Attention Deficit Hyperactivity Disorder (ADHD) and Polyphenols: A Systematic Review
Abstract
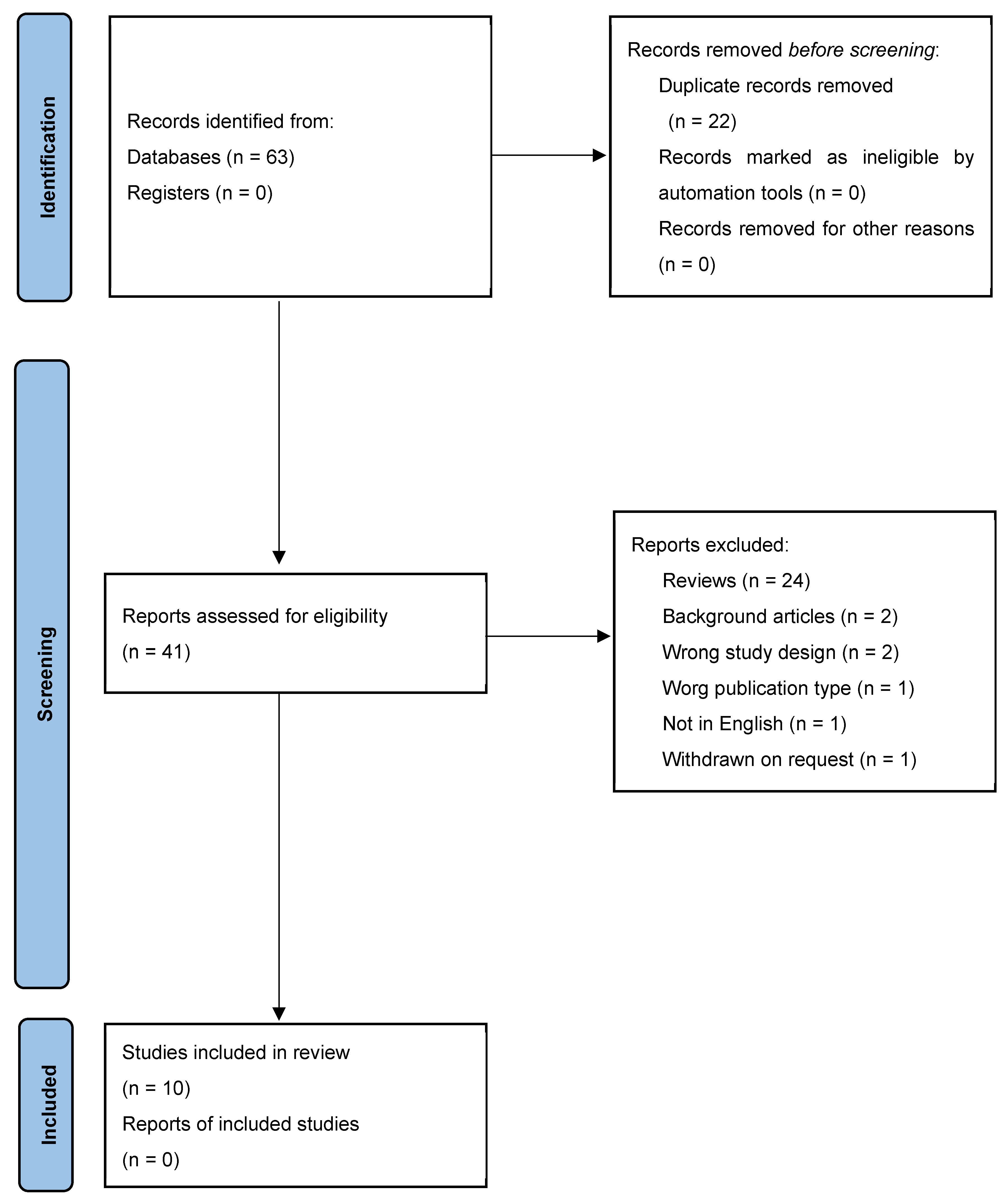
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Extraction of Relevant Data, Quality, and Risk of Bias Assesment
3. Results and Discussion
3.1. Efficacy on ADHD Symptoms: Polyphenols vs. Traditional Drugs and Placebo
3.2. Efficacy of Polyphenols in Rebalancing Oxidative Stress Pathways
3.3. Polyphenols May Be Protective against ADHD
4. Conclusions
5. Limits of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural polyphenols: Chemical classification, definition of classes, subcategories, and structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef]
- Fogliano, V.; Corollaro, M.L.; Vitaglione, P.; Napolitano, A.; Ferracane, R.; Travaglia, F.; Arlorio, M.; Costabile, A.; Klinder, A.; Gibson, G. In vitro bioaccessibility and gut biotransformation of polyphenols present in the water-insoluble cocoa fraction. Mol. Nutr. Food Res. 2011, 55, 44–55. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Gugliandolo, A.; Giacoppo, S.; Ficicchia, M.; Aliquò, A.; Bramanti, P.; Mazzon, E. Eruca sativa seed extract: A novel natural product able to counteract neuroinflammation. Mol. Med. Rep. 2018, 17, 6235–6244. [Google Scholar] [CrossRef]
- Jin, X.; Liu, M.Y.; Zhang, D.F.; Zhong, X.; Du, K.; Qian, P.; Gao, H.; Wei, M.J. Natural products as a potential modulator of microglial polarization in neurodegenerative diseases. Pharmacol. Res. 2019, 145, 104253. [Google Scholar] [CrossRef]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Recent Advances in Nutritional Sciences Glutathione Metabolism and Its Implications for Health 1. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Fão, L.; Mota, S.I.; Rego, A.C. Shaping the Nrf2-ARE-related pathways in Alzheimer’s and Parkinson’s diseases. Ageing Res. Rev. 2019, 54, 100942. [Google Scholar] [CrossRef] [PubMed]
- Rosliuk, D.; Rutkaite, R.; Ivanauskas, L.; Jakstas, V. Interaction between cross-linked cationic starch microgranules and chlorogenic acid isomers in artichoke and green coffee bean aqueous extracts. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2020, 1160, 122385. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Kang, J.B.; Park, D.J.; Kim, M.O.; Koh, P.O. Chlorogenic acid alleviates cerebral ischemia-induced neuroinflammation via attenuating nuclear factor kappa B activation. Neurosci. Lett. 2022, 773, 136495. [Google Scholar] [CrossRef] [PubMed]
- Bagdas, D.; Gul, Z.; Meade, J.A.; Cam, B.; Cinkilic, N.; Gurun, M.S. Pharmacologic Overview of Chlorogenic Acid and its Metabolites in Chronic Pain and Inflammation. Curr. Neuropharmacol. 2019, 18, 216–228. [Google Scholar] [CrossRef]
- Bhandari, R.; Kuhad, A. Resveratrol suppresses neuroinflammation in the experimental paradigm of autism spectrum disorders. Neurochem. Int. 2017, 103, 8–23. [Google Scholar] [CrossRef]
