Anti-Photoaging Effects of Upcycled Citrus junos Seed Anionic Peptides on Ultraviolet-Radiation-Induced Skin Aging in a Reconstructed Skin Model
Abstract
1. Introduction
2. Results
2.1. Separation of LMAPs
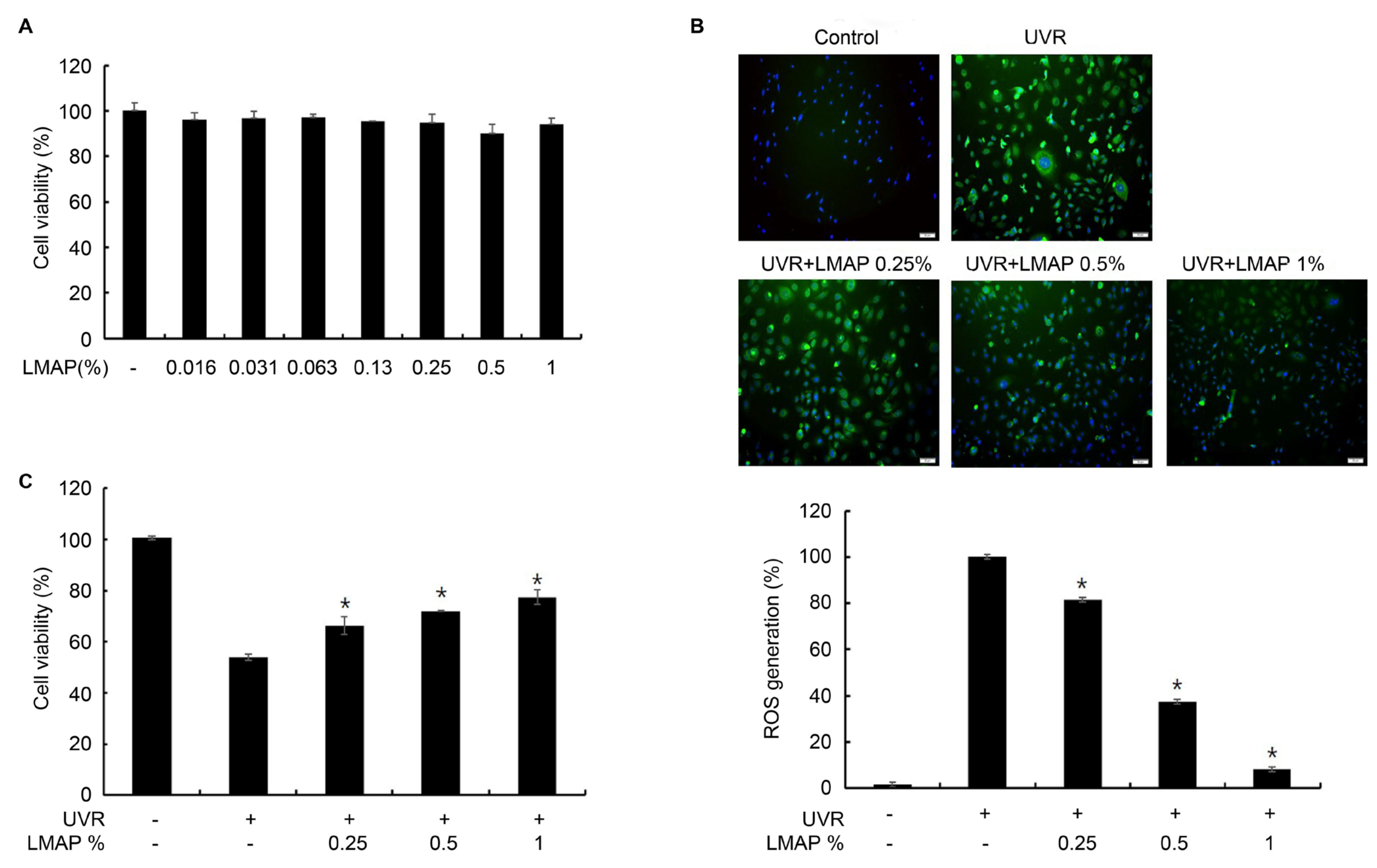
2.2. Antioxidant Effects of LMAPs in Keratinocytes
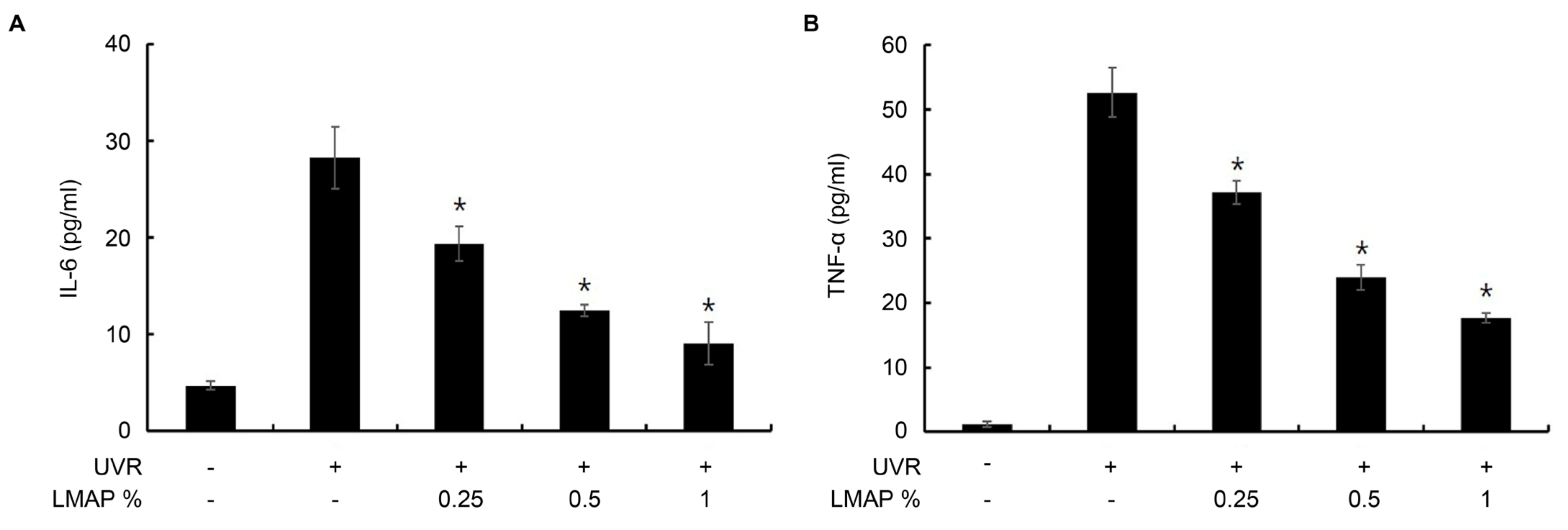
2.3. LMAP Treatment Inhibits UVR-Induced Cytokine Production by Keratinocytes
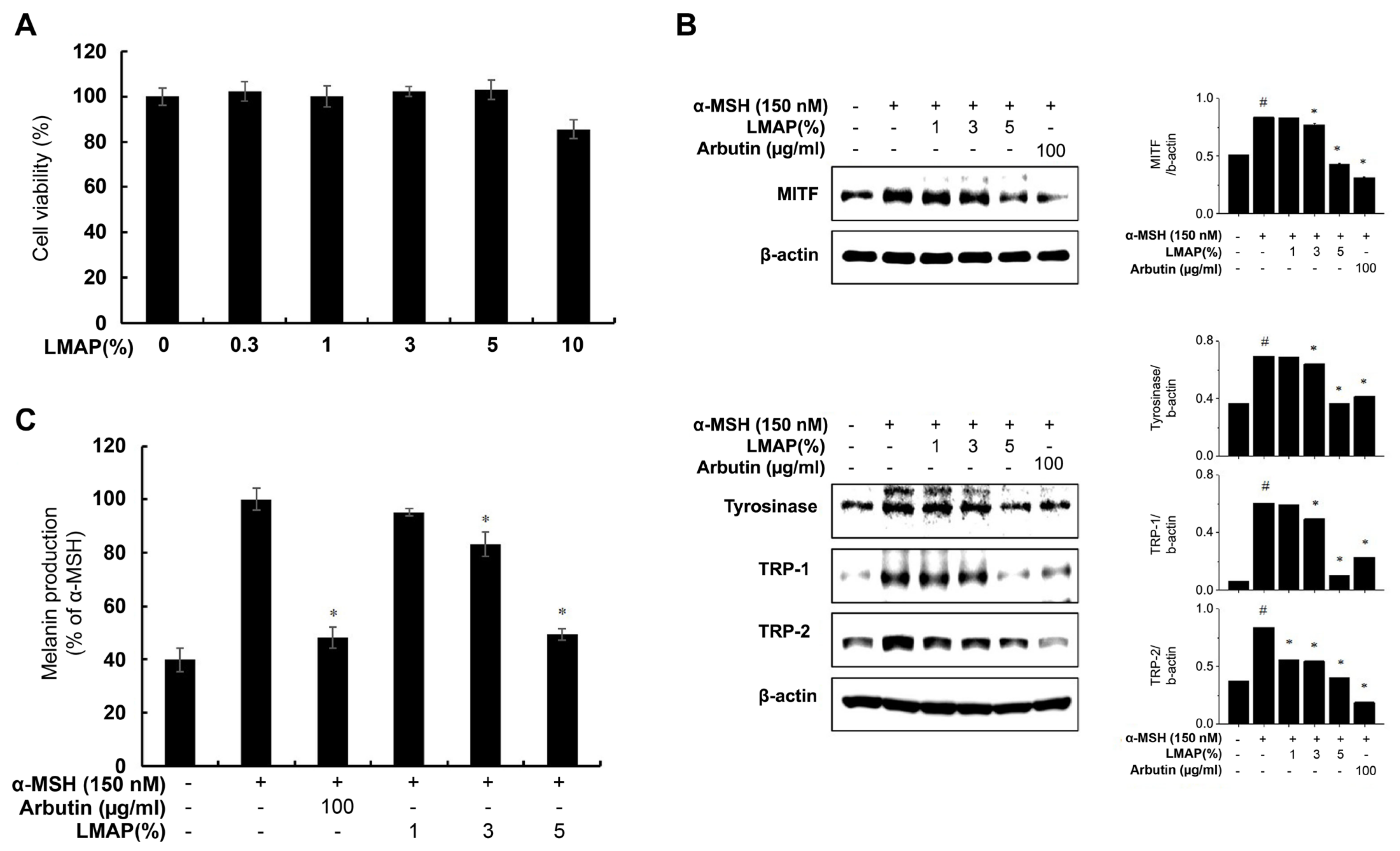
2.4. LMAP Treatment Inhibits Pigmentation following UVR Irradiation
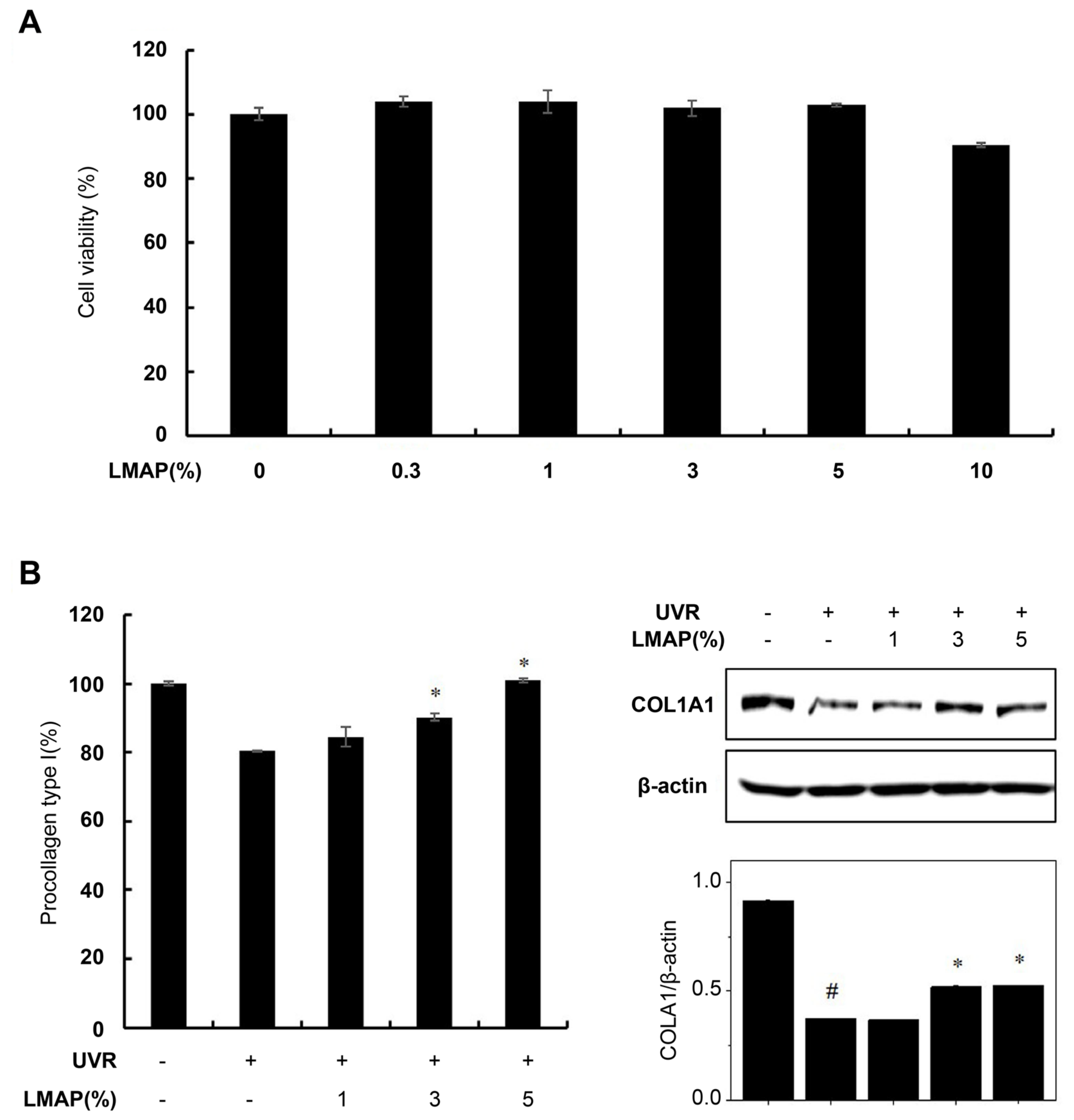
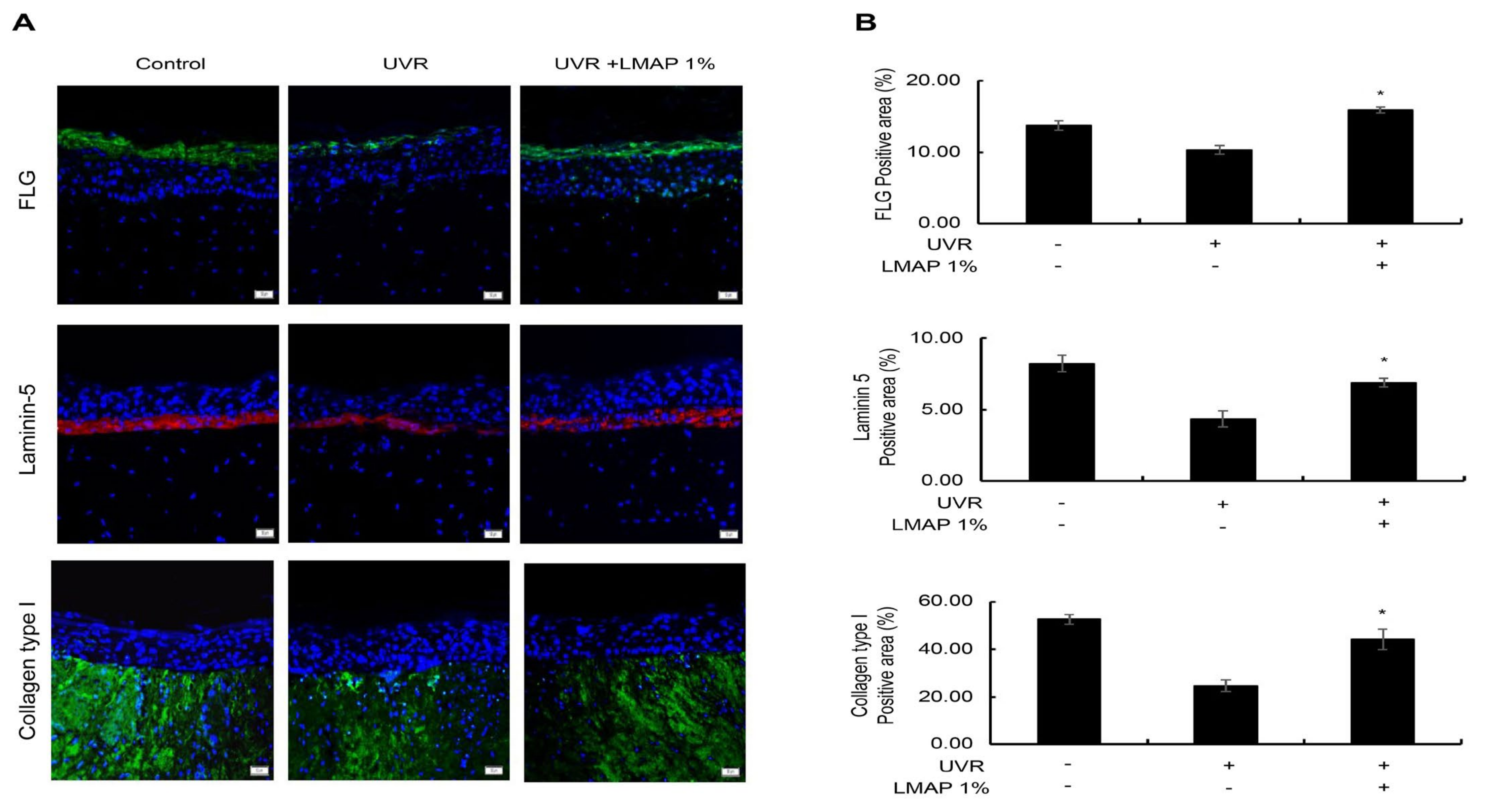
2.5. LMAP Treatment Inhibits Wrinkle Formation following UVR Irradiation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Extraction and Separation of Low-Molecular-Weight Anionic Peptides
4.3. Mass Spectrometry
4.4. Measurement of Zeta Potential
4.5. Cell Culture
4.6. Evaluation of Cell Viability and Measurement of Cellular ROS
4.7. Enzyme Immunoassays for IL-6, TNF-α, and Procollagen Type I
4.8. Western Blotting
4.9. Reconstructed Human Photoaging Skin Model
4.10. Histological Analysis, Melanin Content, and Immunofluorescence Analysis
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ueno, H.; Tanaka, M.; Machmudah, S.; Sasaki, M.; Goto, M. Supercritical carbon dioxide extraction of valuable compounds from Citrus junos seed. Food Bioprocess Technol. 2008, 1, 357–363. [Google Scholar] [CrossRef]
- Ueno, H.; Tanaka, M.; Hosino, M.; Sasaki, M.; Goto, M. Extraction of valuable compounds from the flavedo of Citrus junos using subcritical water. Sep. Purif. Technol. 2008, 62, 513–516. [Google Scholar] [CrossRef]
