An Innovative Approach to a Potential Neuroprotective Sideritis scardica-Clinoptilolite Phyto-Nanocarrier: In Vitro Investigation and Evaluation
Abstract
1. Introduction
2. Results and Discussion
2.1. Screening of Amino Acid from Sideritis Scardica Sample
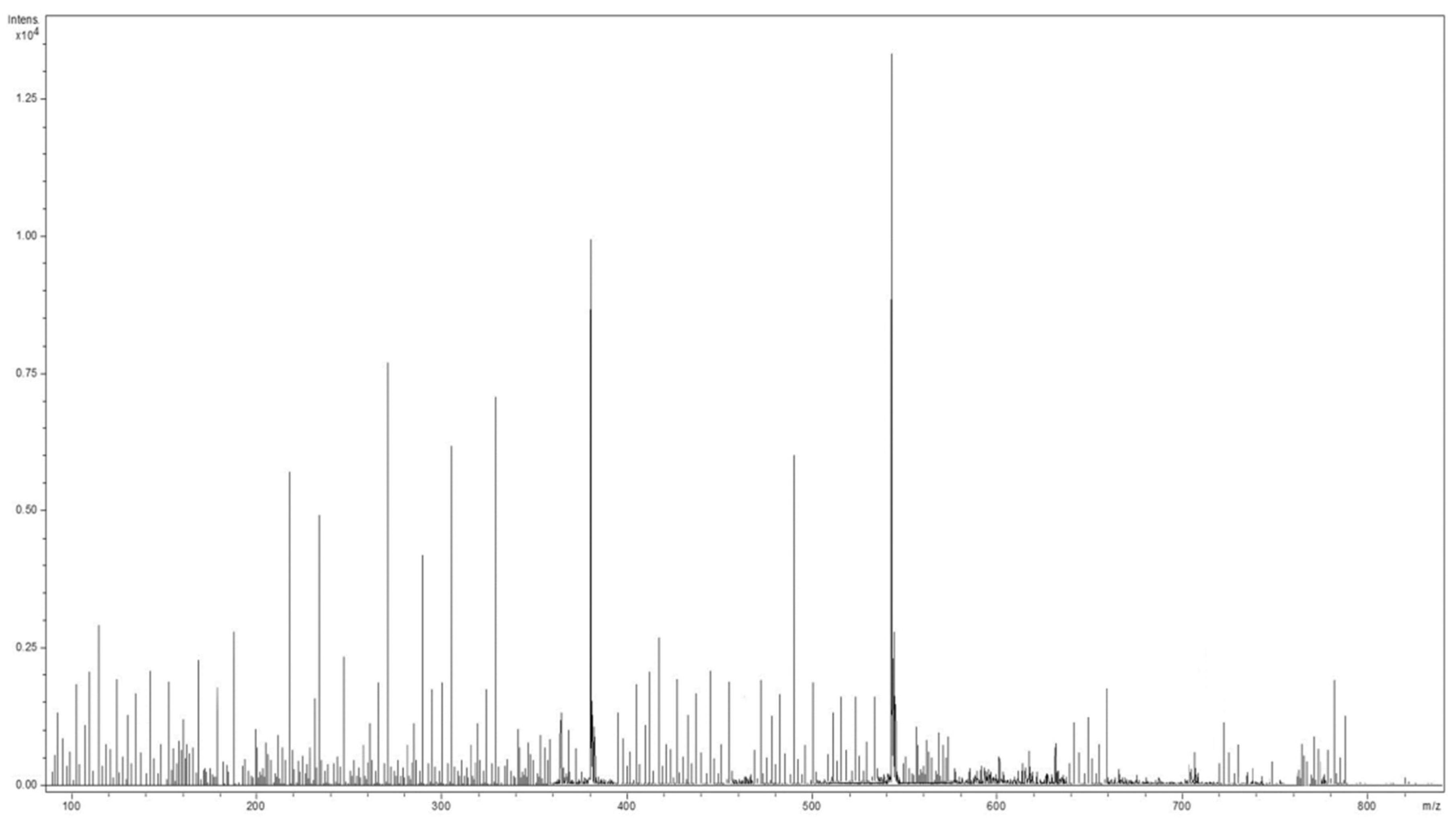
2.2. Mass Spectrometry Analysis of Sideritis Scardica Sample
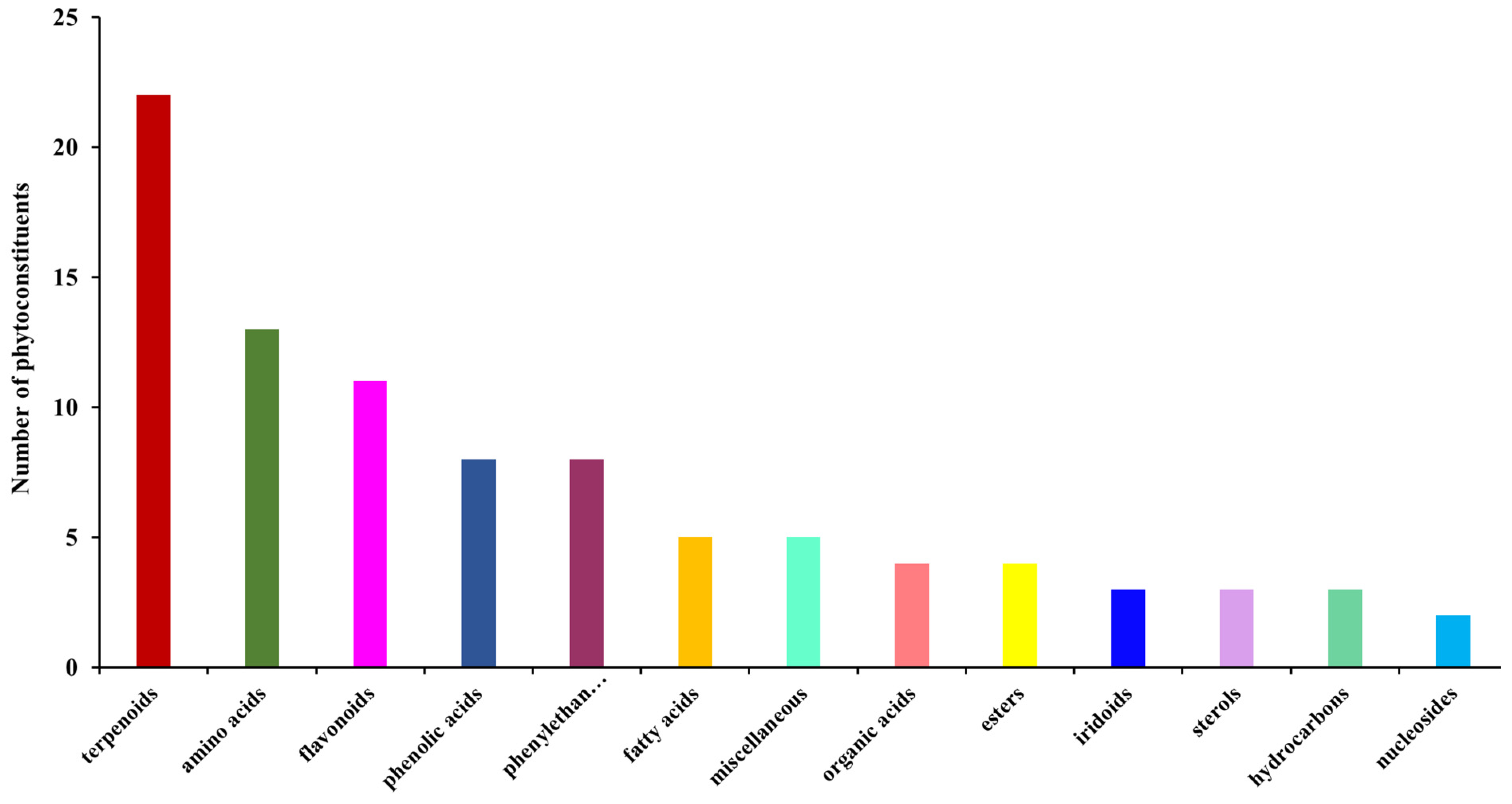
2.3. Screening and Classification of the Differential Metabolites
2.4. Phyto-Nanocarrier System
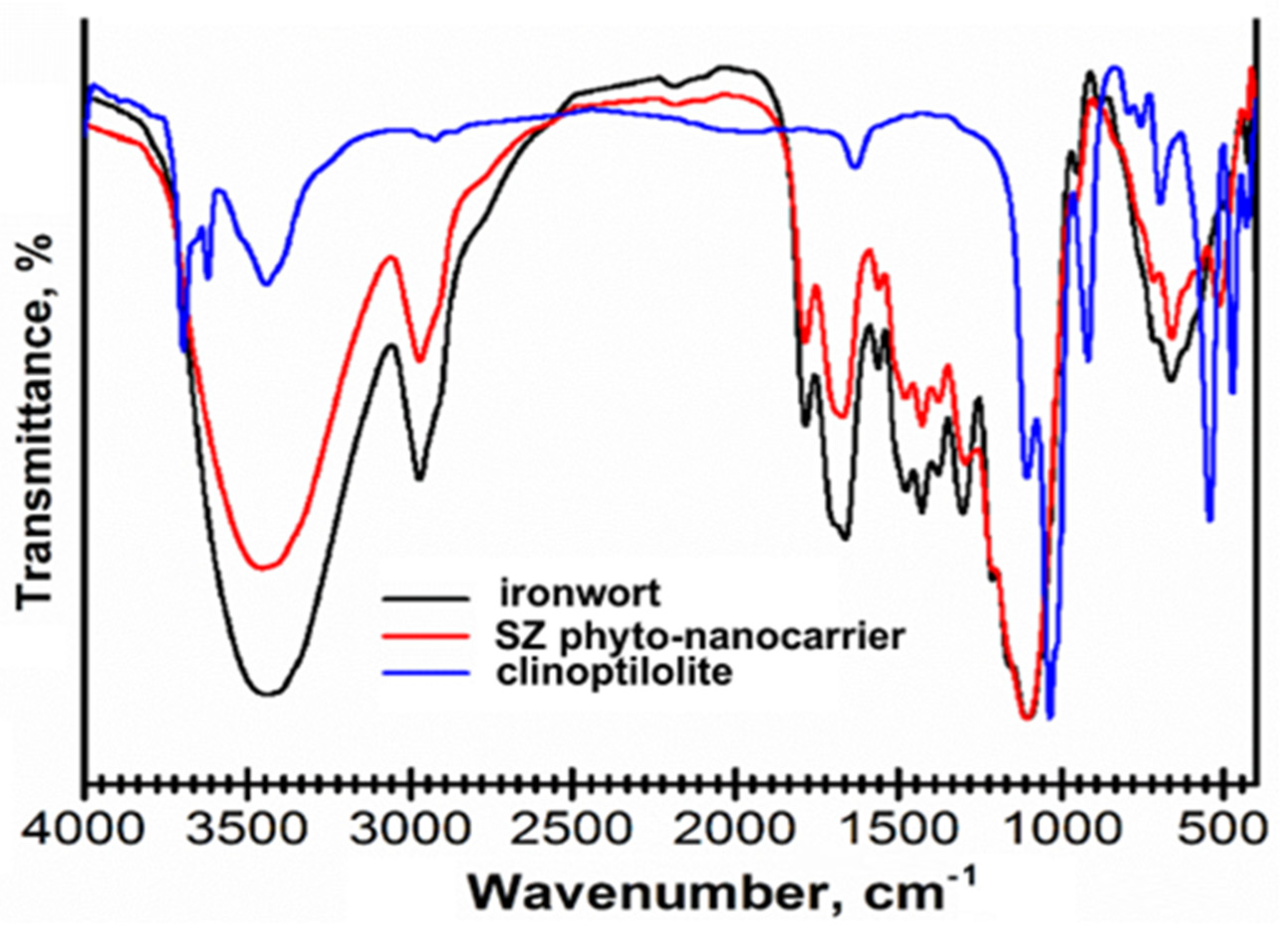
2.4.1. FT-IR Spectroscopy
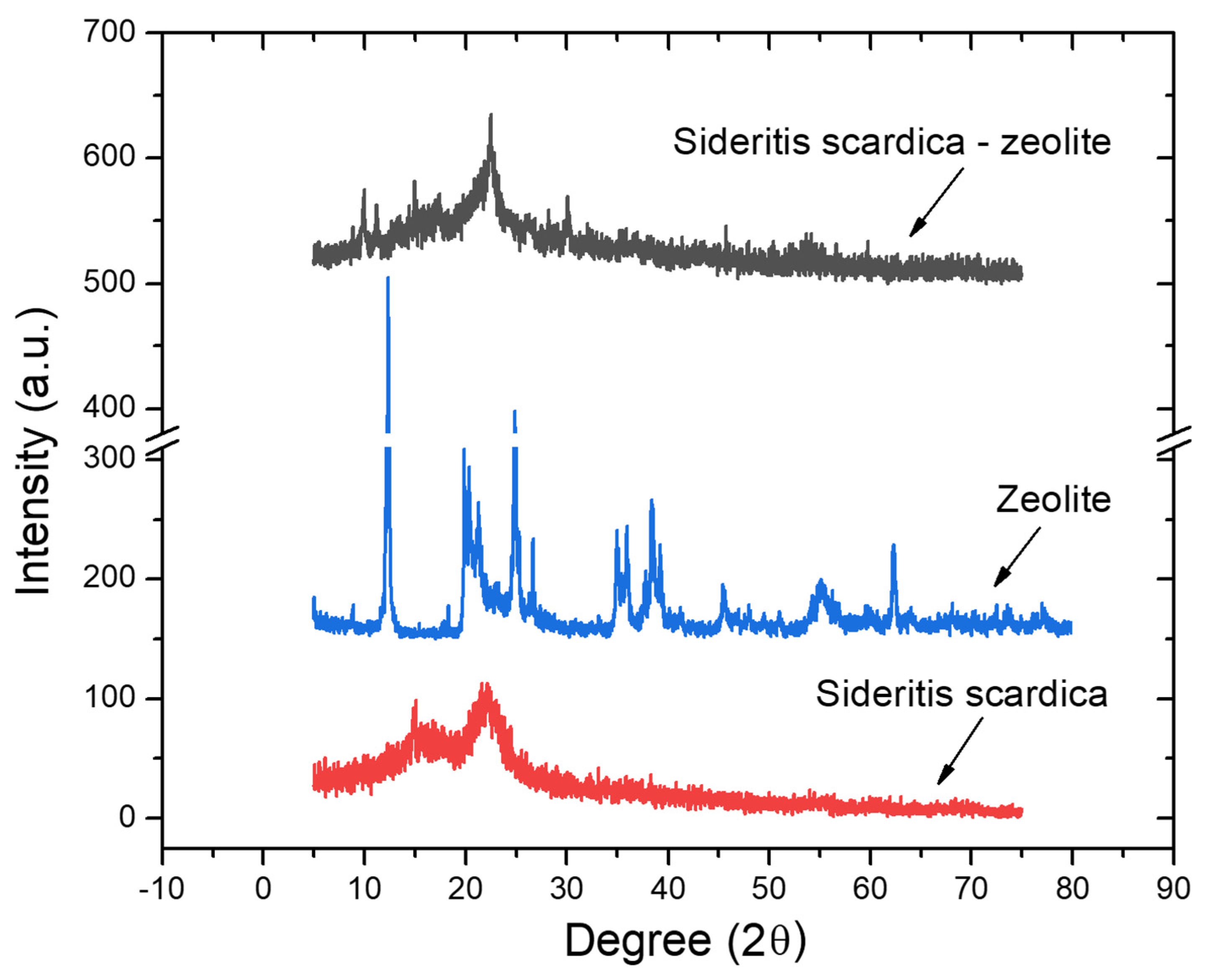
2.4.2. X-ray-Diffraction Spectroscopy
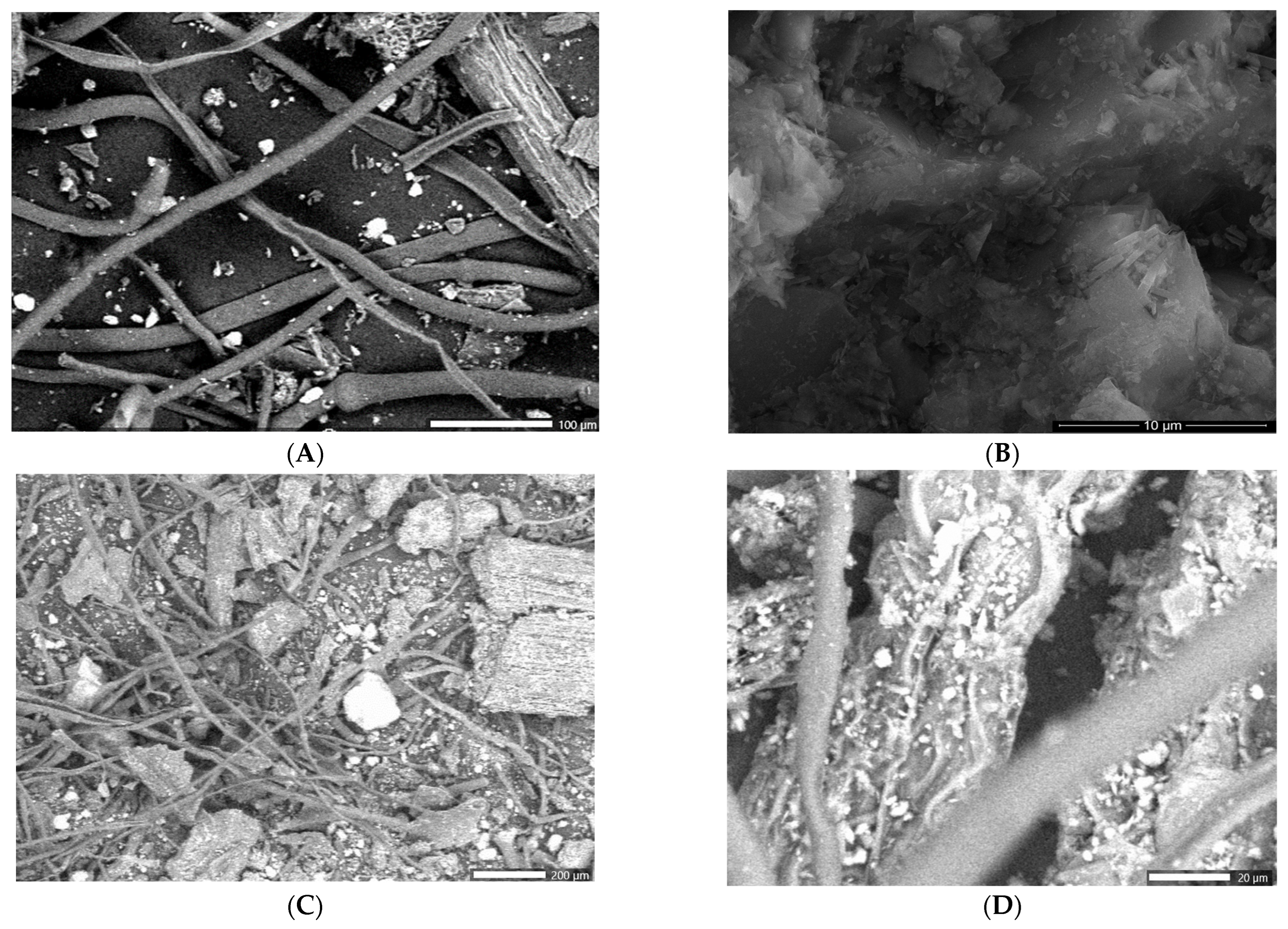
2.4.3. Scanning Electron Microscopy (SEM)
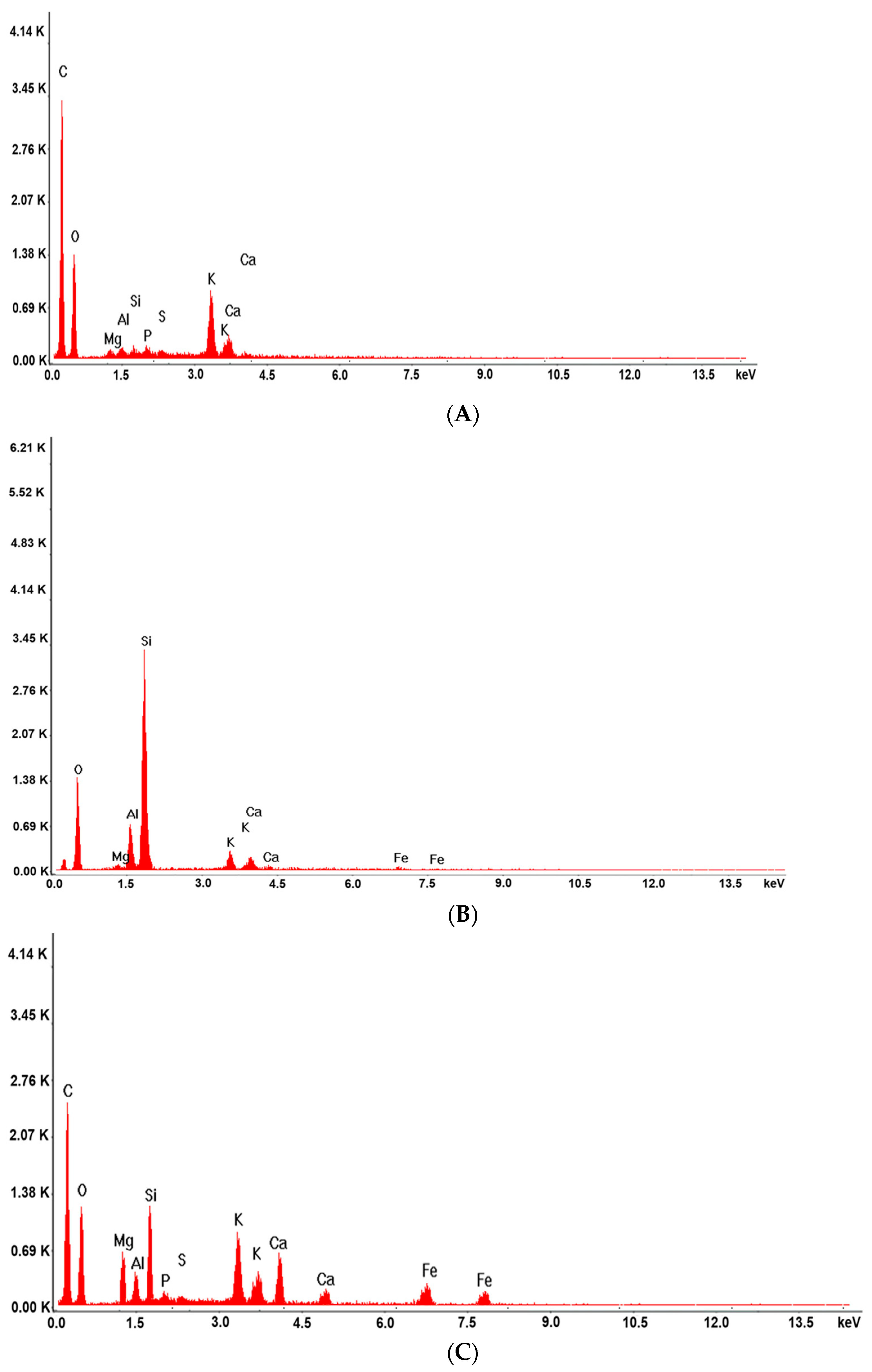
2.4.4. Energy Dispersive X-ray (EDX)
2.5. Dynamic Light Scattering (DLS)
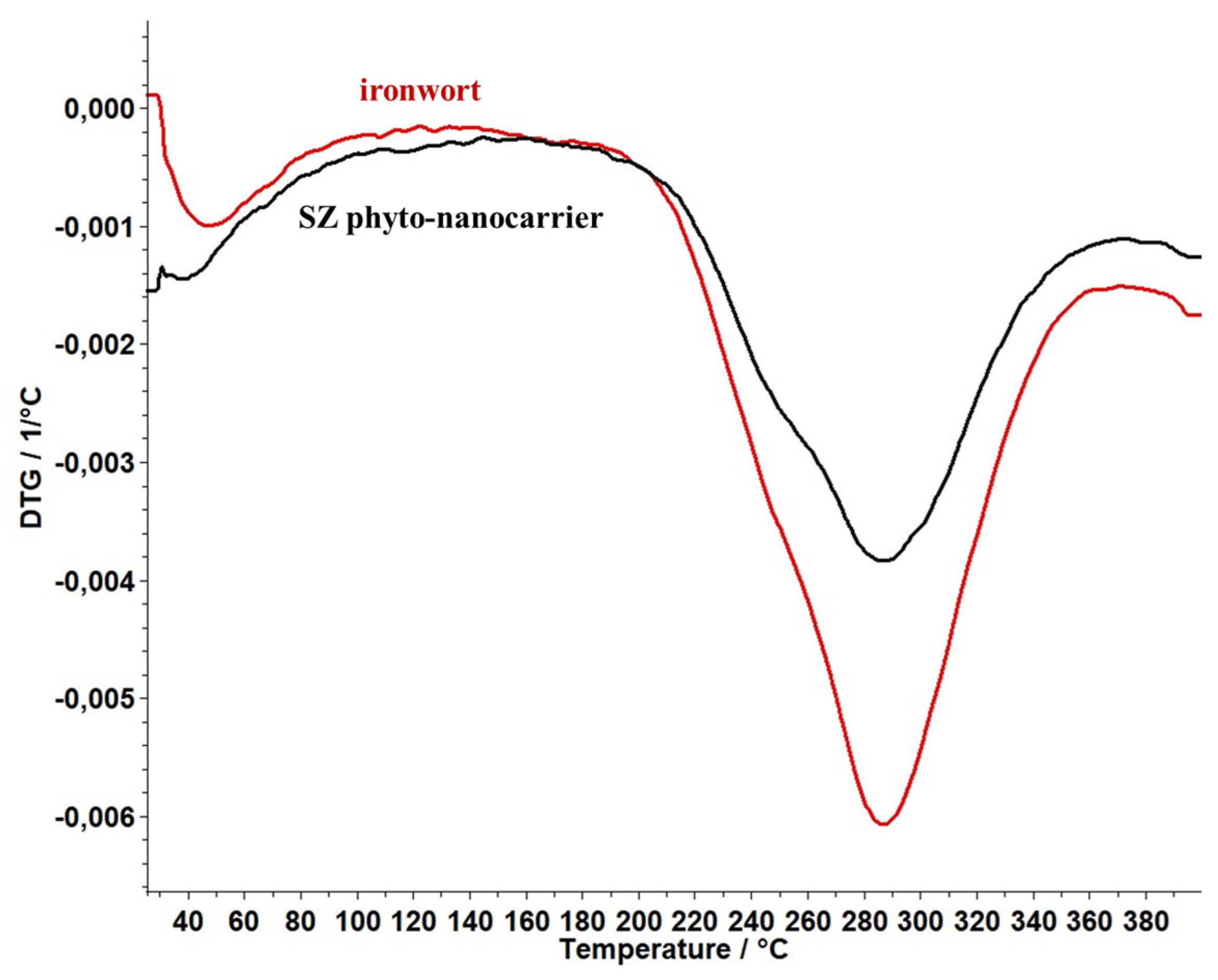
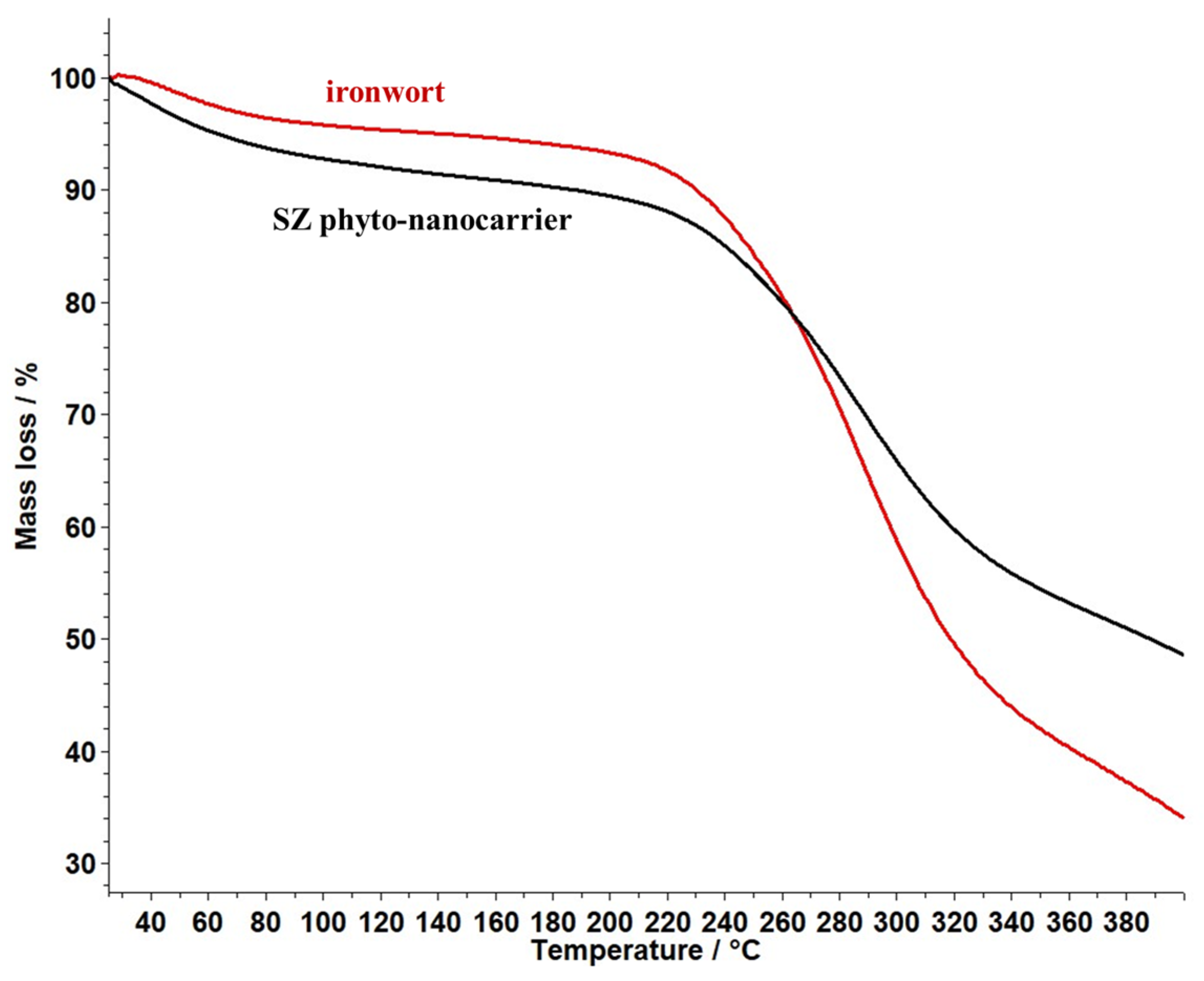
2.6. Thermal Behaviour Study
2.7. Screening of Antioxidant Activity and Total Polyphenolic Contents
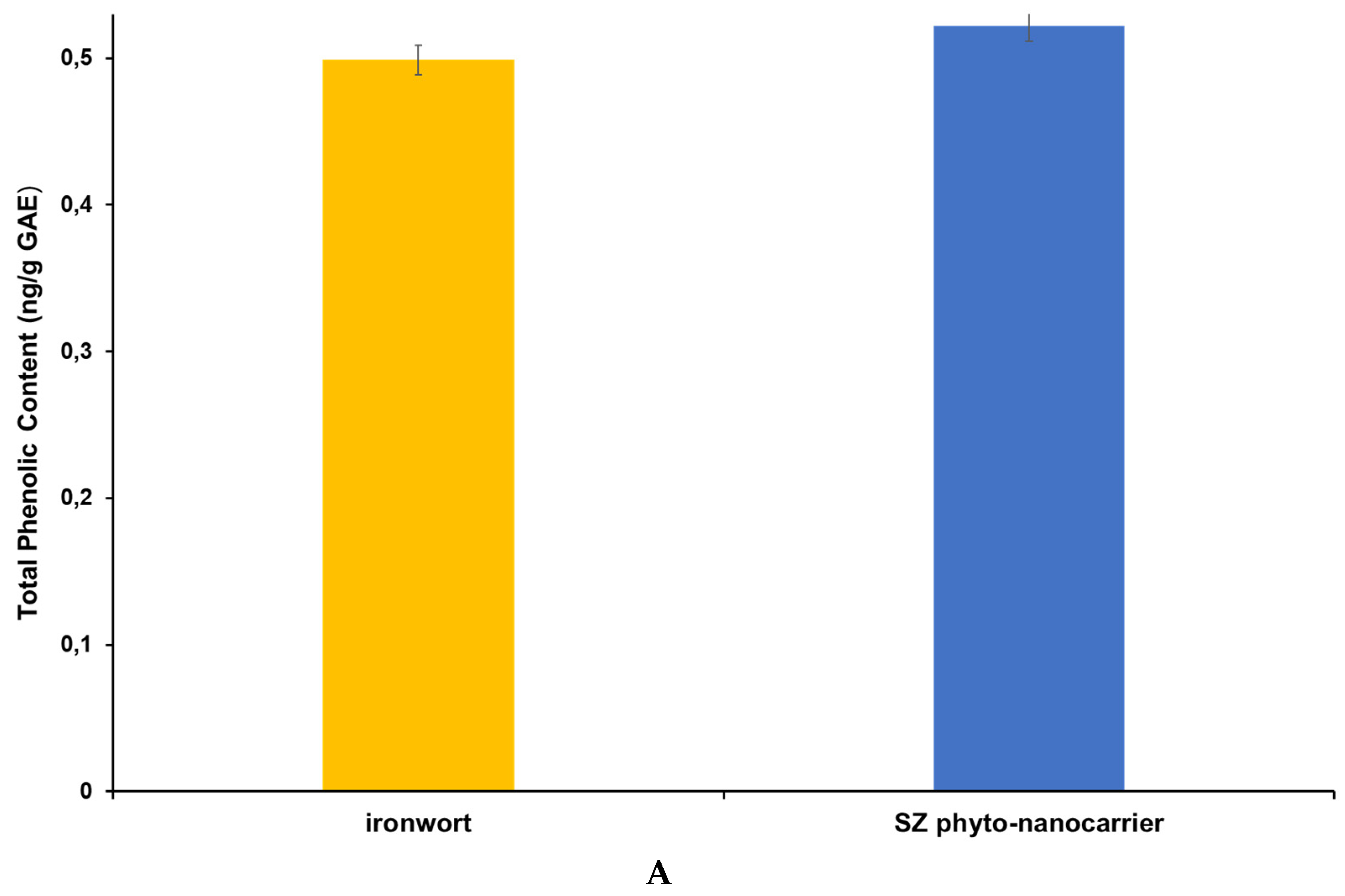
2.7.1. Total Polyphenolic Contents (TPCs) Assay—Folin Ciocalteu
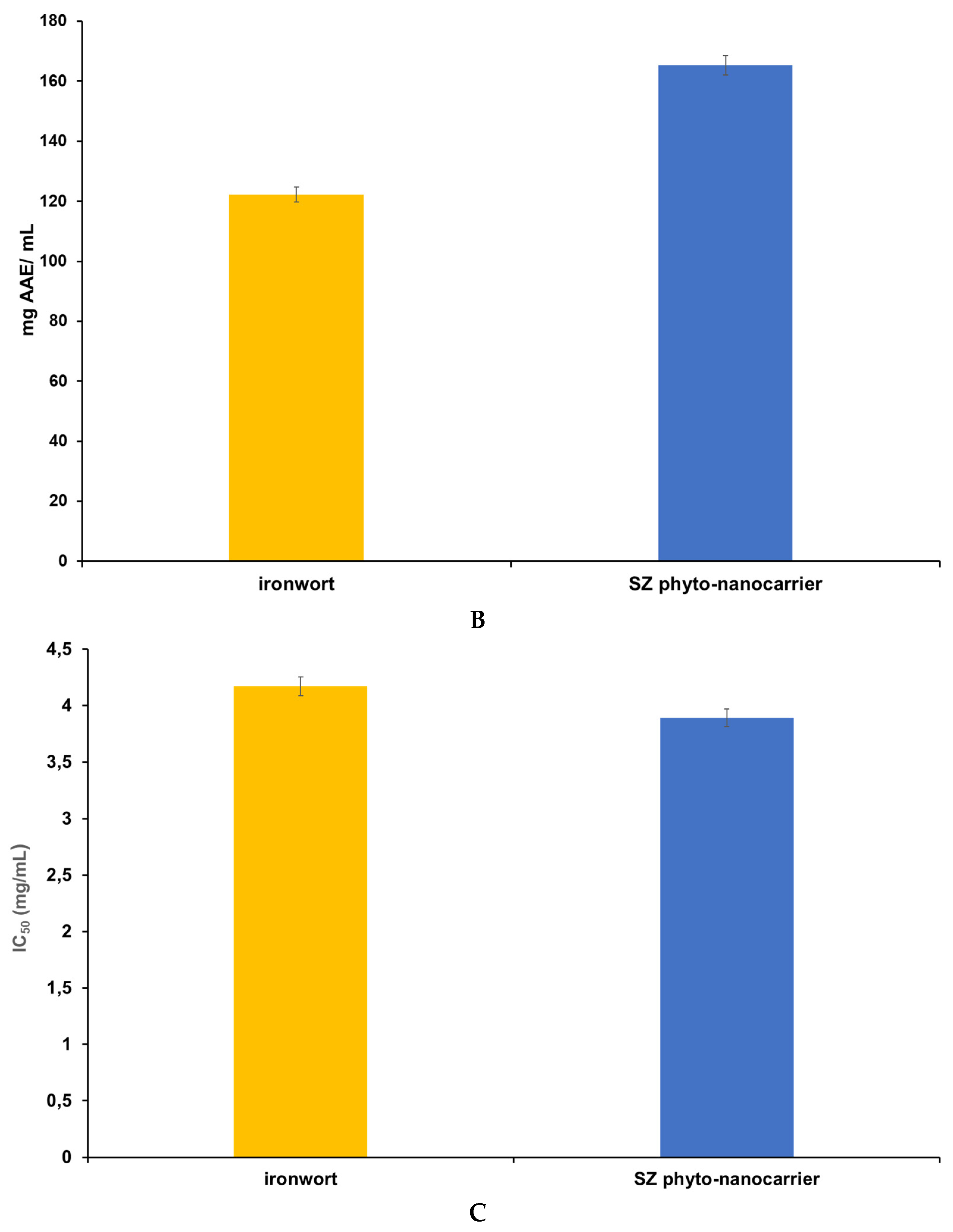
2.7.2. Phosphomolybdate Assay (Total Antioxidant Capacity)
2.7.3. DPPH (1,1-diphenyl-2-picrylhydrazyl) Free-Radical-Scavenging Assay
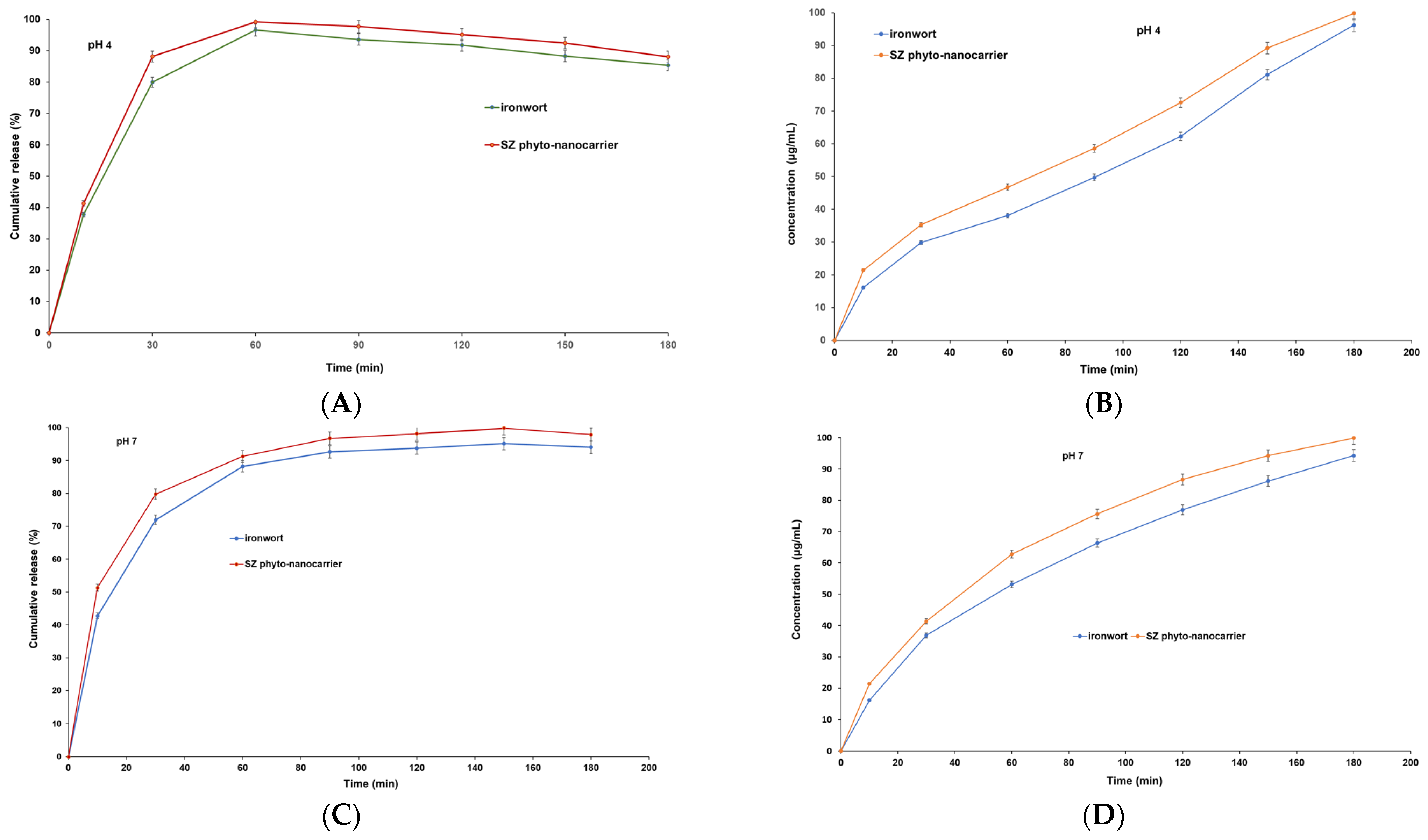
2.8. In Vitro Dissolution Assay
3. Materials and Methods
3.1. Reagents and materials
3.2. Plant Samples’ Preparation for Chemical Screening
3.2.1. GC-MS Analysis (EZ: Faast GC-MS Free Amino Acids Kit)
GC-MS Separation Conditions
3.2.2. Mass Spectrometry
3.3. SZ Phyto-Nanocarrier
3.4. Characterization of Phyto-Nanocarrier and Its Components
3.4.1. Fourier Transform Infrared (FT-IR) Spectroscopy
3.4.2. XRD Spectroscopy
3.4.3. Scanning Electron Microscopy (SEM)
3.4.4. Dynamic Light Scattering (DLS) Particle Size Distribution Analysis
3.5. Thermal Analysis
3.6. Antioxidant Activity and Total Phenolic Content
3.6.1. Total Polyphenol Content Using the Folin–Ciocalteu Method
3.6.2. DPPH Radical Scavenging Assay
3.6.3. Phosphomolybdate Assay (Total Antioxidant Capacity)
3.7. In Vitro Dissolution Test
Preparation of the Curves of the Concentrations for the Compound Dissolution Profile
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ververis, A.; Ioannou, K.; Kyriakou, S.; Violaki, N.; Panayiotidis, M.I.; Plioukas, M.; Christodoulou, K. Sideritis scardica extracts demonstrate neuroprotective activity against Aβ25–35 toxicity. Plants 2023, 12, 1716. [Google Scholar] [CrossRef]
