Bioinformatics and Experimental Validation for Identifying Biomarkers Associated with AMG510 (Sotorasib) Resistance in KRASG12C-Mutated Lung Adenocarcinoma
Abstract
1. Introduction
2. Results
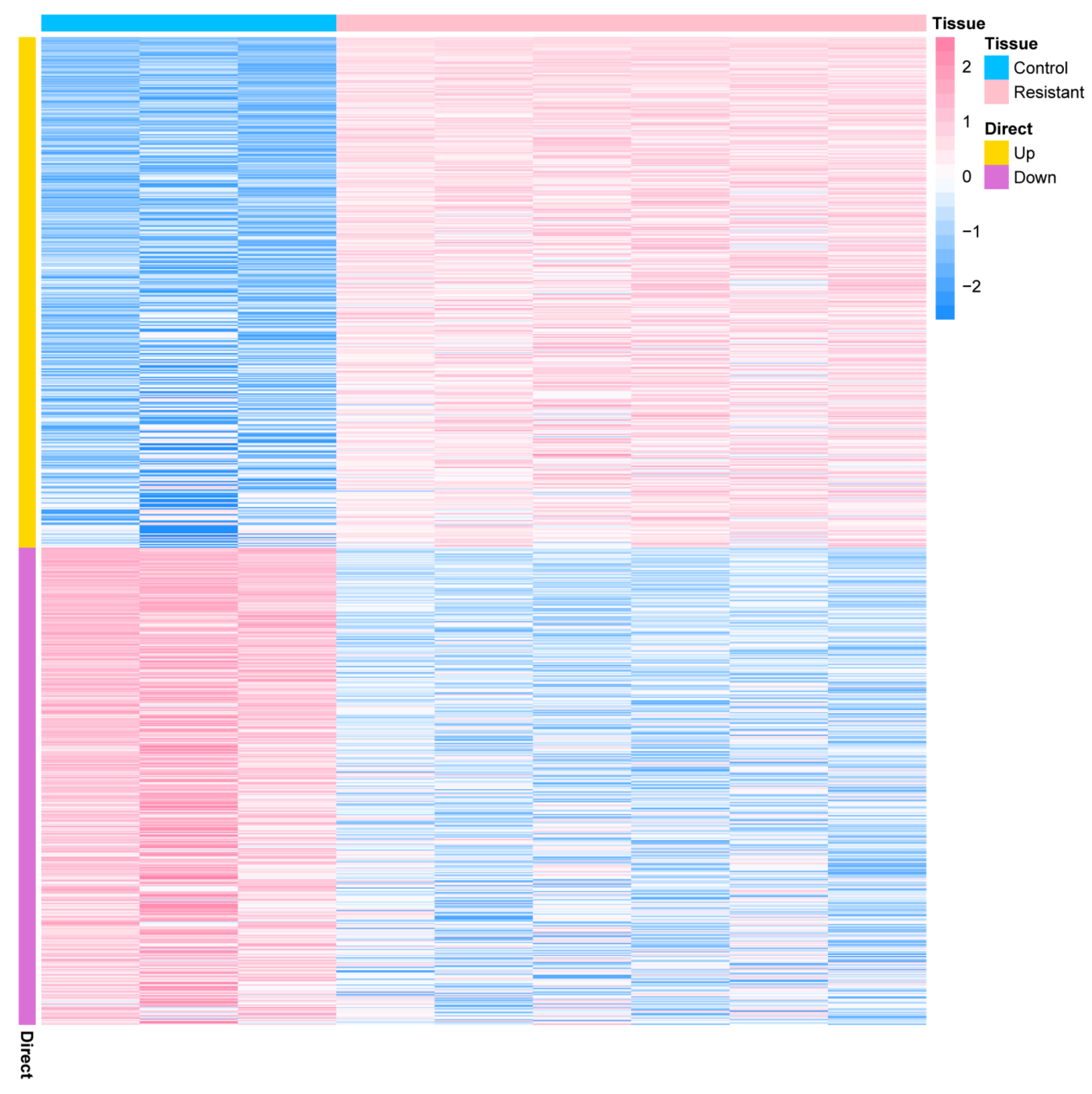
2.1. Identification and Functional Enrichment Analysis of the DEGs in a PDX Model of AMG510 Resistance of LUAD
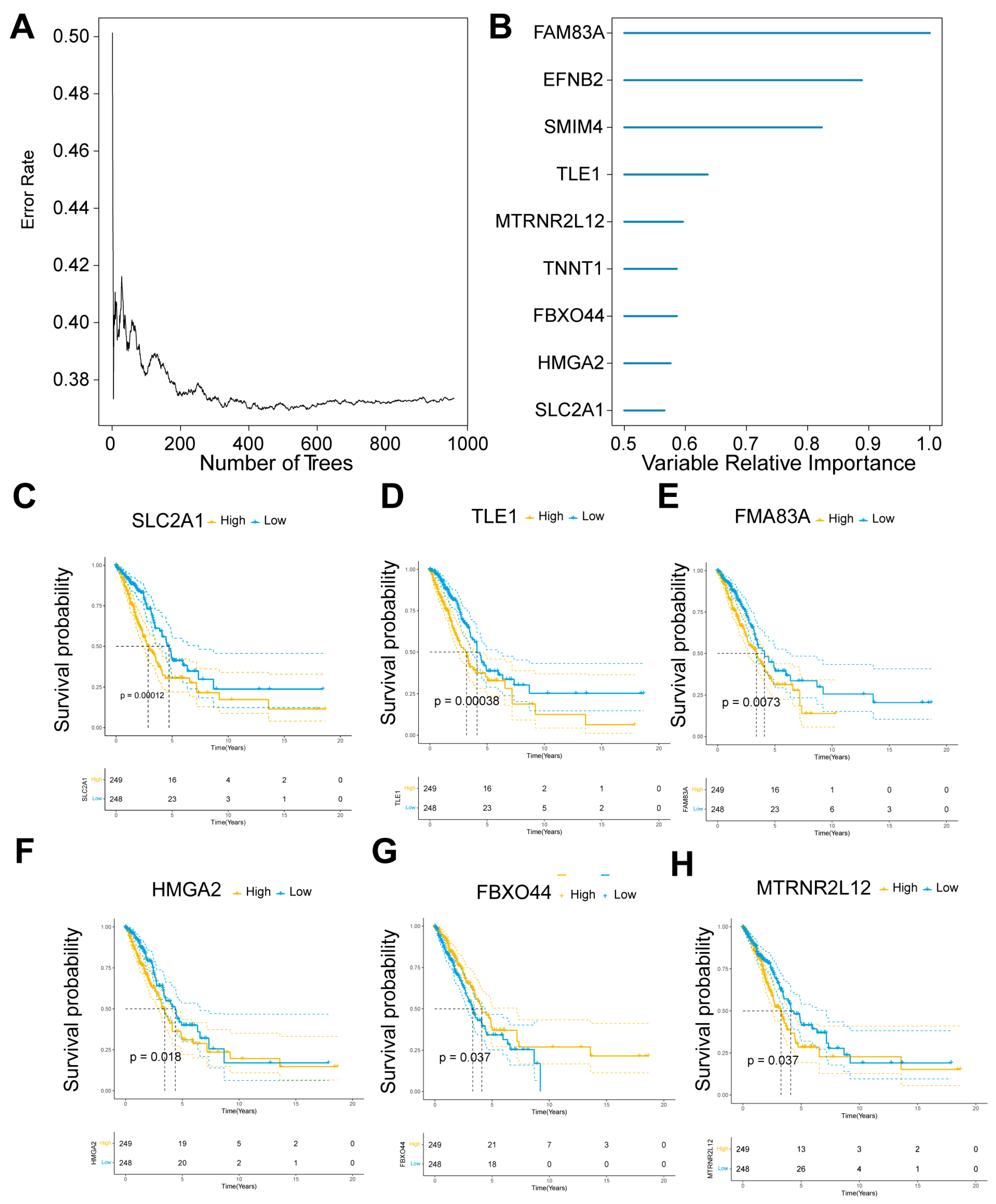
2.2. Key Molecular Screening of AMG510 Resistance in LUAD
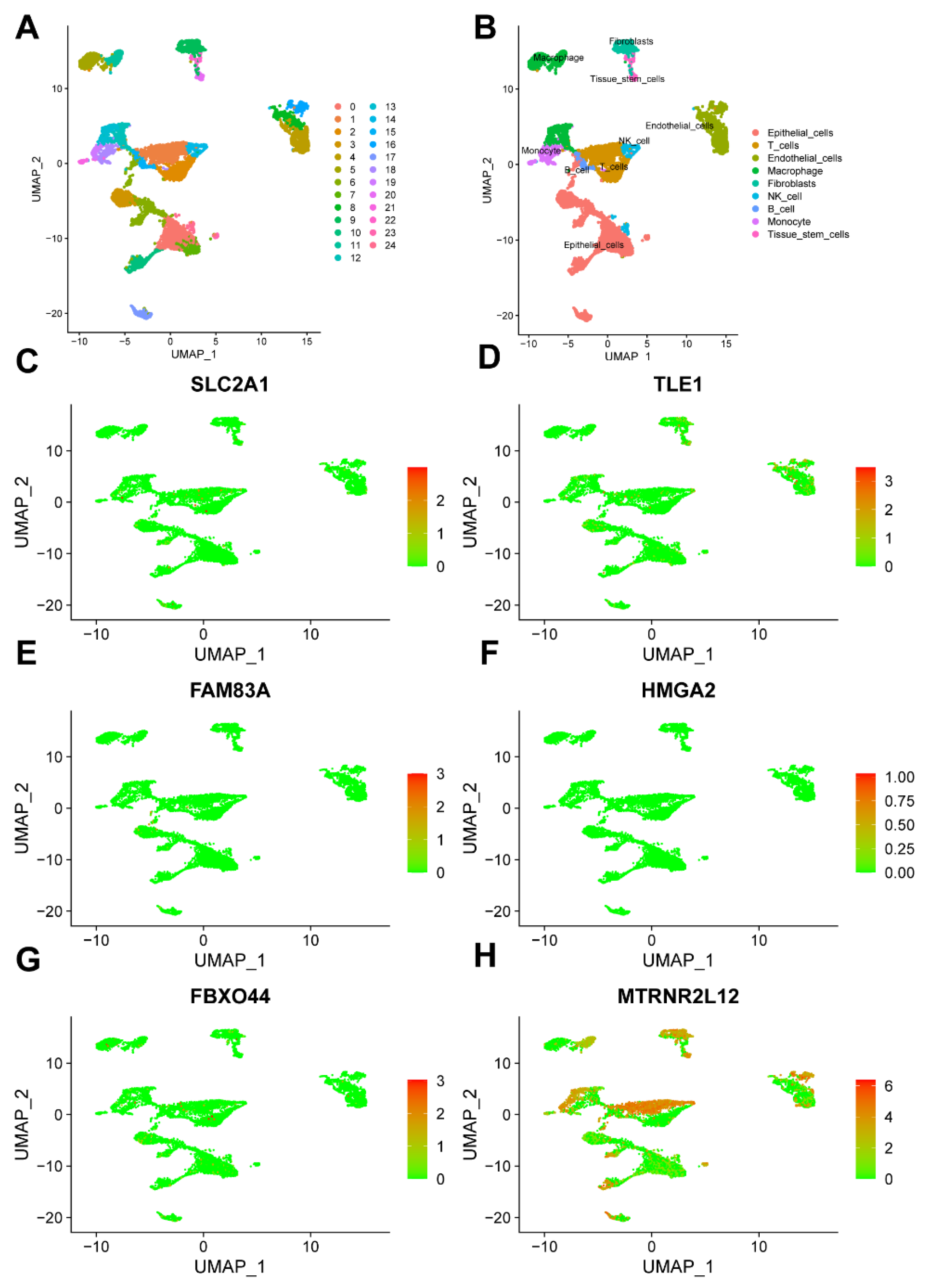
2.3. Key Molecules in AMG510 Resistance in LUAD Associated with Tumor Immune Cell Infiltration
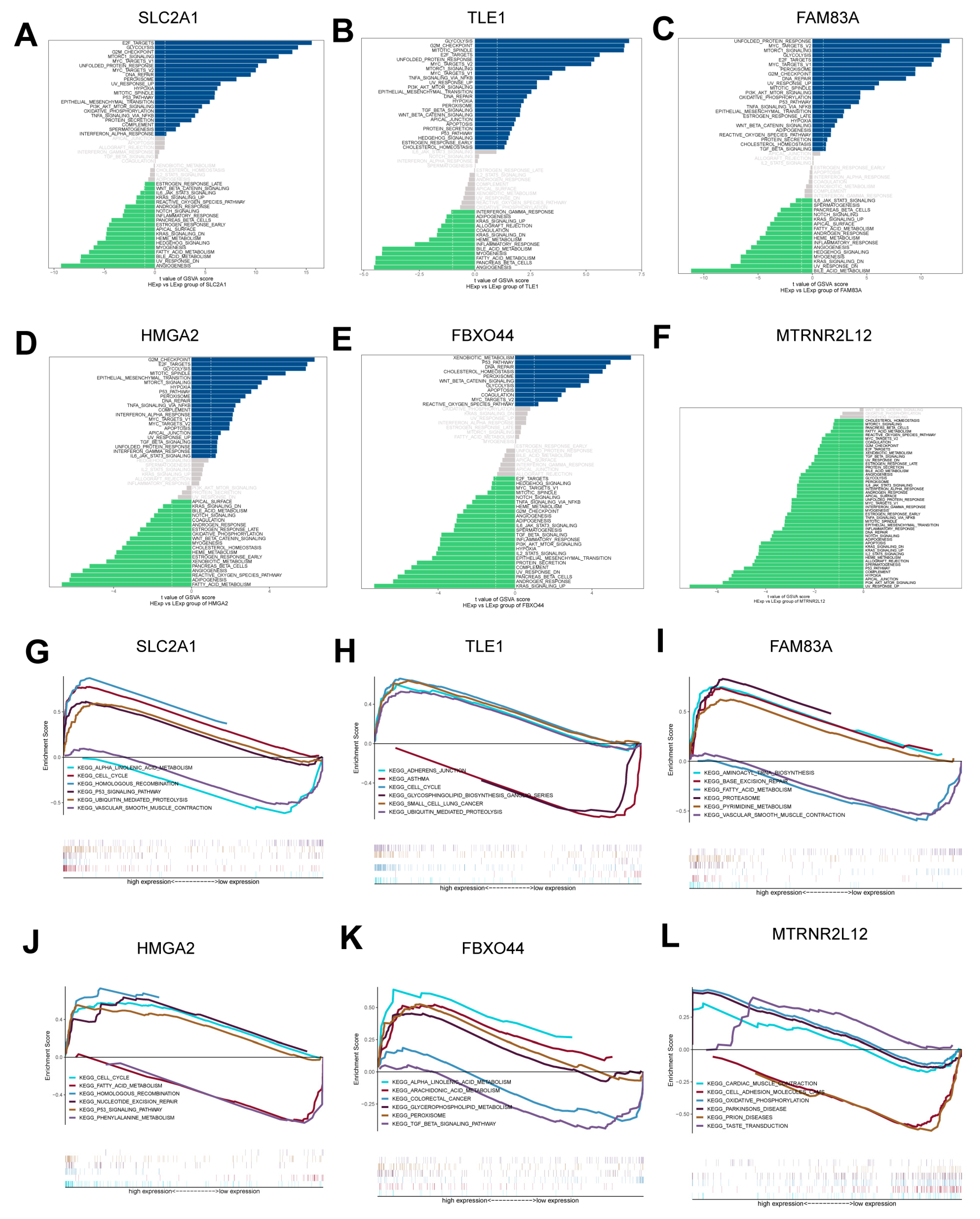
2.4. Pathway Enrichment Analysis of Key Molecules for AMG510 Resistance in LUAD
2.5. Construction of Nomogram and Exploitation of Calibration Curves for Prediction of Prognosis of LUAD Patients by Key Molecules in AMG510 Resistance
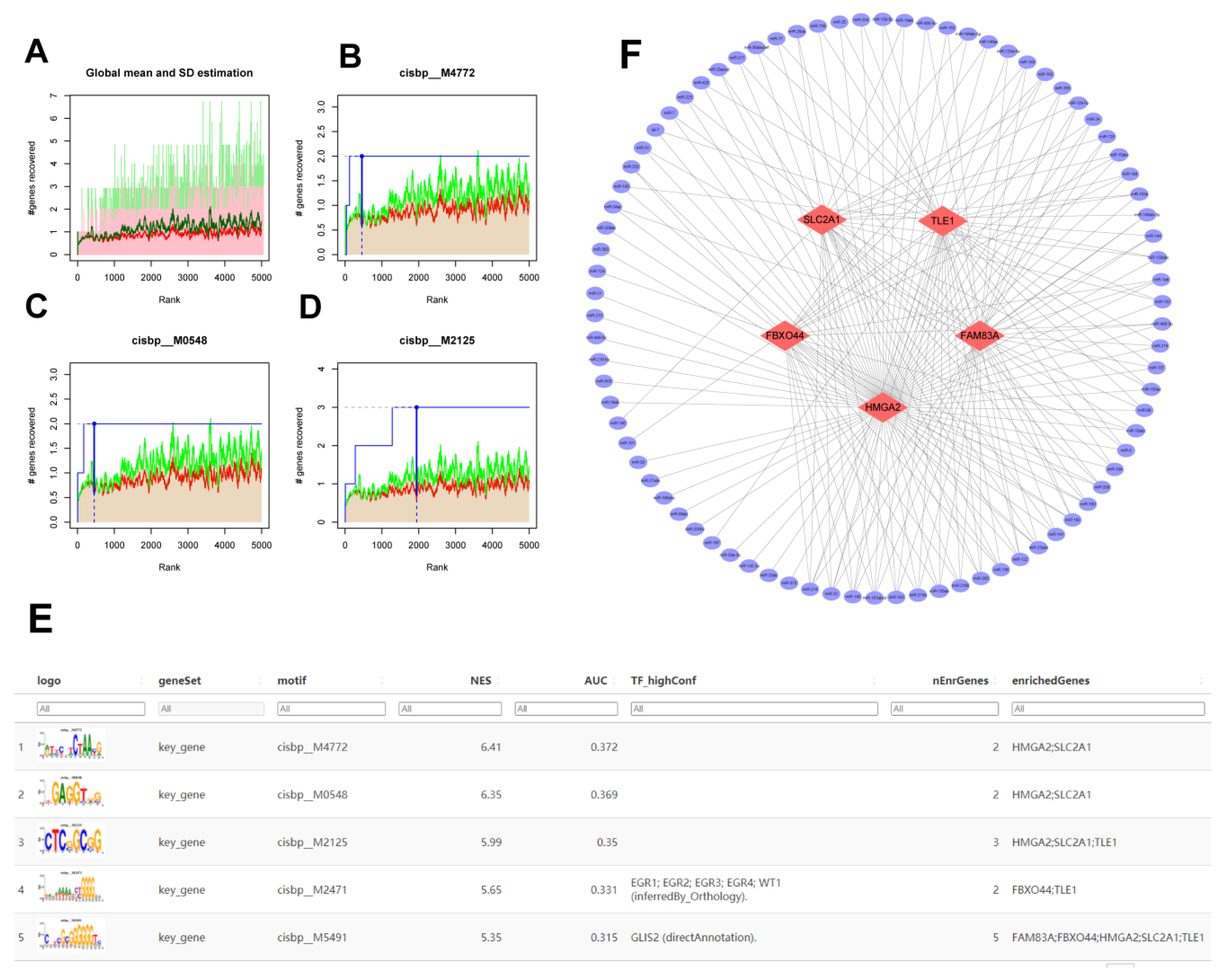
2.6. Analysis of the Regulatory Network of Transcription Factors (TFs) Involved in Key Molecules of AMG510 Resistance
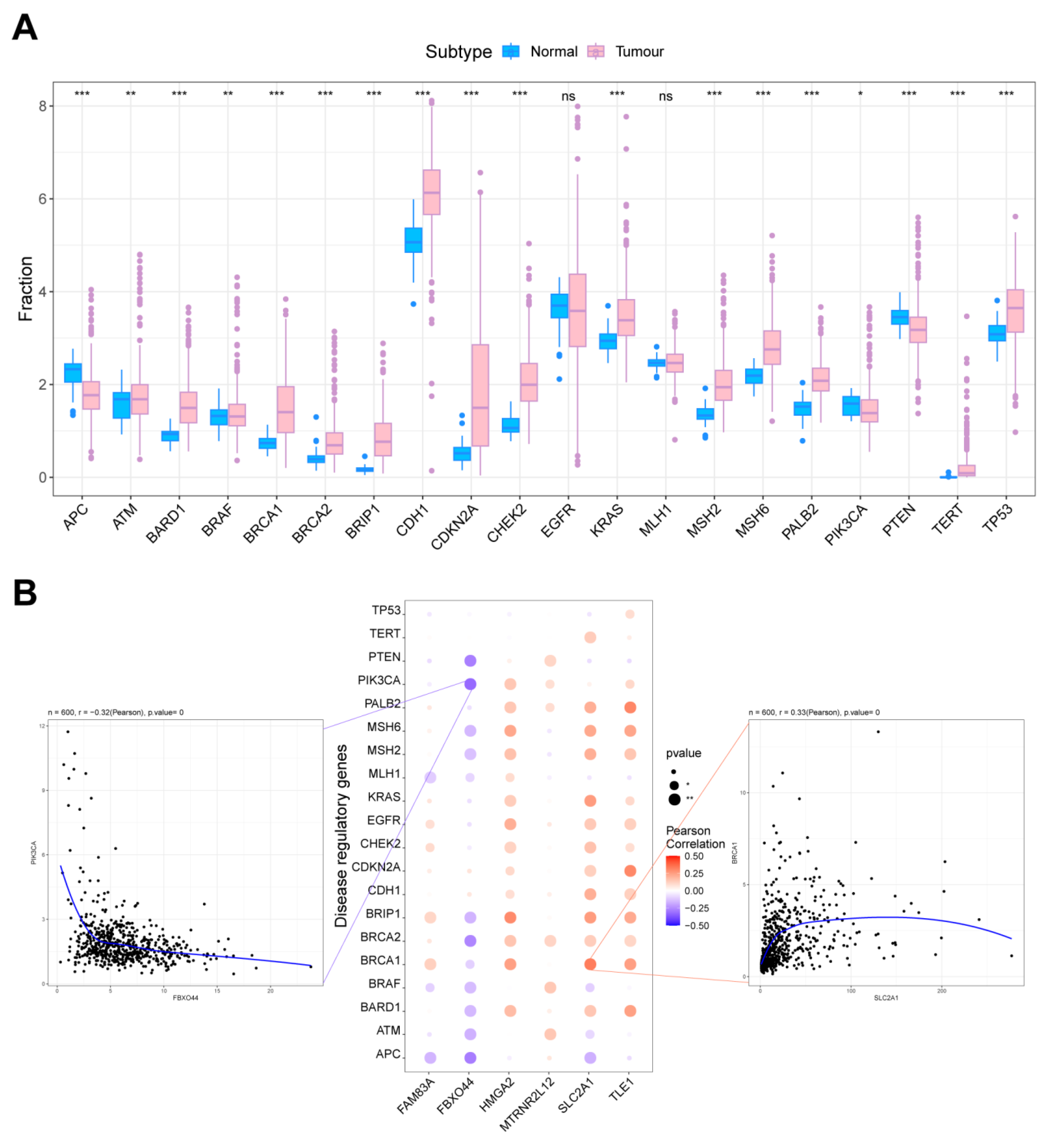
2.7. Correlation Analysis of Key Molecules in AMG510 Resistance with LUAD Oncogenes
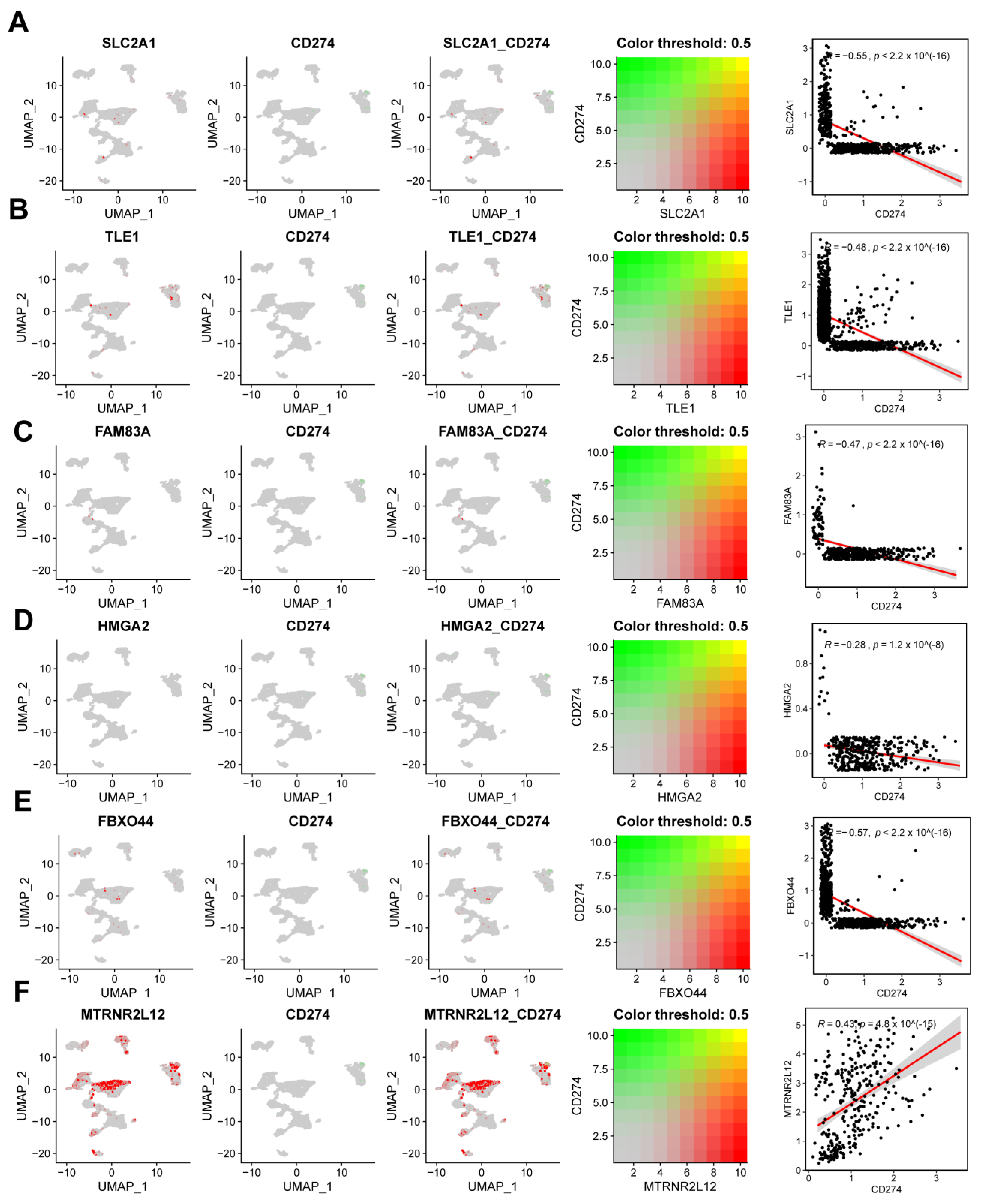
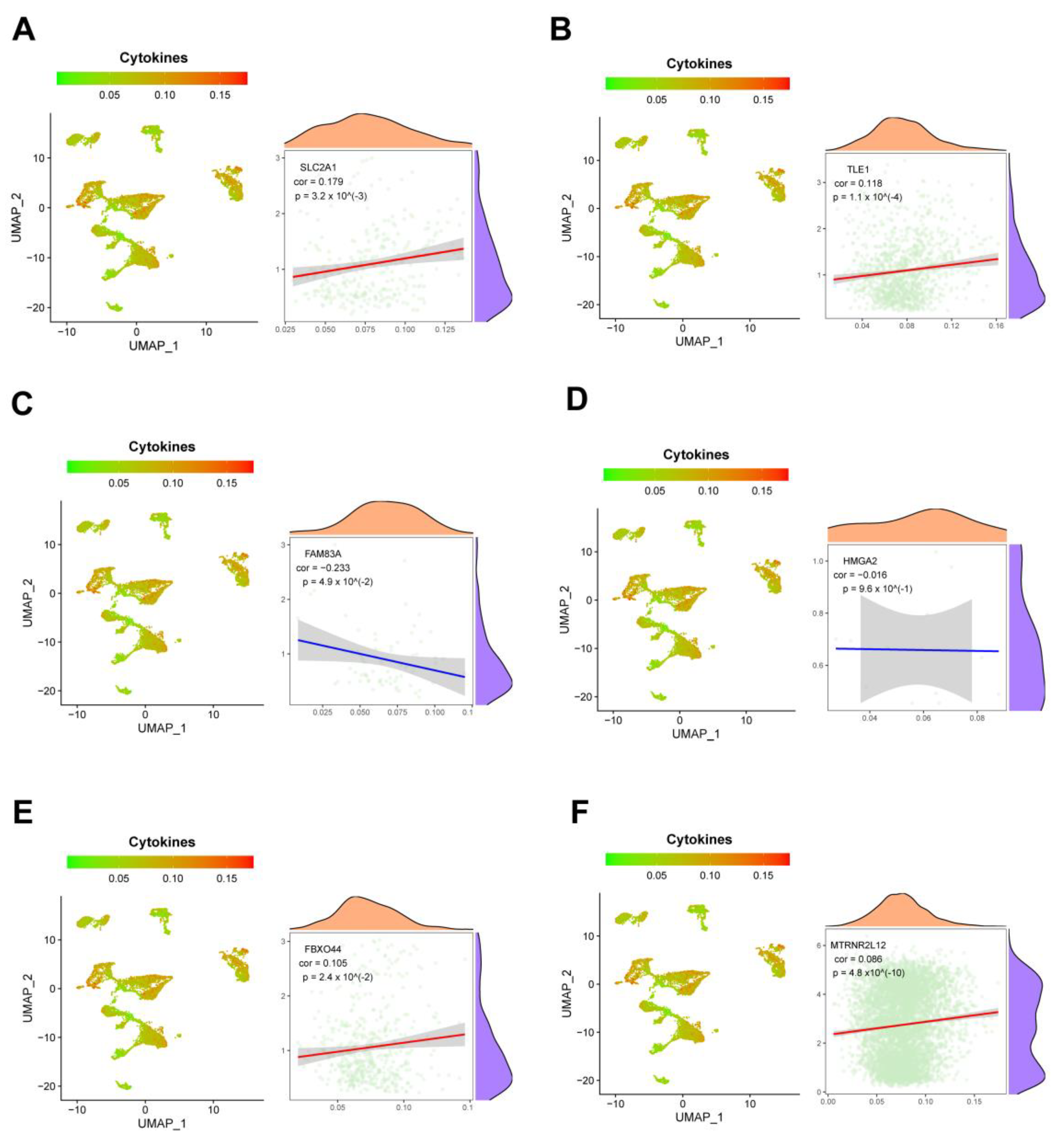
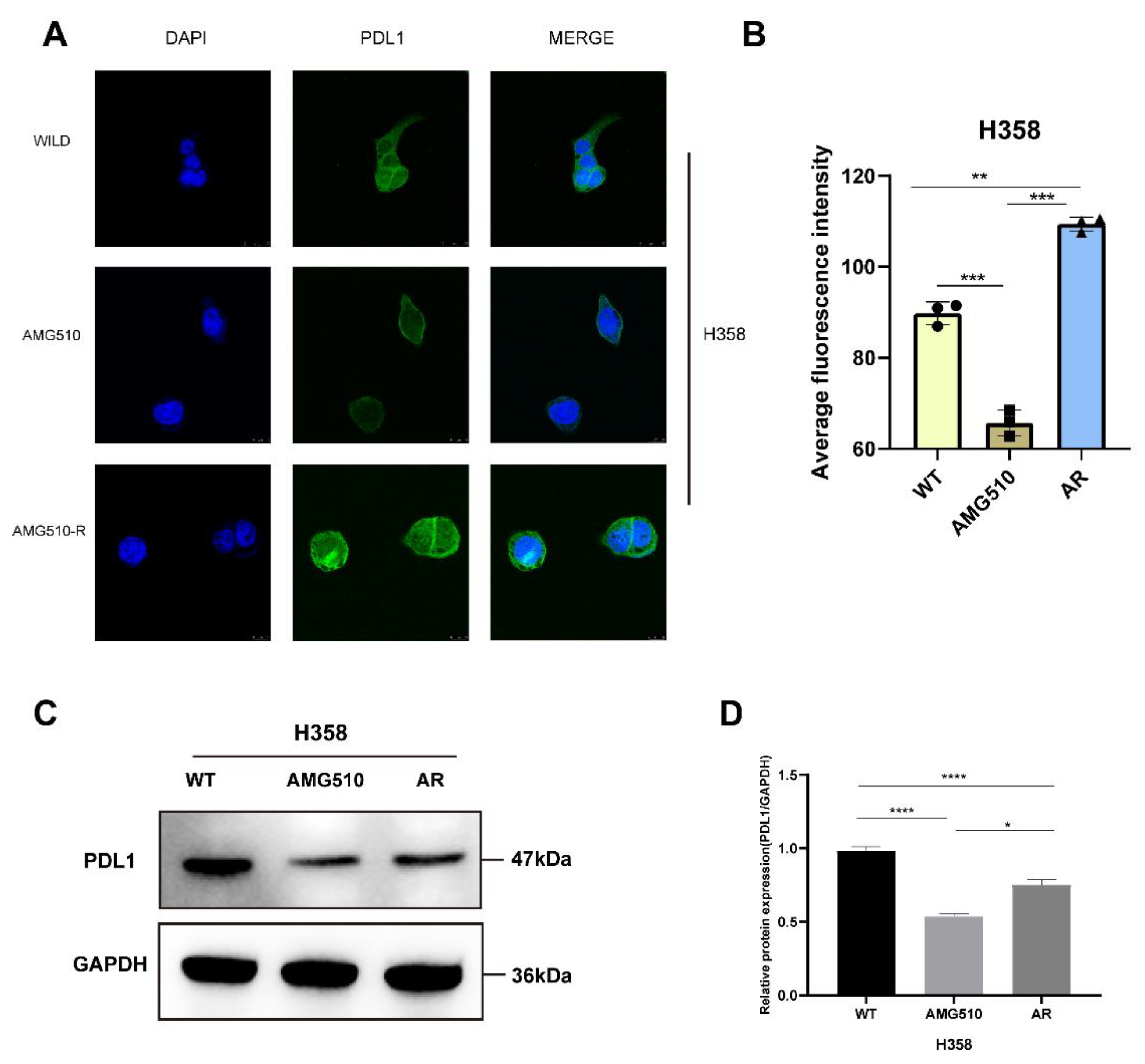
2.8. Single-Cell Analysis Revealed Correlations between Key Molecules in AMG510 Resistance and PD-L1, Cytokines, and Factors
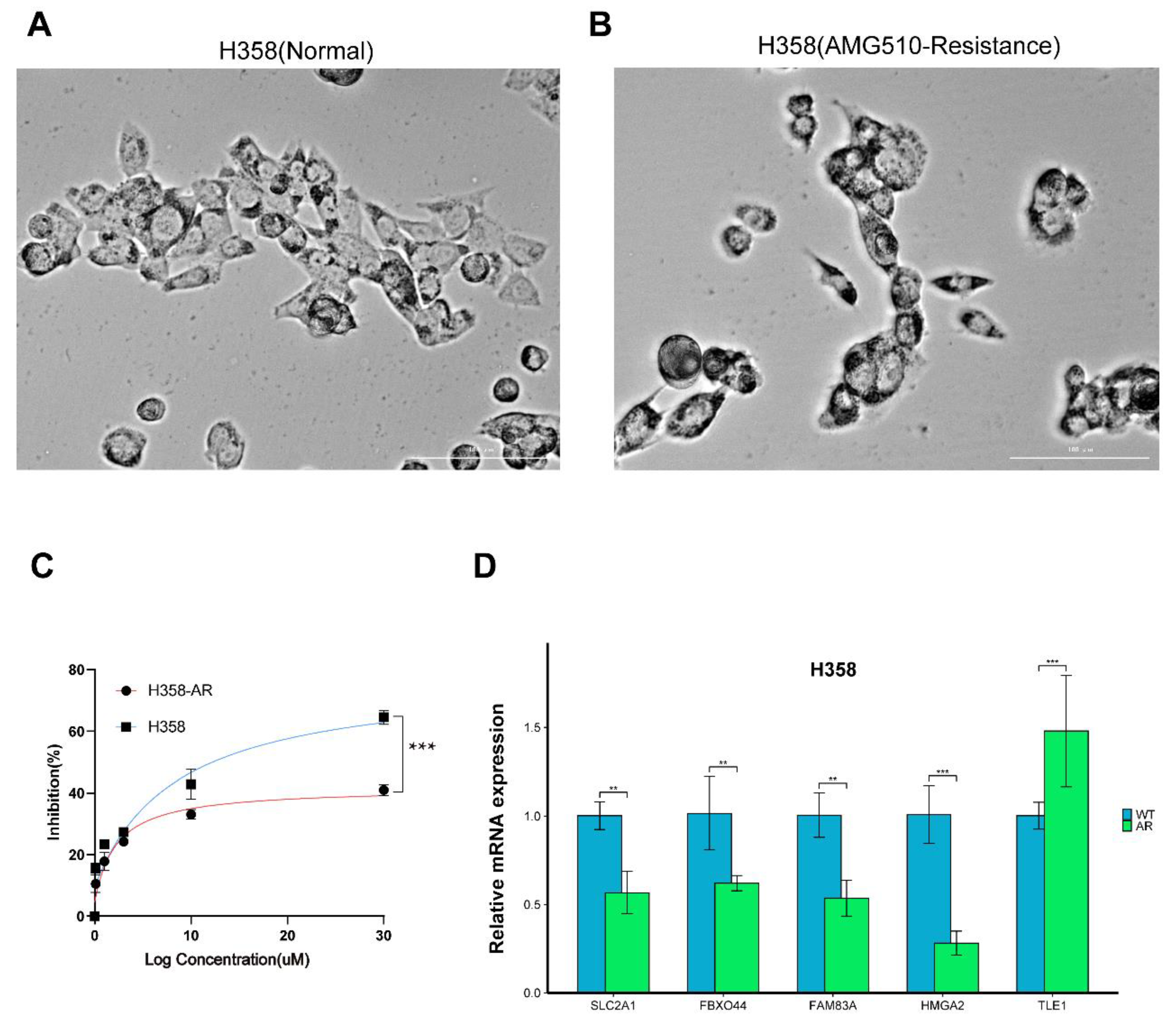
2.9. Construction and Experimental Validation of the AMG510 Treatment-Resistant LUAD Cell Line
3. Discussions