- Kim, J.W.; Jo, E.H.; Moon, J.E.; Cha, H.; Chang, M.H.; Cho, H.T.; Lee, M.K.; Jung, W.S.; Lee, J.H.; Heo, W.; et al. In vitro and in vivo inhibitory effect of Citrus junos Tanaka peel extract against oxidative stress-induced apoptotic death of lung cells. Antioxidants 2020, 9, 1231. [Google Scholar] [CrossRef]
- Song, H.Y.; Jo, A.; Shin, J.; Lim, E.H.; Lee, Y.E.; Jeong, D.E.; Lee, M. Anti-inflammatory activities of isogosferol, a furanocoumarin isolated from Citrus junos seed shells through bioactivity-guided fractionation. Molecules 2019, 24, 4088. [Google Scholar] [CrossRef]
- Suzuki, A.; Hirakawa, E.; Umeki, M.; Sakai, K.; Koya, M.; Oda, H.; Ishikawa, Y. Yuzu, Citrus junos, peels extract ameliorated hepatic steatosis induced by chloretone in rats. Food Sci. Technol. Res. 2021, 27, 281–292. [Google Scholar] [CrossRef]
- Kim, S.Y. Chemical composition and antioxidant activity of crude polysaccharide from citron (Citrus junos Sieb. Ex TANAKA) seed. Prev. Nutr. Food Sci. 2018, 23, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.E.; Hernández-Mendoza, A.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Liceaga, A.M. Potential role of natural bioactive peptides for development of cosmeceutical skin products. Peptides 2019, 122, 170170. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M. Biological activities of protein and marine-derived peptides from byproducts and seaweeds. In Marine Protein and Peptides: Biological Activities and Applications; Kim, S.K., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2013; pp. 139–165. [Google Scholar]