- Ahmad, S.F.; Ansari, M.A.; Nadeem, A.; Bakheet, S.A.; Alzahrani, M.Z.; Alshammari, M.A.; Alanazi, W.A.; Alasmari, A.F.; Attia, S.M. Resveratrol attenuates pro-inflammatory cytokines and activation of JAK1-STAT3 in BTBR T+ Itpr3tf/J autistic mice. Eur. J. Pharmacol. 2018, 829, 70–78. [Google Scholar] [CrossRef]
- Ahmad, S.F.; Ansari, M.A.; Nadeem, A.; Alzahrani, M.Z.; Bakheet, S.A.; Attia, S.M. Resveratrol Improves Neuroimmune Dysregulation Through the Inhibition of Neuronal Toll-Like Receptors and COX-2 Signaling in BTBR T+ Itpr3tf/J Mice. Neuromolecular Med. 2018, 20, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Segal, L.; Penman, M.G.; Piriou, Y. Evaluation of the systemic toxicity and mutagenicity of OLIGOPIN®, procyanidolic oligomers (OPC) extracted from French Maritime Pine Bark extract. Toxicol. Rep. 2018, 5, 531–541. [Google Scholar] [CrossRef]
- Lu, Z.; Pu, C.; Zhang, Y.; Sun, Y.; Liao, Y.; Kang, Z.; Feng, X.; Yue, W. Oxidative Stress and Psychiatric Disorders: Evidence from the Bidirectional Mendelian Randomization Study. Antioxidants 2022, 11, 1386. [Google Scholar] [CrossRef]
- American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Salvi, V.; Ribuoli, E.; Servasi, M.; Orsolini, L.; Volpe, U. Adhd and bipolar disorder in adulthood: Clinical and treatment implications. Medicina 2021, 57, 466. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.A.; Nigg, J.T.; Sullivan, E.L. Neuroinflammation as a risk factor for attention deficit hyperactivity disorder. Pharmacol. Biochem. Behav. 2019, 182, 22–34. [Google Scholar] [CrossRef]
- Li, Y.; Yin, A.; Sun, X.; Zhang, M.; Zhang, J.; Wang, P.; Xie, R.; Li, W.; Fan, Z.; Zhu, Y.; et al. Deficiency of tumor suppressor NDRG2 leads to attention deficit and hyperactive behavior. J. Clin. Investig. 2017, 127, 4270–4284. [Google Scholar] [CrossRef]
- Tripp, G.; Wickens, J.R. Neurobiology of ADHD. Neuropharmacology 2009, 57, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, J. Safety of therapeutic methylphenidate in adults: A systematic review of the evidence. J. Psychopharmacol. 2009, 23, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Weyns, A.S.; Verlaet, A.A.J.; Breynaert, A.; Naessens, T.; Fransen, E.; Verhelst, H.; Van West, D.; Van Ingelghem, I.; Jonckheere, A.I.; Beysen, D.; et al. Clinical Investigation of French Maritime Pine Bark Extract on Attention-Deficit Hyperactivity Disorder as compared to Methylphenidate and Placebo: Part 1: Efficacy in a Randomised Trial. J. Funct. Foods 2022, 97, 105246. [Google Scholar] [CrossRef]
- Hsu, C.D.; Hsieh, L.H.; Chen, Y.L.; Lin, I.C.; Chen, Y.R.; Chen, C.C.; Shirakawa, H.; Yang, S.C. Complementary effects of pine bark extract supplementation on inattention, impulsivity, and antioxidative status in children with attention-deficit hyperactivity disorder: A double-blinded randomized placebo-controlled cross-over study. Phytother. Res. 2021, 35, 3226–3235. [Google Scholar] [CrossRef] [PubMed]
