Soluble Klotho, a Potential Biomarker of Chronic Kidney Disease–Mineral Bone Disorders Involved in Healthy Ageing: Lights and Shadows
Abstract
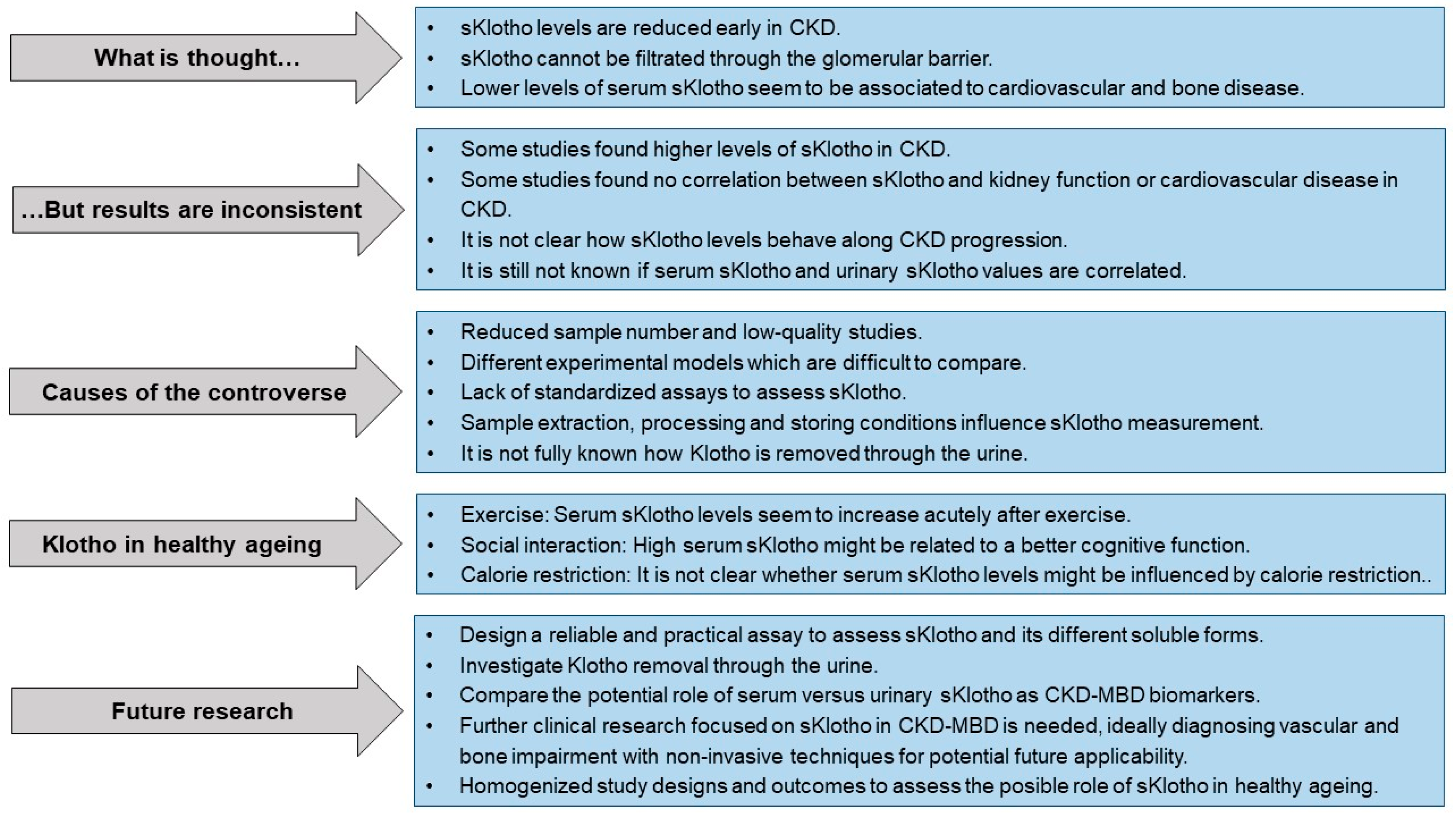
1. The Klotho Protein Discovery: A Scientific Story
2. Systemic Effects of Soluble Klotho
3. Soluble Klotho and CKD
Controversies and Limitations in the Measurement of Serum and Urinary Soluble Klotho
4. Usefulness and Limitations of Measuring Soluble Klotho in Serum and Urine in the Diagnosis of CKD-MBD
5. Soluble Klotho as a Biomarker of Cardiovascular and Bone Alterations
6. Healthy Ageing and Soluble Klotho
6.1. Exercise and Soluble Klotho
6.2. Social Interaction and Soluble Klotho
6.3. Caloric Restriction and Soluble Klotho
7. Klotho as a Strategy for Treatment: Repletion of Soluble Klotho and Klotho-Inducing Drugs
8. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Kurosu, H.; Ogawa, Y.; Miyoshi, M.; Yamamoto, M.; Nandi, A.; Rosenblatt, K.P.; Baum, M.G.; Schiavi, S.; Hu, M.C.; Moe, O.W.; et al. Regulation of fibroblast growth factor-23 signaling by klotho. J. Biol. Chem. 2006, 281, 6120–6123. [Google Scholar] [CrossRef] [PubMed]
- Consortium, A. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat. Genet. 2000, 26, 345–348. [Google Scholar] [CrossRef]
- Kurosu, H.; Yamamoto, M.; Clark, J.D.; Pastor, J.V.; Nandi, A.; Gurnani, P.; McGuinness, O.P.; Chikuda, H.; Yamaguchi, M.; Kawaguchi, H.; et al. Suppression of aging in mice by the hormone Klotho. Science 2005, 309, 1829–1833. [Google Scholar] [CrossRef] [PubMed]
- Kuro, O.M. The Klotho proteins in health and disease. Nat. Rev. Nephrol. 2019, 15, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Nabeshima, Y.; Imura, H. alpha-Klotho: A regulator that integrates calcium homeostasis. Am. J. Nephrol. 2008, 28, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, P.; Krajisnik, T.; Akerstrom, G.; Westin, G.; Larsson, T.E. Type I membrane klotho expression is decreased and inversely correlated to serum calcium in primary hyperparathyroidism. J. Clin. Endocrinol. Metab. 2008, 93, 4152–4157. [Google Scholar] [CrossRef] [PubMed]
- Komaba, H.; Kaludjerovic, J.; Hu, D.Z.; Nagano, K.; Amano, K.; Ide, N.; Sato, T.; Densmore, M.J.; Hanai, J.I.; Olauson, H.; et al. Klotho expression in osteocytes regulates bone metabolism and controls bone formation. Kidney Int. 2017, 92, 599–611. [Google Scholar] [CrossRef]
- Zhu, L.; Stein, L.R.; Kim, D.; Ho, K.; Yu, G.Q.; Zhan, L.; Larsson, T.E.; Mucke, L. Klotho controls the brain-immune system interface in the choroid plexus. Proc. Natl. Acad. Sci. USA 2018, 115, E11388–E11396. [Google Scholar] [CrossRef]
- Takeshita, K.; Fujimori, T.; Kurotaki, Y.; Honjo, H.; Tsujikawa, H.; Yasui, K.; Lee, J.K.; Kamiya, K.; Kitaichi, K.; Yamamoto, K.; et al. Sinoatrial node dysfunction and early unexpected death of mice with a defect of klotho gene expression. Circulation 2004, 109, 1776–1782. [Google Scholar] [CrossRef]
- Witkowski, J.M.; Soroczynska-Cybula, M.; Bryl, E.; Smolenska, Z.; Jozwik, A. Klotho—A common link in physiological and rheumatoid arthritis-related aging of human CD4+ lymphocytes. J. Immunol. 2007, 178, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.; Groen, A.; Molostvov, G.; Lu, T.; Lilley, K.S.; Snead, D.; James, S.; Wilkinson, I.B.; Ting, S.; Hsiao, L.L.; et al. α-Klotho Expression in Human Tissues. J. Clin. Endocrinol. Metab. 2015, 100, E1308–E1318. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.L.; Leaf, E.M.; Hu, M.C.; Takeno, M.M.; Kuro-o, M.; Moe, O.W.; Giachelli, C.M. Vitamin D receptor agonists increase klotho and osteopontin while decreasing aortic calcification in mice with chronic kidney disease fed a high phosphate diet. Kidney Int. 2012, 82, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Donate-Correa, J.; Mora-Fernandez, C.; Martinez-Sanz, R.; Muros-de-Fuentes, M.; Perez, H.; Meneses-Perez, B.; Cazana-Perez, V.; Navarro-Gonzalez, J.F. Expression of FGF23/KLOTHO system in human vascular tissue. Int. J. Cardiol. 2013, 165, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.C.; Shiizaki, K.; Kuro-o, M.; Moe, O.W. Fibroblast growth factor 23 and Klotho: Physiology and pathophysiology of an endocrine network of mineral metabolism. Annu. Rev. Physiol. 2013, 75, 503–533. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, T.; Choi, M.; Moschetta, A.; Peng, L.; Cummins, C.L.; McDonald, J.G.; Luo, G.; Jones, S.A.; Goodwin, B.; Richardson, J.A.; et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005, 2, 217–225. [Google Scholar] [CrossRef]
- Bloch, L.; Sineshchekova, O.; Reichenbach, D.; Reiss, K.; Saftig, P.; Kuro-o, M.; Kaether, C. Klotho is a substrate for alpha-, beta- and gamma-secretase. FEBS Lett. 2009, 583, 3221–3224. [Google Scholar] [CrossRef]
- Hu, M.C.; Shi, M.; Zhang, J.; Addo, T.; Cho, H.J.; Barker, S.L.; Ravikumar, P.; Gillings, N.; Bian, A.; Sidhu, S.S.; et al. Renal Production, Uptake, and Handling of Circulating alphaKlotho. J. Am. Soc. Nephrol. 2016, 27, 79–90. [Google Scholar] [CrossRef]
- Mencke, R.; Harms, G.; Moser, J.; van Meurs, M.; Diepstra, A.; Leuvenink, H.G.; Hillebrands, J.L. Human alternative Klotho mRNA is a nonsense-mediated mRNA decay target inefficiently spliced in renal disease. JCI Insight 2017, 2, e94375. [Google Scholar] [CrossRef]