- Gromkowska-Kępka, K.J.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Socha, K. The impact of ultraviolet radiation on skin photoaging—Review of in vitro studies. J. Cosmet. Dermatol. 2021, 20, 3427–3431. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Plonka, P.M.; Szaflarski, J.P.; Paus, R. How UV light touches the brain and endocrine system through skin, and why. Endocrinology 2018, 159, 1992–2007. [Google Scholar] [CrossRef] [PubMed]
- Friedman, O. Changes associated with the aging face. Facial. Plast. Surg. Clin. N. Am. 2005, 13, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Rittié, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef] [PubMed]
- Rabe, J.H.; Mamelak, A.J.; McElgunn, P.J.; Morison, W.L.; Sauder, D.N. Photoaging: Mechanisms and repair. J. Am. Acad. Dermatol. 2006, 55, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Hasegawa, J.; Asamitsu, K.; Okamoto, T. Prevention of the ultraviolet B-mediated skin photoaging by a nuclear factor κB inhibitor, parthenolide. J. Pharmacol. Exp. Ther. 2005, 315, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Zhang, H.; Zhao, M.; Li, J.; Hu, Y.; Fu, J.; Pi, J.; Wang, H.; Xu, Y. Nrf2 in keratinocytes protects against skin fibrosis via regulating epidermal lesion and inflammatory response. Biochem. Pharmacol. 2020, 174, 113846. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Wei, L.; Che, H.; Shen, Y.; Yang, J.; Mi, K.; Liu, J.; Wu, J.; Yang, H.; Mu, L. A frog peptide ameliorates skin photoaging through scavenging reactive oxygen species. Front. Pharmacol. 2022, 12, 761011. [Google Scholar] [CrossRef] [PubMed]
