Vaping-Dependent Pulmonary Inflammation Is Ca2+ Mediated and Potentially Sex Specific
Abstract
1. Introduction
2. Results
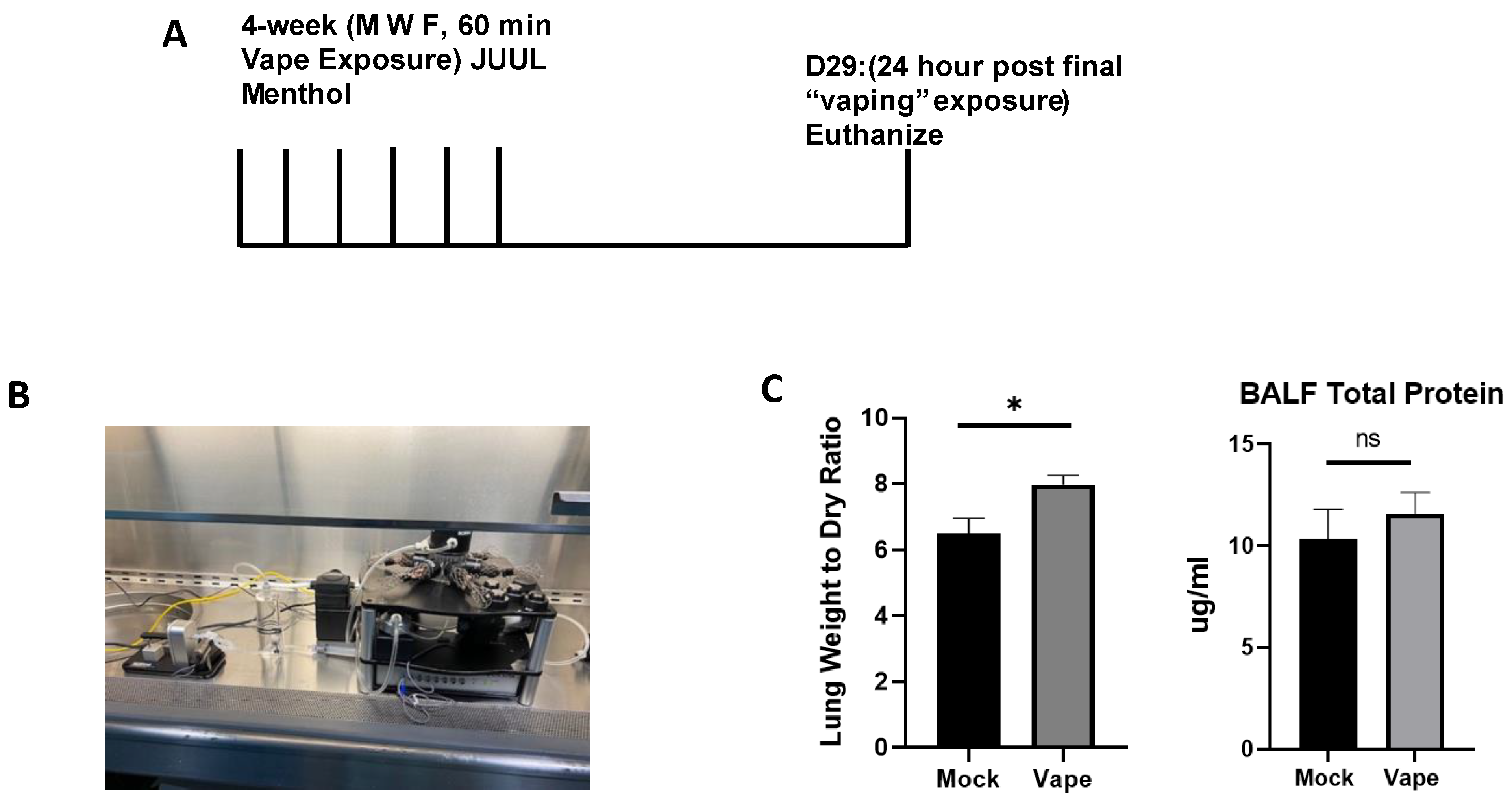
2.1. Establishing an Acute Vaping Mouse Model Using the SCIREQ InExpose System
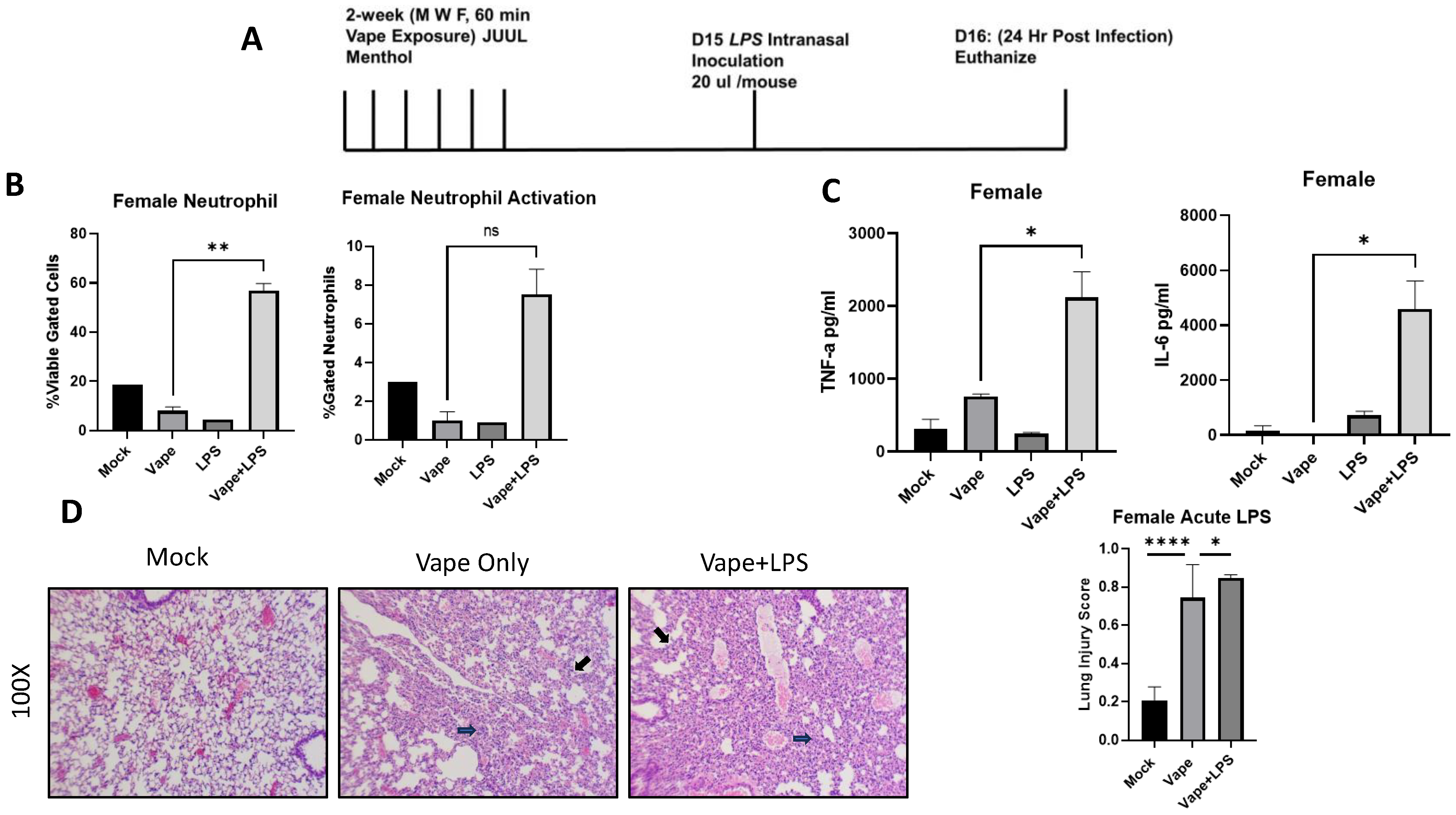
2.2. An Acute Vaping In Vivo Model Illustrates Vaping Exacerbates Lung Cellularity and Inflammation in the Presence of Infection
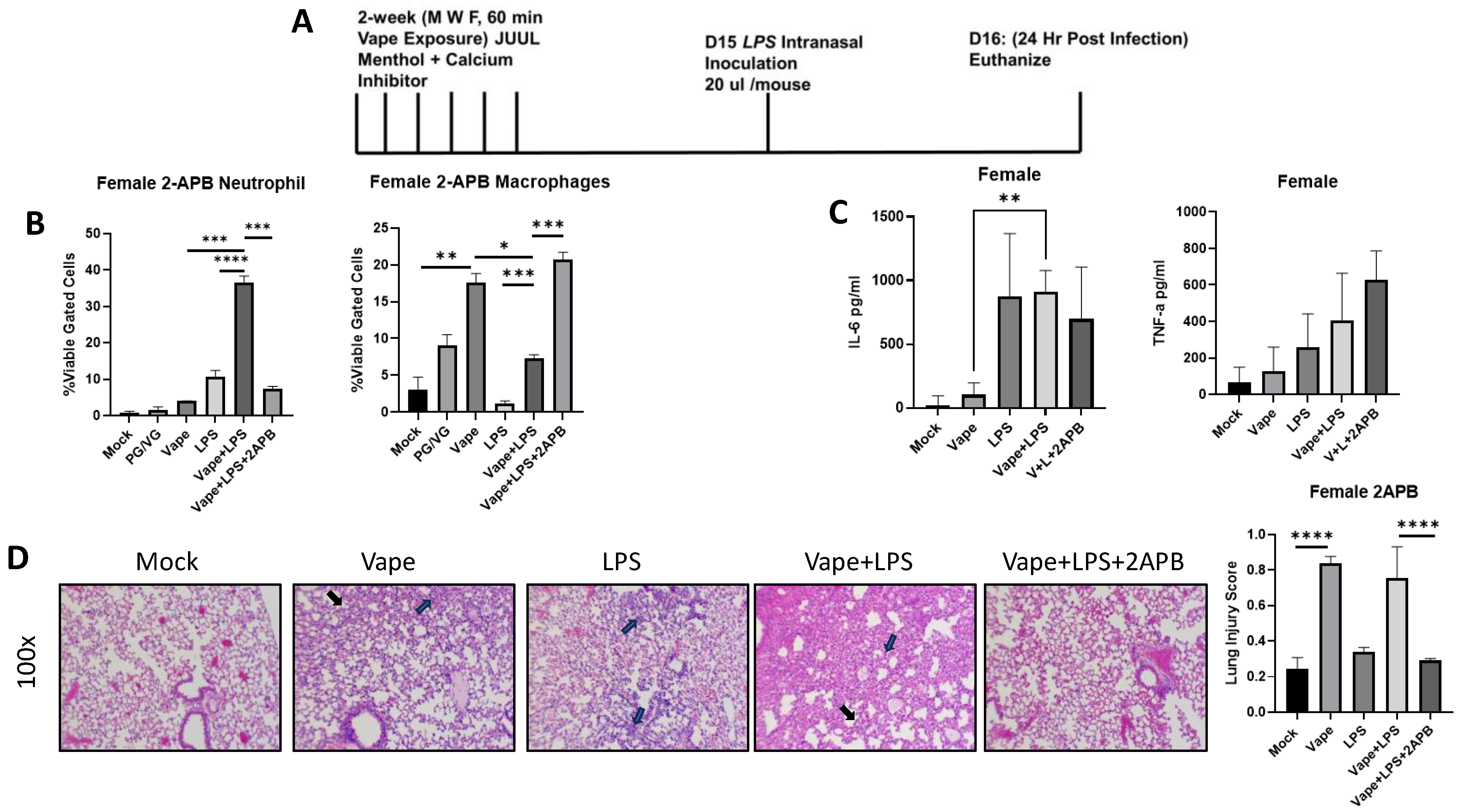
2.3. Treatment with a Ca2+ Inhibitor Mitigates the Alveolar Damage Caused by Vaping and/or Infection
2.4. Male Mouse LPS Exposure Data Demonstrate Differences in Cellularity and Inflammation Compared to Female Data
3. Discussion
4. Materials and Methods
4.1. Mice and Treatment
4.2. Bicinchoninic Acid (BCA) Protein Assay
4.3. Flow Cytometry
4.4. Cytokine Analysis
4.5. Histopathology
4.6. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2019 Chronic Respiratory Diseases Collaborators. Global burden of chronic respiratory diseases and risk factors, 1990–2019: An update from the Global Burden of Disease Study 2019. eClinicalMedicine 2023, 59, 101936. [Google Scholar] [CrossRef]
- Ware, L.B.; Matthay, M.A. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2001, 163, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Ning, Q.M.; Sun, X.N.; Zhao, X.K. Role of mechanical stretching and lipopolysaccharide in early apoptosis and IL-8 of alveolar epithelial type II cells A549. Asian Pac. J. Trop. Med. 2012, 5, 638–644. [Google Scholar] [CrossRef]
- Liu, G.Y. Molecular pathogenesis of Staphylococcus aureus infection. Pediatr. Res. 2009, 65, 71r–77r. [Google Scholar] [CrossRef]
- Hwang, J.H.; Lyes, M.; Sladewski, K.; Enany, S.; McEachern, E.; Mathew, D.P.; Das, S.; Moshensky, A.; Bapat, S.; Pride, D.T.; et al. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. J. Mol. Med. 2016, 94, 667–679. [Google Scholar] [CrossRef]
- Shipman, J.G.; Onyenwoke, R.U.; Sivaraman, V. Calcium-Dependent Pulmonary Inflammation and Pharmacological Interventions and Mediators. Biology 2021, 10, 1053. [Google Scholar] [CrossRef]