- Hu, M.C.; Shi, M.; Zhang, J.; Quiñones, H.; Kuro-o, M.; Moe, O.W. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int. 2010, 78, 1240–1251. [Google Scholar] [CrossRef]
- Xie, J.; Cha, S.K.; An, S.W.; Kuro, O.M.; Birnbaumer, L.; Huang, C.L. Cardioprotection by Klotho through downregulation of TRPC6 channels in the mouse heart. Nat. Commun. 2012, 3, 1238. [Google Scholar] [CrossRef]
- Fernández, Á.F.; Sebti, S.; Wei, Y.; Zou, Z.; Shi, M.; McMillan, K.L.; He, C.; Ting, T.; Liu, Y.; Chiang, W.C.; et al. Disruption of the beclin 1-BCL2 autophagy regulatory complex promotes longevity in mice. Nature 2018, 558, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yoon, J.; An, S.W.; Kuro-o, M.; Huang, C.L. Soluble Klotho Protects against Uremic Cardiomyopathy Independently of Fibroblast Growth Factor 23 and Phosphate. J. Am. Soc. Nephrol. 2015, 26, 1150–1160. [Google Scholar] [CrossRef]
- Hu, M.C.; Shi, M.; Zhang, J.; Quinones, H.; Griffith, C.; Kuro-o, M.; Moe, O.W. Klotho deficiency causes vascular calcification in chronic kidney disease. J. Am. Soc. Nephrol. 2011, 22, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Martín-Vírgala, J.; Fernández-Villabrille, S.; Martín-Carro, B.; Tamargo-Gómez, I.; Navarro-González, J.; Mora-Fernández, C.; Calleros, L.; Astudillo-Cortés, E.; Avello-Llano, N.; Mariño, G.; et al. Serum and urinary soluble α-Klotho as markers of kidney and vascular impairment. Nutrients 2023, 15, 1470. [Google Scholar] [CrossRef]
- Ikushima, M.; Rakugi, H.; Ishikawa, K.; Maekawa, Y.; Yamamoto, K.; Ohta, J.; Chihara, Y.; Kida, I.; Ogihara, T. Anti-apoptotic and anti-senescence effects of Klotho on vascular endothelial cells. Biochem. Biophys. Res. Commun. 2006, 339, 827–832. [Google Scholar] [CrossRef]
- Maekawa, Y.; Ishikawa, K.; Yasuda, O.; Oguro, R.; Hanasaki, H.; Kida, I.; Takemura, Y.; Ohishi, M.; Katsuya, T.; Rakugi, H. Klotho suppresses TNF-alpha-induced expression of adhesion molecules in the endothelium and attenuates NF-kappaB activation. Endocrine 2009, 35, 341–346. [Google Scholar] [CrossRef]
- Zou, D.; Wu, W.; He, Y.; Ma, S.; Gao, J. The role of klotho in chronic kidney disease. BMC Nephrol. 2018, 19, 285. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.C.; Shi, M.; Zhang, J.; Pastor, J.; Nakatani, T.; Lanske, B.; Razzaque, M.S.; Rosenblatt, K.P.; Baum, M.G.; Kuro-o, M.; et al. Klotho: A novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J. 2010, 24, 3438–3450. [Google Scholar] [CrossRef]
- Yu, L.; Meng, W.; Ding, J.; Cheng, M. Klotho inhibits angiotensin II-induced cardiomyocyte hypertrophy through suppression of the AT1R/beta catenin pathway. Biochem. Biophys. Res. Commun. 2016, 473, 455–461. [Google Scholar] [CrossRef]
- Hum, J.M.; O’Bryan, L.M.; Tatiparthi, A.K.; Cass, T.A.; Clinkenbeard, E.L.; Cramer, M.S.; Bhaskaran, M.; Johnson, R.L.; Wilson, J.M.; Smith, R.C.; et al. Chronic Hyperphosphatemia and Vascular Calcification Are Reduced by Stable Delivery of Soluble Klotho. J. Am. Soc. Nephrol. 2017, 28, 1162–1174. [Google Scholar] [CrossRef]
- Kusaba, T.; Okigaki, M.; Matui, A.; Murakami, M.; Ishikawa, K.; Kimura, T.; Sonomura, K.; Adachi, Y.; Shibuya, M.; Shirayama, T.; et al. Klotho is associated with VEGF receptor-2 and the transient receptor potential canonical-1 Ca2+ channel to maintain endothelial integrity. Proc. Natl. Acad. Sci. USA 2010, 107, 19308–19313. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.C.; Kuro-o, M.; Rosenblatt, K.P.; Brobey, R.; Papaconstantinou, J. The ASK1-Signalosome regulates p38 MAPK activity in response to levels of endogenous oxidative stress in the Klotho mouse models of aging. Aging 2010, 2, 597–611. [Google Scholar] [CrossRef]
- Brobey, R.K.; German, D.; Sonsalla, P.K.; Gurnani, P.; Pastor, J.; Hsieh, C.C.; Papaconstantinou, J.; Foster, P.P.; Kuro-o, M.; Rosenblatt, K.P. Klotho Protects Dopaminergic Neuron Oxidant-Induced Degeneration by Modulating ASK1 and p38 MAPK Signaling Pathways. PLoS ONE 2015, 10, e0139914. [Google Scholar] [CrossRef]
- Minamizaki, T.; Konishi, Y.; Sakurai, K.; Yoshioka, H.; Aubin, J.E.; Kozai, K.; Yoshiko, Y. Soluble Klotho causes hypomineralization in Klotho-deficient mice. J. Endocrinol. 2018, 237, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.K.; Ortega, B.; Kurosu, H.; Rosenblatt, K.P.; Kuro, O.M.; Huang, C.L. Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc. Natl. Acad. Sci. USA 2008, 105, 9805–9810. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.K.; Hu, M.C.; Kurosu, H.; Kuro-o, M.; Moe, O.; Huang, C.L. Regulation of renal outer medullary potassium channel and renal K(+) excretion by Klotho. Mol. Pharmacol. 2009, 76, 38–46. [Google Scholar] [CrossRef]
- Dalton, G.; An, S.W.; Al-Juboori, S.I.; Nischan, N.; Yoon, J.; Dobrinskikh, E.; Hilgemann, D.W.; Xie, J.; Luby-Phelps, K.; Kohler, J.J.; et al. Soluble klotho binds monosialoganglioside to regulate membrane microdomains and growth factor signaling. Proc. Natl. Acad. Sci. USA 2017, 114, 752–757. [Google Scholar] [CrossRef]
- Doi, S.; Zou, Y.; Togao, O.; Pastor, J.V.; John, G.B.; Wang, L.; Shiizaki, K.; Gotschall, R.; Schiavi, S.; Yorioka, N.; et al. Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J. Biol. Chem. 2011, 286, 8655–8665. [Google Scholar] [CrossRef]
- Zhou, L.; Li, Y.; Zhou, D.; Tan, R.J.; Liu, Y. Loss of Klotho contributes to kidney injury by derepression of Wnt/β-catenin signaling. J. Am. Soc. Nephrol. 2013, 24, 771–785. [Google Scholar] [CrossRef]
