Alleviative Effect of Geniposide on Lipopolysaccharide-Stimulated Macrophages via Calcium Pathway
Abstract
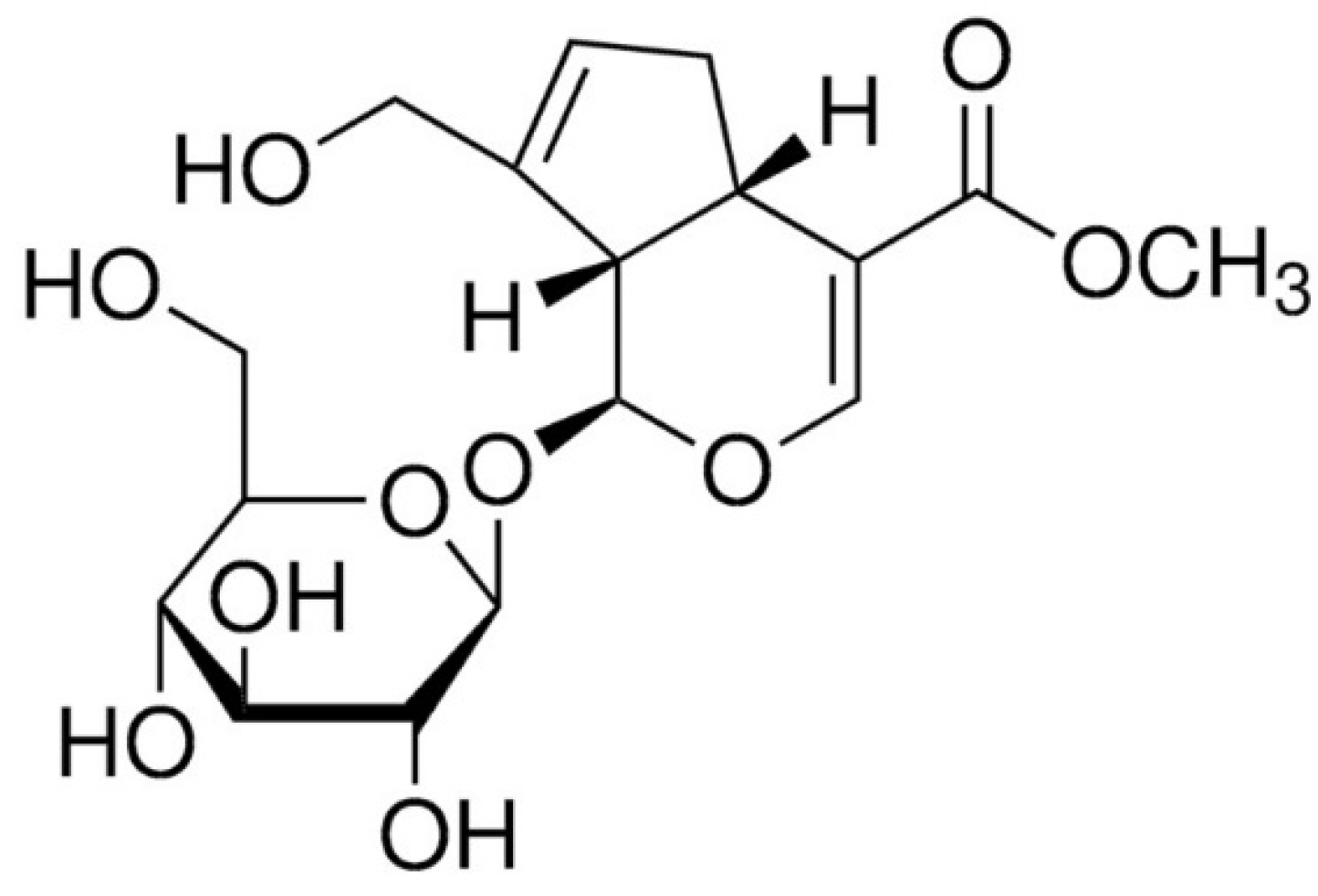
1. Introduction
2. Results
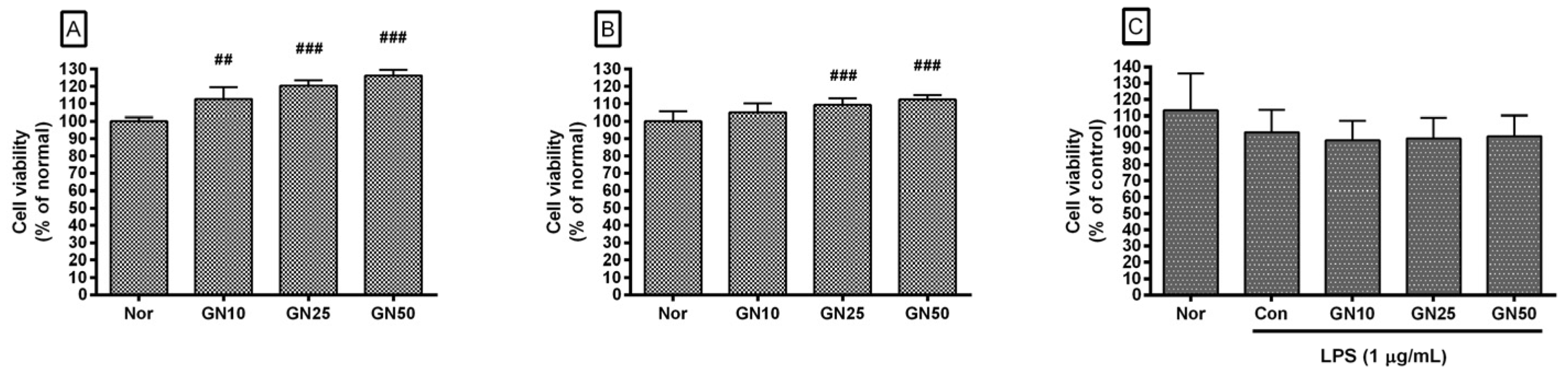
2.1. Effect of Geniposide on Cell Viability
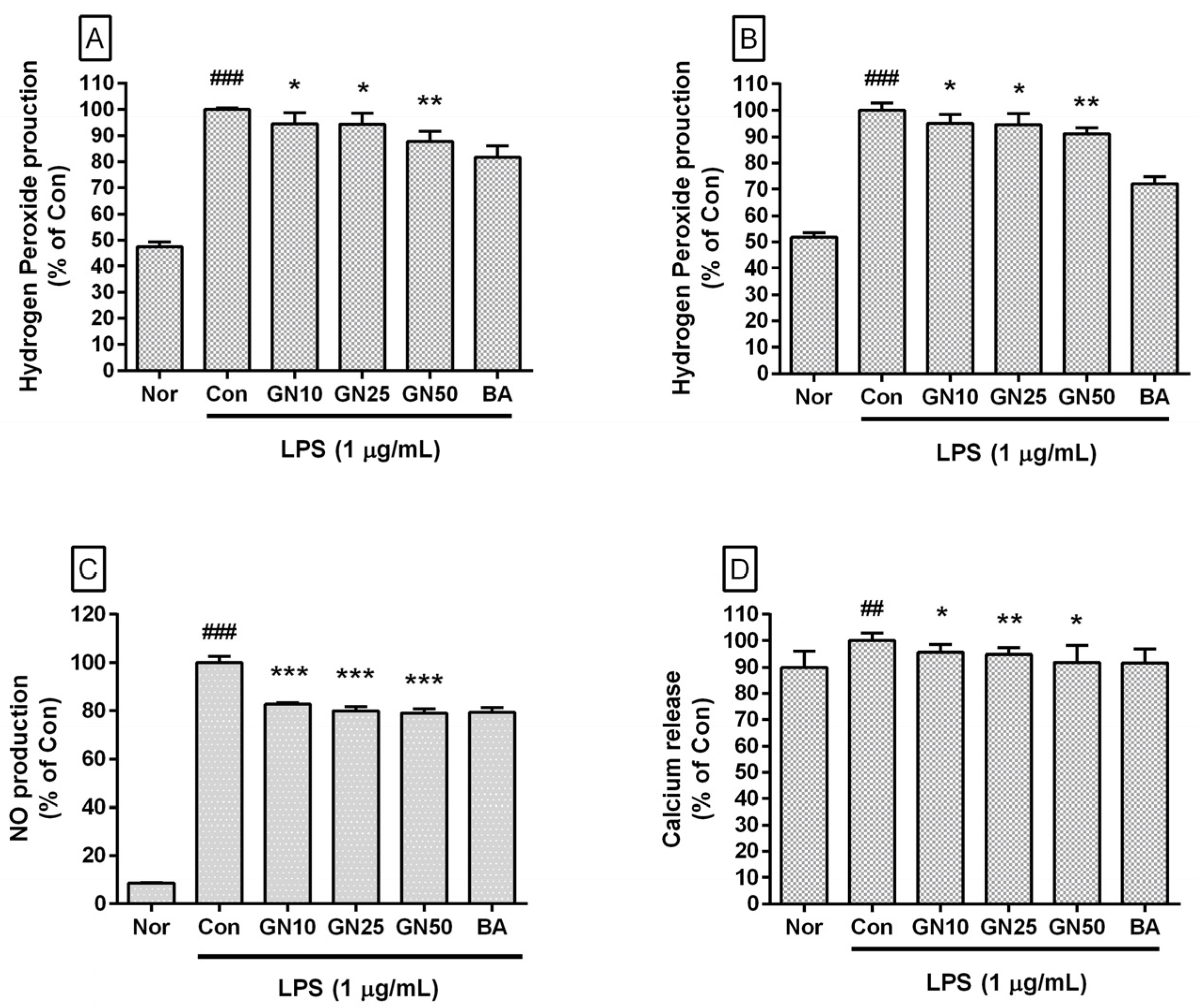
2.2. Effect of Geniposide on the Level of Hydrogen Peroxide, NO, and Ca2+
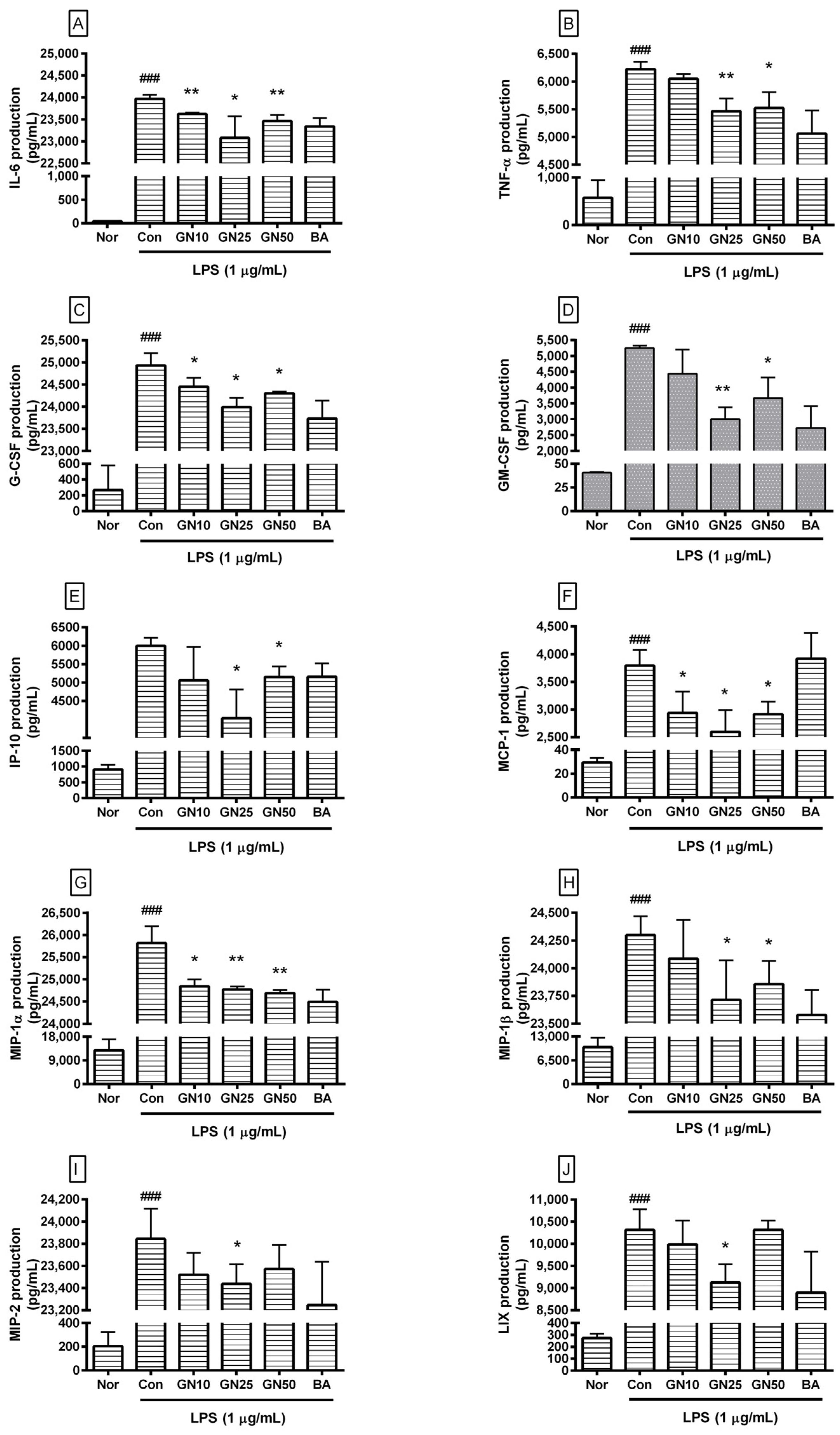
2.3. Effect of Geniposide on Cytokines Production
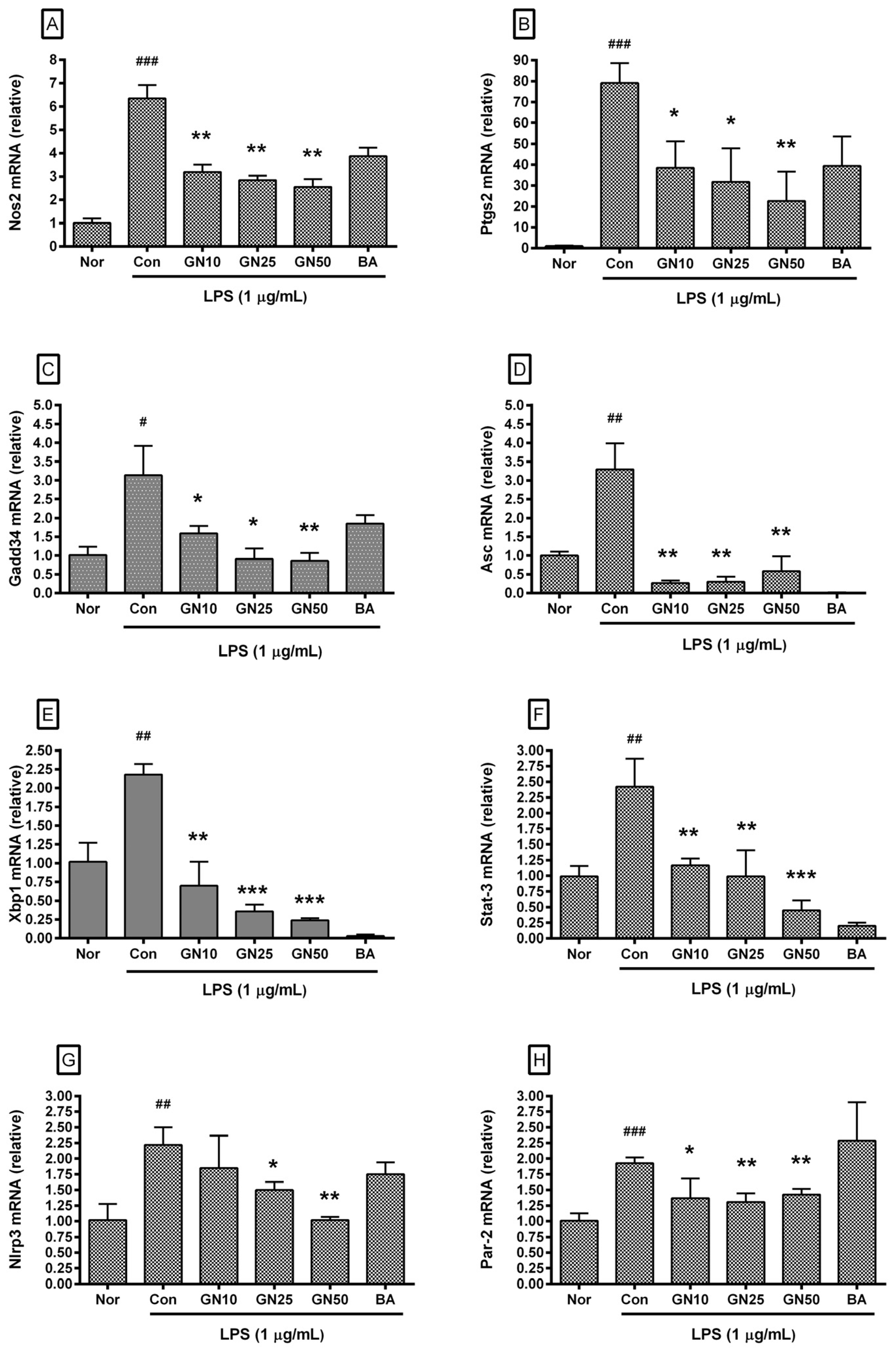
2.4. Effect of Geniposide on Inflammatory Target Genes Expressions
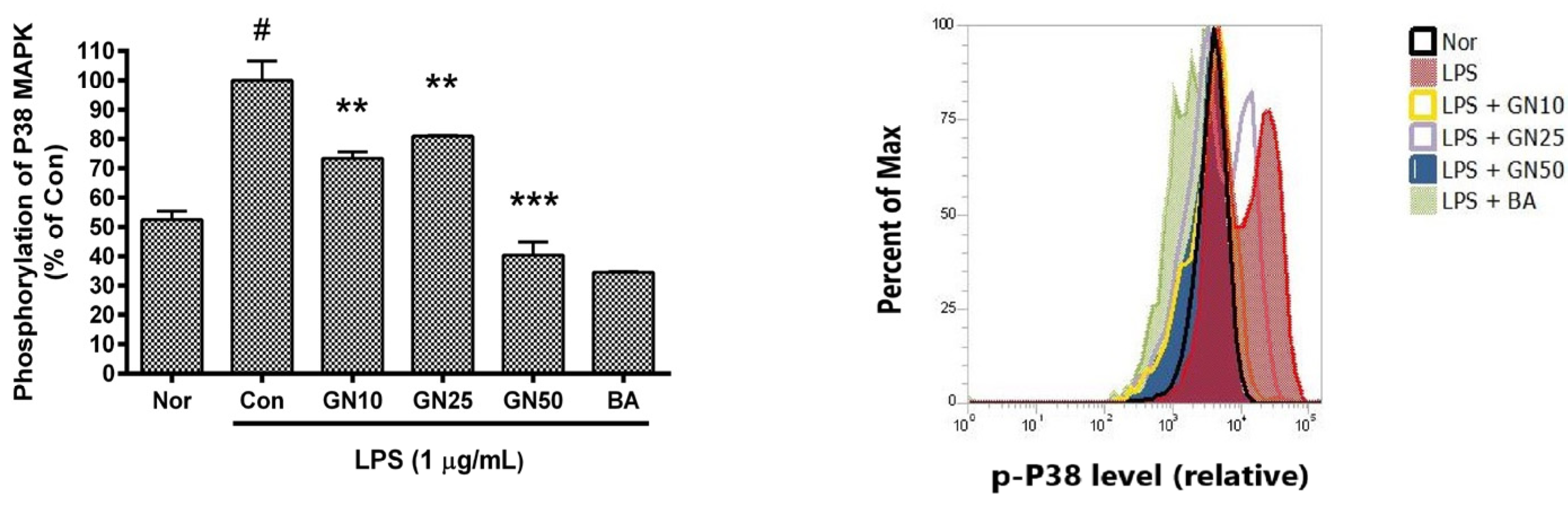
2.5. Effect of Geniposide on P38 MAPK Phosphorylation
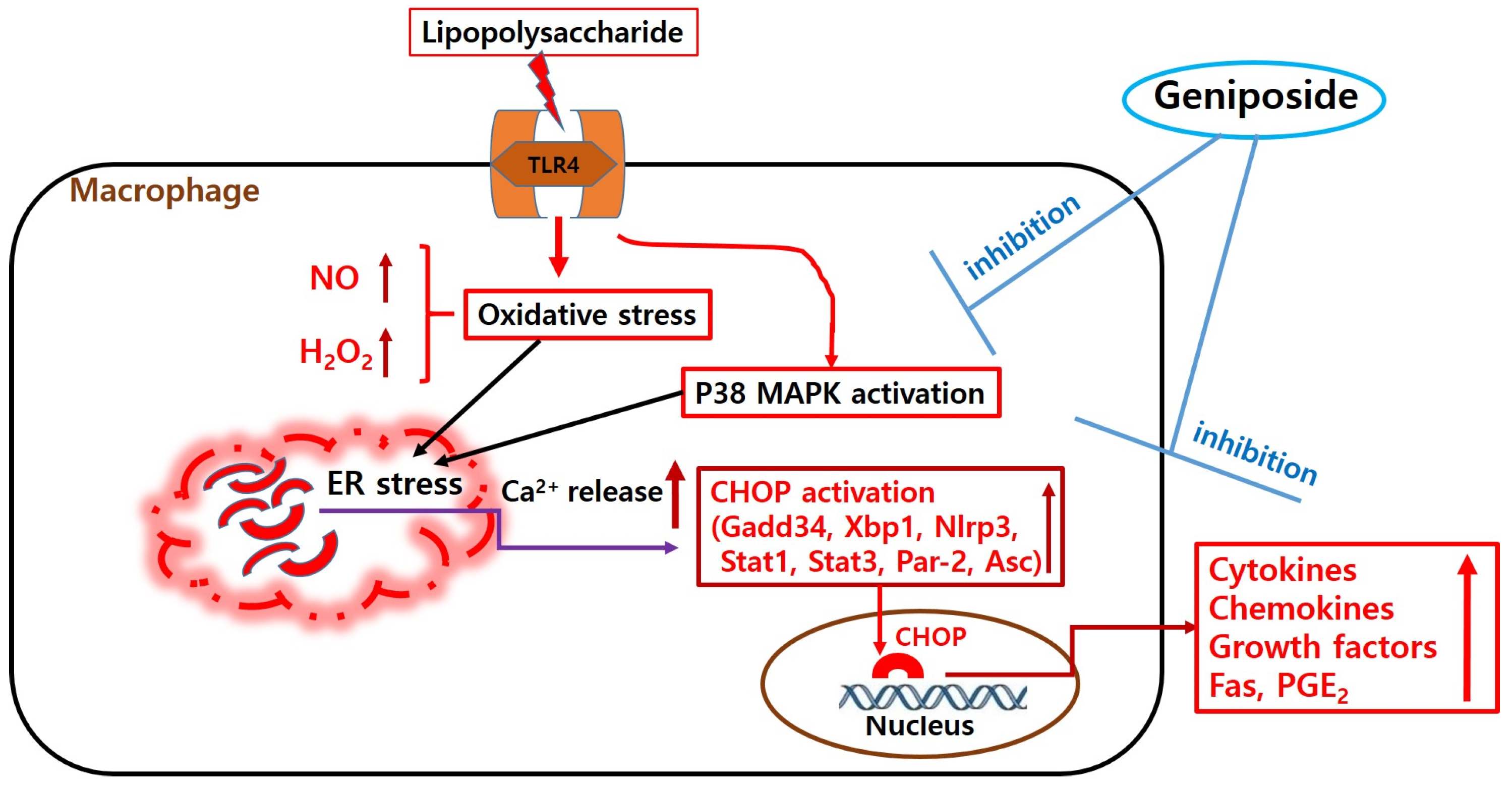
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Cell Culture and Cell Viability
4.2.2. Levels of H2O2, NO, and Ca2+ in Cells
4.2.3. Levels of Inflammatory Cytokines
4.2.4. Quantitative Real-Time RT-PCR for Inflammatory Genes
4.2.5. Phosphorylation of P38 MAPK
4.2.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.C.; Zou, X.B.; Chai, Y.F.; Yao, Y.M. Macrophage Polarization in Inflammatory Diseases. Int. J. Biol. Sci. 2014, 10, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Lariccia, V.; Magi, S.; Serfilippi, T.; Toujani, M.; Gratteri, S.; Amoroso, S. Challenges and Opportunities from Targeting Inflammatory Responses to SARS-CoV-2 Infection: A Narrative Review. J. Clin. Med. 2020, 9, 4021. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, M.; Chen, X.; Montaner, L.J. Cytokine Storm and Leukocyte Changes in Mild Versus Severe SARS-CoV-2 Infection: Review of 3939 COVID-19 Patients in China and Emerging Pathogenesis and Therapy Concepts. J. Leukoc. Biol. 2020, 108, 17–41. [Google Scholar] [CrossRef]
