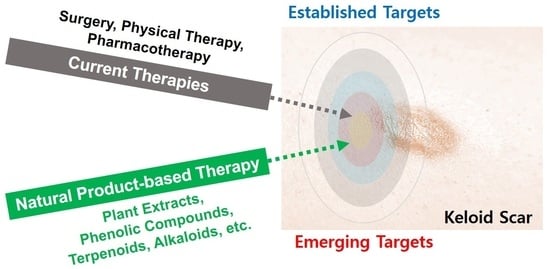
Insights into How Plant-Derived Extracts and Compounds Can Help in the Prevention and Treatment of Keloid Disease: Established and Emerging Therapeutic Targets
Abstract
1. Introduction
2. Methods: Study Search and Selection
3. Established and Emerging Therapeutic Targets
3.1. Cell Proliferation
3.2. Cell Apoptosis
3.3. TGF-β Signaling Pathway
3.4. Collagen Synthesis Pathway
3.5. Nuclear Factor Kappa B (NF-κB) Signaling Pathway
3.6. Wnt Signaling Pathway
3.7. Janus Kinase (JAK)/Signal Transducer and Activator of Transcription (STAT) Signaling Pathway
3.8. Hypoxia-Inducible Factor (HIF)-1α
3.9. Prostaglandin (PG) E2
3.10. Aldo-Keto Reductase Family 1 Member B10 (AKR1B10)
3.11. High-Mobility Group Box 1 (HMGB1)
3.12. Sphingosine-1-Phosphate (S1P) Signaling Pathway
3.13. Activins and Inhibins
3.14. Growth Differentiation Factor (GDF) 9 Signaling Pathway
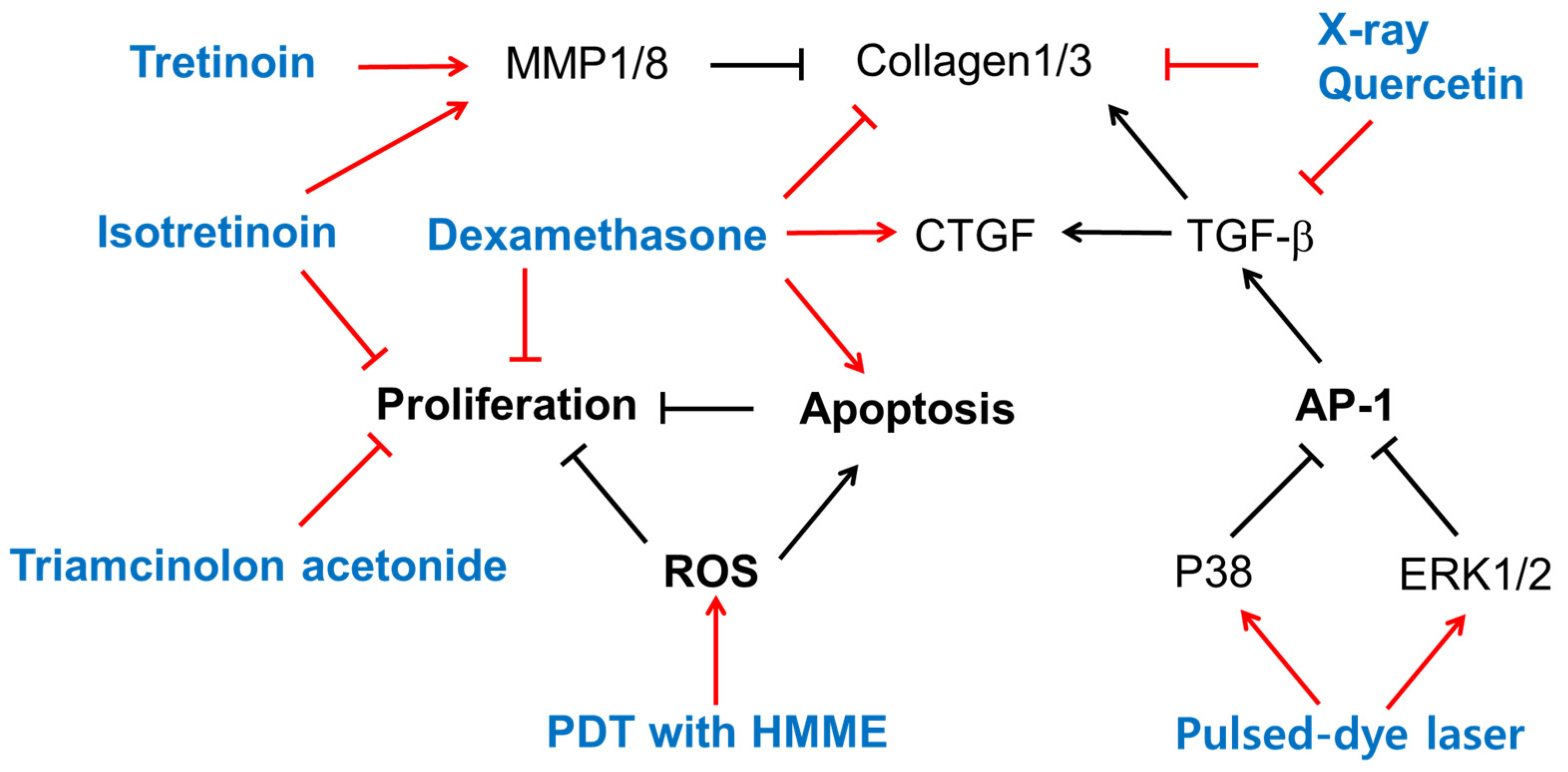
4. Biological Effects of Existing Therapy on Cells
4.1. Retinoids
4.2. Steroids
4.3. Radiation
4.4. Pulsed-Dye Laser
4.5. Photodynamic Therapy (PDT)
5. Biological Activities of Plant Extracts in Cells
6. Biological Activities of Plant-Derived Compounds in Cells
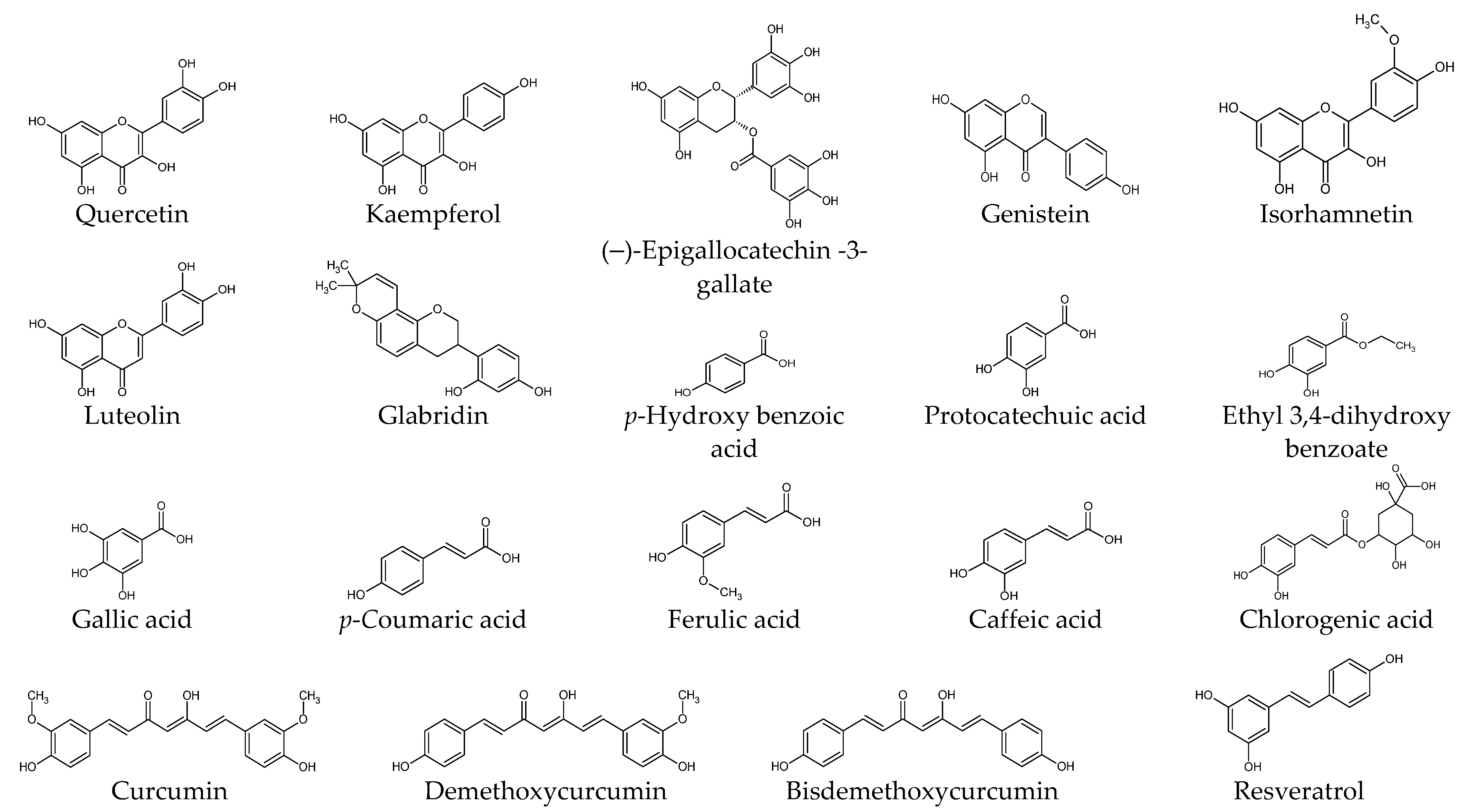
6.1. Phenolic Compounds
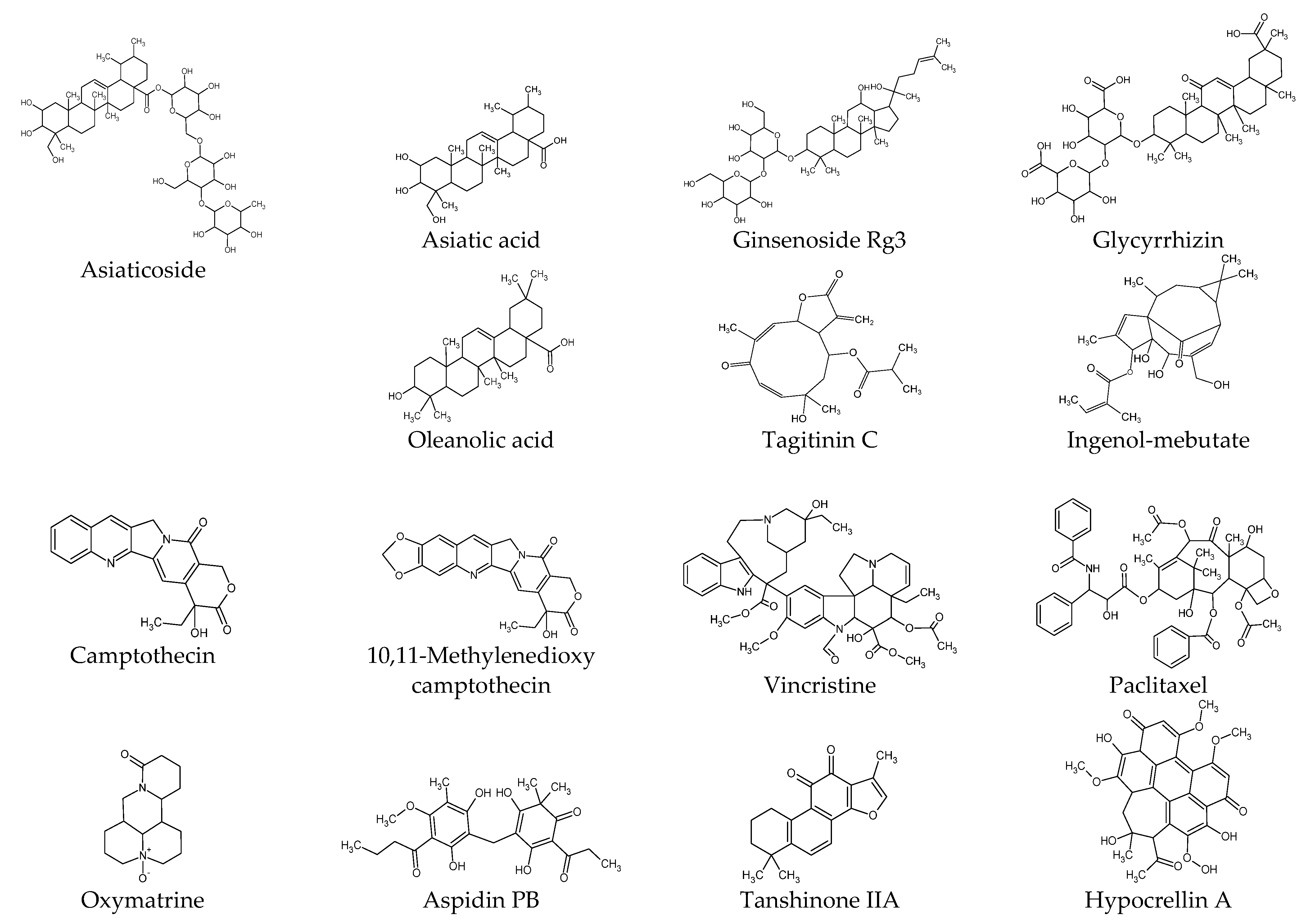
6.2. Terpenoids
6.3. Alkaloids
6.4. Other Compounds
7. Ex Vivo and In Vivo Studies
7.1. Ex Vivo Studies
7.2. In Vivo Studies
8. Clinical Studies
8.1. Silicone Gel versus Tretinoin Cream
8.2. Silicone Gel versus Onion Extract, or Their Combination
8.3. Onion Extract Gel versus Placebo in Adult Patients
8.4. Onion Extract Gel versus Placebo in Pediatric Patients
9. Discussion
9.1. Therapeutic Targets of Plant-Derived Extracts and Compounds
9.2. The Potential of Natural Product-Based Therapy and Future Tasks
9.3. Emerging Therapeutic Targets and Future Perspectives
9.4. Subjective Opinions
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKR1B10 | Aldo-keto reductase family 1 member B10 |
| AKT | protein kinase B |
| AP-1 | activator protein-1 |
| COX | cyclooxygenase |
| CTGF | connective tissue growth factor |
| DKK1 | dickkopf-1 |
| ECM | extracellular matrix |
| EGCG | (–)-epigallocatechin-3-gallate |
| ERK | extracellular signal-regulated kinase |
| FAS | Fas receptor |
| FRAT1 | frequently rearranged in advanced T-cell lymphomas 1 |
| GDF-9 | growth differentiation factor-9 |
| GSK3β | glycogen synthase kinase 3 beta |
| HIF | hypoxia-inducible factor |
| HMGB1 | high-mobility group box 1 |
| HMME | hematoporphyrin monomethyl ether |
| HMOX | heme oxygenase |
| HSP | heat shock protein 1 |
| IGF-1 | insulin-like growth factor 1 |
| IGF-1R | insulin-like growth factor 1 receptor |
| IKK | IκB kinase |
| IL-6 | Interleukin-6 |
| INHBA | inhibin beta A |
| IκBα | NF-κB inhibitor |
| JAK | Janus kinase |
| JNK | c-Jun N-terminal kinase |
| KFs | keloid-derived fibroblast |
| KKs | keloid-derived keratinocyte |
| MAPK | mitogen-activated protein kinase |
| MEK | mitogen-activated protein kinase kinase |
| miRNA | micro RNA |
| MMP | matrix metalloproteinase |
| mTOR | mammalian target of rapamycin |
| NF-κB | nuclear factor kappa B |
| NFs | normal skin-derived fibroblasts |
| NKs | normal skin-derived keratinocytes |
| p70S6K | ribosomal protein S6 kinase |
| PAI-1 | plasminogen activator inhibitor-1 |
| PARP | poly (ADP-ribose) polymerase |
| PG | prostaglandin |
| PI3K | phosphoinositide 3-kinase |
| PKB | protein kinase B |
| PPARγ | peroxisome proliferator–activated receptor gamma |
| PTEN | phosphatase and tensin homolog |
| RAGE | receptors for advanced glycation end-products |
| ROS | reactive oxygen species |
| S1P | sphingosine-1-phosphate |
| S1PR | sphingosine-1-phosphate receptor |
| Se-ZGTP | selenium-containing polysaccharide from Ziyang green tea |
| SFRP | secreted frizzled-related protein |
| shRNA | hairpin RNA |
| siRNA | silencing RNA |
| α-SMA | alpha-smooth muscle actin |
| SMAD | small mothers against decapentaplegic |
| STAT | signal transducer and activator of transcription |
| TGF-β | transforming growth factor beta |
| TGFβR | TGF beta receptor |
| TIMP-1 | tissue inhibitor of metalloproteinase 1 |
| TLR | Toll-like receptor |
| TNF-α | tumor necrosis factor-alpha |
References
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, P. Wound healing and the role of fibroblasts. J. Wound Care 2013, 22, 407–412. [Google Scholar] [PubMed]
- Li, B.; Wang, J.H.C. Fibroblasts and myofibroblasts in wound healing: Force generation and measurement. J. Tissue Viability 2011, 20, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Tracy, L.E.; Minasian, R.A.; Caterson, E.J. Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, M.; Derynck, R.; Miyazono, K. TGF-beta and the TGF-beta Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb. Perspect. Biol. 2016, 8, a021873. [Google Scholar] [CrossRef]