- Yu, L.X.; Li, S.S.; Sha, M.Y.; Kong, J.W.; Ye, J.M.; Liu, Q.F. The controversy of klotho as a potential biomarker in chronic kidney disease. Front. Pharmacol. 2022, 13, 931746. [Google Scholar] [CrossRef]
- Romagnani, P.; Remuzzi, G.; Glassock, R.; Levin, A.; Jager, K.J.; Tonelli, M.; Massy, Z.; Wanner, C.; Anders, H.J. Chronic kidney disease. Nat. Rev. Dis. Primers. 2017, 3, 17088. [Google Scholar] [CrossRef]
- Cannata-Andia, J.B.; Martin-Carro, B.; Martin-Virgala, J.; Rodriguez-Carrio, J.; Bande-Fernandez, J.J.; Alonso-Montes, C.; Carrillo-Lopez, N. Chronic Kidney Disease-Mineral and Bone Disorders: Pathogenesis and Management. Calcif. Tissue Int. 2021, 108, 410–422. [Google Scholar] [CrossRef]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, T.; Yoshizawa, H.; Watanabe, Y.; Numata, A.; Yamazaki, T.; Takeshima, E.; Iwazu, K.; Komada, T.; Otani, N.; Morishita, Y.; et al. Characteristics of urinary and serum soluble Klotho protein in patients with different degrees of chronic kidney disease. BMC Nephrol. 2012, 13, 155. [Google Scholar] [CrossRef]
- Pavik, I.; Jaeger, P.; Ebner, L.; Wagner, C.A.; Petzold, K.; Spichtig, D.; Poster, D.; Wuthrich, R.P.; Russmann, S.; Serra, A.L. Secreted Klotho and FGF23 in chronic kidney disease Stage 1 to 5: A sequence suggested from a cross-sectional study. Nephrol. Dial. Transplant. 2013, 28, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Nam, B.Y.; Kim, D.W.; Kang, M.W.; Han, J.H.; Lee, M.J.; Shin, D.H.; Doh, F.M.; Koo, H.M.; Ko, K.I.; et al. Circulating alpha-klotho levels in CKD and relationship to progression. Am. J. Kidney Dis. 2013, 61, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.J.; Han, D.J.; Lee, J.H.; Jang, S.H.; Kang, J.S.; Gil, H.W.; Park, S.; Lee, E.Y. Soluble klotho as a marker of renal fibrosis and podocyte injuries in human kidneys. PLoS ONE 2018, 13, e0194617. [Google Scholar] [CrossRef]
- Neyra, J.A.; Hu, M.C. Potential application of klotho in human chronic kidney disease. Bone 2017, 100, 41–49. [Google Scholar] [CrossRef]
- Fernandez-Fernandez, B.; Izquierdo, M.C.; Valino-Rivas, L.; Nastou, D.; Sanz, A.B.; Ortiz, A.; Sanchez-Nino, M.D. Albumin downregulates Klotho in tubular cells. Nephrol. Dial. Transplant. 2018, 33, 1712–1722. [Google Scholar] [CrossRef]
- Torregrosa, I.; Montoliu, C.; Urios, A.; Gimenez-Garzo, C.; Tomas, P.; Solis, M.A.; Ramos, C.; Juan, I.; Puchades, M.J.; Saez, G.; et al. Urinary Klotho measured by ELISA as an early biomarker of acute kidney injury in patients after cardiac surgery or coronary angiography. Nefrologia 2015, 35, 172–178. [Google Scholar] [CrossRef]
- Neyra, J.A.; Hu, M.C.; Moe, O.W. Klotho in Clinical Nephrology: Diagnostic and Therapeutic Implications. Clin. J. Am. Soc. Nephrol. 2020, 16, 162–176. [Google Scholar] [CrossRef]
- Seiler, S.; Wen, M.; Roth, H.J.; Fehrenz, M.; Flügge, F.; Herath, E.; Weihrauch, A.; Fliser, D.; Heine, G.H. Plasma Klotho is not related to kidney function and does not predict adverse outcome in patients with chronic kidney disease. Kidney Int. 2013, 83, 121–128. [Google Scholar] [CrossRef]
- Cano, F.J.; Freundlich, M.; Ceballos, M.L.; Rojo, A.P.; Azocar, M.A.; Delgado, I.O.; Ibacache, M.J.; Delucchi, M.A.; Lillo, A.M.; Irarrazabal, C.E.; et al. Longitudinal FGF23 and Klotho axis characterization in children treated with chronic peritoneal dialysis. Clin. Kidney J. 2014, 7, 457–463. [Google Scholar] [CrossRef][Green Version]
- Devaraj, S.; Syed, B.; Chien, A.; Jialal, I. Validation of an immunoassay for soluble Klotho protein: Decreased levels in diabetes and increased levels in chronic kidney disease. Am. J. Clin. Pathol. 2012, 137, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Navarro-González, J.F.; Sánchez-Niño, M.D.; Donate-Correa, J.; Martín-Núñez, E.; Ferri, C.; Pérez-Delgado, N.; Górriz, J.L.; Martínez-Castelao, A.; Ortiz, A.; Mora-Fernández, C. Effects of Pentoxifylline on Soluble Klotho Concentrations and Renal Tubular Cell Expression in Diabetic Kidney Disease. Diabetes Care 2018, 41, 1817–1820. [Google Scholar] [CrossRef] [PubMed]
- Heijboer, A.C.; Blankenstein, M.A.; Hoenderop, J.; de Borst, M.H.; Vervloet, M.G.; on behalf of the NIGRAM Consortium. Laboratory aspects of circulating alpha-Klotho. Nephrol. Dial. Transplant. 2013, 28, 2283–2287. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.L.; Pastor, J.; Carranza, D.; Quinones, H.; Griffith, C.; Goetz, R.; Mohammadi, M.; Ye, J.; Zhang, J.; Hu, M.C.; et al. The demonstration of alphaKlotho deficiency in human chronic kidney disease with a novel synthetic antibody. Nephrol. Dial. Transplant. 2015, 30, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Neyra, J.A.; Moe, O.W.; Pastor, J.; Gianella, F.; Sidhu, S.S.; Sarnak, M.J.; Ix, J.H.; Drew, D.A. Performance of soluble Klotho assays in clinical samples of kidney disease. Clin. Kidney J. 2020, 13, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Adema, A.Y.; Vervloet, M.G.; Blankenstein, M.A.; Heijboer, A.C. alpha-Klotho is unstable in human urine. Kidney Int. 2015, 88, 1442–1444. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, S.; Tripathi, S.; Chandrekar, A.; Waikar, S.S.; Hsiao, L.L. A novel antibody for the detection of alternatively spliced secreted KLOTHO isoform in human plasma. PLoS ONE 2021, 16, e0245614. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, Y.; Hamada, K.; Inoue, K.; Ogata, K.; Ishihara, M.; Kagawa, T.; Inoue, M.; Fujimoto, S.; Ikebe, M.; Yuasa, K.; et al. Serum levels of soluble secreted alpha-Klotho are decreased in the early stages of chronic kidney disease, making it a probable novel biomarker for early diagnosis. Clin. Exp. Nephrol. 2012, 16, 722–729. [Google Scholar] [CrossRef]
