Prognostic Impact of Heat Shock Protein 90 Expression in Women Diagnosed with Cervical Cancer
Abstract
1. Introduction
2. Results
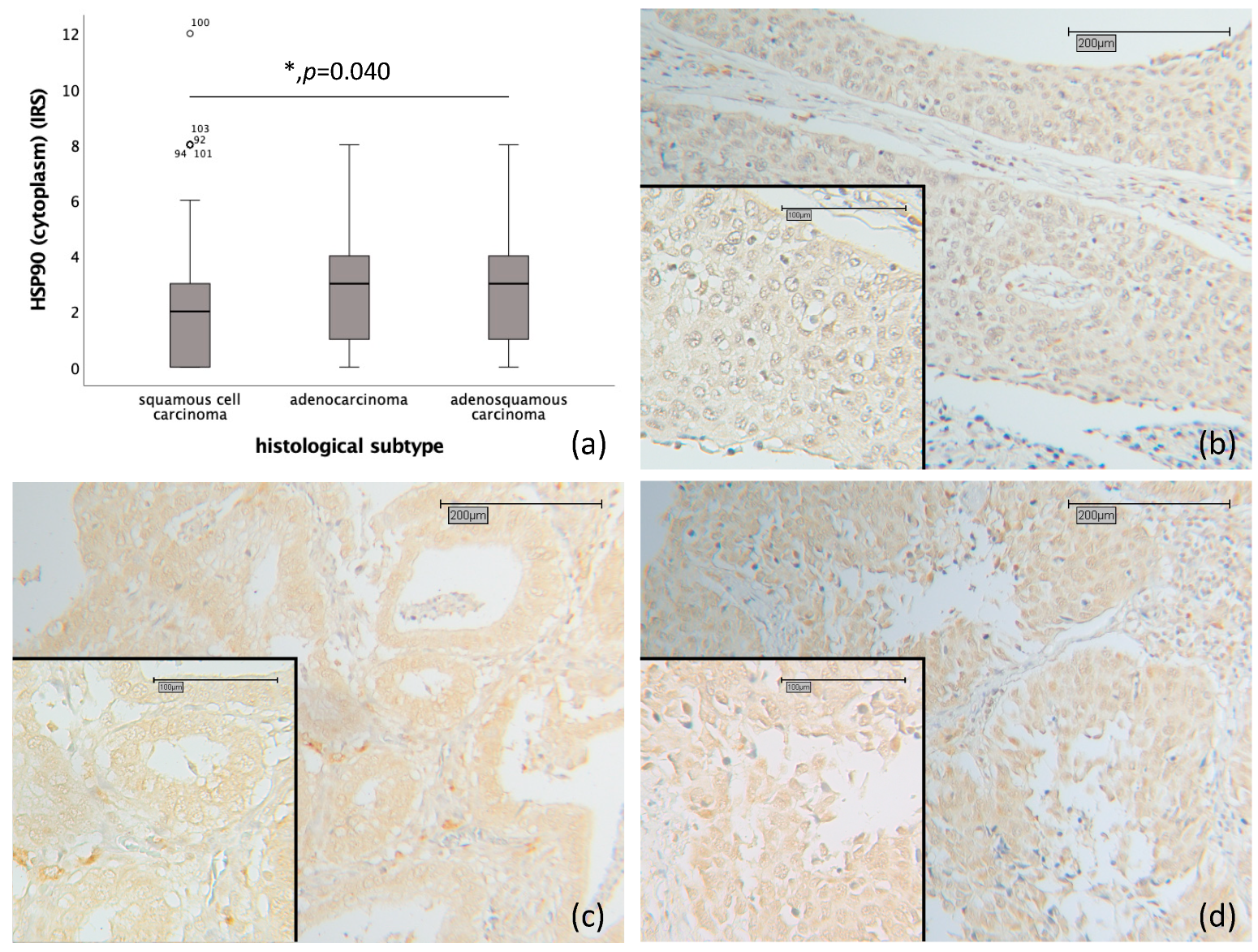
2.1. Correlation of HSP90 with Histology, Grading and TNM- and FIGO-Classification
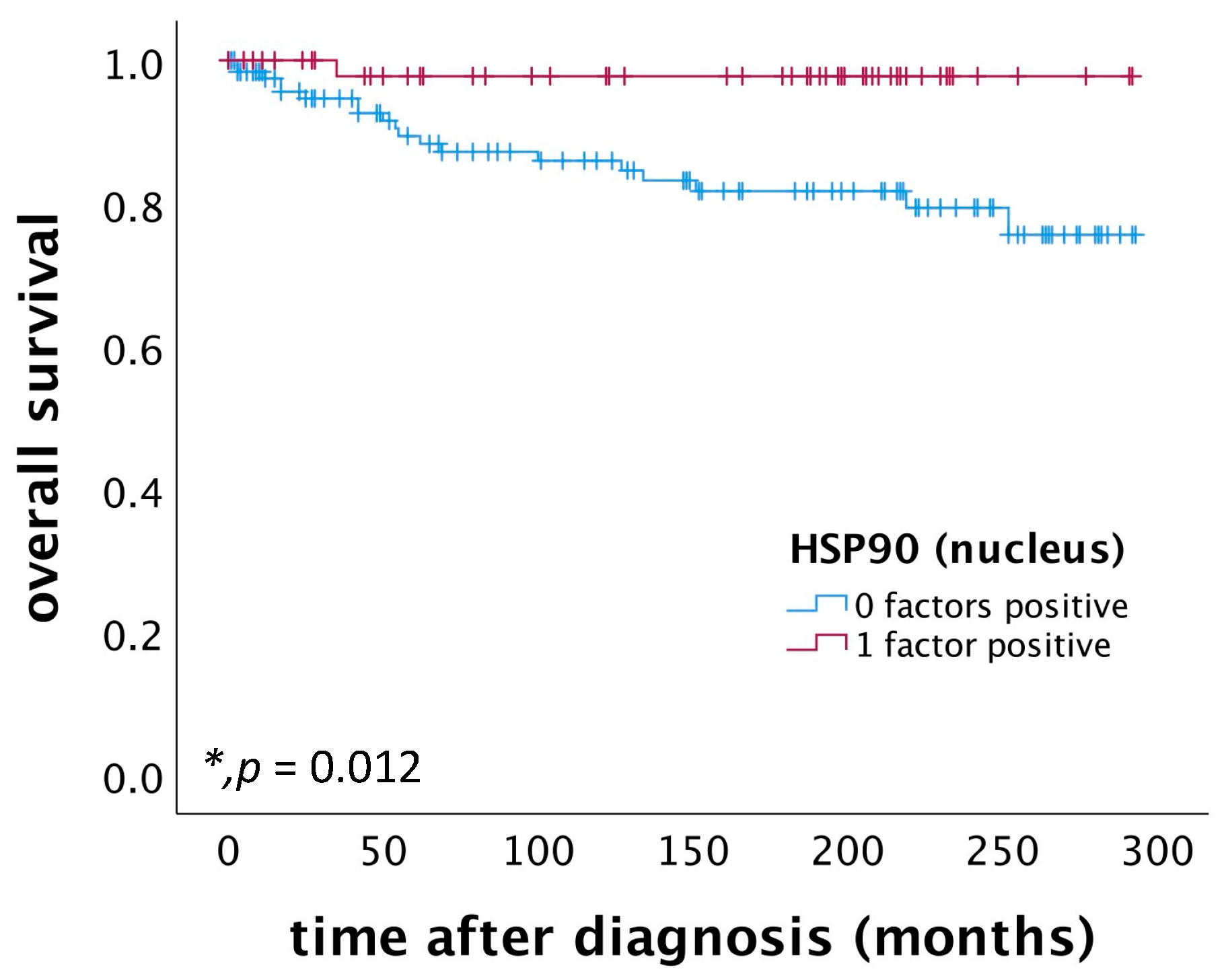
2.2. High Nuclear HSP90 Expression as a Positive Prognostic Factor for OS in Patients Diagnosed with Cervical Squamous Cell Carcinoma
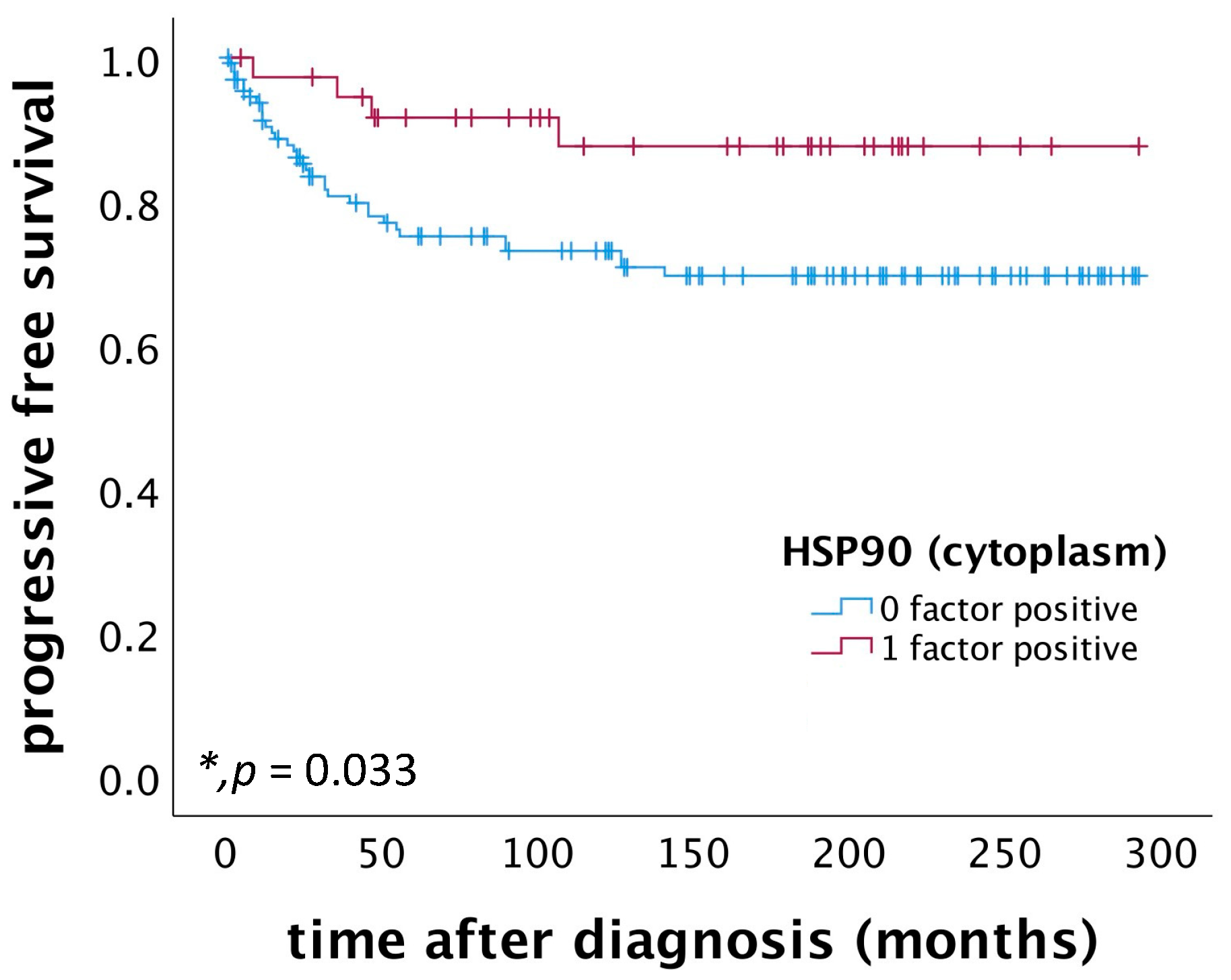
2.3. High Cytoplasmic HSP90 Expression Correlates with Improved PFS in Patients Diagnosed with Cervical Squamous Cell Carcinoma
2.4. Correlation of Nuclear and Cytoplasmic HSP90 Expression with Histopathological Parameters
3. Discussion
4. Materials and Methods
4.1. Characteristics of Patients and Biopsies
4.2. Immunohistochemistry
4.3. Quantification
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| hr-HPV | high-risk human papillomavirus |
| RIPK3 | receptor-interacting serine/threonine-protein kinase 3 |
| MLKL | mixed lineage kinase domain-like protein |
| RIPK1 | receptor-interacting serine/threonine-protein kinase 1 |
| OS | overall survival |
| PFS | progression-free survival |
| HSP | heat shock protein |
| IRS | immunoreactive score |
| TMA | tissue microarray |
| PBS | phosphate buffer saline |
References
- World Health Organization. Cervical Cancer. Updated 20 January 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (accessed on 17 November 2023).