- Aneva, I.; Zhelev, P.; Kozuharova, E.; Danova, K.; Nabavi, S.F.; Behzad, S. Genus Sideritis, section Empedoclia in SouthEastern Europe and Turkey—Studies in ethnopharmacology and recent progress of biological activities. DARU J. Pharm. Sci. 2019, 27, 407–421. [Google Scholar] [CrossRef]
- Moussavi, N.; Azizullah, H.; Malterud, K.E.; Inngjerdingen, K.T.; Wangensteen, H. Immunomodulating polyphenols from Sideritis Scardica. J. Funct. Foods 2022, 96, 105197. [Google Scholar] [CrossRef]
- González-Burgos, E.; Carretero, M.E.; Gómez-Serranillos, M.P. Sideritis spp.: Uses, chemical composition and pharmacological activities—A review. J. Ethnopharmacol. 2011, 135, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Żyżelewicz, D.; Kulbat-Warycha, K.; Oracz, J.; Żyżelewicz, K. Polyphenols and other bioactive compounds of Sideritis plants and their potential biological activity. Molecules 2020, 25, 3763. [Google Scholar] [CrossRef]
- Rahman, H.S.; Othman, H.H.; Hammadi, N.I.; Yeap, S.K.; Amin, K.M.; Abdul Samad, N.; Alitheen, N.B. Novel drug delivery systems for loading of natural plant extracts and their biomedical applications. Int. J. Nanomed. 2020, 15, 2439–2483. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Singh, I.P.; Ahmad, F.; Chatterjee, D.; Bajpai, R.; Sengar, N. Natural products: Drug discovery and development. In Drug Discovery and Development from Targets and Molecules to Medicines; Poduri, R., Ed.; Springer: Singapore, 2021; ISBN 978-981-15-5533-6. [Google Scholar]
- Nath, R.; Roy, R.; Barai, G.; Bairagi, S.; Manna, S.; Chakraborty, R. Modern developments of nano based drug delivery system by combined with phytochemicals-presenting new aspects. Int. J. Sci. Res. Sci. Technol. 2021, 8, 107–129. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Kraljević Pavelić, S.; Simović Medica, J.; Gumbarević, D.; Filošević, A.; Pržulj, N.; Pavelić, K. Critical review on zeolite clinoptilolite safety and medical applications in vivo. Front. Pharmacol. 2018, 9, 1350. [Google Scholar] [CrossRef]
- Serati-Nouri, H.; Jafari, A.; Roshangar, L.; Dadashpour, M.; Pilehvar-Soltanahmadi, Y.; Zarghami, N. Biomedical applications of zeolite-based materials: A review. Mater. Sci. Eng. C 2020, 116, 111225. [Google Scholar] [CrossRef]
- Mastinu, A.; Kumar, A.; Maccarinelli, G.; Bonini, S.A.; Premoli, M.; Aria, F.; Gianoncelli, A.; Memo, M. Zeolite clinoptilolite: Therapeutic virtues of an ancient mineral. Molecules 2019, 24, 1517. [Google Scholar] [CrossRef]
- Souza, I.M.S.; García-Villén, F.; Viseras, C.; Pergher, S.B.C. Zeolites as ingredients of medicinal products. Pharmaceutics 2023, 15, 1352. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cai, Y.; Chen, T.; Bao, X. Zeolites: A series of promising biomaterials in bone tissue engineering. Front. Bioeng. Biotechnol. 2022, 10, 1066552. [Google Scholar] [CrossRef]