- McEachern, E.K.; Hwang, J.H.; Sladewski, K.M.; Nicatia, S.; Dewitz, C.; Mathew, D.P.; Nizet, V.; Crotty Alexander, L.E. Analysis of the effects of cigarette smoke on staphylococcal virulence phenotypes. Infect. Immun. 2015, 83, 2443–2452. [Google Scholar] [CrossRef] [PubMed]
- Crotty Alexander, L.E.; Shin, S.; Hwang, J.H. Inflammatory Diseases of the Lung Induced by Conventional Cigarette Smoke: A Review. Chest 2015, 148, 1307–1322. [Google Scholar] [CrossRef] [PubMed]
- Jerzyński, T.; Stimson, G.V.; Shapiro, H.; Król, G. Estimation of the global number of e-cigarette users in 2020. Harm Reduct. J. 2021, 18, 109. [Google Scholar] [CrossRef]
- Jerzyński, T.; Stimson, G.V. Estimation of the global number of vapers: 82 million worldwide in 2021. Drugs Habits Soc. Policy 2023, 24, 91–103. [Google Scholar] [CrossRef]
- About Electronic Cigarettes (E-Cigarettes). Centers for Disease Control, 2023. Available online: https://www.cdc.gov/tobacco/basic_information/e-cigarettes/about-e-cigarettes.html (accessed on 14 December 2023).
- Results from the Annual National Youth Tobacco Survey. Food and Drug Administration. 2023. Available online: https://www.fda.gov/tobacco-products/youth-and-tobacco/results-annual-national-youth-tobacco-survey (accessed on 14 December 2023).
- Kramarow, E.A.; Elgaddal, N. Current Electronic Cigarette Use Among Adults Aged 18 and Over: United States, 2021. NCHS Data Brief 2023, 475, 1–8. [Google Scholar]
- Silveyra, P.; Fuentes, N.; Rodriguez Bauza, D.E. Sex and Gender Differences in Lung Disease. Adv. Exp. Med. Biol. 2021, 1304, 227–258. [Google Scholar] [CrossRef]
- Orzabal, M.R.; Lunde-Young, E.R.; Ramirez, J.I.; Howe, S.Y.F.; Naik, V.D.; Lee, J.; Heaps, C.L.; Threadgill, D.W.; Ramadoss, J. Chronic exposure to e-cig aerosols during early development causes vascular dysfunction and offspring growth deficits. Transl. Res. 2019, 207, 70–82. [Google Scholar] [CrossRef]