- Young, R.H.; Clement, P.B. Endocervical adenocarcinoma and its variants: Their morphology and differential diagnosis. Histopathology 2002, 41, 185–207. [Google Scholar] [CrossRef]
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): S3-Leitlinie Diagnostik, Therapie und Nachsorge der Patientin mit Zervixkarzinom, Langversion, 2.1, 2021, AWMF-Registernummer: 032/033OL. Available online: https://www.leitlinienprogramm-onkologie.de/leitlinien/zervixkarzinom/ (accessed on 25 February 2022).
- Bosch, F.X.; Lorincz, A.; Muñoz, N.; Meijer, C.J.; Shah, K.V. The causal relation between human papillomavirus and cervical cancer. J. Clin. Pathol. 2002, 55, 244–265. [Google Scholar] [CrossRef]
- Moody, C.A.; Laimins, L.A. Human papillomavirus oncoproteins: Pathways to transformation. Nat. Rev. Cancer 2010, 10, 550–560. [Google Scholar] [CrossRef] [PubMed]
- MMa, W.; Tummers, B.; van Esch, E.M.; Goedemans, R.; Melief, C.J.; Meyers, C.; Boer, J.M.; van der Burg, S.H. Human Papillomavirus Downregulates the Expression of IFITM1 and RIPK3 to Escape from IFNγ- and TNFα-Mediated Antiproliferative Effects and Necroptosis. Front. Immunol. 2016, 7, 496. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Vogelsang, T.L.R.; Kast, V.; Bagnjuk, K.; Eubler, K.; Jeevanandan, S.P.; Schmoeckel, E.; Trebo, A.; Topalov, N.E.; Mahner, S.; Mayr, D.; et al. RIPK1 and RIPK3 are positive prognosticators for cervical cancer patients and C2 ceramide can inhibit tumor cell proliferation in vitro. Front. Oncol. 2023, 13, 1110939. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, Q.; Cai, W.; Ruan, W. Trends of cervical cancer at global, regional, and national level: Data from the Global Burden of Disease study 2019. BMC Public Health 2021, 21, 894. [Google Scholar] [CrossRef] [PubMed]
- Drolet, M.; Bénard, É.; Pérez, N.; Brisson, M. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: Updated systematic review and meta-analysis. Lancet 2019, 394, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Atun, R.; Jaffray, D.A.; Barton, M.B.; Bray, F.; Baumann, M.; Vikram, B.; Hanna, T.P.; Knaul, F.M.; Lievens, Y.; Lui, T.Y.M.; et al. Expanding global access to radiotherapy. Lancet Oncol. 2015, 16, 1153–1186. [Google Scholar] [CrossRef] [PubMed]
- Hartl, F.U.; Hayer-Hartl, M. Converging concepts of protein folding in vitro and in vivo. Nat. Struct. Mol. Biol. 2009, 16, 574–581. [Google Scholar] [CrossRef]
- Saibil, H. Chaperone machines for protein folding, unfolding and disaggregation. Nat. Rev. Mol. Cell Biol. 2013, 14, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Schopf, F.H.; Biebl, M.M.; Buchner, J. The HSP90 chaperone machinery. Nat. Rev. Mol. Cell Biol. 2017, 18, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Hartl, F.U.; Bracher, A.; Hayer-Hartl, M. Molecular chaperones in protein folding and proteostasis. Nature 2011, 475, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Workman, P.; Burrows, F.; Neckers, L.; Rosen, N. Drugging the cancer chaperone HSP90: Combinatorial therapeutic exploitation of oncogene addiction and tumor stress. Ann. N. Y. Acad. Sci. 2007, 1113, 202–216. [Google Scholar] [CrossRef]
