.; Adams, S.F.; Tahirovic, E.; Hagemann, I.S.; Coukos, G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: A meta-analysis. Gynecol. Oncol. 2012, 124, 192–198. [Google Scholar] [CrossRef]
- Palaia, I.; Tomao, F.; Sassu, C.M.; Musacchio, L.; Benedetti Panici, P. Immunotherapy for ovarian cancer: Recent advances and combination therapeutic approaches. OncoTargets Ther. 2020, 2020, 6109–6129. [Google Scholar] [CrossRef]
- Yoon, H.; Kim, A.; Jang, H. Immunotherapeutic Approaches in Ovarian Cancer. Curr. Issues Mol. Biol. 2023, 45, 1233–1249. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xia, B.-R.; Zhang, Z.-C.; Zhang, Y.-J.; Lou, G.; Jin, W.-L. Immunotherapy for ovarian cancer: Adjuvant, combination, and neoadjuvant. Front. Immunol. 2020, 11, 577869. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Hopkins, A.; Jaffee, E.M. Tumor mutational burden and response rate to PD-1 inhibition. N. Engl. J. Med. 2017, 377, 2500–2501. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Solit, D.; Chan, T.; Kurzrock, R. The FDA approval of pembrolizumab for adult and pediatric patients with tumor mutational burden (TMB)≥ 10: A decision centered on empowering patients and their physicians. Ann. Oncol. 2020, 31, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Menezes, M.E.; Bhatia, S.; Wang, X.Y.; Emdad, L.; Sarkar, D.; Fisher, P.B. Gene therapies for cancer: Strategies, challenges and successes. J. Cell. Physiol. 2015, 230, 259–271. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 27 November 2023).
- Áyen, Á.; Jimenez Martinez, Y.; Marchal, J.A.; Boulaiz, H. Recent progress in gene therapy for ovarian cancer. Int. J. Mol. Sci. 2018, 19, 1930. [Google Scholar] [CrossRef]
- Ashburn, T.T.; Thor, K.B. Drug repositioning: Identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004, 3, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Huo, J.-L.; Wang, S.; Yuan, X.-H.; Liu, H.-M. Drug repurposing: Discovery of troxipide analogs as potent antitumor agents. Eur. J. Med. Chem. 2020, 202, 112471. [Google Scholar] [CrossRef]
- Duffy, M.J.; Murray, A.; Synnott, N.C.; O’Donovan, N.; Crown, J. Vitamin D analogues: Potential use in cancer treatment. Crit. Rev. Oncol./Hematol. 2017, 112, 190–197. [Google Scholar] [CrossRef]
- Leyssens, C.; Verlinden, L.; Verstuyf, A. Antineoplastic effects of 1, 25 (OH) 2D3 and its analogs in breast, prostate and colorectal cancer. Endocr.-Relat. Cancer 2013, 20, R31–R47. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, molecular mechanism of action, and pleiotropic effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef]
- Piatek, K.; Schepelmann, M.; Kallay, E. The effect of vitamin D and its analogs in ovarian cancer. Nutrients 2022, 14, 3867. [Google Scholar] [CrossRef] [PubMed]
- Keum, N.; Lee, D.; Greenwood, D.; Manson, J.; Giovannucci, E. Vitamin D supplementation and total cancer incidence and mortality: A meta-analysis of randomized controlled trials. Ann. Oncol. 2019, 30, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fang, F.; Tang, J.; Jia, L.; Feng, Y.; Xu, P.; Faramand, A. Association between vitamin D supplementation and mortality: Systematic review and meta-analysis. BMJ 2019, 366, l4673. [Google Scholar] [CrossRef]
- Kim, H.; Giovannucci, E. Vitamin D status and cancer incidence, survival, and mortality. Sunlight Vitam. D Ski. Cancer 2020, 1268, 39–52. [Google Scholar]
- Srivastava, A.K.; Rizvi, A.; Cui, T.; Han, C.; Banerjee, A.; Naseem, I.; Zheng, Y.; Wani, A.A.; Wang, Q.-E. Depleting ovarian cancer stem cells with calcitriol. Oncotarget 2018, 9, 14481. [Google Scholar] [CrossRef] [PubMed]