- Lee, C.C.; Tsai, C.H.; Chen, C.H.; Yeh, Y.C.; Chung, W.H.; Chen, C.B. An updated review of the immunological mechanisms of keloid scars. Front. Immunol. 2023, 14, 1117630. [Google Scholar] [CrossRef]
- Betarbet, U.; Blalock, T.W. Keloids: A Review of Etiology, Prevention, and Treatment. J. Clin. Aesthet. Dermatol. 2020, 13, 33–43. [Google Scholar]
- Limandjaja, G.C.; Niessen, F.B.; Scheper, R.J.; Gibbs, S. Hypertrophic scars and keloids: Overview of the evidence and practical guide for differentiating between these abnormal scars. Exp. Dermatol. 2021, 30, 146–161. [Google Scholar] [CrossRef]
- Mokos, Z.B.; Jovic, A.; Grgurevic, L.; Dumic-Cule, I.; Kostovic, K.; Ceovic, R.; Marinovic, B. Current Therapeutic Approach to Hypertrophic Scars. Front. Med. 2017, 4, 83. [Google Scholar] [CrossRef]
- Jeschke, M.G.; Wood, F.M.; Middelkoop, E.; Bayat, A.; Teot, L.; Ogawa, R.; Gauglitz, G.G. Scars. Nat. Rev. Dis. Primers 2023, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Gauglitz, G.G.; Korting, H.C.; Pavicic, T.; Ruzicka, T.; Jeschke, M.G. Hypertrophic scarring and keloids: Pathomechanisms and current and emerging treatment strategies. Mol. Med. 2011, 17, 113–125. [Google Scholar] [CrossRef]
- Tan, S.; Khumalo, N.; Bayat, A. Understanding Keloid Pathobiology From a Quasi-Neoplastic Perspective: Less of a Scar and More of a Chronic Inflammatory Disease With Cancer-Like Tendencies. Front. Immunol. 2019, 10, 1810. [Google Scholar] [CrossRef] [PubMed]
- Biazus Soares, G.; Mahmoud, O.; Yosipovitch, G. Pruritus in keloid scars: Mechanisms and treatments. Ital. J. Dermatol. Venerol. 2023, 158, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Al-Attar, A.; Mess, S.; Thomassen, J.M.; Kauffman, C.L.; Davison, S.P. Keloid pathogenesis and treatment. Plast. Reconstr. Surg. 2006, 117, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Barone, N.; Safran, T.; Vorstenbosch, J.; Davison, P.G.; Cugno, S.; Murphy, A.M. Current Advances in Hypertrophic Scar and Keloid Management. Semin. Plast. Surg. 2021, 35, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Ojeh, N.; Bharatha, A.; Gaur, U.; Forde, A.L. Keloids: Current and emerging therapies. Scars Burn. Health 2020, 6, 2059513120940499. [Google Scholar] [CrossRef]
- Walsh, L.A.; Wu, E.; Pontes, D.; Kwan, K.R.; Poondru, S.; Miller, C.H.; Kundu, R.V. Keloid treatments: An evidence-based systematic review of recent advances. Syst. Rev. 2023, 12, 42. [Google Scholar] [CrossRef]
- Ekstein, S.F.; Wyles, S.P.; Moran, S.L.; Meves, A. Keloids: A review of therapeutic management. Int. J. Dermatol. 2021, 60, 661–671. [Google Scholar] [CrossRef]
- Unahabhokha, T.; Sucontphunt, A.; Nimmannit, U.; Chanvorachote, P.; Yongsanguanchai, N.; Pongrakhananon, V. Molecular signalings in keloid disease and current therapeutic approaches from natural based compounds. Pharm. Biol. 2015, 53, 457–463. [Google Scholar] [CrossRef]
- Kleinerman, R.; Kilmer, S.L.; Chotzen, V.A. Mitomycin C in the treatment of keloids: A case and review. J. Drugs Dermatol. 2013, 12, 701–703. [Google Scholar] [PubMed]
- Shah, V.V.; Aldahan, A.S.; Mlacker, S.; Alsaidan, M.; Samarkandy, S.; Nouri, K. 5-Fluorouracil in the Treatment of Keloids and Hypertrophic Scars: A Comprehensive Review of the Literature. Dermatol. Ther. 2016, 6, 169–183. [Google Scholar] [CrossRef]
- Chodon, T.; Sugihara, T.; Igawa, H.H.; Funayama, E.; Furukawa, H. Keloid-derived fibroblasts are refractory to Fas-mediated apoptosis and neutralization of autocrine transforming growth factor-beta1 can abrogate this resistance. Am. J. Pathol. 2000, 157, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Wu, X.; Gao, Z.; Zhou, G.; Zhang, W.J.; Liu, W. Enhanced expression of membrane transporter and drug resistance in keloid fibroblasts. Hum. Pathol. 2012, 43, 2024–2032. [Google Scholar] [CrossRef] [PubMed]
- Nakazono-Kusaba, A.; Takahashi-Yanaga, F.; Miwa, Y.; Morimoto, S.; Furue, M.; Sasaguri, T. PKC412 induces apoptosis through a caspase-dependent mechanism in human keloid-derived fibroblasts. Eur. J. Pharmacol. 2004, 497, 155–160. [Google Scholar] [CrossRef]
- Huu, N.D.; Huu, S.N.; Thi, X.L.; Van, T.N.; Minh, P.P.T.; Minh, T.T.; Van, T.H.; Cam, V.T.; Huyen, M.L.; Hau, K.T.; et al. Successful Treatment of Intralesional Bleomycin in Keloids of Vietnamese Population. Open Access Maced. J. Med. Sci. 2019, 7, 298–299. [Google Scholar] [PubMed]
- Chen, A.D.; Chen, R.F.; Li, Y.T.; Huang, Y.T.; Lin, S.D.; Lai, C.S.; Kuo, Y.R. Triamcinolone Acetonide Suppresses Keloid Formation Through Enhancing Apoptosis in a Nude Mouse Model. Ann. Plast. Surg. 2019, 83, S50–S54. [Google Scholar] [CrossRef]
- Feng, F.; Liu, M.; Pan, L.; Wu, J.; Wang, C.; Yang, L.; Liu, W.; Xu, W.; Lei, M. Biomechanical Regulatory Factors and Therapeutic Targets in Keloid Fibrosis. Front. Pharmacol. 2022, 13, 906212. [Google Scholar] [CrossRef]
- Tie, Y.; Tang, F.; Peng, D.; Zhang, Y.; Shi, H. TGF-β signal transduction: Biology, function and therapy for diseases. Mol. Biomed. 2022, 3, 45. [Google Scholar] [CrossRef]