- Jacobsen, A.V.; Silke, J. The importance of being chaperoned: HSP90 and necroptosis. Cell Chem. Biol. 2016, 23, 205–207. [Google Scholar] [CrossRef]
- Li, D.; Xu, T.; Cao, Y.; Wang, H.; Li, L.; Chen, S.; Wang, X.; Shen, Z. A cytosolic heat shock protein 90 and cochaperone CDC37 complex is required for RIP3 activation during necroptosis. Proc. Natl. Acad. Sci. USA 2015, 112, 5017–5022. [Google Scholar] [CrossRef]
- Li, D.; Li, C.; Li, L.; Chen, S.; Wang, L.; Li, Q.; Wang, X.; Lei, X.; Shen, Z. Natural Product Kongensin A is a Non-Canonical HSP90 Inhibitor that Blocks RIP3-dependent Necroptosis. Cell Chem. Biol. 2016, 23, 257–266. [Google Scholar] [CrossRef]
- Lewis, J.; Devin, A.; Miller, A.; Lin, Y.; Rodriguez, Y.; Neckers, L.; Liu, Z.-G. Disruption of hsp90 function results in degradation of the death domain kinase, receptor-interacting protein (RIP), and blockage of tumor necrosis factor-induced nuclear factor-kappaB activation. J. Biol. Chem. 2000, 275, 10519–10526. [Google Scholar] [CrossRef]
- Zhao, X.M.; Chen, Z.; Zhao, J.B.; Zhang, P.P.; Pu, Y.F.; Jiang, S.H.; Hou, J.J.; Cui, Y.M.; Jia, X.L.; Zhang, S.Q. Hsp90 modulates the stability of MLKL and is required for TNF-induced necroptosis. Cell Death Dis. 2016, 7, e2089. [Google Scholar] [CrossRef]
- Jacobsen, A.V.; Lowes, K.N.; Tanzer, M.C.; Lucet, I.S.; Hildebrand, J.M.; Petrie, E.J.; van Delft, M.F.; Liu, Z.; A Conos, S.; Zhang, J.-G.; et al. HSP90 activity is required for MLKL oligomerisation and membrane translocation and the induction of necroptotic cell death. Cell Death Dis. 2016, 7, e2051. [Google Scholar] [CrossRef]
- Hoter, A.; El-Sabban, M.E.; Naim, H.Y. The HSP90 Family: Structure, Regulation, Function, and Implications in Health and Disease. Int. J. Mol. Sci. 2018, 19, 2560. [Google Scholar] [CrossRef]
- Calderwood, S.K.; Neckers, L. Hsp90 in Cancer: Transcriptional Roles in the Nucleus. Adv. Cancer Res. 2016, 129, 89–106. [Google Scholar]
- Biebl, M.M.; Buchner, J. Structure, Function, and Regulation of the Hsp90 Machinery. Cold Spring Harb. Perspect. Biol. 2019, 11, a034017. [Google Scholar] [CrossRef]
- Birbo, B.; Madu, E.E.; Madu, C.O.; Jain, A.; Lu, Y. Role of HSP90 in Cancer. Int. J. Mol. Sci. 2021, 22, 10317. [Google Scholar] [CrossRef] [PubMed]
- Dimas, D.T.; Perlepe, C.D.; Sergentanis, T.N.; Misitzis, I.; Kontzoglou, K.; Patsouris, E.; Kouraklis, G.; Psaltopoulou, T.; Nonni, A. The Prognostic Significance of Hsp70/Hsp90 Expression in Breast Cancer: A Systematic Review and Meta-analysis. Anticancer. Res. 2018, 38, 1551–1562. [Google Scholar] [PubMed]
- Cheng, Q.; Chang, J.T.; Geradts, J.; Neckers, L.M.; Haystead, T.; Spector, N.L.; Lyerly, H.K. Amplification and high-level expression of heat shock protein 90 marks aggressive phenotypes of human epidermal growth factor receptor 2 negative breast cancer. Breast Cancer Res. 2012, 14, R62. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, S.; Li, Z.; Li, D.; Zhan, Q. High expression of HSP90 is associated with poor prognosis in patients with colorectal cancer. PeerJ 2019, 7, e7946. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, K.; Chandarlapaty, S.; Lake, D.; Gilewski, T.; Robson, M.; Goldfarb, S.; Drullinsky, P.; Sugarman, S.; Wasserheit-Leiblich, C.; Fasano, J.; et al. A phase II open-label study of ganetespib, a novel heat shock protein 90 inhibitor for patients with metastatic breast cancer. Clin. Breast Cancer 2014, 14, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Kong, A.; Rea, D.; Ahmed, S.; Beck, J.T.; López López, R.; Biganzoli, L.; Armstrong, A.C.; Aglietta, M.; Alba, E.; Campone, M.; et al. Phase 1B/2 study of the HSP90 inhibitor AUY922 plus trastuzumab in metastatic HER2-positive breast cancer patients who have progressed on trastuzumab-based regimen. Oncotarget 2016, 7, 37680–37692. [Google Scholar] [CrossRef] [PubMed]
- Barrott, J.J.; Haystead, T.A. Hsp90, an unlikely ally in the war on cancer. FEBS J. 2013, 280, 1381–1396. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Geng, Y.; Lu, X.; Shi, Y.; Wu, G.; Zhang, M.; Shan, B.; Pan, H.; Yuan, J. Chaperone-mediated autophagy is involved in the execution of ferroptosis. Proc. Natl. Acad. Sci. USA 2019, 116, 2996–3005. [Google Scholar] [CrossRef]
- Yu, X.; Mao, M.; Liu, X.; Shen, T.; Li, T.; Yu, H.; Zhang, J.; Chen, X.; Zhao, X.; Zhu, D. A cytosolic heat shock protein 90 and co-chaperone p23 complex activates RIPK3/MLKL during necroptosis of endothelial cells in acute respiratory distress syndrome. J. Mol. Med. 2020, 98, 569–583. [Google Scholar] [CrossRef]
- Marunouchi, T.; Nishiumi, C.; Iinuma, S.; Yano, E.; Tanonaka, K. Effects of Hsp90 inhibitor on the RIP1-RIP3-MLKL pathway during the development of heart failure in mice. Eur. J. Pharmacol. 2021, 898, 173987. [Google Scholar] [CrossRef]
- Walerych, D.; Kudla, G.; Gutkowska, M.; Wawrzynow, B.; Muller, L.; King, F.W.; Helwak, A.; Boros, J.; Zylicz, A.; Zylicz, M. Hsp90 chaperones wild-type p53 tumor suppressor protein. J. Biol. Chem. 2004, 279, 48836–48845. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, T.; Su, W.; Dou, Z.; Zhao, D.; Jin, X.; Lei, H.; Wang, J.; Xie, X.; Cheng, B.; et al. Mutant p53 in cancer: From molecular mechanism to therapeutic modulation. Cell Death Dis. 2022, 13, 974. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Marchenko, N.D.; Schulz, R.; Fischer, V.; Velasco-Hernandez, T.; Talos, F.; Moll, U.M. Functional inactivation of endogenous MDM2 and CHIP by HSP90 causes aberrant stabilization of mutant p53 in human cancer cells. Mol. Cancer Res. 2011, 9, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Schwock, J.; Pham, N.A.; Cao, M.P.; Hedley, D.W. Efficacy of Hsp90 inhibition for induction of apoptosis and inhibition of growth in cervical carcinoma cells in vitro and in vivo. Cancer Chemother. Pharmacol. 2008, 61, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Tummers, B.; Green, D.R. The evolution of regulated cell death pathways in animals and their evasion by pathogens. Physiol. Rev. 2022, 102, 411–454. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Kroemer, G. Cytoplasmic functions of the tumour suppressor p53. Nature 2009, 458, 1127–1130. [Google Scholar] [CrossRef] [PubMed]
- Remmele, W.; Stegner, H.E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 1987, 8, 138–140. [Google Scholar]

| Covariate | Hazard Ratio | 95% CI | p-Value |