- Roy, R.K.; Sharma, U.; Wasson, M.K.; Jain, A.; Hassan, M.I.; Prakash, H. Macrophage Activation Syndrome and COVID 19: Impact of MAPK Driven Immune-Epigenetic Programming by SARS-CoV-2. Front. Immunol. 2021, 12, 763313. [Google Scholar] [CrossRef]
- Bacchiega, B.C.; Bacchiega, A.B.; Usnayo, M.J.; Bedirian, R.; Singh, G.; Pinheiro, G.D. Interleukin 6 Inhibition and Coronary Artery Disease in a High-Risk Population: A Prospective Community-Based Clinical Study. J. Am. Heart Assoc. 2017, 6, e005038. [Google Scholar] [CrossRef]
- Zhou, Y.; Little, P.J.; Xu, S.; Kamato, D. Curcumin Inhibits Lysophosphatidic Acid Mediated MCP-1 Expression via Blocking ROCK Signalling. Molecules 2021, 26, 2320. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Neurath, M.F. Resolution of Chronic Inflammatory Disease: Universal and Tissue-Specific Concepts. Nat. Commun. 2018, 9, 3261. [Google Scholar] [CrossRef]
- Netea, M.G.; Balkwill, F.; Chonchol, M.; Cominelli, F.; Donath, M.Y.; Giamarellos-Bourboulis, E.J.; Golenbock, D.; Gresnigt, M.S.; Heneka, M.T.; Hoffman, H.M.; et al. A Guiding Map for Inflammation. Nat. Immunol. 2017, 18, 826–831. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, Y.; Zhou, R.; Li, Y.; Gao, Y.; Tu, D.; Wilson, B.; Song, S.; Feng, J.; Hong, J.S.; et al. A Novel Role of NLRP3-Generated IL-1beta in the Acute-Chronic Transition of Peripheral Lipopolysaccharide-Elicited Neuroinflammation: Implications for Sepsis-Associated Neurodegeneration. J. Neuroinflamm. 2020, 17, 64. [Google Scholar] [CrossRef]