- Kuittinen, T.; Rovio, P.; Luukkaala, T.; Laurila, M.; Grenman, S.; Kallioniemi, A.; Mäenpää, J. Paclitaxel, carboplatin and 1, 25-D3 inhibit proliferation of ovarian cancer cells in vitro. Anticancer Res. 2020, 40, 3129–3138. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, A.; Naseem, I. Causing DNA damage and stopping DNA repair–Vitamin D supplementation with Poly (ADP-ribose) polymerase 1 (PARP1) inhibitors may cause selective cell death of cancer cells: A novel therapeutic paradigm utilizing elevated copper levels within the tumour. Med. Hypotheses 2020, 144, 110278. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, S.; Guo, B. Vitamin D suppresses ovarian cancer growth and invasion by targeting long non-coding RNA CCAT2. Int. J. Mol. Sci. 2020, 21, 2334. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Park, W.H.; Suh, D.H.; Kim, K.; No, J.H.; Kim, Y.B. Calcitriol Combined with Platinum-based Chemotherapy Suppresses Growth and Expression of Vascular Endothelial Growth Factor of SKOV-3 Ovarian Cancer Cells. Anticancer Res. 2021, 41, 2945–2952. [Google Scholar] [CrossRef]
- Dovnik, A.; Fokter Dovnik, N. Vitamin D and ovarian cancer: Systematic review of the literature with a focus on molecular mechanisms. Cells 2020, 9, 335. [Google Scholar] [CrossRef]
- Guo, Y.; Jiang, F.; Yang, W.; Shi, W.; Wan, J.; Li, J.; Pan, J.; Wang, P.; Qiu, J.; Zhang, Z. Effect of 1α, 25 (OH) 2D3-Treated M1 and M2 Macrophages on Cell Proliferation and Migration Ability in Ovarian Cancer. Nutr. Cancer 2022, 74, 2632–2643. [Google Scholar] [CrossRef]
- Torralba, M.; Farra, R.; Maddaloni, M.; Grassi, M.; Dapas, B.; Grassi, G. Drugs repurposing in high-grade serous ovarian cancer. Curr. Med. Chem. 2020, 27, 7222–7233. [Google Scholar] [CrossRef]
- Goenka, L.; Dubashi, B.; Selvarajan, S.; Ganesan, P. Use of “repurposed” drugs in the treatment of epithelial ovarian cancer: A systematic review. Am. J. Clin. Oncol. 2022, 45, 168–174. [Google Scholar] [CrossRef]
- Islam, S.; Wang, S.; Bowden, N.; Martin, J.; Head, R. Repurposing existing therapeutics, its importance in oncology drug development: Kinases as a potential target. Br. J. Clin. Pharmacol. 2022, 88, 64–74. [Google Scholar] [CrossRef]
- Skorda, A.; Bay, M.L.; Hautaniemi, S.; Lahtinen, A.; Kallunki, T. Kinase inhibitors in the treatment of ovarian cancer: Current state and future promises. Cancers 2022, 14, 6257. [Google Scholar] [CrossRef]
- Puvanenthiran, S.; Essapen, S.; Seddon, A.M.; Modjtahedi, H. Impact of the putative cancer stem cell markers and growth factor receptor expression on the sensitivity of ovarian cancer cells to treatment with various forms of small molecule tyrosine kinase inhibitors and cytotoxic drugs. Int. J. Oncol. 2016, 49, 1825–1838. [Google Scholar] [CrossRef] [PubMed]
- Konstantinopoulos, P.A.; Barry, W.T.; Birrer, M.; Westin, S.N.; Cadoo, K.A.; Shapiro, G.I.; Mayer, E.L.; O’Cearbhaill, R.E.; Coleman, R.L.; Kochupurakkal, B. Olaparib and α-specific PI3K inhibitor alpelisib for patients with epithelial ovarian cancer: A dose-escalation and dose-expansion phase 1b trial. Lancet Oncol. 2019, 20, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; Heitz, F.; Buderath, P.; Powell, M.; Sehouli, J.; Lee, C.M.; Hamilton, A.; Fiorica, J.; Moore, K.N.; Teneriello, M. A randomized, double-blind, placebo-controlled phase 1b/2 study of ralimetinib, a p38 MAPK inhibitor, plus gemcitabine and carboplatin versus gemcitabine and carboplatin for women with recurrent platinum-sensitive ovarian cancer. Gynecol. Oncol. 2020, 156, 23–31. [Google Scholar] [CrossRef]