- Chand, H.S.; Muthumalage, T.; Maziak, W.; Rahman, I. Pulmonary Toxicity and the Pathophysiology of Electronic Cigarette, or Vaping Product, Use Associated Lung Injury. Front. Pharmacol. 2019, 10, 1619. [Google Scholar] [CrossRef]
- Lerner, C.A.; Sundar, I.K.; Yao, H.; Gerloff, J.; Ossip, D.J.; McIntosh, S.; Robinson, R.; Rahman, I. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS ONE 2015, 10, e0116732. [Google Scholar] [CrossRef]
- Patergnani, S.; Danese, A.; Bouhamida, E.; Aguiari, G.; Previati, M.; Pinton, P.; Giorgi, C. Various Aspects of Calcium Signaling in the Regulation of Apoptosis, Autophagy, Cell Proliferation, and Cancer. Int. J. Mol. Sci. 2020, 21, 8323. [Google Scholar] [CrossRef]
- Sassano, M.F.; Ghosh, A.; Tarran, R. Tobacco Smoke Constituents Trigger Cytoplasmic Calcium Release. Appl. Vitro Toxicol. 2017, 3, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Petit, A.; Knabe, L.; Khelloufi, K.; Jory, M.; Gras, D.; Cabon, Y.; Begg, M.; Richard, S.; Massiera, G.; Chanez, P.; et al. Bronchial Epithelial Calcium Metabolism Impairment in Smokers and Chronic Obstructive Pulmonary Disease. Decreased ORAI3 Signaling. Am. J. Respir. Cell Mol. Biol. 2019, 61, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Sivaraman, V.; Parker, D.; Zhang, R.; Jones, M.M.; Onyenwoke, R.U. Vaping Exacerbates Coronavirus-Related Pulmonary Infection in a Murine Model. Front. Physiol. 2021, 12, 634839. [Google Scholar] [CrossRef] [PubMed]
- Onyenwoke, R.U.; Leung, T.; Huang, X.; Parker, D.; Shipman, J.G.; Alhadyan, S.K.; Sivaraman, V. An assessment of vaping-induced inflammation and toxicity: A feasibility study using a 2-stage zebrafish and mouse platform. Food Chem. Toxicol. 2022, 163, 112923. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Jones, M.M.; Parker, D.; Dornsife, R.E.; Wymer, N.; Onyenwoke, R.U.; Sivaraman, V. Acute vaping exacerbates microbial pneumonia due to calcium (Ca2+) dysregulation. PLoS ONE 2021, 16, e0256166. [Google Scholar] [CrossRef]