- Scott, A.J.; O’Dea, K.P.; O’Callaghan, D.; Williams, L.; Dokpesi, J.O.; Tatton, L.; Handy, J.M.; Hogg, P.J.; Takata, M. Reactive Oxygen Species and p38 Mitogen-Activated Protein Kinase Mediate Tumor Necrosis Factor Alpha-Converting Enzyme (TACE/ADAM-17) Activation in Primary Human Monocytes. J. Biol. Chem. 2011, 286, 35466–35476. [Google Scholar] [CrossRef]
- Emadali, A.; Nguyen, D.T.; Rochon, C.; Tzimas, G.N.; Metrakos, P.P.; Chevet, E. Distinct Endoplasmic Reticulum Stress Responses are Triggered during Human Liver Transplantation. J. Pathol. 2005, 207, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Oh-Hashi, K.; Maruyama, W.; Isobe, K. Peroxynitrite Induces GADD34, 45, and 153 VIA p38 MAPK in Human Neuroblastoma SH-SY5Y Cells. Free Radic. Biol. Med. 2001, 30, 213–221. [Google Scholar] [CrossRef]
- Zhou, C.M.; Luo, L.M.; Lin, P.; Pu, Q.; Wang, B.; Qin, S.; Wu, Q.; Yu, X.J.; Wu, M. Annexin A2 Regulates Unfolded Protein Response Via IRE1-XBP1 Axis in Macrophages during P. Aeruginosa Infection. J. Leukoc. Biol. 2021, 110, 375–384. [Google Scholar] [CrossRef]
- Boileau, C.; Amiable, N.; Martel-Pelletier, J.; Fahmi, H.; Duval, N.; Pelletier, J.P. Activation of Proteinase-Activated Receptor 2 in Human Osteoarthritic Cartilage Upregulates Catabolic and Proinflammatory Pathways Capable of Inducing Cartilage Degradation: A Basic Science Study. Arthritis Res. Ther. 2007, 9, R121. [Google Scholar] [CrossRef]
- Koo, H.J.; Lim, K.H.; Jung, H.J.; Park, E.H. Anti-Inflammatory Evaluation of Gardenia Extract, Geniposide and Genipin. J. Ethnopharmacol. 2006, 103, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Cao, J.; Fang, L.; Zhao, H.; Liu, Z.; Ran, J.; Zheng, X.; Li, X.; Zhou, Y.; Ge, D.; et al. Geniposide Suppresses LPS-Induced Nitric Oxide, PGE2 and Inflammatory Cytokine by Downregulating NF-kappaB, MAPK and AP-1 Signaling Pathways in Macrophages. Int. Immunopharmacol. 2014, 20, 298–306. [Google Scholar] [CrossRef]