- Trebatická, J.; Kopasová, S.; Hradečná, Z.; Činovský, K.; Škodáček, I.; Šuba, J.; Shirakawa, H.; Yang, S.C. Treatment of ADHD with French maritime pine bark extract, Pycnogenol®. Eur. Child Adolesc. Psychiatry 2006, 15, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Tenenbaum, S.; Paull, J.C.; Sparrow, E.P.; Dodd, D.K.; Green, L. Hyperactivity Disorder (ADHD). J. Atten. Disord. 2002, 6, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Rafeiy-Torghabeh, M.; Ashraf-Ganjouei, A.; Moradi, K.; Bagheri, S.; Mohammadi, M.R.; Akhondzadeh, S. Resveratrol adjunct to methylphenidate improves symptoms of attention-deficit/hyperactivity disorder: A randomized, double-blinded, placebo-controlled clinical trial. Eur. Child Adolesc. Psychiatry 2020, 30, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Weyns, A.S.; Verlaet, A.A.J.; Van Herreweghe, M.; Breynaert, A.; Fransen, E.; De Meester, I.; Logie, E.; Berghe, W.V.; Verhelst, H.; Van West, D.; et al. Clinical Investigation of French Maritime Pine Bark Extract on Attention-Deficit Hyperactivity Disorder as compared to Methylphenidate and Placebo: Part 2: Oxidative Stress and Immunological Modulation. J. Funct. Foods 2022, 97, 105247. [Google Scholar] [CrossRef]
- Dvořáková, M.; Sivoňová, M.; Trebatická, J.; Škodáček, I.; Waczuliková, I.; Muchová, J.; Duracková, Z. The effect of polyphenolic extract from pine bark, Pycnogenol®, on the level of glutathione in children suffering from attention deficit hyperactivity disorder (ADHD). Redox Rep. 2006, 11, 163–172. [Google Scholar] [CrossRef]
- Dvořáková, M.; Ježová, D.; Blažíček, P.; Trebatická, J.; Škodáček, I.; Šuba, J.; Veta, W.; Rohdewald, P.; Duracková, Z. Urinary catecholamines in children with attention deficit hyperactivity disorder (ADHD): Modulation by a polyphenolic extract from pine bark (Pycnogenol®). Nutr. Neurosci. 2007, 10, 151–157. [Google Scholar] [CrossRef]
- Chovanová, Z.; Muchová, J.; Sivoňová, M.; Dvořáková, M.; Žitňanová, I.; Waczulíková, I.; Trebatická, J.; Skodácek, I.; Duracková, Z. Effect of polyphenolic extract, Pycnogenol®, on the level of 8-oxoguanine in children suffering from attention deficit/hyperactivity disorder. Free Radic. Res. 2006, 40, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Darzi, M.; Abbasi, K.; Ghiasvand, R.; Akhavan Tabib, M.; Rouhani, M.H. The association between dietary polyphenol intake and attention-deficit hyperactivity disorder: A case-control study. BMC Pediatr. 2022, 22, 700. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zou, Y.C.; Wang, M.; Huang, Y.F.; Chen, D.X.; Wei, L.B. Neuropeptide Y levels are associated with nutritional status and cardiovascular events in adults with chronic kidney disease. Eur. J. Clin. Nutr. 2015, 69, 717–721. [Google Scholar] [CrossRef] [PubMed]
| D1 | D2 | D3 | D4 | D5 | Overall | |
|---|---|---|---|---|---|---|
| Weyns, et al., 2022 [24] | + | + | + | + | + | + |
| Hsu, et al., 2021 [25] | + | - | + | + | + | - |
| Trebatická, et al., 2006 [26] | + | + | + | + | + | + |
| Tenenbaum, et al., 2002 [27] | + | + | + | - | + | + |
| Rafeiy-Torghabeh, et al., 2020 [28] | + | + | + | + | + | + |
| Weyns, et al., 2022 [29] | + | + | + | + | + | + |
| Dvořáková, et al., 2006 [30] | + | + | + | + | + | + |
| Dvořáková, et al., 2007 [31] | + | + | + | + | + | + |