- Re, D.B.; Hilpert, M.; Saglimbeni, B.; Strait, M.; Ilievski, V.; Coady, M.; Talayero, M.; Wilmsen, K.; Chesnais, H.; Balac, O.; et al. Exposure to e-cigarette aerosol over two months induces accumulation of neurotoxic metals and alteration of essential metals in mouse brain. Environ. Res. 2021, 202, 111557. [Google Scholar] [CrossRef]
- Scott, A.; Lugg, S.T.; Aldridge, K.; Lewis, K.E.; Bowden, A.; Mahida, R.Y.; Grudzinska, F.S.; Dosanjh, D.; Parekh, D.; Foronjy, R.; et al. Pro-inflammatory effects of e-cigarette vapour condensate on human alveolar macrophages. Thorax 2018, 73, 1161–1169. [Google Scholar] [CrossRef]
- Gómez, A.C.; Rodríguez-Fernández, P.; Villar-Hernández, R.; Gibert, I.; Muriel-Moreno, B.; Lacoma, A.; Prat-Aymerich, C.; Domínguez, J. E-cigarettes: Effects in phagocytosis and cytokines response against Mycobacterium tuberculosis. PLoS ONE 2020, 15, e0228919. [Google Scholar] [CrossRef] [PubMed]
- Zemanick, E.T.; Wagner, B.D.; Harris, J.K.; Wagener, J.S.; Accurso, F.J.; Sagel, S.D. Pulmonary exacerbations in cystic fibrosis with negative bacterial cultures. Pediatr. Pulmonol. 2010, 45, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Jones, M.M.; Dornsife, R.E.; Wu, T.; Sivaraman, V.; Tarran, R.; Onyenwoke, R.U. JUUL e-liquid exposure elicits cytoplasmic Ca(2+) responses and leads to cytotoxicity in cultured airway epithelial cells. Toxicol. Lett. 2021, 337, 46–56. [Google Scholar] [CrossRef]
- Ghosh, A.; Beyazcicek, O.; Davis, E.S.; Onyenwoke, R.U.; Tarran, R. Cellular effects of nicotine salt-containing e-liquids. J. Appl. Toxicol. 2021, 41, 493–505. [Google Scholar] [CrossRef]
- Bengalli, R.; Ferri, E.; Labra, M.; Mantecca, P. Lung Toxicity of Condensed Aerosol from E-CIG Liquids: Influence of the Flavor and the In Vitro Model Used. Int. J. Environ. Res. Public Health 2017, 14, 1254. [Google Scholar] [CrossRef]