- Rotondi, S.; Pasquali, M.; Tartaglione, L.; Muci, M.L.; Mandanici, G.; Leonangeli, C.; Sales, S.; Farcomeni, A.; Mazzaferro, S. Soluble alpha -Klotho Serum Levels in Chronic Kidney Disease. Int. J. Endocrinol. 2015, 2015, 872193. [Google Scholar] [CrossRef] [PubMed]
- Koyama, D.; Sato, Y.; Aizawa, M.; Maki, T.; Kurosawa, M.; Kuro-o, M.; Furukawa, Y. Soluble αKlotho as a candidate for the biomarker of aging. Biochem. Biophys. Res. Commun. 2015, 467, 1019–1025. [Google Scholar] [CrossRef]
- Bob, F.; Schiller, A.; Timar, R.; Lighezan, D.; Schiller, O.; Timar, B.; Bujor, C.G.; Munteanu, M.; Gadalean, F.; Mihaescu, A.; et al. Rapid decline of kidney function in diabetic kidney disease is associated with high soluble Klotho levels. Nefrologia 2019, 39, 250–257. [Google Scholar] [CrossRef]
- Kimura, T.; Akimoto, T.; Watanabe, Y.; Kurosawa, A.; Nanmoku, K.; Muto, S.; Kusano, E.; Yagisawa, T.; Nagata, D. Impact of Renal Transplantation and Nephrectomy on Urinary Soluble Klotho Protein. Transplant. Proc. 2015, 47, 1697–1699. [Google Scholar] [CrossRef]
- Memmos, E.; Sarafidis, P.; Pateinakis, P.; Tsiantoulas, A.; Faitatzidou, D.; Giamalis, P.; Vasilikos, V.; Papagianni, A. Soluble Klotho is associated with mortality and cardiovascular events in hemodialysis. BMC Nephrol. 2019, 20, 217. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, L.; Yin, X.; Ye, J.; Li, S. Correlation Between Soluble Klotho and Vascular Calcification in Chronic Kidney Disease: A Meta-Analysis and Systematic Review. Front. Physiol. 2021, 12, 711904. [Google Scholar] [CrossRef]
- Keles, N.; Caliskan, M.; Dogan, B.; Keles, N.N.; Kalcik, M.; Aksu, F.; Kostek, O.; Aung, S.M.; Isbilen, B.; Oguz, A. Low Serum Level of Klotho Is an Early Predictor of Atherosclerosis. Tohoku J. Exp. Med. 2015, 237, 17–23. [Google Scholar] [CrossRef]
- Donate-Correa, J.; Ferri, C.M.; Martin-Nunez, E.; Perez-Delgado, N.; Gonzalez-Luis, A.; Mora-Fernandez, C.; Navarro-Gonzalez, J.F. Klotho as a biomarker of subclinical atherosclerosis in patients with moderate to severe chronic kidney disease. Sci. Rep. 2021, 11, 15877. [Google Scholar] [CrossRef]
- Yu, L.; Kang, L.; Ren, X.Z.; Diao, Z.L.; Liu, W.H. Circulating alpha-Klotho Levels in Hemodialysis Patients and Their Relationship to Atherosclerosis. Kidney Blood Press Res. 2018, 43, 1174–1182. [Google Scholar] [CrossRef]
- Kitagawa, M.; Sugiyama, H.; Morinaga, H.; Inoue, T.; Takiue, K.; Ogawa, A.; Yamanari, T.; Kikumoto, Y.; Uchida, H.A.; Kitamura, S.; et al. A decreased level of serum soluble Klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease. PLoS ONE 2013, 8, e56695. [Google Scholar] [CrossRef] [PubMed]
- Park, M.Y.; Herrmann, S.M.; Saad, A.; Eirin, A.; Tang, H.; Lerman, A.; Textor, S.C.; Lerman, L.O. Biomarkers of kidney injury and klotho in patients with atherosclerotic renovascular disease. Clin. J. Am. Soc. Nephrol. 2015, 10, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Seiler, S.; Rogacev, K.S.; Roth, H.J.; Shafein, P.; Emrich, I.; Neuhaus, S.; Floege, J.; Fliser, D.; Heine, G.H. Associations of FGF-23 and sKlotho with cardiovascular outcomes among patients with CKD stages 2-4. Clin. J. Am. Soc. Nephrol. 2014, 9, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Savvoulidis, P.; Kalogeropoulos, A.P.; Raptis, V.; Rafailidis, V.; Georgianos, P.I.; Balaskas, E.V.; Kouskouras, K.; Karvounis, H.; Hadjimiltiades, S. Calcification of coronary arteries and aortic valve and circulating a-klotho levels in patients with chronic kidney disease. J. Thorac. Dis. 2020, 12, 431–437. [Google Scholar] [CrossRef]
- Buiten, M.S.; de Bie, M.K.; Bouma-de Krijger, A.; van Dam, B.; Dekker, F.W.; Jukema, J.W.; Rabelink, T.J.; Rotmans, J.I. Soluble Klotho is not independently associated with cardiovascular disease in a population of dialysis patients. BMC Nephrol. 2014, 15, 197. [Google Scholar] [CrossRef] [PubMed]
- Erkus, E.; Buyukterzi, Z.; Karakose, S.; Kurku, H.; Kurtgoz, P.O.; Topal, M.; Guney, I. The relationship of soluble klotho level with uremic cardiomyopathy and ecocardiographic parameters in hemodialysis patients. Semin. Dial. 2021, 34, 157–162. [Google Scholar] [CrossRef]
- Kashimada, K.; Yamashita, T.; Tsuji, K.; Nifuji, A.; Mizutani, S.; Nabeshima, Y.; Noda, M. Defects in growth and bone metabolism in klotho mutant mice are resistant to GH treatment. J. Endocrinol. 2002, 174, 403–410. [Google Scholar] [CrossRef][Green Version]
- Yamashita, T.; Nabeshima, Y.; Noda, M. High-resolution micro-computed tomography analyses of the abnormal trabecular bone structures in klotho gene mutant mice. J. Endocrinol. 2000, 164, 239–245. [Google Scholar] [CrossRef][Green Version]
- Yamashita, T.; Yoshitake, H.; Tsuji, K.; Kawaguchi, N.; Nabeshima, Y.; Noda, M. Retardation in bone resorption after bone marrow ablation in klotho mutant mice. Endocrinology 2000, 141, 438–445. [Google Scholar] [CrossRef]