| Chovanová, et al., 2006 [32] | + | + | + | + | + | + |
| Darzi, et al., 2022 [33] | x | - | + | + | + | - |
Domains
| Evaluation
| |||||
| Study | Study Design | Number of Participants | Main Results |
|---|---|---|---|
| Weyns et al., 2022 [24] | Double-blind randomized clinical trial | 88 |
|
| Hsu et al., 2021 [25] | Double-blinded randomized placebo-controlled cross-over study | 20 |
|
| Trebatická et al., 2006 [26] | Double-blinded randomized placebo-controlled study | 61 |
↓ inattention score (p = 0.00014 and p = 0.0067)
|
| Rafeiy-Torghabeh et al., 2020 [28] | Double-blinded randomized placebo-controlled study. | 66 |
↓ after 4 weeks (33.93 to 10.27) ↓ after 8 weeks (33.93 to 8.50) Time–treatment interaction p = 0.015
↓ after 8 weeks (16.33 to 4.50) Time–treatment interaction p = 0.032
↓ after 8 weeks (17.60 to 4) Time–treatment interaction p = 0.036 |
| Study | Study Design | Number of Participants | Main Results |
|---|---|---|---|
| Hsu et al., 2021 [25] | Double-blinded randomized placebo-controlled cross-over study | 20 |
|
| Dvořáková et al., 2006 [30] | Double-blinded randomized placebo-controlled study | 43 |
↑ GSH level (102.89 to 130.44 μmol/L; p = 0.0054)
↑ TAS levels (1.02 to 1.09 mmol/L; p = 0.002) |
| Dvořáková et al., 2007 [31] | Double-blinded randomized placebo-controlled study | 57 |
|
| Chovanová et al., 2006 [32] | Double-blinded randomized placebo-controlled study | 61 |
↑ TAS in ADHD children after the wash-out period from PBE (1.091 to 1.026; p = 0.0019) |
| Study | Study Design | Number of Patients | Main Results |
|---|---|---|---|
| Darzi et al., 2022 [33] | Case-control study | 200 | Indirect relationship between dietary polyphenol consumption and the risk of ADHD (p < 0.001). The results remained significant after the adjustment for: energy intake BMI, socioeconomic status, age, and gender (p < 0.001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turiaco, F.; Cullotta, C.; Mannino, F.; Bruno, A.; Squadrito, F.; Pallio, G.; Irrera, N. Attention Deficit Hyperactivity Disorder (ADHD) and Polyphenols: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 1536. https://doi.org/10.3390/ijms25031536
Turiaco F, Cullotta C, Mannino F, Bruno A, Squadrito F, Pallio G, Irrera N. Attention Deficit Hyperactivity Disorder (ADHD) and Polyphenols: A Systematic Review. International Journal of Molecular Sciences. 2024; 25(3):1536. https://doi.org/10.3390/ijms25031536
Chicago/Turabian StyleTuriaco, Fabrizio, Chiara Cullotta, Federica Mannino, Antonio Bruno, Francesco Squadrito, Giovanni Pallio, and Natasha Irrera. 2024. "Attention Deficit Hyperactivity Disorder (ADHD) and Polyphenols: A Systematic Review" International Journal of Molecular Sciences 25, no. 3: 1536. https://doi.org/10.3390/ijms25031536
APA StyleTuriaco, F., Cullotta, C., Mannino, F., Bruno, A., Squadrito, F., Pallio, G., & Irrera, N. (2024). Attention Deficit Hyperactivity Disorder (ADHD) and Polyphenols: A Systematic Review. International Journal of Molecular Sciences, 25(3), 1536. https://doi.org/10.3390/ijms25031536