- Peng, D.; Fu, M.; Wang, M.; Wei, Y.; Wei, X. Targeting TGF-β signal transduction for fibrosis and cancer therapy. Mol. Cancer 2022, 21, 104. [Google Scholar] [CrossRef]
- Budi, E.H.; Schaub, J.R.; Decaris, M.; Turner, S.; Derynck, R. TGF-β as a driver of fibrosis: Physiological roles and therapeutic opportunities. J. Pathol. 2021, 254, 358–373. [Google Scholar] [CrossRef] [PubMed]
- Sible, J.C.; Eriksson, E.; Oliver, N. DNA binding proteins from keloid fibroblasts form unique complexes with the human fibronectin promoter. Gene Expr. 1996, 5, 269–283. [Google Scholar] [PubMed]
- Gelse, K.; Poschl, E.; Aigner, T. Collagens—Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.N.; Dive, A.M.; Moharil, R.; Munde, P. Enigmatic insight into collagen. J. Oral. Maxillofac. Pathol. 2016, 20, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef]
- Ito, S.; Nagata, K. Roles of the endoplasmic reticulum-resident, collagen-specific molecular chaperone Hsp47 in vertebrate cells and human disease. J. Biol. Chem. 2019, 294, 2133–2141. [Google Scholar] [CrossRef] [PubMed]
- Salo, A.M.; Myllyharju, J. Prolyl and lysyl hydroxylases in collagen synthesis. Exp. Dermatol. 2021, 30, 38–49. [Google Scholar] [CrossRef]
- Boo, Y.C. Ascorbic Acid (Vitamin C) as a Cosmeceutical to Increase Dermal Collagen for Skin Antiaging Purposes: Emerging Combination Therapies. Antioxidants 2022, 11, 1663. [Google Scholar] [CrossRef]
- Matsui, Y.; Hirata, Y.; Wada, I.; Hosokawa, N. Visualization of Procollagen IV Reveals ER-to-Golgi Transport by ERGIC-independent Carriers. Cell Struct. Funct. 2020, 45, 107–119. [Google Scholar] [CrossRef]
- Malhotra, V.; Erlmann, P. The pathway of collagen secretion. Annu. Rev. Cell Dev. Biol. 2015, 31, 109–124. [Google Scholar] [CrossRef]
- Asgari, M.; Latifi, N.; Heris, H.K.; Vali, H.; Mongeau, L. In vitro fibrillogenesis of tropocollagen type III in collagen type I affects its relative fibrillar topology and mechanics. Sci. Rep. 2017, 7, 1392. [Google Scholar] [CrossRef] [PubMed]
- Majamaa, K.; Gunzler, V.; Hanauske-Abel, H.M.; Myllyla, R.; Kivirikko, K.I. Partial identity of the 2-oxoglutarate and ascorbate binding sites of prolyl 4-hydroxylase. J. Biol. Chem. 1986, 261, 7819–7823. [Google Scholar] [CrossRef]
- Sasaki, T.; Majamaa, K.; Uitto, J. Reduction of collagen production in keloid fibroblast cultures by ethyl-3,4-dihydroxybenzoate. Inhibition of prolyl hydroxylase activity as a mechanism of action. J. Biol. Chem. 1987, 262, 9397–9403. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.Y.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lin, L.B.; Zhang, Z.Q.; Zhang, H.Y.; Hu, H.B. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Cai, J.; Zhang, J.; Zhao, Y.; Xu, B. Abnormal nuclear factor (NF)-kappaB signal pathway and aspirin inhibits tumor necrosis factor alpha-induced NF-kappaB activation in keloid fibroblasts. Dermatol. Surg. 2007, 33, 697–708. [Google Scholar] [PubMed]
- Li, Q.; Cheng, F.; Zhou, K.; Fang, L.; Wu, J.; Xia, Q.; Cen, Y.; Chen, J.; Qing, Y. Increased sensitivity to TNF-alpha promotes keloid fibroblast hyperproliferation by activating the NF-kappaB, JNK and p38 MAPK pathways. Exp. Ther. Med. 2021, 21, 502. [Google Scholar] [CrossRef]
- Petri, J.B.; Haustein, U.F. Lysine acetylsalicylate decreases proliferation and extracellular matrix gene expression rate in keloid fibroblasts in vitro. Eur. J. Dermatol. 2002, 12, 231–235. [Google Scholar]
- Makino, S.; Mitsutake, N.; Nakashima, M.; Saenko, V.A.; Ohtsuru, A.; Umezawa, K.; Tanaka, K.; Hirano, A.; Yamashita, S. DHMEQ, a novel NF-kappaB inhibitor, suppresses growth and type I collagen accumulation in keloid fibroblasts. J. Dermatol. Sci. 2008, 51, 171–180. [Google Scholar] [CrossRef]
- Liu, J.Q.; Xiao, Q.; Xiao, J.N.; Niu, C.X.; Li, Y.Y.; Zhang, X.J.; Zhou, Z.W.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Kikuchi, A.; Kishida, S.; Yamamoto, H. Regulation of Wnt signaling by protein-protein interaction and post-translational modifications. Exp. Mol. Med. 2006, 38, 1–10. [Google Scholar] [CrossRef]
- Lyros, O.; Rafiee, P.; Nie, L.H.; Medda, R.; Jovanovic, N.; Schmidt, J.; Mackinnon, A.; Venu, N.; Shaker, R. Dickkopf-1, the Wnt antagonist, is induced by acidic pH and mediates epithelial cellular senescence in human reflux esophagitis. Am. J. Physiol.Gastrointest. Liver Physiol. 2014, 306, G557–G574. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Akhmetshina, A.; Palumbo, K.; Dees, C.; Bergmann, C.; Venalis, P.; Zerr, P.; Horn, A.; Kireva, T.; Beyer, C.; Zwerina, J.; et al. Activation of canonical Wnt signalling is required for TGF-β-mediated fibrosis. Nat. Commun. 2012, 3, 735. [Google Scholar] [CrossRef] [PubMed]