4. Materials and Methods
4.1. Data Downloads and Analyses
4.2. Randomized Survival Forest Analyses
4.3. Immune Cell Infiltration Analyses
4.4. GSVA Analyses
4.5. GSEA Analyses
4.6. Nomogram Modeling
4.7. miRNA Analyses
4.8. Regulatory Network Analyses of Biomarkers
4.9. Single-Cell Analyses (SCAs)
4.10. Cell Cultures and Reagents
4.11. CCK-8 Assay
4.12. Quantitative Reverse Transcription PCR (qRT-PCR)
4.13. Immunofluorescence
4.14. Western Blotting
4.15. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bade, B.C.; Dela Cruz, C.S. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin. Chest Med. 2020, 41, 1–24. [Google Scholar] [CrossRef]
- Salehi-Rad, R.; Li, R.; Paul, M.K.; Dubinett, S.M.; Liu, B. The Biology of Lung Cancer: Development of More Effective Methods for Prevention, Diagnosis, and Treatment. Clin. Chest Med. 2020, 41, 25–38. [Google Scholar] [CrossRef]
- Minna, J.D.; Roth, J.A.; Gazdar, A.F. Focus on lung cancer. Cancer Cell 2002, 1, 49–52. [Google Scholar] [CrossRef]
- Abu Rous, F.; Singhi, E.K.; Sridhar, A.; Faisal, M.S.; Desai, A. Lung Cancer Treatment Advances in 2022. Cancer Investig. 2023, 41, 12–24. [Google Scholar] [CrossRef]
- Miettinen, O.S. Curability of lung cancer. Expert. Rev. Anticancer Ther. 2007, 7, 399–401. [Google Scholar] [CrossRef][Green Version]
- Yang, Y.; Zhang, H.; Huang, S.; Chu, Q. KRAS Mutations in Solid Tumors: Characteristics, Current Therapeutic Strategy, and Potential Treatment Exploration. J. Clin. Med. 2023, 12, 709. [Google Scholar] [CrossRef]