- Kuzmova, Z.; Kuzma, M.; Gazova, A.; Kovarova, M.; Jackuliak, P.; Killinger, Z.; Kyselovic, J.; Payer, J. Fibroblast Growth Factor 23 and Klotho Are Associated With Trabecular Bone Score but Not Bone Mineral Density in the Early Stages of Chronic Kidney Disease: Results of the Cross-Sectional Study. Physiol. Res. 2021, 70 (Suppl. 1), S43–S51. [Google Scholar] [CrossRef] [PubMed]
- Desbiens, L.C.; Sidibe, A.; Ung, R.V.; Mac-Way, F. FGF23-Klotho Axis and Fractures in Patients Without and With Early CKD: A Case-Cohort Analysis of CARTaGENE. J. Clin. Endocrinol. Metab. 2022, 107, e2502–e2512. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.L.; Mendes, F.; Carias, E.; Rato, F.; Santos, N.; Neves, P.L.; Silva, A.P. FGF23-klotho axis as predictive factors of fractures in type 2 diabetics with early chronic kidney disease. J. Diabetes Complicat. 2020, 34, 107476. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Mendes, M.; Silva, C.; Cotovio, P.; Aires, I.; Navarro, D.; Caeiro, F.; Salvador, R.; Correia, B.; Cabral, G.; et al. Bone densitometry versus bone histomorphometry in renal transplanted patients: A cross-sectional study. Transpl. Int. 2021, 34, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Marchelek-Mysliwiec, M.; Wisniewska, M.; Nowosiad-Magda, M.; Safranow, K.; Kwiatkowska, E.; Banach, B.; Dolegowska, B.; Dolegowska, K.; Stepniewska, J.; Domanski, L.; et al. Association Between Plasma Concentration of Klotho Protein, Osteocalcin, Leptin, Adiponectin, and Bone Mineral Density in Patients with Chronic Kidney Disease. Horm. Metab. Res. 2018, 50, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.C.; Cotovio, P.; Aires, I.; Mendes, M.; Navarro, D.; Silva, C.; Caeiro, F.; Salvador, R.; Correia, B.; Cabral, G.; et al. The Role of Bone Volume, FGF23 and Sclerostin in Calcifications and Mortality; a Cohort Study in CKD Stage 5 Patients. Calcif. Tissue Int. 2022, 110, 215–224. [Google Scholar] [CrossRef]
- Boisvert, N.C.; Holterman, C.E.; Gutsol, A.; Coulombe, J.; Pan, W.; Alexander, R.T.; Gray, D.A.; Kennedy, C.R. Ubiquitin COOH-terminal hydrolase L1 deletion is associated with urinary alpha-klotho deficiency and perturbed phosphate homeostasis. Am. J. Physiol. Physiol. 2018, 315, F353–F363. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.C.; Zhang, L.X. Prevalence and Disease Burden of Chronic Kidney Disease. Adv. Exp. Med. Biol. 2019, 1165, 3–15. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- DePinho, R.A. The age of cancer. Nature 2000, 408, 248–254. [Google Scholar] [CrossRef]
- Watts, E.L.; Matthews, C.E.; Freeman, J.R.; Gorzelitz, J.S.; Hong, H.G.; Liao, L.M.; McClain, K.M.; Saint-Maurice, P.F.; Shiroma, E.J.; Moore, S.C. Association of Leisure Time Physical Activity Types and Risks of All-Cause, Cardiovascular, and Cancer Mortality Among Older Adults. JAMA Netw. Open 2022, 5, e2228510. [Google Scholar] [CrossRef] [PubMed]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Buchman, A.S.; Yu, L.; Boyle, P.A.; Shah, R.C.; Bennett, D.A. Total daily physical activity and longevity in old age. Arch. Intern Med. 2012, 172, 444–446. [Google Scholar] [CrossRef]
- Evans, I.E.M.; Llewellyn, D.J.; Matthews, F.E.; Woods, R.T.; Brayne, C.; Clare, L.; on behalf of the CFAS-Wales Research team. Social isolation, cognitive reserve, and cognition in healthy older people. PLoS ONE 2018, 13, e0201008. [Google Scholar] [CrossRef]
- Cherry, K.E.; Walker, E.J.; Brown, J.S.; Volaufova, J.; LaMotte, L.R.; Welsh, D.A.; Su, L.J.; Jazwinski, S.M.; Ellis, R.; Wood, R.H.; et al. Social engagement and health in younger, older, and oldest-old adults in the Louisiana Healthy Aging Study. J. Appl. Gerontol. 2013, 32, 51–75. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Veronese, N.; Baiamonte, E.; Guarrera, M.; Parisi, A.; Ruffolo, C.; Tagliaferri, F.; Barbagallo, M. Healthy Aging and Dietary Patterns. Nutrients 2022, 14, 889. [Google Scholar] [CrossRef]
- Espuch-Oliver, A.; Vazquez-Lorente, H.; Jurado-Fasoli, L.; de Haro-Munoz, T.; Diaz-Alberola, I.; Lopez-Velez, M.D.S.; de Haro-Romero, T.; Castillo, M.J.; Amaro-Gahete, F.J. References Values of Soluble alpha-Klotho Serum Levels Using an Enzyme-Linked Immunosorbent Assay in Healthy Adults Aged 18-85 Years. J. Clin. Med. 2022, 11, 2415. [Google Scholar] [CrossRef]
- Watson, S.L.; Weeks, B.K.; Weis, L.J.; Harding, A.T.; Horan, S.A.; Beck, B.R. High-Intensity Resistance and Impact Training Improves Bone Mineral Density and Physical Function in Postmenopausal Women With Osteopenia and Osteoporosis: The LIFTMOR Randomized Controlled Trial. J. Bone Miner. Res. 2018, 33, 211–220. [Google Scholar] [CrossRef]
- Thomas, E.; Battaglia, G.; Patti, A.; Brusa, J.; Leonardi, V.; Palma, A.; Bellafiore, M. Physical activity programs for balance and fall prevention in elderly: A systematic review. Medicine 2019, 98, e16218. [Google Scholar] [CrossRef]
- Liu-Ambrose, T.; Davis, J.C.; Best, J.R.; Dian, L.; Madden, K.; Cook, W.; Hsu, C.L.; Khan, K.M. Effect of a Home-Based Exercise Program on Subsequent Falls Among Community-Dwelling High-Risk Older Adults After a Fall: A Randomized Clinical Trial. JAMA 2019, 321, 2092–2100. [Google Scholar] [CrossRef]
- Lozano-Montoya, I.; Correa-Perez, A.; Abraha, I.; Soiza, R.L.; Cherubini, A.; O’Mahony, D.; Cruz-Jentoft, A.J. Nonpharmacological interventions to treat physical frailty and sarcopenia in older patients: A systematic overview—The SENATOR Project ONTOP Series. Clin. Interv. Aging 2017, 12, 721–740. [Google Scholar] [CrossRef]