- Xiaofeng, Y.; Qinren, C.; Jingping, H.; Xiao, C.; Miaomiao, W.; Xiangru, F.; Xianxing, X.; Meixia, H.; Jing, L.; Jingyuan, W.; et al. Geniposide, an Iridoid Glucoside Derived from Gardenia Jasminoides, Protects Against Lipopolysaccharide-Induced Acute Lung Injury in Mice. Planta Med. 2012, 78, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Zhou, F.; Xu, Y.; Liu, X.; Zhang, Y.; Gu, M.; Su, Z.; Zhao, D.; Zhang, L.; Jia, Y. Geniposide Regulates the miR-101/MKP-1/p38 Pathway and Alleviates Atherosclerosis Inflammatory Injury in ApoE(−/−) Mice. Immunobiology 2019, 224, 296–306. [Google Scholar] [CrossRef]
- Jin, Z.; Li, J.; Pi, J.; Chu, Q.; Wei, W.; Du, Z.; Qing, L.; Zhao, X.; Wu, W. Geniposide Alleviates Atherosclerosis by Regulating Macrophage Polarization Via the FOS/MAPK Signaling Pathway. Biomed. Pharmacother. 2020, 125, 110015. [Google Scholar] [CrossRef]
- Kim, D.-H.; Lee, J.-Y.; Kim, Y.-J.; Kim, H.-J.; Park, W. Rubi Fructus Water Extract Alleviates LPS-Stimulated Macrophage Activation via an ERS-Induced Calcium/CHOP Signaling Pathway. Nutrients 2020, 12, 3577. [Google Scholar] [CrossRef]
- Hsieh, S.C.; Fang, S.H.; Rao, Y.K.; Tzeng, Y.M. Inhibition of Pro-Inflammatory Mediators and Tumor Cell Proliferation by Anisomeles Indica Extracts. J. Ethnopharmacol. 2008, 118, 65–70. [Google Scholar] [CrossRef]