- Menyhárd, D.K.; Pálfy, G.; Orgován, Z.; Vida, I.; Keserű, G.M.; Perczel, A. Structural impact of GTP binding on downstream KRAS signaling. Chem. Sci. 2020, 11, 9272–9289. [Google Scholar] [CrossRef]
- Hillig, R.C.; Sautier, B.; Schroeder, J.; Moosmayer, D.; Hilpmann, A.; Stegmann, C.M.; Werbeck, N.D.; Briem, H.; Boemer, U.; Weiske, J.; et al. Discovery of potent SOS1 inhibitors that block RAS activation via disruption of the RAS-SOS1 interaction. Proc. Natl. Acad. Sci. USA 2019, 116, 2551–2560. [Google Scholar] [CrossRef] [PubMed]
- Yen, I.; Shanahan, F.; Merchant, M.; Orr, C.; Hunsaker, T.; Durk, M.; La, H.; Zhang, X.; Martin, S.E.; Lin, E.; et al. Pharmacological Induction of RAS-GTP Confers RAF Inhibitor Sensitivity in KRAS Mutant Tumors. Cancer Cell 2018, 34, 611–625.e7. [Google Scholar] [CrossRef] [PubMed]
- Timar, J.; Kashofer, K. Molecular epidemiology and diagnostics of KRAS mutations in human cancer. Cancer Metastasis Rev. 2020, 39, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Ambrogio, C.; Köhler, J.; Zhou, Z.W.; Wang, H.; Paranal, R.; Li, J.; Capelletti, M.; Caffarra, C.; Li, S.; Lv, Q.; et al. KRAS Dimerization Impacts MEK Inhibitor Sensitivity and Oncogenic Activity of Mutant KRAS. Cell 2018, 172, 857–868.e15. [Google Scholar] [CrossRef]
- Golay, H.G.; Barbie, D.A. Targeting cytokine networks in KRAS-driven tumorigenesis. Expert. Rev. Anticancer Ther. 2014, 14, 869–871. [Google Scholar] [CrossRef]
- Nussinov, R.; Wang, G.; Tsai, C.J.; Jang, H.; Lu, S.; Banerjee, A.; Zhang, J.; Gaponenko, V. Calmodulin and PI3K Signaling in KRAS Cancers. Trends Cancer 2017, 3, 214–224. [Google Scholar] [CrossRef]