- Oggiano, G.; Pokimica, B.; Popovic, T.; Poštić, M. Beneficial properties of zeolite. Ital. J. Food Sci. 2023, 35, 72–78. [Google Scholar] [CrossRef]
- Nikolov, A.; Dobreva, L.; Danova, S.; Miteva-Staleva, J.; Krumova, E.; Rashev, V.; Vilhelmova-Ilieva, N. Natural and modified zeolite clinoptilolite with antimicrobial properties: A review. Acta Microbiol. Bulg. 2023, 39, 147–161. [Google Scholar] [CrossRef]
- Servatan, M.; Zarrintaj, P.; Mahmodi, G.; Kim, S.J.; Ganjali, M.R.; Saeb, M.R.; Mozafari, M. Zeolites in drug delivery: Progress, challenges and opportunities. Drug Discov. Today 2020, 25, 642–656. [Google Scholar] [CrossRef]
- Ferreira, A.P.; Almeida-Aguiar, C.; Costa, S.P.G.; Neves, I.C. Essential oils encapsulated in zeolite structures as delivery systems (EODS): An overview. Molecules 2022, 27, 8525. [Google Scholar] [CrossRef]
- Göktaş, S.; Ülkü, S.; Bayraktar, O. Clinoptilolite-rich mineral as a novel carrier for the active constituents present in Ginkgo biloba leaf extract. Appl. Clay Sci. 2008, 40, 6–14. [Google Scholar] [CrossRef]
- Alsanad, S.; Howard, R.; Williamson, E. An assessment of the impact of herb-drug combinations used by cancer patients. BMC Complement. Altern. Med. 2016, 16, 393. [Google Scholar] [CrossRef] [PubMed]
- Puri, V.; Nagpal, M.; Singh, I.; Singh, M.; Dhingra, G.A.; Huanbutta, K.; Dheer, D.; Sharma, A.; Sangnim, T.A. Comprehensive review on nutraceuticals: Therapy support and formulation challenges. Nutrients 2022, 14, 4637. [Google Scholar] [CrossRef]
- Kıroğlu, O.; Berktaş, F.; Khan, Z.; Dağkıran, M.; Karatas, Y. Self-medication practices with conventional and herbal drugs among ear, nose, and throat patients. Rev. Assoc. Médica Bras. 2022, 68, 1416–1422. [Google Scholar] [CrossRef]
- Sánchez, M.; González-Burgos, E.; Iglesias, I.; Lozano, R.; Gómez-Serranillos, M.P. Current uses and knowledge of medicinal plants in the autonomous community of Madrid (Spain): A descriptive cross-sectional study. BMC Complement. Med. Ther. 2020, 20, 306. [Google Scholar] [CrossRef]
- Pal, S.K.; Shukla, Y. Herbal medicine: Current status and the future. Asian Pac. J. Cancer Prev. 2003, 4, 281–288. [Google Scholar]