- Patel, B.V.; Wilson, M.R.; Takata, M. Resolution of acute lung injury and inflammation: A translational mouse model. Eur. Respir. J. 2012, 39, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ci, X.; Wen, Z.; Peng, L. Diosmetin Alleviates Lipopolysaccharide-Induced Acute Lung Injury through Activating the Nrf2 Pathway and Inhibiting the NLRP3 Inflammasome. Biomol. Ther. 2018, 26, 157–166. [Google Scholar] [CrossRef]
- Crotty Alexander, L.E.; Drummond, C.A.; Hepokoski, M.; Mathew, D.; Moshensky, A.; Willeford, A.; Das, S.; Singh, P.; Yong, Z.; Lee, J.H.; et al. Chronic inhalation of e-cigarette vapor containing nicotine disrupts airway barrier function and induces systemic inflammation and multiorgan fibrosis in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R834–R847. [Google Scholar] [CrossRef] [PubMed]
- Rowell, T.R.; Keating, J.E.; Zorn, B.T.; Glish, G.L.; Shears, S.B.; Tarran, R. Flavored e-liquids increase cytoplasmic Ca(2+) levels in airway epithelia. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 318, L226–L241. [Google Scholar] [CrossRef] [PubMed]
- Lamb, T.; Rahman, I. Pro-inflammatory effects of aerosols from e-cigarette-derived flavoring chemicals on murine macrophages. Toxicol. Rep. 2023, 10, 431–435. [Google Scholar] [CrossRef]
- Maruyama, T.; Kanaji, T.; Nakade, S.; Kanno, T.; Mikoshiba, K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J. Biochem. 1997, 122, 498–505. [Google Scholar] [CrossRef]
- Chokshi, R.; Fruasaha, P.; Kozak, J.A. 2-aminoethyl diphenyl borinate (2-APB) inhibits TRPM7 channels through an intracellular acidification mechanism. Channels 2012, 6, 362–369. [Google Scholar] [CrossRef]
- Bootman, M.D.; Collins, T.J.; Mackenzie, L.; Roderick, H.L.; Berridge, M.J.; Peppiatt, C.M. 2-aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J 2002, 16, 1145–1150. [Google Scholar] [CrossRef]
- Paczosa, M.K.; Mecsas, J. Klebsiella pneumoniae: Going on the Offense with a Strong Defense. Microbiol. Mol. Biol. Rev. 2016, 80, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Sun, Y.; Sukumaran, P.; Quenum Zangbede, F.O.; Jondle, C.N.; Sharma, A.; Evans, D.L.; Chauhan, P.; Szlabick, R.E.; Aaland, M.O.; et al. M1 Macrophage Polarization Is Dependent on TRPC1-Mediated Calcium Entry. iScience 2018, 8, 85–102. [Google Scholar] [CrossRef]