- Rodriguez-Viciana, P.; Tetsu, O.; Oda, K.; Okada, J.; Rauen, K.; McCormick, F. Cancer targets in the Ras pathway. Cold Spring Harb. Symp. Quant. Biol. 2005, 70, 461–467. [Google Scholar] [CrossRef]
- Skoulidis, F.; Li, B.T.; Dy, G.K.; Price, T.J.; Falchook, G.S.; Wolf, J.; Italiano, A.; Schuler, M.; Borghaei, H.; Barlesi, F.; et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N. Engl. J. Med. 2021, 384, 2371–2381. [Google Scholar] [CrossRef]
- Zheng, X.; Luo, J.; Liu, W.; Ashby, C.R., Jr.; Chen, Z.S.; Lin, L. Sotorasib: A treatment for non-small cell lung cancer with the KRAS G12C mutation. Drugs Today 2022, 58, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Fakih, M.G.; Kopetz, S.; Kuboki, Y.; Kim, T.W.; Munster, P.N.; Krauss, J.C.; Falchook, G.S.; Han, S.W.; Heinemann, V.; Muro, K.; et al. Sotorasib for previously treated colorectal cancers with KRAS(G12C) mutation (CodeBreaK100): A prespecified analysis of a single-arm, phase 2 trial. Lancet Oncol. 2022, 23, 115–124. [Google Scholar] [CrossRef]
- Veluswamy, R.; Mack, P.C.; Houldsworth, J.; Elkhouly, E.; Hirsch, F.R. KRAS G12C-Mutant Non-Small Cell Lung Cancer: Biology, Developmental Therapeutics, and Molecular Testing. J. Mol. Diagn. 2021, 23, 507–520. [Google Scholar] [CrossRef]