- Alam, S.; Sarker, M.R.; Afrin, S.; Richi, F.T.; Zhao, C.; Zhou, J.-R.; Mohamed, I.N. Traditional herbal medicines, bioactive metabolites, and plant products against COVID-19: Update on clinical trials and mechanism of actions. Front. Pharmacol. 2021, 12, 671498. [Google Scholar] [CrossRef] [PubMed]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Wang, Y.; Wei, D.; Zhang, L.; Zhang, B.; Xiao, H.; Song, H.; Mao, X. Nanoparticle-based drug delivery systems with platinum drugs for overcoming cancer drug resistance. J. Mater. Chem. B 2021, 9, 5173–5194. [Google Scholar] [CrossRef] [PubMed]
- Chiangjong, W.; Chutipongtanate, S.; Hongeng, S. Anticancer peptide: Physicochemical property, functional aspect and trend in clinical application (Review). Int. J. Oncol. 2020, 57, 678–696. [Google Scholar] [CrossRef] [PubMed]
- Emran, T.B.; Shahriar, A.; Mahmud, A.R.; Rahman, T.; Abir, M.H.; Siddiquee, M.; Ahmed, H.; Rahman, N.; Nainu, F.; Wahyudin, E.; et al. Multidrug resistance in cancer: Understanding molecular mechanisms, immunoprevention and therapeutic approaches. Front. Oncol. 2022, 12, 891652. [Google Scholar] [CrossRef]
- Ahmad, R.; Srivastava, S.; Ghosh, S.; Khare, S.K. Phytochemical delivery through nanocarriers: A review. Colloids Surf. B Biointerfaces 2021, 197, 111389. [Google Scholar] [CrossRef]
- David J Newman, Natural products and drug discovery. Natl. Sci. Rev. 2022, 9, nwac206. [CrossRef] [PubMed]
- Choudhury, F.K.; Pandey, P.; Meitei, R.; Cardona, D.; Gujar, A.C.; Shulaev, V. GC-MS/MS Profiling of Plant Metabolites. In Plant Metabolic Engineering; Methods in Molecular Biology; Shulaev, V., Ed.; Humana: New York, NY, USA, 2022; Volume 2396. [Google Scholar]
- Proestos, C.; Sereli, D.; Komaitis, M. Determination of phenolic compounds in aromatic plants by RP-HPLC and GC-MS. Food Chem. 2006, 95, 44–52. [Google Scholar] [CrossRef]
- Phenomenex, E.Z. Faast-Easy Fast Amino Acid Sampling Testing Kit-User Guide; Torrance, CA, USA 411 Madrid Avenue, Torrance, CA90501–1430, USA. Available online: http://www.phenomenex.com (accessed on 24 June 2023).
- Trikka, F.; Michailidou, S.; Makris, A.; Argiriou, A. Biochemical fingerprint of Greek sideritis spp.: Implications for potential drug discovery and advanced breeding strategies. Med. Aromat. Plants 2019, 8, 1–11. [Google Scholar] [CrossRef]
- Segneanu, A.-E.; Grozescu, I.; Cziple, F.; Berki, D.; Damian, D.; Niculite, C.M.; Florea, A.; Leabu, M. Helleborus purpurascens—Amino acid and peptide analysis linked to the chemical and antiproliferative properties of the extracted compounds. Molecules 2015, 20, 22170–22187. [Google Scholar] [CrossRef]