- Fiuza-Luces, C.; Santos-Lozano, A.; Joyner, M.; Carrera-Bastos, P.; Picazo, O.; Zugaza, J.L.; Izquierdo, M.; Ruilope, L.M.; Lucia, A. Exercise benefits in cardiovascular disease: Beyond attenuation of traditional risk factors. Nat. Rev. Cardiol. 2018, 15, 731–743. [Google Scholar] [CrossRef]
- Cai, H.; Li, G.; Hua, S.; Liu, Y.; Chen, L. Effect of exercise on cognitive function in chronic disease patients: A meta-analysis and systematic review of randomized controlled trials. Clin. Interv. Aging 2017, 12, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Lenze, E.J.; Voegtle, M.; Miller, J.P.; Ances, B.M.; Balota, D.A.; Barch, D.; Depp, C.A.; Diniz, B.S.; Eyler, L.T.; Foster, E.R.; et al. Effects of Mindfulness Training and Exercise on Cognitive Function in Older Adults: A Randomized Clinical Trial. JAMA 2022, 328, 2218–2229. [Google Scholar] [CrossRef] [PubMed]
- Gaitan, J.M.; Moon, H.Y.; Stremlau, M.; Dubal, D.B.; Cook, D.B.; Okonkwo, O.C.; van Praag, H. Effects of Aerobic Exercise Training on Systemic Biomarkers and Cognition in Late Middle-Aged Adults at Risk for Alzheimer’s Disease. Front. Endocrinol. 2021, 12, 660181. [Google Scholar] [CrossRef]
- Matsubara, T.; Miyaki, A.; Akazawa, N.; Choi, Y.; Ra, S.G.; Tanahashi, K.; Kumagai, H.; Oikawa, S.; Maeda, S. Aerobic exercise training increases plasma Klotho levels and reduces arterial stiffness in postmenopausal women. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H348–H355. [Google Scholar] [CrossRef]
- King, K.E.; McCormick, J.J.; Notley, S.R.; Fujii, N.; Kenny, G.P. Serum Klotho Concentrations in Young and Older Men During Prolonged Exercise in Temperate and Hot Conditions. Curr. Aging Sci. 2022, 15, 180–185. [Google Scholar] [CrossRef]
- Tan, S.J.; Chu, M.M.; Toussaint, N.D.; Cai, M.M.; Hewitson, T.D.; Holt, S.G. High-intensity physical exercise increases serum alpha-klotho levels in healthy volunteers. J. Circ. Biomark. 2018, 7, 1849454418794582. [Google Scholar] [CrossRef]
- Correa, H.L.; Neves, R.V.P.; Deus, L.A.; Souza, M.K.; Haro, A.S.; Costa, F.; Silva, V.L.; Santos, C.A.R.; Moraes, M.R.; Simoes, H.G.; et al. Blood Flow Restriction Training Blunts Chronic Kidney Disease Progression in Humans. Med. Sci. Sports Exerc. 2021, 53, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Neves, R.V.P.; Correa, H.L.; Deus, L.A.; Reis, A.L.; Souza, M.K.; Simoes, H.G.; Navalta, J.W.; Moraes, M.R.; Prestes, J.; Rosa, T.S. Dynamic not isometric training blunts osteo-renal disease and improves the sclerostin/FGF23/Klotho axis in maintenance hemodialysis patients: A randomized clinical trial. J. Appl. Physiol. 2021, 130, 508–516. [Google Scholar] [CrossRef]
- Saghiv, M.S.; Sira, D.B.; Goldhammer, E.; Sagiv, M. The effects of aerobic and anaerobic exercises on circulating soluble-Klotho and IGF-I in young and elderly adults and in CAD patients. J. Circ. Biomark. 2017, 6, 1849454417733388. [Google Scholar] [CrossRef]
- Morishima, T.; Ochi, E. Effect of combined aerobic and resistance exercise on serum Klotho secretion in healthy young men -a pilot study. Curr. Res. Physiol. 2022, 5, 246–250. [Google Scholar] [CrossRef]
- Santos-Dias, A.; MacKenzie, B.; Oliveira-Junior, M.C.; Moyses, R.M.; Consolim-Colombo, F.M.; Vieira, R.P. Longevity protein klotho is induced by a single bout of exercise. Br. J. Sports Med. 2017, 51, 549–550. [Google Scholar] [CrossRef]
- Dziechciaz, M.; Filip, R. Biological psychological and social determinants of old age: Bio-psycho-social aspects of human aging. Ann. Agric. Environ. Med. 2014, 21, 835–838. [Google Scholar] [CrossRef]
- Pichora-Fuller, M.K.; Mick, P.; Reed, M. Hearing, Cognition, and Healthy Aging: Social and Public Health Implications of the Links between Age-Related Declines in Hearing and Cognition. Semin. Hear. 2015, 36, 122–139. [Google Scholar] [CrossRef]
- Wu, F.; Sheng, Y. Social support network, social support, self-efficacy, health-promoting behavior and healthy aging among older adults: A pathway analysis. Arch. Gerontol. Geriatr. 2019, 85, 103934. [Google Scholar] [CrossRef]
- Semba, R.D.; Moghekar, A.R.; Hu, J.; Sun, K.; Turner, R.; Ferrucci, L.; O’Brien, R. Klotho in the cerebrospinal fluid of adults with and without Alzheimer’s disease. Neurosci. Lett. 2014, 558, 37–40. [Google Scholar] [CrossRef]
- Chen, C.D.; Sloane, J.A.; Li, H.; Aytan, N.; Giannaris, E.L.; Zeldich, E.; Hinman, J.D.; Dedeoglu, A.; Rosene, D.L.; Bansal, R.; et al. The antiaging protein Klotho enhances oligodendrocyte maturation and myelination of the CNS. J. Neurosci. 2013, 33, 1927–1939. [Google Scholar] [CrossRef] [PubMed]
- Linghui, D.; Simin, Y.; Zilong, Z.; Yuxiao, L.; Shi, Q.; Birong, D. The relationship between serum klotho and cognitive performance in a nationally representative sample of US adults. Front. Aging Neurosci. 2023, 15, 1053390. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Ma, L.; Wu, C. Association between Serum Klotho and Physical Frailty in Middle-Aged and Older Adults: Finding From the National Health and Nutrition Examination Survey. J. Am. Med. Dir. Assoc. 2023, 24, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Sanz, B.; Arrieta, H.; Rezola-Pardo, C.; Fernandez-Atutxa, A.; Garin-Balerdi, J.; Arizaga, N.; Rodriguez-Larrad, A.; Irazusta, J. Low serum klotho concentration is associated with worse cognition, psychological components of frailty, dependence, and falls in nursing home residents. Sci. Rep. 2021, 11, 9098. [Google Scholar] [CrossRef] [PubMed]