- Sun, Y.; Niu, W.; Du, F.; Du, C.; Li, S.; Wang, J.; Li, L.; Wang, F.; Hao, Y.; Li, C. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J. Hematol. Oncol. 2016, 9, 105. [Google Scholar] [CrossRef]
- Li, L.; Kong, F.; Zhang, L.; Li, X.; Fu, X.; Wang, X.; Wu, J.; Zhang, F.; Ren, L.; Zhang, M. Apatinib, a novel VEGFR-2 tyrosine kinase inhibitor, for relapsed and refractory nasopharyngeal carcinoma: Data from an open-label, single-arm, exploratory study. Investig. New Drugs 2020, 38, 1847–1853. [Google Scholar] [CrossRef]
- Zhang, M.; Jang, H.; Nussinov, R. PI3K inhibitors: Review and new strategies. Chem. Sci. 2020, 11, 5855–5865. [Google Scholar] [CrossRef] [PubMed]
- Orbegoso, C.; Marquina, G.; George, A.; Banerjee, S. The role of Cediranib in ovarian cancer. Expert Opin. Pharmacother. 2017, 18, 1637–1648. [Google Scholar] [CrossRef]
- Abdelgalil, A.A.; Al-Kahtani, H.M.; Al-Jenoobi, F.I. Erlotinib. In Profiles of Drug Substances, Excipients and Related Methodology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 45, pp. 93–117. [Google Scholar]
- Liu, M.; Liu, H.; Chen, J. Mechanisms of the CDK4/6 inhibitor palbociclib (PD 0332991) and its future application in cancer treatment. Oncol. Rep. 2018, 39, 901–911. [Google Scholar] [CrossRef]
- Miyamoto, S.; Kakutani, S.; Sato, Y.; Hanashi, A.; Kinoshita, Y.; Ishikawa, A. Drug review: Pazopanib. Jpn. J. Clin. Oncol. 2018, 48, 503–513. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Adnane, L.; Newell, P.; Villanueva, A.; Llovet, J.M.; Lynch, M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol. Cancer Ther. 2008, 7, 3129–3140. [Google Scholar] [CrossRef]
- Papaetis, G.S.; Syrigos, K.N. Sunitinib: A multitargeted receptor tyrosine kinase inhibitor in the era of molecular cancer therapies. BioDrugs 2009, 23, 377–389. [Google Scholar] [CrossRef]
- Glassman, D.; Bateman, N.W.; Lee, S.; Zhao, L.; Yao, J.; Tan, Y.; Ivan, C.; Rangel, K.M.; Zhang, J.; Conrads, K.A. Molecular correlates of venous thromboembolism (VTE) in ovarian cancer. Cancers 2022, 14, 1496. [Google Scholar] [CrossRef]
- Liz-Pimenta, J.; Tavares, V.; Neto, B.V.; Santos, J.M.; Guedes, C.B.; Araújo, A.; Khorana, A.A.; Medeiros, R. Thrombosis and cachexia in cancer: Two partners in crime? Crit. Rev. Oncol. Hematol. 2023, 186, 103989. [Google Scholar] [CrossRef] [PubMed]
- Swier, N.; Versteeg, H.H. Reciprocal links between venous thromboembolism, coagulation factors and ovarian cancer progression. Thromb. Res. 2017, 150, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Tamura, R.; Yoshihara, K.; Enomoto, T. Therapeutic strategies focused on cancer-associated hypercoagulation for ovarian clear cell carcinoma. Cancers 2022, 14, 2125. [Google Scholar] [CrossRef] [PubMed]
- Unruh, D.; Horbinski, C. Beyond thrombosis: The impact of tissue factor signaling in cancer. J. Hematol. Oncol. 2020, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- Koizume, S.; Miyagi, Y. Tissue factor in cancer-associated thromboembolism: Possible mechanisms and clinical applications. Br. J. Cancer 2022, 127, 2099–2107. [Google Scholar] [CrossRef]
- Davie, E.W.; Fujikawa, K.; Kisiel, W. The coagulation cascade: Initiation, maintenance, and regulation. Biochemistry 1991, 30, 10363–10370. [Google Scholar] [CrossRef] [PubMed]
- Wojtukiewicz, M.Z.; Sierko, E.; Klementt, P.; Rak, J. The hemostatic system and angiogenesis in malignancy. Neoplasia 2001, 3, 371–384. [Google Scholar] [CrossRef]