- Sato, M. Upregulation of the Wnt/beta-catenin pathway induced by transforming growth factor-beta in hypertrophic scars and keloids. Acta Derm. Venereol. 2006, 86, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Chua, A.W.; Gan, S.U.; Ting, Y.; Fu, Z.; Lim, C.K.; Song, C.; Sabapathy, K.; Phan, T.T. Keloid fibroblasts are more sensitive to Wnt3a treatment in terms of elevated cellular growth and fibronectin expression. J. Dermatol. Sci. 2011, 64, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Yin, Q.; Wolkerstorfer, A.; Lapid, O.; Niessen, F.B.; Van Zuijlen, P.P.M.; Gibbs, S. The JAK-STAT pathway in keloid pathogenesis: A systematic review with qualitative synthesis. Exp. Dermatol. 2023, 32, 588–598. [Google Scholar] [CrossRef]
- Lee, Y.I.; Shim, J.E.; Kim, J.; Lee, W.J.; Kim, J.W.; Nam, K.H.; Lee, J.H. WNT5A drives interleukin-6-dependent epithelial-mesenchymal transition via the JAK/STAT pathway in keloid pathogenesis. Burn. Trauma. 2022, 10, tkac023. [Google Scholar] [CrossRef]
- Park, G.; Yoon, B.S.; Moon, J.H.; Kim, B.; Jun, E.K.; Oh, S.; Kim, H.; Song, H.J.; Noh, J.Y.; Oh, C.; et al. Green tea polyphenol epigallocatechin-3-gallate suppresses collagen production and proliferation in keloid fibroblasts via inhibition of the STAT3-signaling pathway. J. Investig. Dermatol. 2008, 128, 2429–2441. [Google Scholar] [CrossRef]
- Hong, Y.K.; Wu, C.H.; Lin, Y.C.; Huang, Y.L.; Hung, K.S.; Pai, T.P.; Liu, Y.T.; Chen, T.C.; Chan, H.; Hsu, C.K. ASC-J9 Blocks Cell Proliferation and Extracellular Matrix Production of Keloid Fibroblasts through Inhibiting STAT3 Signaling. Int. J. Mol. Sci. 2022, 23, 5549. [Google Scholar] [CrossRef]
- Kang, Y.; Roh, M.R.; Rajadurai, S.; Rajadurai, A.; Kumar, R.; Njauw, C.N.; Zheng, Z.; Tsao, H. Hypoxia and HIF-1alpha Regulate Collagen Production in Keloids. J. Investig. Dermatol. 2020, 140, 2157–2165. [Google Scholar] [CrossRef] [PubMed]
- Lei, R.; Li, J.; Liu, F.; Li, W.H.; Zhang, S.Z.; Wang, Y.; Chu, X.; Xu, J.H. HIF-1α promotes the keloid development through the activation of TGF-β/Smad and TLR4/MyD88/NF-κB pathways. Cell Cycle 2019, 18, 3239–3250. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; Zhang, M.; Guan, E.; Han, Q.; Liu, Y.; Long, X.; Long, F.; Zhao, R.C.; Huang, J.; Liu, Z.; et al. Resveratrol inhibits proliferation and promotes apoptosis of keloid fibroblasts by targeting HIF-1alpha. J. Plast. Surg. Hand Surg. 2020, 54, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.K.; Zhang, M.Z.; Zhang, W.C.; Li, Z.J.; Si, L.B.; Long, X.; Yu, N.Z.; Wang, X.J. Role of HIF-1alpha in pathogenic mechanisms of keloids. J. Cosmet. Dermatol. 2023, 22, 1436–1448. [Google Scholar] [CrossRef]
- Hayashi, T.; Nishihira, J.; Koyama, Y.; Sasaki, S.; Yamamoto, Y. Decreased prostaglandin E2 production by inflammatory cytokine and lower expression of EP2 receptor result in increased collagen synthesis in keloid fibroblasts. J. Investig. Dermatol. 2006, 126, 990–997. [Google Scholar] [CrossRef]
- Myles, M.E.; Russell, J.D.; Trupin, J.S.; Smith, J.C.; Russell, S.B. Keloid fibroblasts are refractory to inhibition of DNA synthesis by phorbol esters. Altered response is accompanied by reduced sensitivity to prostaglandin E2 and altered down-regulation of phorbol ester binding sites. J. Biol. Chem. 1992, 267, 9014–9020. [Google Scholar] [CrossRef]
- Jumper, N.; Hodgkinson, T.; Arscott, G.; Har-Shai, Y.; Paus, R.; Bayat, A. The Aldo-Keto Reductase AKR1B10 Is Up-Regulated in Keloid Epidermis, Implicating Retinoic Acid Pathway Dysregulation in the Pathogenesis of Keloid Disease. J. Investig. Dermatol. 2016, 136, 1500–1512. [Google Scholar] [CrossRef]
- Chen, R.; Kang, R.; Tang, D. The mechanism of HMGB1 secretion and release. Exp. Mol. Med. 2022, 54, 91–102. [Google Scholar] [CrossRef]
- Li, L.C.; Gao, J.; Li, J. Emerging role of HMGB1 in fibrotic diseases. J. Cell Mol. Med. 2014, 18, 2331–2339. [Google Scholar] [CrossRef]
- Jeon, Y.R.; Roh, H.; Jung, J.H.; Ahn, H.M.; Lee, J.H.; Yun, C.O.; Lee, W.J. Antifibrotic Effects of High-Mobility Group Box 1 Protein Inhibitor (Glycyrrhizin) on Keloid Fibroblasts and Keloid Spheroids through Reduction of Autophagy and Induction of Apoptosis. Int. J. Mol. Sci. 2019, 20, 4134. [Google Scholar] [CrossRef]
- Kim, J.; Park, J.C.; Lee, M.H.; Yang, C.E.; Lee, J.H.; Lee, W.J. High-Mobility Group Box 1 Mediates Fibroblast Activity via RAGE-MAPK and NF-kappaB Signaling in Keloid Scar Formation. Int. J. Mol. Sci. 2017, 19, 76. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.H.; Song, Y.K.; Chung, H.; Ko, H.M.; Lee, S.H.; Jo, D.I.; Kim, B.; Lee, D.H.; Kim, S.H. Association between sphingosine-1-phosphate-induced signal transduction via mitogen-activated protein kinase pathways and keloid formation. Arch. Dermatol. Res. 2019, 311, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.H.; Liu, B.; Liu, S.J.; Zhang, J.Z.; Lin, S.F. Sphingosine-1-phosphate receptor 2 mediates endothelial cells dysfunction by PI3K-Akt pathway under high glucose condition. Eur. J. Pharmacol. 2016, 776, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Cao, X.; Liu, H.; Huang, W.; Zhang, L.; Jiang, T. Isorhamnetin attenuates the proliferation, invasion, migration and fibrosis of keloid fibroblasts by targeting S1PR1. Exp. Ther. Med. 2023, 26, 310. [Google Scholar] [CrossRef] [PubMed]