- Kostadinova, E.; Nikolova, D.; Alipieva, K.; Stefova, M.; Stefkov, G.; Evstatieva, L.; Matevski, V.; Bankova, V. Chemical constituents of the essential oils of Sideritis scardica Griseb. and Sideritis raeseri Boiss and Heldr. from Bulgaria and Macedonia. Nat. Prod. Res. 2007, 21, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Kaparakou, E.H.; Daferera, D.; Kanakis, C.D.; Skotti, E.; Kokotou, M.G.; Tarantilis, P.A. Chemical composition of the essential oils of three popular Sideritis species cultivated in Greece using GC-MS analysis. Biomolecules 2023, 13, 1157. [Google Scholar] [CrossRef]
- Mróz, M.; Bartoszek, A.; Kusznierewicz, B. Comparative study on assisted solvent extraction techniques for the extraction of biologically active compounds from Sideritis raeseri and Sideritis scardica. Molecules 2023, 28, 4207. [Google Scholar] [CrossRef]
- Lytra, K.; Tomou, E.; Chrysargyris, A.; Drouza, C.; Skaltsa, H.; Tzortzakis, N. Traditionally used Sideritis cypria Post.: Phytochemistry, nutritional content, bioactive compounds of cultivated populations. Front. Pharmacol. 2020, 11, 529498. [Google Scholar] [CrossRef]
- Tadić, V.; Bojović, D.; Arsić, I.; Đorđević, S.; Aksentijevic, K.; Stamenić, M.; Janković, S. Chemical and antimicrobial evaluation of supercritical and conventional Sideritis scardica Griseb., Lamiaceae extracts. Molecules 2012, 17, 2683–2703. [Google Scholar] [CrossRef]
- Qazimi, B.; Stefkov, G.; Karapandzova, M.; Cvetkovikj, I.; Kulevanova, S. Aroma compounds of mountain tea (Sideritis scardica and S. raeseri) from western Balkan. Nat. Prod. Commun. 2014, 9, 1369–1372. [Google Scholar] [CrossRef] [PubMed]
- Todorova, M.; Trendafilova, A. Sideritis scardica Griseb., an endemic species of Balkan peninsula: Traditional uses, cultivation, chemical composition, biological activity. J. Ethnopharmacol. 2014, 152, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Romanucci, V.; Di Fabio, G.; D’Alonzo, D.; Guaragna, A.; Scapagnini, G.; Zarrelli, A. Traditional uses, chemical composition and biological activities of Sideritis raeseri Boiss. & Heldr. J. Sci. Food Agric. 2016, 97, 373–383. [Google Scholar] [PubMed]
- Deveci, E.; Tel-Çayan, G.; Usluer, Ö.; Duru, M.E. Chemical composition, antioxidant, anticholinesterase and anti-tyrosinase activities of essential oils of two Sideritis species from Turkey. Iran. J. Pharm. Res. IJPR 2019, 18, 903–913. [Google Scholar] [PubMed]
- EMA/HMPC/39455/2015. Committee on Herbal Medicinal Products (HMPC) Assessment report on Sideritis scardica Griseb. Sideritis clandestina (Bory & Chaub.) Hayek; Sideritis raeseri Boiss. & Heldr.; Sideritis syriaca L., herba, 2016:1–30. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-sideritis-scardica-griseb-sideritis-clandestina-bory-chaub-hayek-sideritis-raeseri-boiss-heldr-sideritis-syriaca-l-herba_en.pdf (accessed on 24 June 2023).