- Sim, W.-J.; Kim, J.; Baek, K.-S.; Lim, W.; Lim, T.-G. Porcine placenta peptide inhibits UVB-induced skin wrinkle formation and dehydration: Insights into MAPK signaling pathways from in vitro and in vivo studies. Int. J. Mol. Sci. 2024, 25, 83. [Google Scholar] [CrossRef]
- Xiao, Z.; Liang, P.; Chen, J.; Chen, M.F.; Gong, F.; Li, C.; Zhou, C.; Hong, P.; Yang, P.; Qian, Z.J. A peptide YGDEY from tilapia gelatin hydrolysates inhibits UVB-mediated skin photoaging by regulating MMP-1 and MMP-9 expression in HaCaT cells. Photochem. Photobiol. 2019, 95, 1424–1432. [Google Scholar] [CrossRef]
- Zeng, Q.; Jiang, J.; Wang, J.; Zhou, Q.; Zhang, X. N-terminal acetylation and C-terminal amidation of Spirulina platensis-derived hexapeptide: Anti-photoaging activity and proteomic analysis. Mar. Drugs 2019, 17, 520. [Google Scholar] [CrossRef]
- Yao, W.; Yong, J.; Lv, B.; Guo, S.; You, L.; Cheung, P.C.-K.; Kulikouskaya, V.I. Enhanced in vitro anti-photoaging effect of degraded seaweed polysaccharides by UV/H2O2 treatment. Mar. Drugs 2023, 21, 430. [Google Scholar] [CrossRef]
- Ko, H.J.; Kim, J.; Ahn, M.; Kim, J.H.; Lee, G.S.; Shin, T. Ergothioneine alleviates senescence of fibroblasts induced by UVB damage of keratinocytes via activation of the Nrf2/HO-1 pathway and HSP70 in keratinocytes. Exp. Cell Res. 2021, 400, 112516. [Google Scholar] [CrossRef]
- Jung, Y.R.; Kim, D.H.; Kim, S.R.; An, H.J.; Lee, E.K.; Tanaka, T.; Kim, N.D.; Yokozawa, T.; Park, J.N.; Chung, H.Y. Anti-wrinkle effect of magnesium lithospermate B from Salvia miltiorrhiza Bunge: Inhibition of MMPs via NF-κb signaling. PLoS ONE 2014, 9, e102689. [Google Scholar] [CrossRef] [PubMed]