- Tseng, T.H.; Chu, C.Y.; Huang, J.M.; Shiow, S.J.; Wang, C.J. Crocetin Protects Against Oxidative Damage in Rat Primary Hepatocytes. Cancer Lett. 1995, 97, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Liaw, J.; Chao, Y.C. Effect of in Vitro and in Vivo Aerosolized Treatment with Geniposide on Tracheal Permeability in Ovalbumin-Induced Guinea Pigs. Eur. J. Pharmacol. 2001, 433, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Hsu, J.D.; Wang, C.J. Inhibition of 12-O-Tetradecanoylphorbol-13-Acetate-Caused Tumor Promotion in Benzoa.Pyrene-Initiated CD-1 Mouse Skin by Geniposide. Anticancer Res. 1995, 15, 411–416. [Google Scholar] [PubMed]
- Park, E.H.; Joo, M.H.; Kim, S.H.; Lim, C.J. Antiangiogenic Activity of Gardenia Jasminoides Fruit. Phytother. Res. 2003, 17, 961–962. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hou, J.; Zhang, P.; Li, D.; Zhang, C.; Liu, J. Geniposide Reduces Inflammatory Responses of Oxygen-Glucose Deprived Rat Microglial Cells Via Inhibition of the TLR4 Signaling Pathway. Neurochem. Res. 2012, 37, 2235–2248. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; He, J.L.; Li, W.M.; Yang, Z.; Wang, Y.X.; Yin, J.; Du, Y.G.; Yu, C. Geniposide Inhibits Interleukin-6 and Interleukin-8 Production in Lipopolysaccharide-Induced Human Umbilical Vein Endothelial Cells by Blocking p38 and ERK1/2 Signaling Pathways. Inflamm. Res. 2010, 59, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.A. Management of Sepsis. N. Engl. J. Med. 2006, 355, 1699–1713. [Google Scholar] [CrossRef]
- Spite, M.; Norling, L.V.; Summers, L.; Yang, R.; Cooper, D.; Petasis, N.A.; Flower, R.J.; Perretti, M.; Serhan, C.N. Resolvin D2 is a Potent Regulator of Leukocytes and Controls Microbial Sepsis. Nature 2009, 461, 1287–1291. [Google Scholar] [CrossRef]