- Bojovic, D.; Jankovic, S.; Potpara, Z.; Tadic, V. Summary of the phytochemical research performed to date on Sideritis species. Serbian J. Exp. Clin. Res. 2011, 12, 109–122. [Google Scholar] [CrossRef]
- Begas, E.; Kilindris, T.; Kouvaras, E.; Tsioutsioumi, A.; Kouretas, D.; Asprodini, E.K. Dietary effects of Sideritis scardica “mountain tea” on human in vivo activities of xenobiotic metabolizing enzymes in healthy subjects. Food Chem. Toxicol. 2018, 122, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Segneanu, A.E.; Marin, C.N.; Ghirlea, O.F.; Feier, C.V.I.; Muntean, C.; Grozescu, I. Artemisia annua growing wild in Romania—A metabolite profile approach to target a drug delivery system based on magnetite nanoparticles. Plants 2021, 10, 2245. [Google Scholar] [CrossRef]
- Dash, D.K.; Tyagi, C.K.; Sahu, A.K.; Tripathi, V. Revisiting the Medicinal Value of Terpenes and Terpenoids. In Revisiting Plant Biostimulants; Meena, V., Ed.; IntechOpen: London, UK, 2022. [Google Scholar]
- Liga, S.; Paul, C.; Péter, F. Flavonoids: Overview of biosynthesis, biological activity, and current extraction techniques. Plants 2023, 12, 2732. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Gu, C.; Mao, X.; Chen, D.; Yu, B.; Yang, Q. Isoleucine plays an important role for maintaining immune function. Curr. Protein Pept. Sci. 2019, 20, 644–651. [Google Scholar] [CrossRef]
- Dhama, K.; Karthik, K.; Khandia, R.; Munjal, A.; Tiwari, R.; Rana, R.; Khurana, S.K.; Ullah, S.; Khan, R.U.; Alagawany, M.; et al. Medicinal and therapeutic potential of herbs and plant metabolites/Extracts countering viral pathogens-current knowledge and future prospects. Curr. Drug Metab. 2018, 19, 236–263. [Google Scholar] [CrossRef]
- Lieu, E.L.; Nguyen, T.; Rhyne, S.; Kim, J. Amino acids in cancer. Exp. Mol. Med. 2020, 52, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Liu, X.; Cheng, C.; Yu, W.; Yi, P. Metabolism of amino acids in cancer. Front. Cell Dev. Biol. 2021, 8, 603837. [Google Scholar] [CrossRef]
- Pant, A.; Yang, Z. Asparagine: An Achilles heel of virus replication? ACS Infect. Dis. 2020, 6, 2301–2303. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Ji, K.; Ge, X.; Zhu, J.; Ren, M.; Mi, H. Methionine played a positive role in improving the intestinal digestion capacity, anti-inflammatory reaction and oxidation resistance of grass carp, Ctenopharyngodon idella, fry. Fish Shellfish. Immunol. 2022, 128, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Melano, I.; Kuo, L.; Lo, C.; Sung, W.; Tien, N.; Su, C. Effects of basic amino acids and their derivatives on SARS-CoV-2 and influenza-a virus infection. Viruses 2021, 13, 1301. [Google Scholar] [CrossRef] [PubMed]
- Aiyelabola, T.; Isabirye, D.; Akinkunmi, E.O.; Ogunkunle, O.; Ojo, I. Synthesis, characterization, and antimicrobial activities of coordination compounds of aspartic acid. J. Chem. 2016, 2016, 7317015. [Google Scholar] [CrossRef]
- Ji, Y.; Hou, Y.; Blachier, F.; Wu, Z. Editorial: Amino acids in intestinal growth and health. Front. Nutr. 2023, 10, 1172548. [Google Scholar] [CrossRef]
- Cheng, J.; Tang, J.C.; Pan, M.X.; Chen, S.F.; Zhao, D.; Zhang, Y.; Liao, H.B.; Zhuang, Y.; Lei, R.X.; Wang, S.; et al. L-lysine confers neuroprotection by suppressing inflammatory response via microRNA-575/PTEN signaling after mouse intracerebral hemorrhage injury. Exp. Neurol. 2020, 327, 113214. [Google Scholar] [CrossRef]
- Theodosis-Nobelos, P.; Papagiouvannis, G.; Tziona, P.; Kourounakis, P.N.; Rekka, E.A. Antioxidant serine-(NSAID) hybrids with anti-inflammatory and hypolipidemic potency. Molecules 2021, 26, 4060. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, D.; Cui, C.; Hao, J.; Zhang, Z.; Guo, L. Research progress and trends of phenylethanoid glycoside delivery systems. Foods 2022, 11, 769. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, P.; Cheng, G.; Zhang, Y. A brief review of phenolic compounds identified from plants: Their extraction, analysis, and biological activity. Nat. Prod. Commun. 2022, 17, 1934578X211069721. [Google Scholar] [CrossRef]
- Wang, C.; Gong, X.; Bo, A.; Zhang, L.; Zhang, M.; Zang, E.; Zhang, C.; Li, M. Iridoids: Research advances in their phytochemistry, biological activities, and pharmacokinetics. Molecules 2020, 25, 287. [Google Scholar] [CrossRef] [PubMed]
- Baumel, B.S.; Doraiswamy, P.M.; Sabbagh, M.; Wurtman, R. Potential neuroregenerative and neuroprotective effects of uridine/choline-enriched multinutrient dietary intervention for mild cognitive impairment: A narrative review. Neurol. Ther. 2021, 10, 43–60. [Google Scholar] [CrossRef]
- Chojnowski, K.; Opielka, M.; Nazar, W.; Kowianski, P.; Smolenski, R.T. Neuroprotective effects of guanosine in ischemic stroke—Small steps towards effective therapy. Int. J. Mol. Sci. 2021, 22, 6898. [Google Scholar] [CrossRef]
- Das, K.; Gezici, S. Secondary plant metabolites, their separation and identification, and role in human disease prevention. Ann. Phytomed. Int. J. 2018, 7, 13–24. [Google Scholar] [CrossRef]
- He, M.; Ding, N. Plant unsaturated fatty acids: Multiple roles in stress response. Front. Plant Sci. 2020, 11, 562785. [Google Scholar] [CrossRef]
- Vezza, T.; Canet, F.; De Marañón, A.M.; Bañuls, C.; Rocha, M.; Víctor, V.M. Phytosterols: Nutritional health players in the management of obesity and its related disorders. Antioxidants 2020, 9, 1266. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Lin, Q.; Zhao, H.; Li, X.; Sang, S.; McClements, D.J.; Long, J.; Jin, Z.; Wang, J.; Qiu, C. Bioaccessibility and bioavailability of phytochemicals: Influencing factors, improvements, and evaluations. Food Hydrocoll. 2023, 135, 108165. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Scumaci, D.; Giuzio, F.; Saturnino, C.; Aquaro, S.; Rosano, C.; Sinicropi, M.S. Multidrug’Resistance (MDR): A Widespread Phenomenon in Pharmacological Therapies. Molecules 2022, 27, 616. [Google Scholar] [CrossRef]
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A review of the common neurodegenerative disorders: Current therapeutic approaches and the potential role of nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and applications. In Analytical Techniques in the Sciences; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2004; ISBN 9780470854273. [Google Scholar]
- Yadav, N.; Yadav, R.; Goyal, A. Chemistry of terpenoids. Int. J. Pharm. Sci. Rev. Res. 2014, 27, 272–278. [Google Scholar]
- Segneanu, A.-E.; Marin, C.N.; Herea, D.D.; Stanusoiu, I.; Muntean, C.; Grozescu, I. Romanian Viscum album L.—Untargeted low-molecular metabolomic approach to engineered Viscum–AuNPs carrier assembly. Plants 2022, 11, 1820. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Yang, B. Phenylethanoid glycosides: Research advances in their phytochemistry, pharmacological activity and pharmacokinetics. Molecules 2016, 21, 991. [Google Scholar] [CrossRef]
- Akcos, Y.; Ezer, N.; Çalis, I.; Demirdamar, R.; Tel, B.C. Polyphenolic compounds of Sideritis lycia and their anti-inflammatory activity. Pharm. Biol. 1999, 37, 118–122. [Google Scholar] [CrossRef]
- Olivier, D.K.; Shikanga, E.A.; Combrinck, S.; Krause, R.W.M.; Regnier, T.; Dlamini, T.P. Phenylethanoid glycosides from Lippia Javanica. South Afr. J. Bot. 2010, 76, 58–63. [Google Scholar] [CrossRef]
- Scarsini, M.; Thurotte, A.; Veidl, B.; Amiard, F.; Niepceron, F.; Badawi, M.; Lagarde, F.; Schoefs, B.; Marchand, J. Metabolite quantification by Fourier Transform Infrared Spectroscopy in diatoms: Proof of Concept on Phaeodactylum tricornutum. Front. Plant Sci. 2021, 12, 756421. [Google Scholar] [CrossRef]
- Topală, C.M.; Tătarua, L.D.; Ducu, C. ATR-FTIR spectra fingerprinting of medicinal herbs extracts prepared using microwave extraction. Arab. J. Med. Aromat. Plants AJMAP 2017, 3, 1–9. [Google Scholar]
- Filopoulou, A.; Vlachou, S.; Boyatzis, S.C. Fatty acids and their metal salts: A review of their infrared spectra in light of their presence in cultural heritage. Molecules 2021, 26, 6005. [Google Scholar] [CrossRef] [PubMed]
- Krysa, M.; Szymańska-Chargot, M.; Zdunek, A. FT-IR and FT-Raman fingerprints of flavonoids—A review. Food Chem. 2022, 393, 133430. [Google Scholar] [CrossRef] [PubMed]
- Bensemmane, N.; Bouzidi, N.; Daghbouche, Y.; Garrigues, S.; Guardia, M.; El Hattab, M. Quantification of phenolic acids by partial least squares Fourier-transform infrared (PLS-FTIR) in extracts of medicinal plants. Phytochem. Anal. 2021, 32, 206–221. [Google Scholar] [CrossRef]
- Akbari, S.; Abdurahman, N.H.; Fayaz, F.; Noory, V. Iridoids of fenugreek (Trigonella-foenum-graecum L.) seed extract detected via LC-QTOF-MS analysis. Future Foods 2021, 4, 100067. [Google Scholar] [CrossRef]
- Pang, M.; Jiang, S.; Cao, L.; Pan, L. Novel synthesis of steryl esters from phytosterols and amino acid. J. Agric. Food Chem. 2011, 59, 10732–10736. [Google Scholar] [CrossRef]
- Mukesh, K.K.; Manoj, S.; Agrawal, R.D. Isolation and identification of phytosterols from leaves of Maytenus emarginata plant. Int. J. Educ. Mod. Manag. Appl. Sci. Soc. Sci. (IJEMMASSS) 2021, 3, 291–296. [Google Scholar]