- Namwanje, M.; Brown, C.W. Activins and Inhibins: Roles in Development, Physiology, and Disease. Cold Spring Harb. Perspect. Biol. 2016, 8, a021881. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Chan, S.Y.; Lim, I.J.; Phillips, D.J.; Phan, T.T. The role of the activin system in keloid pathogenesis. Am. J. Physiol.-Cell Physiol. 2007, 292, C1331–C1338. [Google Scholar] [CrossRef]
- Ham, S.; Harrison, C.; de Kretser, D.; Wallace, E.M.; Southwick, G.; Temple-Smith, P. Potential treatment of keloid pathogenesis with follistatin 288 by blocking the activin molecular pathway. Exp. Dermatol. 2021, 30, 402–408. [Google Scholar] [CrossRef]
- Fu, M.; Peng, D.; Lan, T.; Wei, Y.; Wei, X. Multifunctional regulatory protein connective tissue growth factor (CTGF): A potential therapeutic target for diverse diseases. Acta Pharm Sin B. 2022, 12, 1740–1760. [Google Scholar] [CrossRef]
- Mazerbourg, S.; Klein, C.; Roh, J.; Kaivo-Oja, N.; Mottershead, D.G.; Korchynskyi, O.; Ritvos, O.; Hsueh, A.J.W. Growth differentiation factor-9 signaling is mediated by the type I receptor, activin receptor-like kinase 5. Mol. Endocrinol. 2004, 18, 653–665. [Google Scholar] [CrossRef]
- Gao, Z.; Wu, X.L.; Song, N.; Zhang, L.; Liu, W. Differential expression of growth differentiation factor-9 in keloids. Burns 2010, 36, 1289–1295. [Google Scholar] [CrossRef]
- Jiang, Z.; Yu, Q.; Xia, L.; Zhang, Y.; Wang, X.; Wu, X.; Gao, Z. Growth Differentiation Factor-9 Promotes Fibroblast Proliferation and Migration in Keloids through the Smad2/3 Pathway. Cell. Physiol. Biochem. 2016, 40, 207–218. [Google Scholar] [CrossRef]
- Wu, X.; Bian, D.; Dou, Y.; Gong, Z.; Tan, Q.; Xia, Y.; Dai, Y. Asiaticoside hinders the invasive growth of keloid fibroblasts through inhibition of the GDF-9/MAPK/Smad pathway. J. Biochem. Mol. Toxicol. 2017, 31, e21922. [Google Scholar] [CrossRef] [PubMed]
- Abergel, R.P.; Meeker, C.A.; Oikarinen, H.; Oikarinen, A.I.; Uitto, J. Retinoid modulation of connective tissue metabolism in keloid fibroblast cultures. Arch. Dermatol. 1985, 121, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Uchida, G.; Yoshimura, K.; Kitano, Y.; Okazaki, M.; Harii, K. Tretinoin reverses upregulation of matrix metalloproteinase-13 in human keloid-derived fibroblasts. Exp. Dermatol. 2003, 12 (Suppl. S2), 35–42. [Google Scholar] [CrossRef] [PubMed]
- Syed, F.; Bagabir, R.A.; Paus, R.; Bayat, A. Ex vivo evaluation of antifibrotic compounds in skin scarring: EGCG and silencing of PAI-1 independently inhibit growth and induce keloid shrinkage. Lab. Investig. 2013, 93, 946–960. [Google Scholar] [CrossRef] [PubMed]
- Cruz, N.I.; Korchin, L. Inhibition of human keloid fibroblast growth by isotretinoin and triamcinolone acetonide in vitro. Ann. Plast. Surg. 1994, 33, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Zeng, X.; Zhang, F.Q.; Wang, X.J. Influence of quercetin and x-ray on collagen synthesis of cultured human keloid-derived fibroblasts. Chin. Med. Sci. J. 2006, 21, 179–183. [Google Scholar]
- Kuo, Y.R.; Wu, W.S.; Wang, F.S. Flashlamp pulsed-dye laser suppressed TGF-beta1 expression and proliferation in cultured keloid fibroblasts is mediated by MAPK pathway. Lasers Surg. Med. 2007, 39, 358–364. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Li, L.; Li, X. Photodynamic Therapy-Induced Apoptosis of Keloid Fibroblasts is Mediated by Radical Oxygen Species In Vitro. Clin. Lab. 2015, 61, 1257–1266. [Google Scholar] [CrossRef]
- He, S.; Yang, Y.; Liu, X.; Huang, W.; Zhang, X.; Yang, S.; Zhang, X. Compound Astragalus and Salvia miltiorrhiza extract inhibits cell proliferation, invasion and collagen synthesis in keloid fibroblasts by mediating transforming growth factor-beta/Smad pathway. Br. J. Dermatol. 2012, 166, 564–574. [Google Scholar] [CrossRef]
- Bratkov, V.M.; Shkondrov, A.M.; Zdraveva, P.K.; Krasteva, I.N. Flavonoids from the Genus Astragalus: Phytochemistry and Biological Activity. Pharmacogn. Rev. 2016, 10, 11–32. [Google Scholar] [PubMed]
- Abd Elrahim Abd Elkader, H.T.; Essawy, A.E.; Al-Shami, A.S. Astragalus species: Phytochemistry, biological actions and molecular mechanisms underlying their potential neuroprotective effects on neurological diseases. Phytochemistry 2022, 202, 113293. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.Y.; Sun, C.T.; Wan, H.T.; Shou, Q.Y.; Han, B.; Sheng, M.M.; Li, L.Q.; Kai, G.Y. Bioactive components and molecular mechanisms of Salvia miltiorrhiza Bunge in promoting blood circulation to remove blood stasis. J. Ethnopharmacol. 2023, 317, 116697. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Lee, J.S.; Bae, G.Y.; Kim, J.J.; Chin, Y.W.; Bahk, Y.Y.; Min, H.G.; Cha, H.J. Extract of Aneilema keisak inhibits transforming growth factor-beta-dependent signalling by inducing Smad2 downregulation in keloid fibroblasts. Exp. Dermatol. 2013, 22, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.B.L.; Kwan, Y.M. The biological activities of the spiderworts (Tradescantia). Food Chem. 2020, 317, 126411. [Google Scholar] [CrossRef]