- Hu, Z.; Murakami, T.; Suzuki, K.; Tamura, H.; Kuwahara-Arai, K.; Iba, T.; Nagaoka, I. Antimicrobial Cathelicidin Peptide LL-37 Inhibits the LPS/ATP-Induced Pyroptosis of Macrophages by Dual Mechanism. PLoS ONE 2014, 9, e85765. [Google Scholar] [CrossRef]
- Sun, J.; Bhatia, M. Blockade of Neurokinin-1 Receptor Attenuates CC and CXC Chemokine Production in Experimental Acute Pancreatitis and Associated Lung Injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G143–G153. [Google Scholar] [CrossRef] [PubMed]
- Dayer, J.M.; Choy, E. Therapeutic Targets in Rheumatoid Arthritis: The Interleukin-6 Receptor. Rheumatology 2010, 49, 15–24. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, C.B.; Cardell, L.O. Long-Term Exposure to IL-1beta Enhances Toll-IL-1 Receptor-Mediated Inflammatory Signaling in Murine Airway Hyperresponsiveness. Eur. Cytokine Netw. 2009, 20, 148–156. [Google Scholar]
- Sheller, J.R.; Polosukhin, V.V.; Mitchell, D.; Cheng, D.S.; Peebles, R.S.; Blackwell, T.S. Nuclear Factor Kappa B Induction in Airway Epithelium Increases Lung Inflammation in Allergen-Challenged Mice. Exp. Lung Res. 2009, 35, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Brahmachari, S.; Pahan, K. Role of Cytokine p40 Family in Multiple Sclerosis. Minerva Med. 2008, 99, 105–118. [Google Scholar] [PubMed]
- Srivastava, M.; Jung, S.; Wilhelm, J.; Fink, L.; Buhling, F.; Welte, T.; Bohle, R.M.; Seeger, W.; Lohmeyer, J.; Maus, U.A. The Inflammatory versus Constitutive Trafficking of Mononuclear Phagocytes into the Alveolar Space of Mice is Associated with Drastic Changes in their Gene Expression Profiles. J. Immunol. 