- Zhen, M.C.; Wang, F.Q.; Wu, S.F.; Zhao, Y.L.; Liu, P.G.; Yin, Z.Y. Identification of mTOR as a primary resistance factor of the IAP antagonist AT406 in hepatocellular carcinoma cells. Oncotarget 2017, 8, 9466–9475. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chatterjee, S.; Huang, E.H.; Christie, I.; Kurland, B.F.; Burns, T.F. Acquired Resistance to the Hsp90 Inhibitor, Ganetespib, in KRAS-Mutant NSCLC Is Mediated via Reactivation of the ERK-p90RSK-mTOR Signaling Network. Mol. Cancer Ther. 2017, 16, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chang, J.; Liu, M.; Yuan, J.; Zhang, J.; Qin, J.; Xia, X.; Wang, Y. Quantitative proteomics profiling reveals activation of mTOR pathway in trastuzumab resistance. Oncotarget 2017, 8, 45793–45806. [Google Scholar] [CrossRef]
- Murugan, A.K. mTOR: Role in cancer, metastasis and drug resistance. Semin. Cancer Biol. 2019, 59, 92–111. [Google Scholar] [CrossRef]
- Rodrik-Outmezguine, V.S.; Okaniwa, M.; Yao, Z.; Novotny, C.J.; McWhirter, C.; Banaji, A.; Won, H.; Wong, W.; Berger, M.; de Stanchina, E.; et al. Overcoming mTOR resistance mutations with a new-generation mTOR inhibitor. Nature 2016, 534, 272–276. [Google Scholar] [CrossRef]
- Kohno, K.; Uchiumi, T.; Niina, I.; Wakasugi, T.; Igarashi, T.; Momii, Y.; Yoshida, T.; Matsuo, K.; Miyamoto, N.; Izumi, H. Transcription factors and drug resistance. Eur. J. Cancer 2005, 41, 2577–2586. [Google Scholar] [CrossRef]
- Yu, G.; Chen, Y.; Hu, Y.; Zhou, Y.; Ding, X.; Zhou, X. Roles of transducin-like enhancer of split (TLE) family proteins in tumorigenesis and immune regulation. Front. Cell Dev. Biol. 2022, 10, 1010639. [Google Scholar] [CrossRef]