- Tang, Z.M.; Cao, Y.; Ding, J.C.; Zhai, X.X.; Jing, M.Q.; Wang, M.M.; Lu, L. Wubeizi Ointment Suppresses Keloid Formation through Modulation of the mTOR Pathway. Biomed. Res. Int. 2020, 2020, 3608372. [Google Scholar] [CrossRef]
- Ding, J.C.; Tang, Z.M.; Zhai, X.X.; Chen, X.H.; Li, J.G.; Zhang, C.X. The effects of Wubeizi ointment on the proliferation of keloid-derived fibroblasts. Cell Biochem. Biophys. 2015, 71, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.X.; Ding, J.C.; Tang, Z.M.; Li, J.G.; Chen, X.H.; Zhang, C.X. Effect of Wubeizi ointment aqueous solution on the expression of type I and III procollagen genes in keloid fibroblasts. Exp. Ther. Med. 2017, 13, 503–506. [Google Scholar] [CrossRef]
- Tang, Z.M.; Ding, J.C.; Zhai, X.X.; Jing, M.Q.; Guan, Z.Q.; Li, Y.C. MicroRNA-21 may be involved in the therapeutic effects of Galla chinensis ointment on keloid. J. Int. Med. Res. 2020, 48, 300060520909602. [Google Scholar] [CrossRef]
- Ren, Y.Y.; Zhang, X.R.; Li, T.N.; Zeng, Y.J.; Wang, J.; Huang, Q.W. Galla Chinensis, a Traditional Chinese Medicine: Comprehensive review of botany, traditional uses, chemical composition, pharmacology and toxicology. J. Ethnopharmacol. 2021, 278, 114247. [Google Scholar] [CrossRef]
- Chen, P.Y.; Shih, T.H.; Chang, K.C.; Wang, J.S.; Yang, C.M.; Chang, Y.S. Potential of galled leaves of Goji (Lycium chinense) as functional food. BMC Nutr. 2020, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Xia, M.; Lan, J.; Yang, L.; Wang, Z.; Wang, R.; Tao, H.; Shi, Y. A comprehensive review on the ethnobotany, phytochemistry, pharmacology and quality control of the genus Lycium in China. Food Funct. 2023, 14, 2998–3025. [Google Scholar] [CrossRef] [PubMed]
- Widiatmoko, A.; Fitri, L.E.; Endharti, A.T.; Murlistyarini, S.; Brahmanti, H.; Yuniaswan, A.P.; Ekasari, D.P.; Rasyidi, F.; Nahlia, N.L.; Safitri, P.R. Inhibition Effect of Physalis angulata Leaf Leaf Extract on Viability, Collagen Type I, and Tissue Inhibitor of Metalloproteinase 1 (TIMP-1) but Not Plasminogen Activator Inhibitor-1 (PAI-1) of Keloid Fibroblast Culture. Clin. Cosmet. Investig. Dermatol. 2023, 16, 2365–2373. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna Pillai, J.; Wali, A.F.; Menezes, G.A.; Rehman, M.U.; Wani, T.A.; Arafah, A.; Zargar, S.; Mir, T.M. Chemical Composition Analysis, Cytotoxic, Antimicrobial and Antioxidant Activities of Physalis angulata L.: A Comparative Study of Leaves and Fruit. Molecules 2022, 27, 1480. [Google Scholar] [CrossRef] [PubMed]
- Pikuła, M.; Żebrowska, M.E.; Pobłocka-Olech, L.; Krauze-Baranowska, M.; Sznitowska, M.; Trzonkowski, P. Effect of enoxaparin and onion extract on human skin fibroblast cell line—Therapeutic implications for the treatment of keloids. Pharm. Biol. 2013, 52, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Jenwitheesuk, K.; Surakunprapha, P.; Jenwitheesuk, K.; Kuptarnond, C.; Prathanee, S.; Intanoo, W. Role of silicone derivative plus onion extract gel in presternal hypertrophic scar protection: A prospective randomized, double blinded, controlled trial. Int. Wound J. 2012, 9, 397–402. [Google Scholar] [CrossRef]
- Zhang, Q.; Kelly, A.P.; Wang, L.; French, S.W.; Tang, X.; Duong, H.S.; Messadi, D.V.; Le, A.D. Green tea extract and (-)-epigallocatechin-3-gallate inhibit mast cell-stimulated type I collagen expression in keloid fibroblasts via blocking PI-3K/AkT signaling pathways. J. Investig. Dermatol. 2006, 126, 2607–2613. [Google Scholar] [CrossRef]
- Phan, T.T.; See, P.; Tran, E.; Nguyen, T.T.; Chan, S.Y.; Lee, S.T.; Huynh, H. Suppression of insulin-like growth factor signalling pathway and collagen expression in keloid-derived fibroblasts by quercetin: Its therapeutic potential use in the treatment and/or prevention of keloids. Br. J. Dermatol. 2003, 148, 544–552. [Google Scholar] [CrossRef]
- Phan, T.T.; Lim, I.J.; Sun, L.; Chan, S.Y.; Bay, B.H.; Tan, E.K.; Lee, S.T. Quercetin inhibits fibronectin production by keloid-derived fibroblasts. Implication for the treatment of excessive scars. J. Dermatol. Sci. 2003, 33, 192–194. [Google Scholar] [CrossRef]
- Phan, T.T.; Lim, I.J.; Chan, S.Y.; Tan, E.K.; Lee, S.T.; Longaker, M.T. Suppression of transforming growth factor beta/smad signaling in keloid-derived fibroblasts by quercetin: Implications for the treatment of excessive scars. J. Trauma. 2004, 57, 1032–1037. [Google Scholar] [CrossRef]
- Phan, T.T.; Sun, L.; Bay, B.H.; Chan, S.Y.; Lee, S.T. Dietary compounds inhibit proliferation and contraction of keloid and hypertrophic scar-derived fibroblasts in vitro: Therapeutic implication for excessive scarring. J. Trauma. 2003, 54, 1212–1224. [Google Scholar] [CrossRef] [PubMed]