2005, 175, 1884–1893. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, R.; Feng, L.; Guo, W.; Cao, N.; Qian, C.; Teng, P.; Wang, L.; Wu, X.; Sun, Y.; et al. A Novel Chromone Derivative with Anti-Inflammatory Property Via Inhibition of ROS-Dependent Activation of TRAF6-ASK1-p38 Pathway. PLoS ONE 2012, 7, e37168. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Z.; Ron, D. Stress-Induced Phosphorylation and Activation of the Transcription Factor CHOP (GADD153) by p38 MAP Kinase. Science 1996, 272, 1347–1349. [Google Scholar] [CrossRef]
- Endo, M.; Mori, M.; Akira, S.; Gotoh, T. C/EBP Homologous Protein (CHOP) is Crucial for the Induction of Caspase-11 and the Pathogenesis of Lipopolysaccharide-Induced Inflammation. J. Immunol. 2006, 176, 6245–6253. [Google Scholar] [CrossRef]
- Stout, B.A.; Melendez, K.; Seagrave, J.; Holtzman, M.J.; Wilson, B.; Xiang, J.; Tesfaigzi, Y. STAT1 Activation Causes Translocation of Bax to the Endoplasmic Reticulum during the Resolution of Airway Mucous Cell Hyperplasia by IFN-Gamma. J. Immunol. 2007, 178, 8107–8116. [Google Scholar] [CrossRef]
- Tabas, I.; Seimon, T.; Timmins, J.; Li, G.; Lim, W. Macrophage Apoptosis in Advanced Atherosclerosis. Ann. N. Y. Acad. Sci. 2009, 1173 (Suppl. S1), E40–E45. [Google Scholar] [CrossRef] [PubMed]
- Timmins, J.M.; Ozcan, L.; Seimon, T.A.; Li, G.; Malagelada, C.; Backs, J.; Backs, T.; Bassel-Duby, R.; Olson, E.N.; Anderson, M.E.; et al. Calcium/calmodulin-Dependent Protein Kinase II Links ERS with Fas and Mitochondrial Apoptosis Pathways. J. Clin. Investig. 2009, 119, 2925–2941. [Google Scholar] [CrossRef] [PubMed]