- Faller, C.; Bracher, M.; Dami, N.; Roguet, R. Predictive ability of reconstructed human epidermis equivalents for the assessment of skin irritation of cosmetics. Toxicol. In Vitro 2002, 16, 557–572. [Google Scholar] [CrossRef] [PubMed]
- Kirsch-Volders, M.; Decordier, I.; Elhajouji, A.; Plas, G.; Aardema, M.J.; Fenech, M. In vitro genotoxicity testing using the micronucleus assay in cell lines, human lymphocytes and 3D human skin models. Mutagenesis 2011, 26, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Gillbro, J.M.; Olsson, M.J. The melanogenesis and mechanisms of skin-lightening agents--existing and new approaches. Int. J. Cosmet. Sci. 2011, 33, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.J.; Kim, J.H.; Lee, G.S.; Shin, T. Sulforaphane controls the release of paracrine factors by keratinocytes and thus mitigates particulate matter-induced premature skin aging by suppressing melanogenesis and maintaining collagen homeostasis. Phytomedicine 2020, 77, 153276. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Huang, J.; Lu, J.; Hu, S.; Pei, S.; Ouyang, Y.; Ding, Y.; Hu, Y.; Kang, L.; Huang, L.; et al. Ganoderma lucidum polysaccharide reduces melanogenesis by inhibiting the paracrine effects of keratinocytes and fibroblasts via IL-6/STAT3/FGF2 pathway. J. Cell. Physiol. 2019, 234, 22799–22808. [Google Scholar] [CrossRef] [PubMed]