- Jurzak, M.; Adamczyk, K.; Antonczak, P.; Garncarczyk, A.; Kusmierz, D.; Latocha, M. Evaluation of genistein ability to modulate CTGF mRNA/protein expression, genes expression of TGFbeta isoforms and expression of selected genes regulating cell cycle in keloid fibroblasts in vitro. Acta Pol. Pharm. 2014, 71, 972–986. [Google Scholar] [PubMed]
- Zhang, X.; Liu, W.; Wei, S. Luteolin affects keloid fibroblast proliferation and apoptosis by regulating FRAT1 gene expression. Cell Mol. Biol. 2020, 66, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Qian, D.; Tang, D.D.; Liu, J.; Wang, L.Y.; Chen, W.; Wu, C.J.; Peng, W. Glabridin from Glycyrrhiza glabra Possesses a Therapeutic Role against Keloid via Attenuating PI3K/Akt and Transforming Growth Factor-beta1/SMAD Signaling Pathways. J. Agric. Food Chem. 2022, 70, 10782–10793. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.C.; Chen, M.J.; Yu, Y.M.; Ko, S.Y.; Chang, C.C. Suppression of TGF-beta1/SMAD pathway and extracellular matrix production in primary keloid fibroblasts by curcuminoids: Its potential therapeutic use in the chemoprevention of keloid. Arch. Dermatol. Res. 2010, 302, 717–724. [Google Scholar] [CrossRef]
- Ikeda, K.; Torigoe, T.; Matsumoto, Y.; Fujita, T.; Sato, N.; Yotsuyanagi, T. Resveratrol inhibits fibrogenesis and induces apoptosis in keloid fibroblasts. Wound Repair. Regen. 2013, 21, 616–623. [Google Scholar] [CrossRef]
- Tang, B.; Zhu, B.; Liang, Y.Y.; Bi, L.K.; Hu, Z.C.; Chen, B.; Zhang, K.; Zhu, J.Y. Asiaticoside suppresses collagen expression and TGF-beta/Smad signaling through inducing Smad7 and inhibiting TGF-beta RI and TGF-beta RII in keloid fibroblasts. Arch. Dermatol. Res. 2011, 303, 563–572. [Google Scholar] [CrossRef]
- Bian, D.; Zhang, J.; Wu, X.; Dou, Y.; Yang, Y.; Tan, Q.; Xia, Y.; Gong, Z.; Dai, Y. Asiatic acid isolated from Centella asiatica inhibits TGF-beta1-induced collagen expression in human keloid fibroblasts via PPAR-gamma activation. Int. J. Biol. Sci. 2013, 9, 1032–1042. [Google Scholar] [CrossRef]
- Tang, M.; Bian, W.; Cheng, L.; Zhang, L.; Jin, R.; Wang, W.; Zhang, Y. Ginsenoside Rg3 inhibits keloid fibroblast proliferation, angiogenesis and collagen synthesis in vitro via the TGF-beta/Smad and ERK signaling pathways. Int. J. Mol. Med. 2018, 41, 1487–1499. [Google Scholar]
- Ranti, I.; Wahyuningsih, M.S.H.; Wirohadidjojo, Y.W. The antifibrotic effect of isolate tagitinin C from tithonia diversifolia (Hemsley) A. Gray on keloid fibroblast cell. Pan Afr. Med. J. 2018, 30, 264. [Google Scholar]
- De Felice, B.; Manfellotto, F.; Garbi, C.; Santoriello, M.; Nacca, M. miR-34 modulates apoptotic gene expression in Ingenol mebutate treated keloid fibroblasts. Mol. Med. Rep. 2018, 17, 7081–7088. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, D.; Yuan, X.; Jin, Z.; Pi, L. Oleanolic acid regulates the proliferation and extracellular matrix of keloid fibroblasts by mediating the TGF-beta1/SMAD signaling pathway. J. Cosmet. Dermatol. 2023, 22, 2083–2089. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Y.; Gao, W.Y.; Li, X.; Yi, C.G.; Zheng, Y.; Li, Y.; Xiao, B.; Ma, X.J.; Yan, L.; Lu, K.H.; et al. Effect of camptothecin on collagen synthesis in fibroblasts from patients with keloid. Ann. Plast. Surg. 2009, 63, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Cheng, X.; Wang, Z.; Wang, J.; Gao, T.; Li, P.; Kong, M.; Chen, X. Transdermal delivery of 10,11-methylenedioxycamptothecin by hyaluronic acid based nanoemulsion for inhibition of keloid fibroblast. Carbohydr. Polym. 2014, 112, 376–386. [Google Scholar] [CrossRef]
- Fan, D.L.; Zhao, W.J.; Wang, Y.X.; Han, S.Y.; Guo, S. Oxymatrine inhibits collagen synthesis in keloid fibroblasts via inhibition of transforming growth factor-beta1/Smad signaling pathway. Int. J. Dermatol. 2012, 51, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, L.; Huang, W.; Jin, M.; Wang, Q.; Gao, Z.; Jin, Z. Improving the anti-keloid outcomes through liposomes loading paclitaxel-cholesterol complexes. Int. J. Nanomed. 2019, 14, 1385–1400. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Li, G.; Li, S. Aspidin PB, a novel natural anti-fibrotic compound, inhibited fibrogenesis in TGF-beta1-stimulated keloid fibroblasts via PI-3K/Akt and Smad signaling pathways. Chem. Biol. Interact. 2015, 238, 66–73. [Google Scholar] [CrossRef]
- Chen, G.; Liang, Y.; Liang, X.; Li, Q.; Liu, D. Tanshinone IIA Inhibits Proliferation and Induces Apoptosis through the Downregulation of Survivin in Keloid Fibroblasts. Ann. Plast. Surg. 2016, 76, 180–186. [Google Scholar] [CrossRef]
- Lu, L.; Chai, L.; Wang, W.; Yuan, X.; Li, S.; Cao, C. A Selenium-Enriched Ziyang Green Tea Polysaccharide Induces Bax-Dependent Mitochondrial Apoptosis and Inhibits TGF-beta1-Stimulated Collagen Expression in Human Keloid Fibroblasts via NG2 Inactivation. Biol. Trace Elem. Res. 2017, 176, 270–277. [Google Scholar] [CrossRef]
- Niu, T.; Tian, Y.; Shi, Y.; Guo, G.; Tong, Y.; Wang, G. Antifibrotic effects of Hypocrellin A combined with LED red light irradiation on keloid fibroblasts by counteracting the TGF-beta/Smad/autophagy/apoptosis signalling pathway. Photodiagn. Photodyn. Ther. 2021, 34, 102202. [Google Scholar] [CrossRef]