- McCay, C.M.; Crowell, M.F.; Maynard, L.A. The effect of retarded growth upon the length of life span and upon the ultimate body size. 1935. Nutrition 1989, 5, 155–171; discussion 172. [Google Scholar] [PubMed]
- Maegawa, S.; Lu, Y.; Tahara, T.; Lee, J.T.; Madzo, J.; Liang, S.; Jelinek, J.; Colman, R.J.; Issa, J.J. Caloric restriction delays age-related methylation drift. Nat. Commun. 2017, 8, 539. [Google Scholar] [CrossRef] [PubMed]
- Mattison, J.A.; Colman, R.J.; Beasley, T.M.; Allison, D.B.; Kemnitz, J.W.; Roth, G.S.; Ingram, D.K.; Weindruch, R.; de Cabo, R.; Anderson, R.M. Caloric restriction improves health and survival of rhesus monkeys. Nat. Commun. 2017, 8, 14063. [Google Scholar] [CrossRef]
- Berrigan, D.; Perkins, S.N.; Haines, D.C.; Hursting, S.D. Adult-onset calorie restriction and fasting delay spontaneous tumorigenesis in p53-deficient mice. Carcinogenesis 2002, 23, 817–822. [Google Scholar] [CrossRef]
- Acosta-Rodriguez, V.; Rijo-Ferreira, F.; Izumo, M.; Xu, P.; Wight-Carter, M.; Green, C.B.; Takahashi, J.S. Circadian alignment of early onset caloric restriction promotes longevity in male C57BL/6J mice. Science 2022, 376, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Anderson, R.M. Nutrition, longevity and disease: From molecular mechanisms to interventions. Cell 2022, 185, 1455–1470. [Google Scholar] [CrossRef]
- Green, C.L.; Lamming, D.W.; Fontana, L. Molecular mechanisms of dietary restriction promoting health and longevity. Nat. Rev. Mol. Cell. Biol. 2022, 23, 56–73. [Google Scholar] [CrossRef]
- Il’yasova, D.; Fontana, L.; Bhapkar, M.; Pieper, C.F.; Spasojevic, I.; Redman, L.M.; Das, S.K.; Huffman, K.M.; Kraus, W.E.; Investigators, C.S. Effects of 2 years of caloric restriction on oxidative status assessed by urinary F2-isoprostanes: The CALERIE 2 randomized clinical trial. Aging Cell 2018, 17, e12719. [Google Scholar] [CrossRef]
- Ravussin, E.; Redman, L.M.; Rochon, J.; Das, S.K.; Fontana, L.; Kraus, W.E.; Romashkan, S.; Williamson, D.A.; Meydani, S.N.; Villareal, D.T.; et al. A 2-Year Randomized Controlled Trial of Human Caloric Restriction: Feasibility and Effects on Predictors of Health Span and Longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1097–1104. [Google Scholar] [CrossRef]
- Miyazaki, T.; Takenaka, T.; Inoue, T.; Sato, M.; Hanyu, M.; Eiki, Y.; Nodera, M.; Yanagisawa, H.; Ohno, Y.; Shibazaki, S.; et al. Klotho Expression is Induced by Calorie Restriction in Adult Male Rats. Trace Nutr. Res. 2010, 27, 92–96. [Google Scholar] [CrossRef]
- Shafie, A.; Rahimi, A.M.; Ahmadi, I.; Nabavizadeh, F.; Ranjbaran, M.; Ashabi, G. High-protein and low-calorie diets improved the anti-aging Klotho protein in the rats’ brain: The toxic role of high-fat diet. Nutr. Metab. 2020, 17, 86. [Google Scholar] [CrossRef]
- De-la, O.A.; Jurado-Fasoli, L.; Gracia-Marco, L.; Henriksson, P.; Castillo, M.J.; Amaro-Gahete, F.J. Association of Energy and Macronutrients Intake with S-Klotho Plasma Levels in Middle-Aged Sedentary Adults: A Cross-Sectional Study. J. Nutr. Health Aging 2022, 26, 360–366. [Google Scholar] [CrossRef]
- Cui, J.; Yang, Z.; Wang, J.; Yin, S.; Xiao, Y.; Bai, Y.; Wang, J. A cross-sectional analysis of association between visceral adiposity index and serum anti-aging protein Klotho in adults. Front. Endocrinol. 2023, 14, 1082504. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.; Kuro, O.M.; Chen, C.H.; Sue, Y.M.; Chen, Y.C.; Wu, H.H.; Cheng, C.Y. The secreted Klotho protein restores phosphate retention and suppresses accelerated aging in Klotho mutant mice. Eur. J. Pharmacol. 2013, 698, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Flores, B.; Gillings, N.; Bian, A.; Cho, H.J.; Yan, S.; Liu, Y.; Levine, B.; Moe, O.W.; Hu, M.C. alphaKlotho Mitigates Progression of AKI to CKD through Activation of Autophagy. J. Am. Soc. Nephrol. 2016, 27, 2331–2345. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Guo, H.; Meng, S.; Zhu, B.; Fang, J.; Huang, J.; Chen, J.; Wang, Y.; Wang, L.; Yao, X.; et al. Klotho ameliorates diabetic nephropathy by activating Nrf2 signaling pathway in podocytes. Biochem. Biophys. Res. Commun. 2021, 534, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wu, Y.L.; Huang, C.L. Deficiency of Soluble alpha-Klotho as an Independent Cause of Uremic Cardiomyopathy. Vitam. Horm. 2016, 101, 311–330. [Google Scholar] [CrossRef]
- Chen, K.; Wang, S.; Sun, Q.W.; Zhang, B.; Ullah, M.; Sun, Z. Klotho Deficiency Causes Heart Aging via Impairing the Nrf2-GR Pathway. Circ. Res. 2021, 128, 492–507. [Google Scholar] [CrossRef] [PubMed]
- Kawarazaki, W.; Mizuno, R.; Nishimoto, M.; Ayuzawa, N.; Hirohama, D.; Ueda, K.; Kawakami-Mori, F.; Oba, S.; Marumo, T.; Fujita, T. Salt causes aging-associated hypertension via vascular Wnt5a under Klotho deficiency. J. Clin. Investig. 2020, 130, 4152–4166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, L.; Lin, W.; Yin, S.; Duan, A.; Liu, Z.; Cao, W. Rhein reverses Klotho repression via promoter demethylation and protects against kidney and bone injuries in mice with chronic kidney disease. Kidney Int. 2017, 91, 144–156. [Google Scholar] [CrossRef]