- Duval, C.; Chagnoleau, C.; Pouradier, F.; Sextius, P.; Condom, E.; Bernerd, F. Human skin model containing melanocytes: Essential role of keratinocyte growth factor for constitutive pigmentation functional response to α-melanocyte stimulating hormone and forskolin. Tissue Eng. Part C Methods 2012, 18, 947–957. [Google Scholar] [CrossRef] [PubMed]
- An, K.N.; Sun, Y.L.; Luo, Z.P. Flexibility of type I collagen and mechanical property of connective tissue. Biorheology 2004, 41, 239–246. [Google Scholar]
- Sin, J.W.; Kwon, S.H.; Choi, J.Y.; Na, J.I.; Huh, C.H.; Choi, H.R.; Park, K.C. Molecular mechanisms of dermal aging and antiaging approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef]
- Imokawa, G. Mechanism of UVB-induced wrinkling of the skin: Paracrine cytokine linkage between keratinocytes and fibroblasts leading to the stimulation of elastase. J. Investig. Dermatol. Symp. Proc. 2009, 14, 36–43. [Google Scholar] [CrossRef]
- McDaniel, D.H.; Dover, J.S.; Wortzman, M.; Nelson, D.B. In vitro and in vivo evaluation of a moisture treatment cream containing three critical elements of natural skin moisturization. J. Cosmet. Dermatol. 2020, 19, 1121–1128. [Google Scholar] [CrossRef]
- Contet-Audonneau, J.L.; Jeanmaire, C.; Pauly, G. A histological study of human wrinkle structures: Comparison between sun-exposed areas of the face, with or without wrinkles, and sun-protected areas. Br. J. Dermatol. 1999, 140, 1038–1047. [Google Scholar] [CrossRef]
- Kezic, S.; Kammeyer, A.; Calkoen, F.; Fluhr, J.W.; Bos, J.D. Natural moisturizing factor components in the stratum corneum as biomarkers of filaggrin genotype: Evaluation of minimally invasive methods. Br. J. Dermatol. 2009, 161, 1098–1104. [Google Scholar] [CrossRef]