- Khorana, A.A.; Ahrendt, S.A.; Ryan, C.K.; Francis, C.W.; Hruban, R.H.; Hu, Y.C.; Hostetter, G.; Harvey, J.; Taubman, M.B. Tissue factor expression, angiogenesis, and thrombosis in pancreatic cancer. Clin. Cancer Res. 2007, 13, 2870–2875. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, X.; Chen, Q.; Chen, X.; Teng, J.; Wang, C.; Li, M.; Fan, L. Down-regulation of tissue factor inhibits invasion and metastasis of non-small cell lung cancer. J. Cancer 2020, 11, 1195. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Xue, Y.; Liu, L.; Zhang, X.; Pei, J.; Zhang, Y.; Wang, Y.; Yu, K. Tissue factor overexpression in triple-negative breast cancer promotes immune evasion by impeding T-cell infiltration and effector function. Cancer Lett. 2023, 565, 216221. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.G.; Prendergast, E.; Geddings, J.E.; Walts, A.E.; Agadjanian, H.; Hisada, Y.; Karlan, B.Y.; Mackman, N.; Walsh, C.S. Evaluation of venous thrombosis and tissue factor in epithelial ovarian cancer. Gynecol. Oncol. 2017, 146, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.J.; Morinaga, L.T.K.; Alves, J.L.J.; Castro, M.A.; Calderaro, D.; Jardim, C.V.P.; Souza, R. Cancer-associated thrombosis: The when, how and why. Eur. Respir. Rev. 2019, 28, 180119. [Google Scholar] [CrossRef] [PubMed]
- Vlodavsky, I.; Sanderson, R.; Ilan, N. Heparanase: From Basic Research to Clinical Applications; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Tavares, V.; Neto, B.V.; Marques, I.S.; Assis, J.; Pereira, D.; Medeiros, R. Cancer-associated thrombosis: What about microRNAs targeting the tissue factor coagulation pathway? Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 2023, 1879, 189053. [Google Scholar] [CrossRef] [PubMed]
- Markham, A. Tisotumab vedotin: First approval. Drugs 2021, 81, 2141–2147. [Google Scholar] [CrossRef] [PubMed]
- de Bono, J.S.; Concin, N.; Hong, D.S.; Thistlethwaite, F.C.; Machiels, J.-P.; Arkenau, H.-T.; Plummer, R.; Jones, R.H.; Nielsen, D.; Windfeld, K. Tisotumab vedotin in patients with advanced or metastatic solid tumours (InnovaTV 201): A first-in-human, multicentre, phase 1–2 trial. Lancet Oncol. 2019, 20, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Lorusso, D.; Gennigens, C.; González-Martín, A.; Randall, L.; Cibula, D.; Lund, B.; Woelber, L.; Pignata, S.; Forget, F. Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): A multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021, 22, 609–619. [Google Scholar] [CrossRef]
- Lin, R.J.; Afshar-Kharghan, V.; Schafer, A.I. Paraneoplastic thrombocytosis: The secrets of tumor self-promotion. Blood J. Am. Soc. Hematol. 2014, 124, 184–187. [Google Scholar] [CrossRef]
- Koenen, R.R. The prowess of platelets in immunity and inflammation. Thromb. Haemost. 2016, 116, 605–612. [Google Scholar] [CrossRef]
- Davis, A.N.; Afshar-Kharghan, V.; Sood, A.K. Platelet effects on ovarian cancer. Semin. Oncol. 2014, 41, 378–384. [Google Scholar] [CrossRef]
- Rajkumar, A.; Szallasi, A. Paraneoplastic thrombocytosis in breast cancer. Anticancer Res. 2013, 33, 4545–4546. [Google Scholar] [PubMed]
- Stone, R.L.; Nick, A.M.; McNeish, I.A.; Balkwill, F.; Han, H.D.; Bottsford-Miller, J.; Rupaimoole, R.; Armaiz-Pena, G.N.; Pecot, C.V.; Coward, J. Paraneoplastic thrombocytosis in ovarian cancer. N. Engl. J. Med. 2012, 366, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Levin, J.; Conley, C.L. Thrombocytosis associated with malignant disease. Arch. Intern. Med. 1964, 114, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Zeimet, A.G.; Marth, C.; Müller-Holzner, E.; Daxenbichler, G.; Dapunt, O. Significance of thrombocytosis in patients with epithelial ovarian cancer. Am. J. Obstet. Gynecol. 1994, 170, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Raungkaewmanee, S.; Tangjitgamol, S.; Manusirivithaya, S.; Srijaipracharoen, S.; Thavaramara, T. Platelet to lymphocyte ratio as a prognostic factor for epithelial ovarian cancer. J. Gynecol. Oncol. 2012, 23, 265. [Google Scholar] [CrossRef] [PubMed]