- Hu, S.; Chen, Z.; Gu, J.; Tan, L.; Zhang, M.; Lin, W. TLE2 is associated with favorable prognosis and regulates cell growth and gemcitabine sensitivity in pancreatic cancer. Ann. Transl. Med. 2020, 8, 1017. [Google Scholar] [CrossRef] [PubMed]
- Shin, T.H.; Brynczka, C.; Dayyani, F.; Rivera, M.N.; Sweetser, D.A. TLE4 regulation of wnt-mediated inflammation underlies its role as a tumor suppressor in myeloid leukemia. Leuk. Res. 2016, 48, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Khoo, B.L.; Chaudhuri, P.K.; Ramalingam, N.; Tan, D.S.; Lim, C.T.; Warkiani, M.E. Single-cell profiling approaches to probing tumor heterogeneity. Int. J. Cancer 2016, 139, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Lawson, D.A.; Kessenbrock, K.; Davis, R.T.; Pervolarakis, N.; Werb, Z. Tumour heterogeneity and metastasis at single-cell resolution. Nat. Cell Biol. 2018, 20, 1349–1360. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Fan, J.; He, Y.; Xiong, A.; Yu, J.; Li, Y.; Zhang, Y.; Zhao, W.; Zhou, F.; Li, W.; et al. Single-cell profiling of tumor heterogeneity and the microenvironment in advanced non-small cell lung cancer. Nat. Commun. 2021, 12, 2540. [Google Scholar] [CrossRef] [PubMed]
- Leader, A.M.; Grout, J.A.; Maier, B.B.; Nabet, B.Y.; Park, M.D.; Tabachnikova, A.; Chang, C.; Walker, L.; Lansky, A.; Le Berichel, J.; et al. Single-cell analysis of human non-small cell lung cancer lesions refines tumor classification and patient stratification. Cancer Cell 2021, 39, 1594–1609.e12. [Google Scholar] [CrossRef]