- Segneanu, A.; Velciov, S.M.; Olariu, S.; Cziple, F.; Damian, D.; Grozescu, I. Bioactive molecules profile from natural compounds. In Amino Acid—New Insights and Roles in Plant and Animal; Asao, T., Asaduzzaman, M., Eds.; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Olejniczak, A.B.; Sut, A.; Wróblewski, A.E.; Leśnikowski, Z.J. Infrared spectroscopy of nucleoside and DNA-oligonucleotide conjugates labeled with carborane or metallacarborane cage. Vib. Spectrosc. 2005, 39, 177–185. [Google Scholar] [CrossRef]
- Mozgawa, W.; Król, M.; Barczyk, K. FT-IR studies of zeolites from different structural groups. Chemik 2011, 65, 667–674. [Google Scholar]
- Korkuna, O.; Leboda, R.; Skubiszewska-Zieba, J.; Vrublevska, T.; Gunko, V.M.; Ryczkowski, J. Structural and physicochemical properties of natural zeolites: Clinoptilolite and mordenite. Microporous Mesoporous Mater. 2006, 87, 243–254. [Google Scholar] [CrossRef]
- Segneanu, A.E.; Trusca, R.; Cepan, C.; Mihailescu, M.; Muntean, C.; Herea, D.D.; Grozescu, I.; Salifoglou, A. Innovative low-cost composite nanoadsorbents based on eggshell waste for nickel removal from aqueous media. Nanomaterials 2023, 1, 2572. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Chiang, Y.W.; Santos, R.M. X-ray diffraction techniques for mineral characterization: A review for engineers of the fundamentals, applications, and research directions. Minerals 2022, 12, 205. [Google Scholar] [CrossRef]
- Senila, M.; Neag, E.; Cadar, O.; Hoaghia, M.A.; Roman, M.; Moldovan, A.; Hosu, A.; Lupas, A.; Kovacs, E.D. Characteristics of volcanic tuff from Macicasu (Romania) and its capacity to remove ammonia from contaminated Air. Molecules 2022, 27, 3503. [Google Scholar] [CrossRef] [PubMed]
- Güngör, D.; Özen, S. Development and characterization of clinoptilolite-, mordenite-, and analcime-based geopolymers: A comparative study. Case Stud. Constr. Mater. 2021, 15, e00576. [Google Scholar] [CrossRef]
- Segneanu, A.E.; Vlase, G.; Lukinich-Gruia, A.T.; Herea, D.D.; Grozescu, I. Untargeted metabolomic approach of Curcuma longa to neurodegenerative phytocarrier system based on silver nanoparticles. Antioxidants 2022, 11, 2261. [Google Scholar] [CrossRef]
- Goetze, J.; Yarulina, I.; Gascon, J.; Kapteijn, F.; Weckhuysen, B.M. Revealing lattice expansion of small-pore zeolite catalysts during the methanol-to-olefins process using combined operando X-ray diffraction and UV–vis spectroscopy. ACS Catal. 2018, 8, 2060–2070. [Google Scholar] [CrossRef]
- Kadja, G.T.; Culsum, N.T.; Putri, R.M. Recent advances in the utilization of zeolite-based materials for controlled drug delivery. Results Chem. 2022, 5, 100910. [Google Scholar] [CrossRef]
- Abukhadra, R.A.; Adlii, A.; Khim, J.; Ajarem, J.; Allam, A. Insight into the technical qualification of the sonocogreen CaO/Clinoptilolite nanocomposite (CaO (NP) /Clino) as an advanced delivery system for 5-fluorouracil: Equilibrium and cytotoxicity. ACS Omega 2021, 6, 31982–31992. [Google Scholar] [CrossRef]
- Díaz, M.J.; Ruiz-Montoya, M.; Palma, A.; de-Paz, M.V. Thermogravimetry applicability in compost and composting research: A review. Appl. Sci. 2021, 11, 1692. [Google Scholar] [CrossRef]
- Fernandes, F.H.A.; Santana, C.P.; Santos, R.L.; Correia, L.P.; Conceição, M.M.; Macêdo, R.O.; Medeiros, A.C.D. Thermal characterization of dried extract of medicinal plant by DSC and analytical techniques. J. Therm. Anal. Calorim. 2012, 113, 443–447. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Talalay, P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol. Nutr. Food Res. 2008, 52, S128–S138. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Valgimigli, L. Methods to measure the antioxidant activity of phytochemicals and plant extracts. J. Agric. Food Chem. 2018, 66, 3324–3329. [Google Scholar] [CrossRef]
- Losada-Barreiro, S.; Sezgin-Bayindir, Z.; Paiva-Martins, F.; Bravo-Díaz, C. Biochemistry of antioxidants: Mechanisms and pharmaceutical applications. Biomedicines 2022, 10, 3051. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch.Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Flieger, J.; Flieger, W.; Baj, J.; Maciejewski, R. Antioxidants: Classification, natural sources, activity/capacity measurements, and usefulness for the synthesis of nanoparticles. Materials 2021, 14, 4135. [Google Scholar] [CrossRef] [PubMed]
- Mbinda, W.; Musangi, C. Antioxidant activity, total phenolic and total flavonoid contents of stem back and root methanolic extracts of Calotropis procera. J. Phytopharm. 2019, 8, 161–166. [Google Scholar] [CrossRef]
- Segneanu, A.E.; Vlase, G.; Vlase, T.; Sicoe, C.A.; Ciocalteu, M.V.; Herea, D.D.; Ghirlea, O.F.; Grozescu, I.; Nanescu, V. Wild-grown Romanian Helleborus purpurascens approach to novel chitosan phyto-nanocarriers—Metabolite profile and antioxidant properties. Plants 2023, 12, 3479. [Google Scholar] [CrossRef]
- El-Sawi, S.A.; Motawae, H.M.; Abdelkawy, M.A.; Fekry, M.I.; Youssef, H.F.; Farghaly, A.A.; Maamoun, M.A. Novel anticancer drug delivery system based on zeolite encapsulating Hamelia patens leaf and flower extracts. Indian J. Nat. Prod. Resour. 2021, 12, 181–194. [Google Scholar]
- Mbamalu, O.N.; Syce, J.; Samsodien, H. Short communication. challenges relating to comparison of flavonoid glycosides dissolution profiles from Sutherlandia frutescens products. Acta Pharm. 2017, 67, 137–146. [Google Scholar] [CrossRef]
- Pranskuniene, Z.; Bernatoniene, J.; Kalveniene, Z.; Masteikova, R.; Mekas, T.; Velziene, S.; Ivanauskas, K.; Suchockas, V.; Mintauckiene, I.; Savickas, A. New formula herbal pellets demonstrate a uniform and stable release of the active ingredients in vitro. Acta Pol Pharm. 2013, 70, 727–736. [Google Scholar]
- Sharma, R.; Ashawat, M.S.; Verma, C.P.S.; Kumari, N. A fast release tablet containing Herbal extracts (ginger, cinnamon, turmeric, long pepper and punarnava). Asian J. Pharm. Tech. 2020, 10, 231–240. [Google Scholar] [CrossRef]
- Osei-Asare, C.; Akuffo Owusu, F.W.; Entsie, P.; Kwansima Annan, A.; Akosua Gyamaa, R.; Makafui Amenuke, E. Formulation and in vitro evaluation of oral capsules from liquid herbal antimalarials marketed in Ghana. Hindawi J. Trop. Med. 2021, 2021, 6694664. [Google Scholar] [CrossRef]
- Hussain, A.; Attique, F.; Naqvi, S.A.R.; Ali, A.; Ibrahim, M.; Hussain, H.; Zafar, F.; Iqbal, R.S.; Ayub, M.A.; Assiri, M.A.; et al. Nanoformulation of Curcuma longa root extract and evaluation of its dissolution potential. ACS Omega 2022, 8, 1088–1096. [Google Scholar] [CrossRef]
- Chowdary, H.V.; Venkaiah, A.; Malik, S.V.; Reddy, H.S.; Achari, S.V.; Raju, P.Y. Dissolution test as a quality control tool for herbal formulations—A comprehensive review. Int. J. Innov. Pharm. Res. 2014, 5, 364–369. [Google Scholar]
- Lex, T.R.; Rodriguez, J.D.; Zhang, L.; Jiang, W.; Gao, Z. Development of in vitro dissolution testing methods to simulate fed conditions for immediate release solid oral dosage forms. AAPS J. 2022, 24, 40. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jin, X.; Ren, X.; Zhu, Y.; Liu, Z.; Gao, X. Comparative in vitro dissolution of two commercially available Er-Zhi-Wan herbal medicinal products. Indian J. Pharm. Sci. 2015, 77, 391–398. [Google Scholar]
- Aswathy, K.; Asdaq, S.M.B.; Saritha, C.; Thomas, L.; Haridas, N.; Viswanad, V.; Sahu, R.K.; Fattepur, S.; Alamri, A.S.; Alsanie, W.F.; et al. Formulation and in-vitro characterization of fast-disintegrating herbal extract sublingual immunotherapy tablet for peanut-induced allergic asthma. Saudi J. Biol. Sci. 2022, 29, 1283–1297. [Google Scholar] [CrossRef] [PubMed]
- Medina-Remón, A.; Barrionuevo-González, A.; Zamora-Ros, R.; Andres-Lacueva, C.; Estruch, R.; Martínez-González, M.Á.; Diez-Espino, J.; Lamuela-Raventos, R.M. Rapid Folin–Ciocalteu method using microtiter 96-well plate cartridges for solid phase extraction to assess urinary total phenolic compounds, as a biomarker of total polyphenols intake. Anal. Chim. Acta 2009, 634, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Surat, P. pH in the Human Body. News-Medical, 10 October 2022. Available online: https://www.news-medical.net/health/pH-in-the-Human-Body.aspx (accessed on 16 November 2023).
- Anumolu, P.D.; Gurrala, S.; Venkata Satya, S.C.; Polisetty, S.V.; Ravindran, A.; Achanta, R. Development of a discriminative and biorelevant dissolution test method for atorvastatin/fenofibrate combination with appliance of derivative spectrophotometry. Turk. J. Pharm. Sci. 2019, 16, 62–68. [Google Scholar] [CrossRef]
- Mureşan, M.; Olteanu, D.; Filip, G.A.; Clichici, S.; Baldea, I.; Jurca, T.; Pallag, A.; Marian, E.; Frum, A.; Gligor, F.G.; et al. Comparative study of the pharmacological properties and biological effects of Polygonum aviculare L. herba extract-entrapped liposomes versus quercetin-wntrapped liposomes on doxorubicin-induced toxicity on HUVECs. Pharmaceutics 2021, 13, 1418. [Google Scholar] [CrossRef]
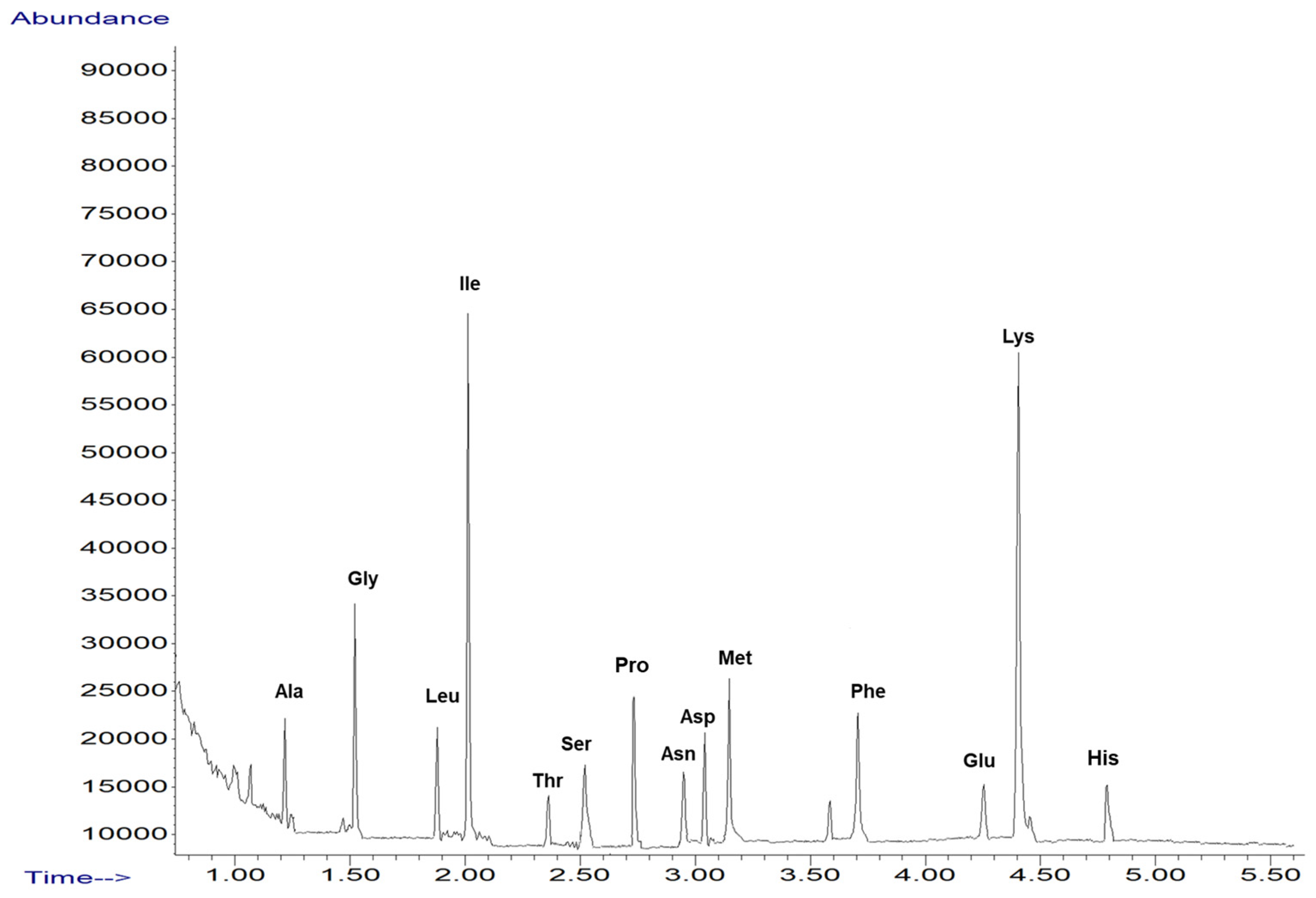

| Proposed Structure | Abbreviation | SIM (Selected Ion Monitoring) |