- Young, G.H.; Wu, V.C. KLOTHO methylation is linked to uremic toxins and chronic kidney disease. Kidney Int. 2012, 81, 611–612. [Google Scholar] [CrossRef]
- Li, Y.; Chen, F.; Wei, A.; Bi, F.; Zhu, X.; Yin, S.; Lin, W.; Cao, W. Klotho recovery by genistein via promoter histone acetylation and DNA demethylation mitigates renal fibrosis in mice. J. Mol. Med. 2019, 97, 541–552. [Google Scholar] [CrossRef]
- Mora-Fernández, C.; Sánchez-Niño, M.D.; Donate-Correa, J.; Martín-Núñez, E.; Pérez-Delgado, N.; Valiño-Rivas, L.; Fernández-Fernández, B.; Ortiz, A.; Navarro-González, J.F. Sodium-glucose co-transporter-2 inhibitors increase Klotho in patients with diabetic kidney disease: A clinical and experimental study. Biomed. Pharmacother. = Biomed. Pharmacother. 2022, 154, 113677. [Google Scholar] [CrossRef]
- Mizusaki, K.; Hasuike, Y.; Kimura, T.; Nagasawa, Y.; Kuragano, T.; Yamada, Y.; Nojima, M.; Yamamoto, S.; Nakanishi, T.; Ishihara, M. Inhibition of the Mammalian Target of Rapamycin May Augment the Increase in Soluble Klotho Levels in Renal Transplantation Recipients. Blood Purif. 2019, 47 (Suppl. 2), 12–18. [Google Scholar] [CrossRef]
- Yoon, H.E.; Lim, S.W.; Piao, S.G.; Song, J.H.; Kim, J.; Yang, C.W. Statin upregulates the expression of klotho, an anti-aging gene, in experimental cyclosporine nephropathy. Nephron Exp. Nephrol. 2012, 120, e123–e133. [Google Scholar] [CrossRef]
- Yang, H.C.; Deleuze, S.; Zuo, Y.; Potthoff, S.A.; Ma, L.J.; Fogo, A.B. The PPARgamma agonist pioglitazone ameliorates aging-related progressive renal injury. J. Am. Soc. Nephrol. 2009, 20, 2380–2388. [Google Scholar] [CrossRef] [PubMed]
- Ritter, C.S.; Zhang, S.; Delmez, J.; Finch, J.L.; Slatopolsky, E. Differential expression and regulation of Klotho by paricalcitol in the kidney, parathyroid, and aorta of uremic rats. Kidney Int. 2015, 87, 1141–1152. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Lin, S.; Tang, R.; Veeraragoo, P.; Peng, W.; Wu, R. Role of Fosinopril and Valsartan on Klotho Gene Expression Induced by Angiotensin II in Rat Renal Tubular Epithelial Cells. Kidney Blood Press. Res. 2010, 33, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.E.; Ghee, J.Y.; Piao, S.; Song, J.H.; Han, D.H.; Kim, S.; Ohashi, N.; Kobori, H.; Kuro-o, M.; Yang, C.W. Angiotensin II blockade upregulates the expression of Klotho, the anti-ageing gene, in an experimental model of chronic cyclosporine nephropathy. Nephrol. Dial. Transplant. 2011, 26, 800–813. [Google Scholar] [CrossRef]
- Safarpour, Y.; Vaziri, N.D.; Jabbari, B. Movement Disorders in Chronic Kidney Disease—A Descriptive Review. J. Stroke Cerebrovasc. Dis. 2021, 30, 105408. [Google Scholar] [CrossRef] [PubMed]
- Prud’homme, G.J.; Glinka, Y.; Kurt, M.; Liu, W.; Wang, Q. The anti-aging protein Klotho is induced by GABA therapy and exerts protective and stimulatory effects on pancreatic beta cells. Biochem. Biophys. Res. Commun. 2017, 493, 1542–1547. [Google Scholar] [CrossRef] [PubMed]
- Son, D.O.; Liu, W.; Li, X.; Prud’homme, G.J.; Wang, Q. Combined effect of GABA and glucagon-like peptide-1 receptor agonist on cytokine-induced apoptosis in pancreatic β-cell line and isolated human islets. J. Diabetes 2019, 11, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Neyra, J.A.; Hu, M.C. alphaKlotho and Chronic Kidney Disease. Vitam. Horm. 2016, 101, 257–310. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Vírgala, J.; Martín-Carro, B.; Fernández-Villabrille, S.; Ruiz-Torres, M.P.; Gómez-Alonso, C.; Rodríguez-García, M.; Fernández-Martín, J.L.; Alonso-Montes, C.; Panizo, S.; Cannata-Andía, J.B.; et al. Soluble Klotho, a Potential Biomarker of Chronic Kidney Disease–Mineral Bone Disorders Involved in Healthy Ageing: Lights and Shadows. Int. J. Mol. Sci. 2024, 25, 1843. https://doi.org/10.3390/ijms25031843
Martín-Vírgala J, Martín-Carro B, Fernández-Villabrille S, Ruiz-Torres MP, Gómez-Alonso C, Rodríguez-García M, Fernández-Martín JL, Alonso-Montes C, Panizo S, Cannata-Andía JB, et al. Soluble Klotho, a Potential Biomarker of Chronic Kidney Disease–Mineral Bone Disorders Involved in Healthy Ageing: Lights and Shadows. International Journal of Molecular Sciences. 2024; 25(3):1843. https://doi.org/10.3390/ijms25031843
Chicago/Turabian StyleMartín-Vírgala, Julia, Beatriz Martín-Carro, Sara Fernández-Villabrille, María Piedad Ruiz-Torres, Carlos Gómez-Alonso, Minerva Rodríguez-García, José Luis Fernández-Martín, Cristina Alonso-Montes, Sara Panizo, Jorge B. Cannata-Andía, and et al. 2024. "Soluble Klotho, a Potential Biomarker of Chronic Kidney Disease–Mineral Bone Disorders Involved in Healthy Ageing: Lights and Shadows" International Journal of Molecular Sciences 25, no. 3: 1843. https://doi.org/10.3390/ijms25031843
APA StyleMartín-Vírgala, J., Martín-Carro, B., Fernández-Villabrille, S., Ruiz-Torres, M. P., Gómez-Alonso, C., Rodríguez-García, M., Fernández-Martín, J. L., Alonso-Montes, C., Panizo, S., Cannata-Andía, J. B., Naves-Díaz, M., & Carrillo-López, N. (2024). Soluble Klotho, a Potential Biomarker of Chronic Kidney Disease–Mineral Bone Disorders Involved in Healthy Ageing: Lights and Shadows. International Journal of Molecular Sciences, 25(3), 1843. https://doi.org/10.3390/ijms25031843