- Allensworth, S.; Langstraat, C.; Martin, J.; Lemens, M.; McGree, M.; Weaver, A.; Dowdy, S.; Podratz, K.; Bakkum-Gamez, J. Evaluating the prognostic significance of preoperative thrombocytosis in epithelial ovarian cancer. Gynecol. Oncol. 2013, 130, 499–504. [Google Scholar] [CrossRef]
- Patel, D.; Thankachan, S.; Sreeram, S.; Kavitha, K.; Suresh, P.S. The role of tumor-educated platelets in ovarian cancer: A comprehensive review and update. Pathol.-Res. Pract. 2022, 241, 154267. [Google Scholar] [CrossRef]
- Green, G.A. Understanding NSAIDs: From aspirin to COX-2. Clin. Cornerstone 2001, 3, 50–59. [Google Scholar] [CrossRef]
- Langley, R.E. Clinical evidence for the use of aspirin in the treatment of cancer. Ecancermedicalscience 2013, 7, 297. [Google Scholar] [PubMed]
- Man, X.; Wang, B.; Tan, Y.; Yang, X.; Zhang, S. Aspirin use and mortality in women with ovarian cancer: A meta-analysis. Front. Oncol. 2021, 10, 575831. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lichtenberger, L.M.; Taylor, M.; Bottsford-Miller, J.N.; Haemmerle, M.; Wagner, M.J.; Lyons, Y.; Pradeep, S.; Hu, W.; Previs, R.A. Antitumor and antiangiogenic effects of aspirin-PC in ovarian cancer. Mol. Cancer Ther. 2016, 15, 2894–2904. [Google Scholar] [CrossRef] [PubMed]
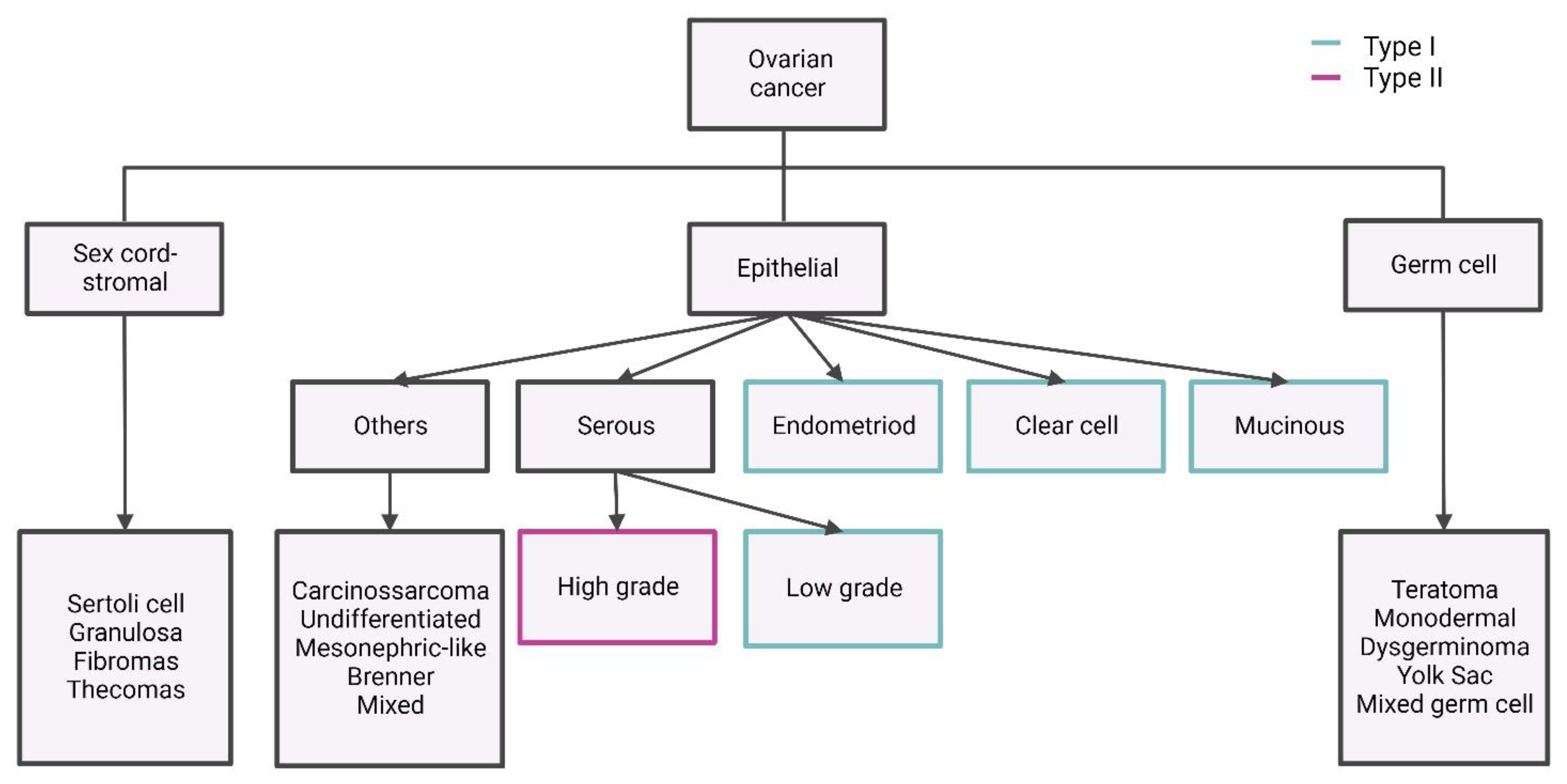
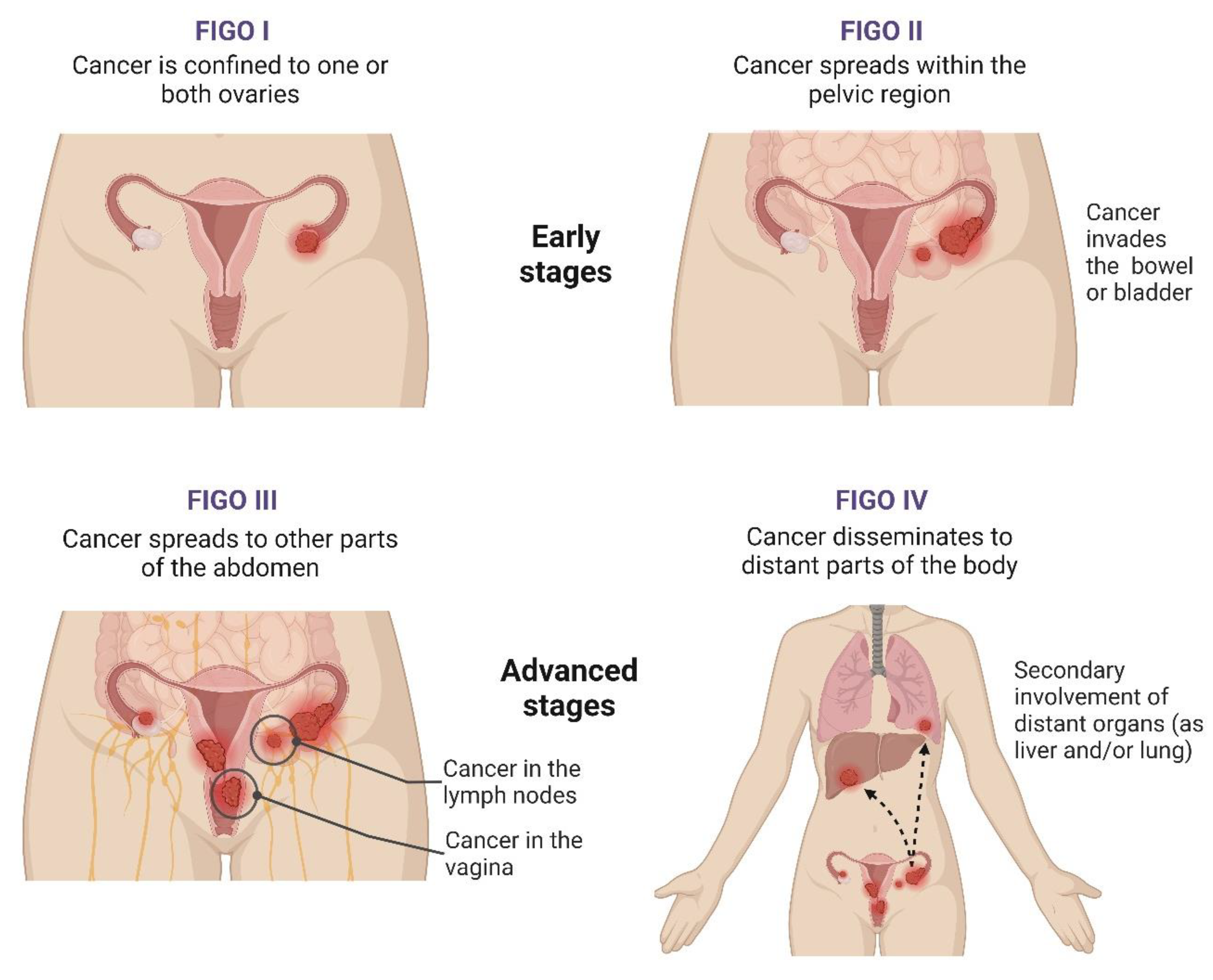
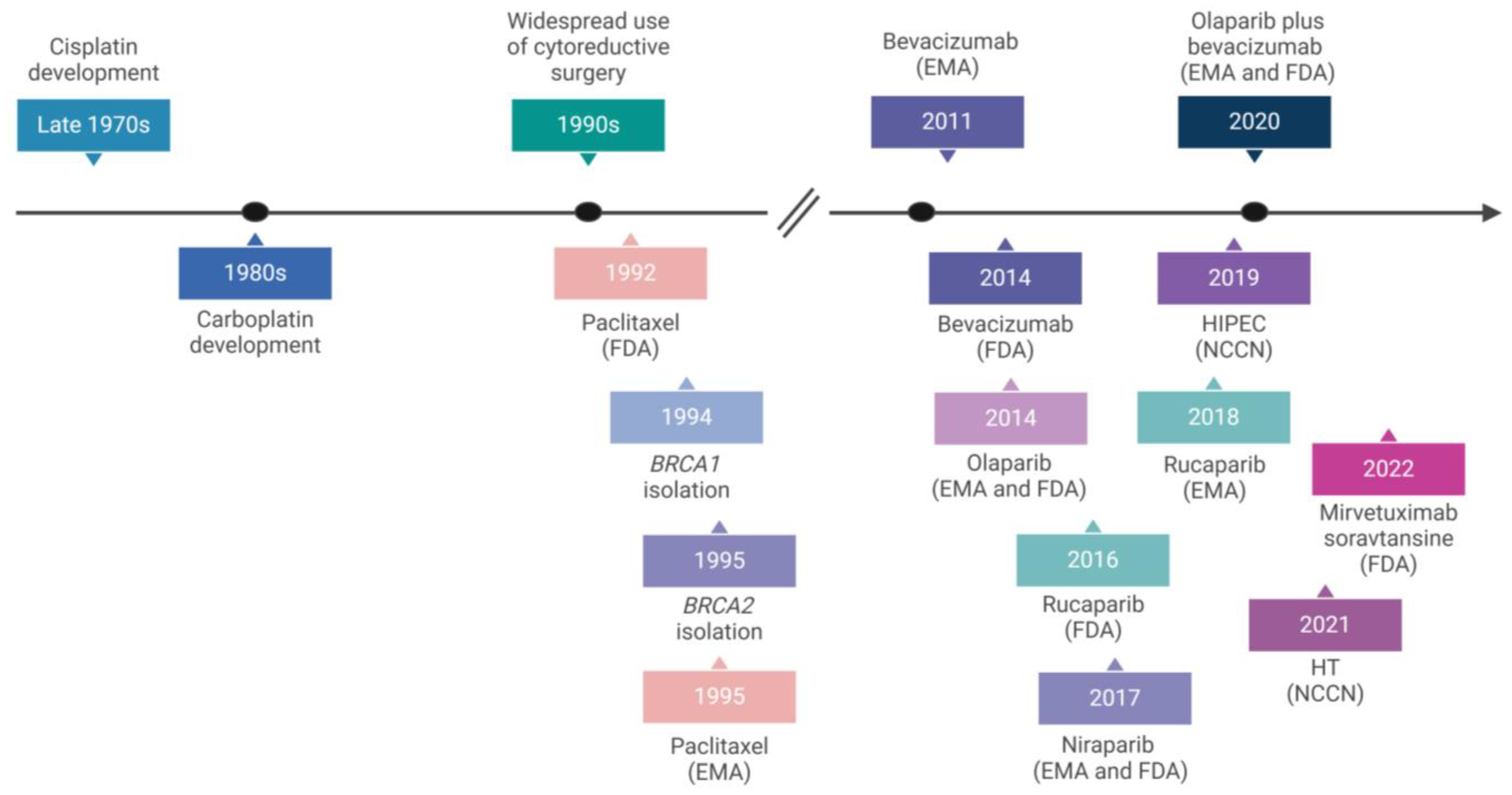
| Authors (Year) | Number of Cases | Methods | HGSC Clusters and/or Main Features | Main Findings | References |
|---|---|---|---|---|---|
| Macintyre et al., (2018) | 132 patients 112 patients (Pan-Cancer Analysis of Whole Genomes) 415 patients (TCGA) | Genome sequencing | Signatures of copy number variations: Signature 1—telomere shortening and RAS/MAPK activation; Signature 2—tandem duplication; Signature 3—BRCA1/2-related HRR deficiency; Signature 4—whole genome duplication; Signature 5—subclonal catastrophic chromothriptic-like events; Signature 6—focal amplification; Signature 7—non-BRCA1/2-related HRR deficiency. | Signature 1—platinum-resistant recurrence and poor survival; Signature 2—poor survival; Signatures 3 and 7—prolonged survival; Signatures 4, 5 and 6—unclear implications. | [97] |
| Harris et al., (2019) | Not reported | Tumour xenografting DNA and RNA NGS DNA fingerprinting Immunohistochemistry | DNA alternations in genes involved in the ERBB2 pathway. | Deregulation in the ERBB2 pathway—favourable results by combining platinum-based chemotherapy with anti-HER2 drugs. | [98] |
| Li et al., (2019) | Seven patients | Tumour xenografting RNA and whole exome sequencing Immunohistochemistry | Deregulation of AKT3, HLA-DPA1, PIK3R5 and SAP25 expression; POLR2A and TMEM205 mutations. | Features associated with the acquisition of chemoresistance to carboplatin and paclitaxel. | [99] |
| McDonald et al., (2019) | 450 patients with chemoresistance (TCGA) | Genome-wide cluster analysis Pathway enrichment analysis | Cluster 1—growth factor signalling; Cluster 2—cell survival; Cluster 3—cellular senescence. | Best therapeutic options: Cluster 1—tyrosine kinases or angiokinase inhibitors; Cluster 2—mTOR inhibitors; Cluster 3—deacetylase inhibitors. | [100] |