|---|---|---|
| alanine | Ala | 130, 70 |
| glycine | Gly | 116, 74 |
| leucine | Leu | 172, 86 |
| isoleucine | Ile | 172, 130 |
| threonine | Thr | 160, 101 |
| serine | Ser | 146, 203 |
| proline | Pro | 156, 243 |
| asparagine | Asn | 155, 69 |
| aspartic acid | Asp | 216, 130 |
| methionine | Met | 203, 277 |
| phenylalanine | Phe | 206, 190 |
| glutamic acid | Glu | 230, 170 |
| lysine | Lys | 170, 128 |
| histidine | His | 282, 168 |
| No | Detected m/z | Theoretic m/z | Formula | Tentative of Identification | Category | Ref. |
|---|---|---|---|---|---|---|
| 1 | 76.08 | 75.07 | C2H5NO2 | glycine | amino acids | [37,38,51] |
| 2 | 90.11 | 89.09 | C3H7NO2 | alanine | [37] | |
| 3 | 106.07 | 105.09 | C3H7NO3 | serine | [37] | |
| 4 | 116.11 | 115.13 | C5H9NO2 | proline | [37] | |
| 5 | 120.13 | 119.12 | C4H9NO3 | threonine | [37] | |
| 6 | 132.15 | 131.17 | C6H13NO2 | isoleucine | [37] | |
| 7 | 133.13 | 132.12 | C4H8N2O3 | asparagine | [37] | |
| 8 | 134.11 | 133.10 | C4H7NO4 | aspartic acid | [37] | |
| 9 | 147.18 | 146.19 | C6H14N2O2 | lysine | [37,38,51] | |
| 10 | 148.14 | 147.13 | C5H9NO4 | glutamic acid | [37] | |
| 11 | 149.23 | 149.21 | C5H11NO2S | methionine | [37,38,51] | |
| 12 | 156.17 | 155.15 | C6H9N3O2 | histidine | [37,38,51] | |
| 13 | 166.17 | 165.19 | C9H11NO2 | phenylalanine | [37] | |
| 14 | 271.25 | 270.24 | C15H10O5 | apigenin | flavonoids | [5,45,46,48] |
| 15 | 285.27 | 284.26 | C16H12O5 | genkwanin | [41] | |
| 16 | 287.23 | 286.24 | C15H10O6 | luteolin | [45,46,48] | |
| 17 | 301.27 | 300.26 | C16H12O6 | chrysoeriol | [5,45,48] | |
| 18 | 303.25 | 302.23 | C15H10O7 | hypolaetin | [48] | |
| 19 | 315.28 | 314.29 | C17H14O6 | cirsimaritin | [41,49] | |
| 20 | 317.25 | 316.26 | C16H12O7 | nepetin | [49] | |
| 21 | 329.28 | 328.30 | C18H16O6 | salvigenin | [49] | |
| 22 | 343.35 | 342.34 | C16H22O8 | coniferin | [41] | |
| 23 | 345.21 | 344.22 | C18H16O7 | eupatorin | [41] | |
| 24 | 651.61 | 650.60 | C30H34O16 | tremasperin | [41] | |
| 25 | 165.17 | 164.16 | C9H8O3 | p-coumaric acid | phenolic acids | [45] |
| 26 | 169.14 | 168.15 | C8H8O4 | vanillic acid | [45] | |
| 27 | 171.13 | 170.12 | C7H6O5 | gallic acid | [45] | |
| 28 | 181.15 | 180.16 | C9H8O4 | caffeic acid | [45] | |
| 29 | 193.15 | 192.17 | C7H12O6 | quinic acid | [48] | |
| 30 | 195.17 | 194.18 | C10H10O4 | ferulic acid | [45] | |
| 31 | 199.15 | 198.17 | C9H10O5 | syringic acid | [45] | |
| 32 | 355.33 | 354.31 | C16H18O9 | chlorogenic acid | [5,45] | |
| 33 | 135.21 | 134.22 | C10H14 | o-cymene | terpenoids | [42] |
| 34 | 137.24 | 136.23 | C10H16 | α-thujene | [43,44] | |
| 35 | 149.19 | 148.20 | C10H12O | anethone | [43] | |
| 36 | 151.21 | 150.22 | C10H14O | tymol | [43] | |
| 37 | 153.25 | 152.23 | C10H16O | camphor | [43] | |
| 38 | 154.25 | 154.25 | C10H18O | borneol | [43] | |
| 39 | 157.25 | 156.26 | C10H20O | menthol | [43] | |
| 40 | 191.27 | 190.28 | C13H18O | damascenone | [47] | |
| 41 | 193.31 | 192.30 | C13H20O | beta-damascone | [43] | |
| 42 | 199.31 | 198.30 | C15H18 | cadalene | [43] | |
| 43 | 202.34 | 202.33 | C15H22 | α-curcumene | [46] | |
| 44 | 205.35 | 204.35 | C15H24 | humulene | [43] | |
| 45 | 219.31 | 218.33 | C15H22O | germacrone | [48] | |
| 46 | 221.34 | 220.35 | C15H24O | spatulenol | [40,43] | |
| 47 | 223.36 | 222.37 | C15H26O | valeranone | [43] | |
| 48 | 297.49 | 296.50 | C20H40O | phytol | [43] | |
| 49 | 305.49 | 304.50 | C20H32O2 | sideridiol | [45] | |
| 50 | 321.49 | 320.50 | C20H32O3 | sideroxol | [45,48] | |
| 51 | 323.51 | 322.50 | C20H34O3 | andalusol | [49] | |
| 52 | 333.41 | 332.40 | C20H28O4 | carnosic acid | [41] | |
| 53 | 347.49 | 346.50 | C22H34O3 | siderol | [45,48] | |
| 54 | 363.51 | 362.50 | C22H34O4 | eubol | [45,48] | |
| 55 | 463.41 | 462.40 | C20H30O12 | forsythoside | phenylethanoid glycosides | [48] |
| 56 | 625.59 | 624.60 | C29H36O15 | verbascoside | [5] | |
| 57 | 639.58 | 638.60 | C30H38O15 | leucosceptoside A | [48,50] | |
| 58 | 653.58 | 652.60 | C31H40O15 | martynoside | [48,50] | |
| 59 | 669.62 | 668.60 | C31H40O16 | verbascoside | [48] | |
| 60 | 757.71 | 756.70 | C34H44O19 | lavandulifolioside | [48,50] | |
| 61 | 771.68 | 770.70 | C35H46O19 | alyssonoside | [48,50] | |
| 62 | 787.70 | 786.70 | C35H46O20 | echinacoside | [48,50] | |
| 63 | 375.33 | 374.34 | C16H22O10 | geniposidic acid | iridoids | [41] |
| 64 | 449.39 | 448.40 | C19H28O12 | barlerin | [41] | |
| 65 | 525.51 | 524.50 | C21H32O15 | melittoside | [42] | |
| 66 | 173.27 | 172.26 | C10H20O2 | capric acid | fatty acids | [43] |
| 67 | 201.33 | 200.32 | C12H24O2 | lauric acid | [43] | |
| 68 | 257.41 | 256.42 | C16H32O2 | palmitic acid | [43] | |
| 69 | 281.39 | 280.40 | C18H32O2 | linoleic acid | [43] | |
| 70 | 283.51 | 282.50 | C18H34O2 | oleic acid | [43,46] | |
| 71 | 401.69 | 400.70 | C28H48O | campesterol | sterols | [48] |
| 72 | 413.69 | 412.70 | C29H48O | stigmasterol | [48] | |
| 73 | 415.71 | 414.70 | C29H50O | β sitosterol | [48] | |
| 74 | 245.21 | 244.20 | C9H12N2O6 | uridine | nucleosides | [41] |
| 75 | 284.25 | 283.24 | C10H13N5O5 | guanisine | nucleosides | [41] |
| 76 | 103.15 | 102.17 | C6H14O | hexanol | alcohols | [40] |
| 77 | 129.19 | 128.21 | C8H16O | oct-1-en-3-ol | [40] | |
| 78 | 271.49 | 270.50 | C18H38O | 2-octadecanol | [43] | |
| 79 | 207.27 | 206.28 | C13H18O2 | hexylbenzoate | esters | [39] |
| 80 | 213.25 | 212.24 | C14H12O2 | benzylbenzoate | [39] | |
| 81 | 295.51 | 294.50 | C19H34O2 | methyl lineoleate | [43] | |
| 82 | 313.51 | 312.50 | C20H40O2 | stearyl acetate | [39] | |
| 83 | 143.27 | 142.28 | C10H22 | decane | hydrocarbons | [43] |
| 84 | 325.59 | 324.60 | C23H48 | tricosane | [43] | |
| 85 | 353.71 | 352.70 | C25H52 | pentacosane | [43] | |
| 86 | 121.17 | 120.15 | C8H8O | acetophenone | ketones | [40] |
| 87 | 165.25 | 164.24 | C11H16O | cis-jasmone | [46] | |
| 88 | 123.11 | 122.12 | C7H6O2 | benzoic acid | organic acids | [40] |
| 89 | 135.07 | 134.09 | C4H6O5 | malic acid | [41] | |
| 90 | 151.07 | 150.09 | C4H6O6 | tartaric acid | [41] | |
| 91 | 201.21 | 200.23 | C10H16O4 | camphoric acid | [42] |
| Phytoconstituent | Wavenumber (cm−1) | Ref. |
|---|---|---|
| terpenoids | 2940, 1745, 1700, 1650, 810 | [78,79] |
| phenylethanoid glycosides | 3390, 2853, 2925, 1720, 1700, 1628, 1602, 1520, 1454,1385, 1280 | [80,81,82] |
| fatty acids | 3600, 3020–3010, 2960, 2925, 2875, 2850, 2560, 1702, 1350, 1245, 724 | [83,84,85] |
| flavonoids | 4000–3124, 3140–2980, 1655, 1644, 1620, 1585, 1496, 1465, 1415, 1370, 1275, 1080, 770, 535 | [86] |
| phenolic acids | 1800–1650, 1730, 1640, 1625, 1518, 1440, 1412, 1365, 1310, 1260, 1165, 1090, 945, 805 | [79,87] |
| iridoids | 1740–1458, 1377, 1221–914 | [88] |
| phytosterols | 3425, 3350, 2935, 2832, 1752, 1642, 1465, 1385, 1190, 1065, 990, 950, 880, 741 | [89,90] |
| amino acids | 3400; 3330–3130; 2985, 2360–2530, 2130; 1725–1755 1690, 1675, 1665, 1650, 1645, 1630, 1625, 1610, 1500–1600 | [91] |
| nucleoside | 3350, 3105, 2925, 2800, 1670, 1470, 1395, 1270, 1210, 1140. 1095, 1055, 980, 905, 830, 770, 570, 450 | [92] |
| Sample | Diameters (µm) | Width (µm) |
|---|---|---|
| ironwort | 1.1710 | 0.4540 |
| 0.2609 | 0.2383 | |
| clinoptilolite | 0.2052 | 0.3530 |
| SZ phyto-nanocarrier | 0.4880 | 0.2170 |
| 0.1299 | 0.0637 |
| Sample Name | Total Phenolic Content (mg/g GAE) | mg AAE/mL | IC50 (mg/mL) |
|---|---|---|---|
| ironwort | 0.4988 ± 0.072 | 122.23 ± 0.015 | 4.17 ± 0.019 |
| SZ phyto-nanocarrier | 0.5219 ± 0.010 | 165.37 ± 0.023 | 3.89 ± 0.034 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segneanu, A.-E.; Vlase, G.; Vlase, T.; Bita, A.; Bejenaru, C.; Buema, G.; Bejenaru, L.E.; Dumitru, A.; Boia, E.R. An Innovative Approach to a Potential Neuroprotective Sideritis scardica-Clinoptilolite Phyto-Nanocarrier: In Vitro Investigation and Evaluation. Int. J. Mol. Sci. 2024, 25, 1712. https://doi.org/10.3390/ijms25031712
Segneanu A-E, Vlase G, Vlase T, Bita A, Bejenaru C, Buema G, Bejenaru LE, Dumitru A, Boia ER. An Innovative Approach to a Potential Neuroprotective Sideritis scardica-Clinoptilolite Phyto-Nanocarrier: In Vitro Investigation and Evaluation. International Journal of Molecular Sciences. 2024; 25(3):1712. https://doi.org/10.3390/ijms25031712
Chicago/Turabian StyleSegneanu, Adina-Elena, Gabriela Vlase, Titus Vlase, Andrei Bita, Cornelia Bejenaru, Gabriela Buema, Ludovic Everard Bejenaru, Andrei Dumitru, and Eugen Radu Boia. 2024. "An Innovative Approach to a Potential Neuroprotective Sideritis scardica-Clinoptilolite Phyto-Nanocarrier: In Vitro Investigation and Evaluation" International Journal of Molecular Sciences 25, no. 3: 1712. https://doi.org/10.3390/ijms25031712
APA StyleSegneanu, A.-E., Vlase, G., Vlase, T., Bita, A., Bejenaru, C., Buema, G., Bejenaru, L. E., Dumitru, A., & Boia, E. R. (2024). An Innovative Approach to a Potential Neuroprotective Sideritis scardica-Clinoptilolite Phyto-Nanocarrier: In Vitro Investigation and Evaluation. International Journal of Molecular Sciences, 25(3), 1712. https://doi.org/10.3390/ijms25031712