|---|---|---|---|
| patient’s age (<49 years vs. ≥49 years) | 1.372 | 0.540–3.488 | 0.507 |
| tumor grading | 0.986 | 0.895–1.086 | 0.769 |
| extent of the primary tumor [7] | 2.510 | 1.048–6.015 | 0.039 * |
| nodal status (pNX/0 vs. pN1) | 2.311 | 0.813–6.570 | 0.116 |
| FIGO classification | 0.407 | 0.171–0.971 | 0.043 * |
| positive nuclear HSP90 expression | 0.127 | 0.017–0.957 | 0.045 * |
| Covariate | Hazard Ratio | 95% CI | p-Value |
|---|---|---|---|
| patient’s age (<49 years vs. ≥49 years) | 1.032 | 0.525–2.030 | 0.927 |
| tumor grading | 0.986 | 0.902–1.077 | 0.751 |
| extent of the primary tumor [7] | 2.104 | 1.096–4.038 | 0.025 * |
| nodal status (pNX/0 vs. pN1) | 2.438 | 1.167–5.091 | 0.018 * |
| FIGO classification | 0.496 | 0.259–0.950 | 0.034 * |
| positive cytoplasmic HSP90 expression | 0.316 | 0.108–0.923 | 0.035 * |
| Number of Cases (Total Number of Cases: n = 250) | % | |
|---|---|---|
| Histopathological tumor subtype | ||
| squamous cell carcinoma | 194 | 77.6 |
| adenocarcinoma | 34 | 13.6 |
| adenosquamous carcinoma | 15 | 6.0 |
| unknown | 7 | 2.8 |
| Tumor grading | ||
| G1 | 21 | 8.4 |
| G2 | 143 | 57.2 |
| G3 | 78 | 31.2 |
| unknown | 8 | 3.2 |
| Extent of primary tumor [7] | ||
| pT1 | 107 | 42.8 |
| pT2 | 126 | 50.4 |
| pT3 | 9 | 3.6 |
| pT4 | 1 | 0.4 |
| unknown | 7 | 2.8 |
| Regional lymph node involvement (pN) | ||
| pN0 | 145 | 58.0 |
| pN1 | 98 | 39.2 |
| unknown | 7 | 2.8 |
| Presence of distant metastatic spread (pM) | ||
| pM0 | 2 | 0.8 |
| pM1 | 7 | 2.8 |
| pMX | 235 | 94 |
| unknown | 6 | 2.4 |
| FIGO classification | ||
| FIGO I | 79 | 31.6 |
| FIGO II | 64 | 25.6 |
| FIGO III | 93 | 37.2 |
| FIGO IV | 7 | 2.8 |
| unknown | 7 | 2.8 |
| Progression | ||
| none | 180 | 72.0 |
| at least one | 63 | 25.2 |
| unknown | 7 | 2.8 |
| Survival | ||
| censured | 211 | 84.4 |
| dead (tumor-dependent) | 33 | 13.2 |
| unknown | 6 | 2.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vogelsang, T.L.R.; Schmoeckel, E.; Topalov, N.E.; Ganster, F.; Mahner, S.; Jeschke, U.; Vattai, A. Prognostic Impact of Heat Shock Protein 90 Expression in Women Diagnosed with Cervical Cancer. Int. J. Mol. Sci. 2024, 25, 1571. https://doi.org/10.3390/ijms25031571
Vogelsang TLR, Schmoeckel E, Topalov NE, Ganster F, Mahner S, Jeschke U, Vattai A. Prognostic Impact of Heat Shock Protein 90 Expression in Women Diagnosed with Cervical Cancer. International Journal of Molecular Sciences. 2024; 25(3):1571. https://doi.org/10.3390/ijms25031571
Chicago/Turabian StyleVogelsang, Tilman L. R., Elisa Schmoeckel, Nicole Elisabeth Topalov, Franziska Ganster, Sven Mahner, Udo Jeschke, and Aurelia Vattai. 2024. "Prognostic Impact of Heat Shock Protein 90 Expression in Women Diagnosed with Cervical Cancer" International Journal of Molecular Sciences 25, no. 3: 1571. https://doi.org/10.3390/ijms25031571
APA StyleVogelsang, T. L. R., Schmoeckel, E., Topalov, N. E., Ganster, F., Mahner, S., Jeschke, U., & Vattai, A. (2024). Prognostic Impact of Heat Shock Protein 90 Expression in Women Diagnosed with Cervical Cancer. International Journal of Molecular Sciences, 25(3), 1571. https://doi.org/10.3390/ijms25031571