| Hao et al., (2021) | Two patients (four matched pair samples of primary and metastatic tumours) | Single-cell RNA sequencing | Cluster EC1—glycolysis/gluconeogenesis; Cluster EC2—cytokine–cytokine receptor interaction; Cluster EC3—nucleotide and amino acid metabolism; Cluster EC4—immune response Cluster EC5—DNA repair and drug metabolism | Cluster EC5 may be most aggressive and resistant to chemotherapy and PARP inhibitors. | [101] |
| Li et al., (2021) | 66 tumour cells 568 tumour samples and 7 normal ovary samples (TCGA) | Single-cell RNA sequencing | Differently expressed genes | Low expression of ANP32E, EGFL6, GPRC5A, PMP22 and STAT1—prolonged survival; Low expression of ANP32E, CYB5R3 and FBXO21—prolonged PFS | [102] |
| Clinical Trial | Trial Identifier | Immunotherapeutic Agent | Combination | N * | Phase | Setting | Reaction to Platinum | Completion Date * |
|---|---|---|---|---|---|---|---|---|
| Pembrolizumab and lenvatinib for the treatment of serous ovarian cancer patients | NCT05114421 | pembrolizumab (anti-PD-1) | lenvatinib | 30 | II | First-line treatment Recurrent disease | NA NR | January 2024 |
| Neoadjuvant dendritic cell vaccination for ovarian cancer (NEODOC) | NCT05773859 | Specialised Cross-Presenting Dendritic Cells Vaccinations | Standard-of-care treatment | 10 | I/II | First-line treatment | NA | October2024 |
| Systemic immune checkpoint blockade and intraperitoneal chemo-immunotherapy in recurrent ovarian cancer | NCT03734692 | rintatolimod (immune system stimulant) pembrolizumab (anti-PD-1) | carboplatin | 45 | I/II | Recurrent disease | Sensitive | December 2024 |
| Durvalumab and tremelimumab in treating participants with recurrent or refractory ovarian, primary peritoneal, or fallopian tube cancer | NCT03026062 | durvalumab (anti-PDL1) tremelimumab (anti-CTLA-4) | - | 120 | II | Refractory diseaseRecurrent disease | Resistant | December 2024 |
| Pembrolizumab and carboplatin for the treatment of recurrent ovarian, fallopian tube, or primary peritoneal cancer | NCT04387227 | pembrolizumab (anti-PD-1) | carboplatin | 22 | II | Recurrent disease | NR | April 2025 |
| PD-1 antibody combined neoadjuvant chemotherapy for ovarian cancer | NCT04815408 | tislelizumab (anti-PD-1) | paclitaxel and carboplatin | 40 | II | First-line treatment | NA | April 2025 |
| A clinical study on oncolytic virus injection (R130) for the treatment of relapsed/refractory ovarian cancer | NCT05801783 | Recombinant oncolytic herpes simplex virus type 1 (R130) | - | 10 | I | Refractory diseaseRecurrent disease | NR | December 2025 |
| OSE2101 alone or in combination with pembrolizumab vs. BSC in patient with platinum-sensitive-recurrent OC (TEDOVA) | NCT04713514 | OSE2101 (cancer vaccine) pembrolizumab (anti-PD-1) | - | 180 | II | Recurrent disease | Sensitive | December 2025 |
| Safety and efficacy of anti-CD47, ALX148 in combination with liposomal doxorubicin and pembrolizumab in recurrent platinum-resistant ovarian cancer | NCT05467670 | pembrolizumab (anti-PD-1) ALX148 (anti-CD47) | PLD | 31 | II | Recurrent disease | Resistant | December 2027 |
| Approach | Therapeutical Agent | Trial Identifier (Status) | Combination | N Participants | Phase | Setting | Reaction to Platinum |
|---|---|---|---|---|---|---|---|
| Replacement of tumour suppressor genes | Ad5CMV-p53 vector (Inserting TP53) | NCT00003588 (completed) | - | 30 | I | Recurrent disease | Resistant |
| NCT00003450 (completed) | - | - | I | Recurrent disease | NR | ||