- Marionnet, C.; Pierrard, C.; Vioux-Chagnoleau, C.; Sok, J.; Asselineau, D.; Bernerd, F. Interactions between fibroblasts and keratinocytes in morphogenesis of dermal epidermal junction in a model of reconstructed skin. J. Investig. Dermatol. 2006, 126, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Iriyama, S.; Yasuda, M.; Nishikawa, S.; Takai, E.; Hosoi, J.; Amano, S. Decrease of laminin-511 in the basement membrane due to photoaging reduces epidermal stem/progenitor cells. Sci. Rep. 2020, 10, 12592. [Google Scholar] [CrossRef]
- Riley, P.A. Melanin. Int. J. Biochem. Cell Biol. 1997, 29, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Asselineau, D.; Bernhard, B.; Bailly, C.; Darmon, M. Epidermal morphogenesis and induction of the 67 kD keratin polypeptide by culture of human keratinocytes at the liquid-air interface. Exp. Cell Res. 1985, 159, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Bernerd, F.; Asselineau, D. Successive alteration and recovery of epidermal differentiation and morphogenesis after specific UVB-damages in skin reconstructed in vitro. Dev. Biol. 1997, 183, 123–138. [Google Scholar] [CrossRef]


| Name | No. | Zeta Potential (mV) | Mobility (cm2/Vs) | E Field (V/cm) |
|---|---|---|---|---|
| Low-molecular-weight peptides | 1 | −1.08 | −8.438 × 10−6 | −16.19 |
| 2 | −0.63 | −4.902 × 10−6 | −16.19 | |
| 3 | −0.81 | −6.326 × 10−6 | −16.19 | |
| Average | −0.84 | −6.555 × 10−6 | −16.19 | |
| STD | 0.23 | 1.779 × 10−6 | 0.00 | |
| Low-molecular-weight anionic peptides | 1 | −8.96 | −6.989 × 10−5 | −16.03 |