- Bryan, N.B.; Dorfleutner, A.; Rojanasakul, Y.; Stehlik, C. Activation of Inflammasomes Requires Intracellular Redistribution of the Apoptotic Speck-Like Protein Containing a Caspase Recruitment Domain. J. Immunol. 2009, 182, 3173–3182. [Google Scholar] [CrossRef] [PubMed]
- Menu, P.; Mayor, A.; Zhou, R.; Tardivel, A.; Ichijo, H.; Mori, K.; Tschopp, J. ER Stress Activates the NLRP3 Inflammasome Via an UPR-Independent Pathway. Cell. Death Dis. 2012, 3, e261. [Google Scholar] [CrossRef]
- Lim, Y.J.; Yi, M.H.; Choi, J.A.; Lee, J.; Han, J.Y.; Jo, S.H.; Oh, S.M.; Cho, H.J.; Kim, D.W.; Kang, M.W.; et al. Roles of Endoplasmic Reticulum Stress-Mediated Apoptosis in M1-Polarized Macrophages during Mycobacterial Infections. Sci. Rep. 2016, 6, 37211. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-J.; Park, W. Alleviative Effect of Geniposide on Lipopolysaccharide-Stimulated Macrophages via Calcium Pathway. Int. J. Mol. Sci. 2024, 25, 1728. https://doi.org/10.3390/ijms25031728
Kim H-J, Park W. Alleviative Effect of Geniposide on Lipopolysaccharide-Stimulated Macrophages via Calcium Pathway. International Journal of Molecular Sciences. 2024; 25(3):1728. https://doi.org/10.3390/ijms25031728
Chicago/Turabian StyleKim, Hyun-Ju, and Wansu Park. 2024. "Alleviative Effect of Geniposide on Lipopolysaccharide-Stimulated Macrophages via Calcium Pathway" International Journal of Molecular Sciences 25, no. 3: 1728. https://doi.org/10.3390/ijms25031728
APA StyleKim, H.-J., & Park, W. (2024). Alleviative Effect of Geniposide on Lipopolysaccharide-Stimulated Macrophages via Calcium Pathway. International Journal of Molecular Sciences, 25(3), 1728. https://doi.org/10.3390/ijms25031728