| Suicide gene therapy | Ad5.SSTR/TK.RGD vector (HSV-TK + GCV) | NCT00964756 (completed) | - | 11 | I | Recurrent disease | NR |
| Genetic immunopotentiation | p53MVA vaccine (Modified vaccinia virus Ankara expressing tumour protein p53) | NCT02275039 (completed) | gemcitabine | 12 | I | Recurrent disease | NR |
| NYESO-1(C259)-transduced autologous T cells | NCT01567891 (completed) | - | 9 | I/II | Refractory disease Recurrent disease | NR | |
| Vigil™ tumour cell vaccine | NCT01309230 (completed) | - | 145 | II | First-line treatment | NA | |
| Gene-modified lymphocytes with MOv-PBL | NCT00019136 (completed) | aldesleukin | 13–50 * | I | Residual disease Recurrent disease | NA NR | |
| CDX-1401 vaccine | NCT03206047 (active) | atezolizumab and guadecitabine | 75 * | I/II | Recurrent disease | Resistant | |
| ALVAC(2)-NY-ESO-1 (M)/TRICOM vaccine | NCT01536054 (completed) | sirolimus and sargramostim | 7 | I | Maintenance therapy Recurrent disease | NA NR | |
| NCT00803569 (completed) | sargramostim | 13 | I | Maintenance therapy Recurrent disease | NA NR | ||
| Cancer virotherapy | MV-CEA MV-NIS | NCT00408590 (completed) | - | 37 | I | Refractory disease Recurrent disease | NR |
| Inhibitor | Target | Trial Identifier (Status) | Combination | N Participants | Phase | Setting | Reaction to Platinum |
|---|---|---|---|---|---|---|---|
| anlotinib | VEGFRs, FGFRs, PDGFRs, c-Kit and RET kinases [230] | NCT05188781 (completed) | pembrolizumab | 34 | II | Refractory or recurrent disease | NR |
| NCT05130515 (completed) | niraparib | 6 | II | Recurrent disease | Resistant | ||
| NCT02584478 (active) | paclitaxel, PLD, topotecan and carboplatin | 294 | III | Recurrent or metastatic disease | NR | ||
| apatinib | VEGFR-2 [231] | NCT02867956 (completed) | etoposide | 35 | II | Refractory or recurrent disease | Resistant |
| NCT03075462 (completed) | fuzuloparib | 98 | I | Recurrent | Sensitive | ||
| NCT04348032 (active) | PLD | 152 | II | Recurrent disease | Resistant | ||
| NCT04229615 (active) | fuzuloparib | 690 | III | Maintenance therapy | Sensitive | ||
| alpelisib | PI3K [232] | NCT04729387 (Active) | olaparib, paclitaxel and PLD | 358 | III | Refractory or recurrent disease | Resistant |
| cediranib | VEGFRs [233] | NCT00275028 (completed) | - | 47 | II | Recurrent disease | Resistant |
| NCT00278343 (completed) | - | 74 | II | Refractory or recurrent disease | NR | ||
| NCT02340611 (completed) | olaparib | 4 | II | Recurrent disease | NR | ||
| NCT02889900 (completed) | olaparib | 62 | II | Recurrent disease | Resistant | ||
| NCT02681237 (completed) | olaparib | 34 | NA | Recurrent disease | NR | ||
| NCT03117933 (active) | olaparib and paclitaxel | 139 | II | Recurrent disease | Resistant | ||
| NCT02502266 (active) | olaparib, paclitaxel, PLD and topotecan | 562 | II/III | Recurrent disease | Resistant | ||
| NCT02345265 (active) | olaparib | 72 | II | Recurrent disease | NR | ||
| NCT02446600 (active) | olaparib | 579 | III | Recurrent disease | Sensitive | ||
| NCT01116648 (active) | olaparib | 155 | I/II | Recurrent disease | Sensitive | ||
| erlotinib | EGFR [234] | NCT00217529 (completed) | docetaxel and carboplatin | 30 * | I/II | First-line treatment | NA |
| NCT00263822 (completed) | - | 835 | III | Maintenance therapy | NR | ||
| NCT00030446 (completed) | carboplatin | 50 | II | Recurrent disease | NR | ||
| NCT00126542 (completed) | bevacizumab | 35 | II | Recurrent or metastatic disease | NR | ||
| NCT00130520 (completed) | bevacizumab | 40 | II | Recurrent disease | Resistant | ||