- Tedesco, S.; Scattolini, V.; Albiero, M.; Bortolozzi, M.; Avogaro, A.; Cignarella, A.; Fadini, G.P. Mitochondrial Calcium Uptake Is Instrumental to Alternative Macrophage Polarization and Phagocytic Activity. Int. J. Mol. Sci. 2019, 20, 4966. [Google Scholar] [CrossRef]
- Matute-Bello, G.; Downey, G.; Moore, B.B.; Groshong, S.D.; Matthay, M.A.; Slutsky, A.S.; Kuebler, W.M. An official American Thoracic Society workshop report: Features and measurements of experimental acute lung injury in animals. Am. J. Respir. Cell Mol. Biol. 2011, 44, 725–738. [Google Scholar] [CrossRef]

| Antibody | Detection Channel (Excitation) | Use |
|---|---|---|
| SiglecF | FITC (488 nm) | Alveolar Macrophage |
| Ly6G | PE (488 nm) | Neutrophil |
| F4/80 | APC-AF750 (638) | All Macrophages |
| CD11b | APC (638 nm) | Activation Marker |
| Live/Dead blue | PB450 (405 nm) | Cell Viability |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shipman, J.G.; Onyenwoke, R.U.; Sivaraman, V. Vaping-Dependent Pulmonary Inflammation Is Ca2+ Mediated and Potentially Sex Specific. Int. J. Mol. Sci. 2024, 25, 1785. https://doi.org/10.3390/ijms25031785
Shipman JG, Onyenwoke RU, Sivaraman V. Vaping-Dependent Pulmonary Inflammation Is Ca2+ Mediated and Potentially Sex Specific. International Journal of Molecular Sciences. 2024; 25(3):1785. https://doi.org/10.3390/ijms25031785
Chicago/Turabian StyleShipman, Jeffrey G., Rob U. Onyenwoke, and Vijay Sivaraman. 2024. "Vaping-Dependent Pulmonary Inflammation Is Ca2+ Mediated and Potentially Sex Specific" International Journal of Molecular Sciences 25, no. 3: 1785. https://doi.org/10.3390/ijms25031785
APA StyleShipman, J. G., Onyenwoke, R. U., & Sivaraman, V. (2024). Vaping-Dependent Pulmonary Inflammation Is Ca2+ Mediated and Potentially Sex Specific. International Journal of Molecular Sciences, 25(3), 1785. https://doi.org/10.3390/ijms25031785