- Zhang, A.; Miao, K.; Sun, H.; Deng, C.X. Tumor heterogeneity reshapes the tumor microenvironment to influence drug resistance. Int. J. Biol. Sci. 2022, 18, 3019–3033. [Google Scholar] [CrossRef] [PubMed]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in tumor progression and regression: A review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Xu, A.; Xu, J. Roles of PD-1/PD-L1 Pathway: Signaling, Cancer, and Beyond. Adv. Exp. Med. Biol. 2020, 1248, 33–59. [Google Scholar] [PubMed]
- Taylor, J.M. Random Survival Forests. J. Thorac. Oncol. 2011, 6, 1974–1975. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Khodadoust, M.S.; Liu, C.L.; Newman, A.M.; Alizadeh, A.A. Profiling Tumor Infiltrating Immune Cells with CIBERSORT. Methods Mol. Biol. 2018, 1711, 243–259. [Google Scholar]
- Holtsträter, C.; Schrörs, B.; Bukur, T.; Löwer, M. Bioinformatics for Cancer Immunotherapy. Methods Mol. Biol. 2020, 2120, 1–9. [Google Scholar]
- Hänzelmann, S.; Castelo, R.; Guinney, J. GSVA: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013, 14, 7. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Iasonos, A.; Schrag, D.; Raj, G.V.; Panageas, K.S. How to build and interpret a nomogram for cancer prognosis. J. Clin. Oncol. 2008, 26, 1364–1370. [Google Scholar] [CrossRef]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J. Cell Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S.A.; Jolma, A.; Campitelli, L.F.; Das, P.K.; Yin, Y.; Albu, M.; Chen, X.; Taipale, J.; Hughes, T.R.; Weirauch, M.T. The Human Transcription Factors. Cell 2018, 172, 650–665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, D.; Peng, M.; Tang, L.; Ouyang, J.; Xiong, F.; Guo, C.; Tang, Y.; Zhou, Y.; Liao, Q.; et al. Single-cell RNA sequencing in cancer research. J. Exp. Clin. Cancer Res. 2021, 40, 81. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, P.; Cheng, W.; Qi, X.; Zhang, P.; Xiong, J.; Li, J. Bioinformatics and Experimental Validation for Identifying Biomarkers Associated with AMG510 (Sotorasib) Resistance in KRASG12C-Mutated Lung Adenocarcinoma. Int. J. Mol. Sci. 2024, 25, 1555. https://doi.org/10.3390/ijms25031555
Lin P, Cheng W, Qi X, Zhang P, Xiong J, Li J. Bioinformatics and Experimental Validation for Identifying Biomarkers Associated with AMG510 (Sotorasib) Resistance in KRASG12C-Mutated Lung Adenocarcinoma. International Journal of Molecular Sciences. 2024; 25(3):1555. https://doi.org/10.3390/ijms25031555
Chicago/Turabian StyleLin, Peng, Wei Cheng, Xin Qi, Pinglu Zhang, Jianshe Xiong, and Jing Li. 2024. "Bioinformatics and Experimental Validation for Identifying Biomarkers Associated with AMG510 (Sotorasib) Resistance in KRASG12C-Mutated Lung Adenocarcinoma" International Journal of Molecular Sciences 25, no. 3: 1555. https://doi.org/10.3390/ijms25031555
APA StyleLin, P., Cheng, W., Qi, X., Zhang, P., Xiong, J., & Li, J. (2024). Bioinformatics and Experimental Validation for Identifying Biomarkers Associated with AMG510 (Sotorasib) Resistance in KRASG12C-Mutated Lung Adenocarcinoma. International Journal of Molecular Sciences, 25(3), 1555. https://doi.org/10.3390/ijms25031555