| NCT00059787 (completed) | carboplatin and paclitaxel | 56 | II | First-line treatment | NA | ||
| NCT00520013 (completed) | bevacizumab, paclitaxel and carboplatin | 60 | II | Maintenance therapy | NR | ||
| palbociclib | CDK4 and CDK6 [235] | NCT01536743 (completed) | - | 26 | II | Recurrent disease | NR |
| pazopanib | VEGFRs, PDGFRs and FGFRs [236] | NCT00281632 (completed) | - | 35 | II | Refractory disease | Refractory |
| NCT01227928 (completed) | - | 145 | II | Maintenance therapy | NA | ||
| NCT01238770 (completed) | cyclophosphamide | 10 | I/II | Recurrent disease | Resistant | ||
| NCT01644825 (completed) | paclitaxel | 72 | II | Refractory or recurrent disease | Resistant | ||
| NCT01262014 (completed) | - | 28 | II | Recurrent disease | Resistant | ||
| NCT01608009 (completed) | paclitaxel | 16 | I | Recurrent disease | Resistant | ||
| NCT00866697 (completed) | - | 940 | III | Maintenance therapy | NA | ||
| NCT01468909 (completed) | paclitaxel | 106 | II | Recurrent disease | Sensitive | ||
| NCT01402271 (completed) | paclitaxel and carboplatin | 88 | I/II | Refractory or recurrent disease | Resistant | ||
| NCT01610206 (completed) | gemcitabine | 148 | II | Recurrent disease | NR | ||
| NCT02383251 (completed) | paclitaxel | 118 | II | Refractory or recurrent disease | Resistant | ||
| sorafenib | Raf serine/threonine kinases, VEGFRs and PDGFR-β [237] | NCT00096395 (completed) | gemcitabine | 33 | II | Recurrent disease | NR |
| NCT00093626 (completed) | - | 73 | II | Recurrent disease | Resistant | ||
| NCT00096200 (completed) | carboplatin and paclitaxel | 44 | II | Recurrent disease | Sensitive | ||
| NCT00791778 (completed) | - | 246 | II | Maintenance therapy | NA | ||
| NCT00390611 (completed) | paclitaxel and carboplatin | 85 | II | First-line treatment | NA | ||
| NCT00436215 (completed) | bevacizumab | 55 | II | Refractory or recurrent disease | Resistant | ||
| NCT01047891 (completed) | topotecan | 174 | II | Recurrent disease | Resistant | ||
| sunitinib | VEGFR1, VEGFR2, PDGFR-α, PDGFR-β, c-Kit, FLT3, RET and CSF1R [238] | NCT00388037 (completed) | - | 31 | II | Recurrent disease | NR |
| NCT00768144 (completed) | - | 36 | II | Refractory or recurrent disease | NR | ||
| NCT01824615 (completed) | - | 30 | II | Recurrent disease | Resistant | ||
| NCT00979992 (completed) | - | 35 | II | Recurrent disease | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavares, V.; Marques, I.S.; Melo, I.G.d.; Assis, J.; Pereira, D.; Medeiros, R. Paradigm Shift: A Comprehensive Review of Ovarian Cancer Management in an Era of Advancements. Int. J. Mol. Sci. 2024, 25, 1845. https://doi.org/10.3390/ijms25031845
Tavares V, Marques IS, Melo IGd, Assis J, Pereira D, Medeiros R. Paradigm Shift: A Comprehensive Review of Ovarian Cancer Management in an Era of Advancements. International Journal of Molecular Sciences. 2024; 25(3):1845. https://doi.org/10.3390/ijms25031845
Chicago/Turabian StyleTavares, Valéria, Inês Soares Marques, Inês Guerra de Melo, Joana Assis, Deolinda Pereira, and Rui Medeiros. 2024. "Paradigm Shift: A Comprehensive Review of Ovarian Cancer Management in an Era of Advancements" International Journal of Molecular Sciences 25, no. 3: 1845. https://doi.org/10.3390/ijms25031845
APA StyleTavares, V., Marques, I. S., Melo, I. G. d., Assis, J., Pereira, D., & Medeiros, R. (2024). Paradigm Shift: A Comprehensive Review of Ovarian Cancer Management in an Era of Advancements. International Journal of Molecular Sciences, 25(3), 1845. https://doi.org/10.3390/ijms25031845