| 2 | −8.77 | −6.840 × 10−5 | −16.02 | |
| 3 | −8.43 | −6.574 × 10−5 | −16.02 | |
| Average | −8.72 | −6.801 × 10−5 | −16.02 | |
| STD | 0.27 | 2.102 × 10−6 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, H.-J.; Sim, S.-A.; Park, M.-H.; Ryu, H.-S.; Choi, W.-Y.; Park, S.-M.; Lee, J.-N.; Hyun, C.-G. Anti-Photoaging Effects of Upcycled Citrus junos Seed Anionic Peptides on Ultraviolet-Radiation-Induced Skin Aging in a Reconstructed Skin Model. Int. J. Mol. Sci. 2024, 25, 1711. https://doi.org/10.3390/ijms25031711
Ko H-J, Sim S-A, Park M-H, Ryu H-S, Choi W-Y, Park S-M, Lee J-N, Hyun C-G. Anti-Photoaging Effects of Upcycled Citrus junos Seed Anionic Peptides on Ultraviolet-Radiation-Induced Skin Aging in a Reconstructed Skin Model. International Journal of Molecular Sciences. 2024; 25(3):1711. https://doi.org/10.3390/ijms25031711
Chicago/Turabian StyleKo, Hyun-Ju, Su-An Sim, Mi-Hee Park, Hwa-Sun Ryu, Won-Yeong Choi, Sung-Min Park, Jung-No Lee, and Chang-Gu Hyun. 2024. "Anti-Photoaging Effects of Upcycled Citrus junos Seed Anionic Peptides on Ultraviolet-Radiation-Induced Skin Aging in a Reconstructed Skin Model" International Journal of Molecular Sciences 25, no. 3: 1711. https://doi.org/10.3390/ijms25031711
APA StyleKo, H.-J., Sim, S.-A., Park, M.-H., Ryu, H.-S., Choi, W.-Y., Park, S.-M., Lee, J.-N., & Hyun, C.-G. (2024). Anti-Photoaging Effects of Upcycled Citrus junos Seed Anionic Peptides on Ultraviolet-Radiation-Induced Skin Aging in a Reconstructed Skin Model. International Journal of Molecular Sciences, 25(3), 1711. https://doi.org/10.3390/ijms25031711