- Jurzak, M.; Adamczyk, K. Influence of genistein on c-Jun, c-Fos and Fos-B of AP-1 subunits expression in skin keratinocytes, fibroblasts and keloid fibroblasts cultured in vitro. Acta Pol. Pharm. 2013, 70, 205–213. [Google Scholar] [PubMed]
- Bellaye, P.S.; Burgy, O.; Bonniaud, P.; Kolb, M. HSP47: A potential target for fibrotic diseases and implications for therapy. Expert. Opin. Ther. Targets. 2021, 25, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Khaiwa, N.; Maarouf, N.R.; Darwish, M.H.; Alhamad, D.W.M.; Sebastian, A.; Hamad, M.; Omar, H.A.; Orive, G.; Al-Tel, T.H. Camptothecin’s journey from discovery to WHO Essential Medicine: Fifty years of promise. Eur. J. Med. Chem. 2021, 223, 113639. [Google Scholar] [CrossRef] [PubMed]
- Skubnik, J.; Pavlickova, V.S.; Ruml, T.; Rimpelova, S. Vincristine in Combination Therapy of Cancer: Emerging Trends in Clinics. Biology 2021, 10, 849. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Cheng, S.X.; Zhang, S.; Sun, H.T.; Xu, Z.W. Vincristine induces cell cycle arrest and apoptosis in SH-SY5Y human neuroblastoma cells. Int. J. Mol. Med. 2013, 31, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Niu, T.H.; Tian, Y.; Wang, G.Y.; Guo, G.J.; Tong, Y.; Shi, Y. Inhibition of ROS-NF-κB-dependent autophagy enhances Hypocrellin A united LED red light-induced apoptosis in squamous carcinoma A431 cells. Cell. Signal. 2020, 69, 109550. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fu, R.; Li, L.; Yang, G.; Ding, S.; Zhong, Z.; Zhou, S. Co-delivery of dexamethasone and green tea polyphenols using electrospun ultrafine fibers for effective treatment of keloid. Pharm. Res. 2014, 31, 1632–1643. [Google Scholar] [CrossRef]
- Kwon, S.Y.; Park, S.D.; Park, K. Comparative effect of topical silicone gel and topical tretinoin cream for the prevention of hypertrophic scar and keloid formation and the improvement of scars. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1025–1033. [Google Scholar] [CrossRef]
- Hosnuter, M.; Payasli, C.; Isikdemir, A.; Tekerekoglu, B. The effects of onion extract on hypertrophic and keloid scars. J. Wound Care 2007, 16, 251–254. [Google Scholar] [CrossRef]
- Wananukul, S.; Chatpreodprai, S.; Peongsujarit, D.; Lertsapcharoen, P. A prospective placebo-controlled study on the efficacy of onion extract in silicone derivative gel for the prevention of hypertrophic scar and keloid in median sternotomy wound in pediatric patients. J. Med. Assoc. Thai. 2013, 96, 1428–1433. [Google Scholar]
- Wheatley, S.P.; Altieri, D.C. Survivin at a glance. J. Cell Sci. 2019, 132, 223826. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Wei, X.H.; He, J.Y.; Cao, Q.Q.; Du, D.Y.; Zhan, X.M.; Zeng, Y.Q.; Yuan, S.T.; Sun, L. The comprehensive landscape of miR-34a in cancer research. Cancer Metastasis Rev. 2021, 40, 925–948. [Google Scholar] [CrossRef] [PubMed]
- Curtin, N.J.; Szabo, C. Poly(ADP-ribose) polymerase inhibition: Past, present and future. Nat. Rev. Drug Discov. 2020, 19, 711–736. [Google Scholar] [CrossRef] [PubMed]
- Sillen, M.; Declerck, P.J. A Narrative Review on Plasminogen Activator Inhibitor-1 and Its (Patho)Physiological Role: To Target or Not to Target? Int. J. Mol. Sci. 2021, 22, 2721. [Google Scholar] [CrossRef] [PubMed]
- Ginarte, M.; Peteiro, C.; Toribio, J. Keloid formation induced by isotretinoin therapy. Int. J. Dermatol. 1999, 38, 228–229. [Google Scholar]
- Bernestein, L.J.; Geronemus, R.G. Keloid formation with the 585-nm pulsed dye laser during isotretinoin treatment. Arch. Dermatol. 1997, 133, 111–112. [Google Scholar] [CrossRef]
- Xia, W.; Phan, T.T.; Lim, I.J.; Longaker, M.T.; Yang, G.P. Complex epithelial-mesenchymal interactions modulate transforming growth factor-β expression in keloid-derived cells. Wound Repair. Regen. 2004, 12, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Phan, T.T.; Lim, I.J.; Longaker, M.T.; Yang, G.P. Differential transcriptional responses of keloid and normal keratinocytes to serum stimulation. J. Surg. Res. 2006, 135, 156–163. [Google Scholar] [CrossRef]
- Hansen, M.; Rubinsztein, D.C.; Walker, D.W. Autophagy as a promoter of longevity: Insights from model organisms. Nat. Rev. Mol. Cell Biol. 2018, 19, 579–593. [Google Scholar] [CrossRef]
- Aman, Y.; Schmauck-Medina, T.; Hansen, M.; Morimoto, R.I.; Simon, A.K.; Bjedov, I.; Palikaras, K.; Simonsen, A.; Johansen, T.; Tavernarakis, N.; et al. Autophagy in healthy aging and disease. Nat. Aging 2021, 1, 634–650. [Google Scholar] [CrossRef]
- Okuno, R.; Ito, Y.; Eid, N.; Otsuki, Y.; Kondo, Y.; Ueda, K. Upregulation of autophagy and glycolysis markers in keloid hypoxic-zone fibroblasts: Morphological characteristics and implications. Histol. Histopathol. 2018, 33, 1075–1087. [Google Scholar] [PubMed]
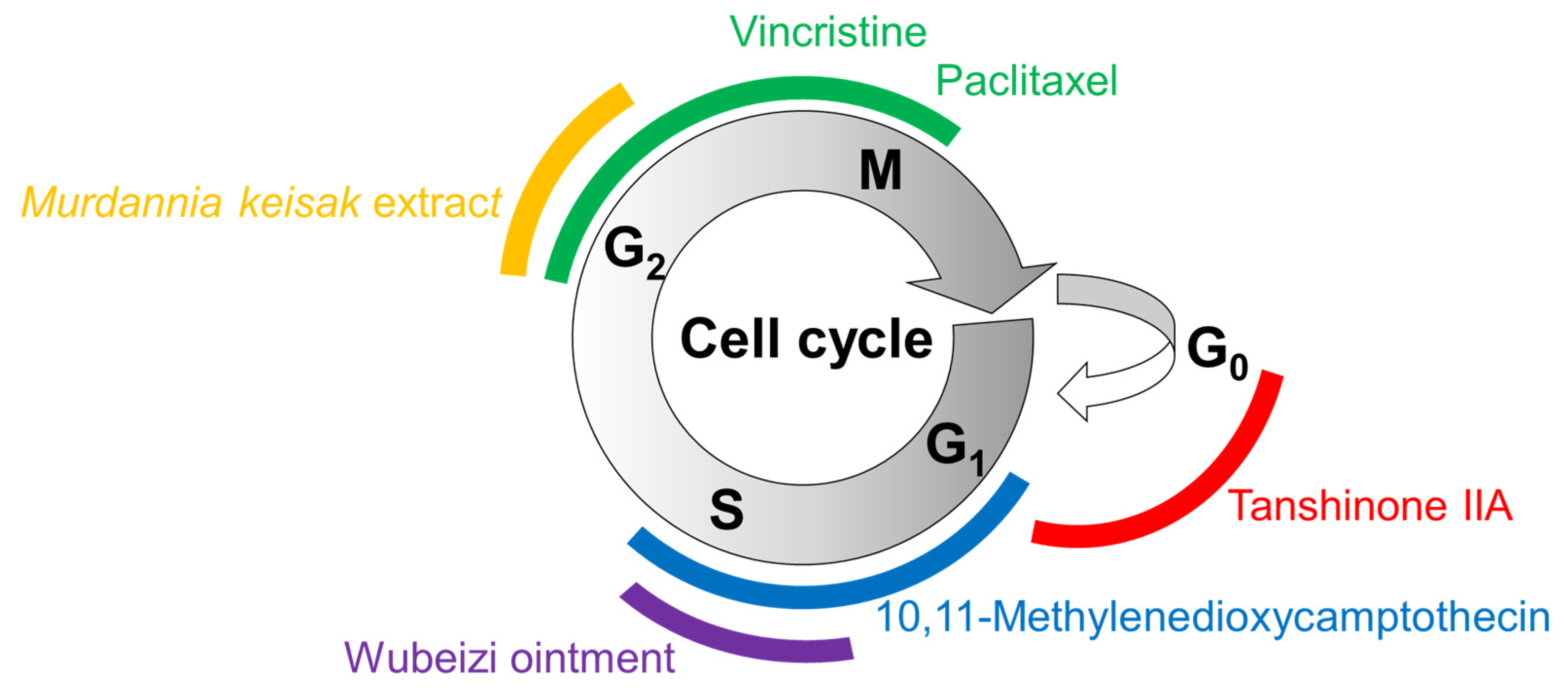
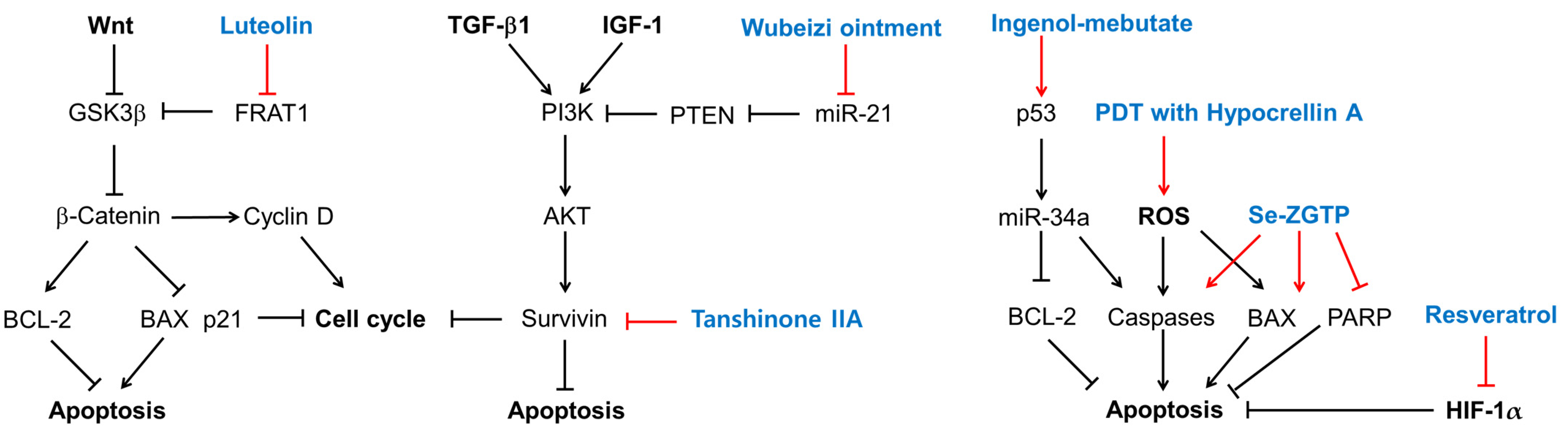
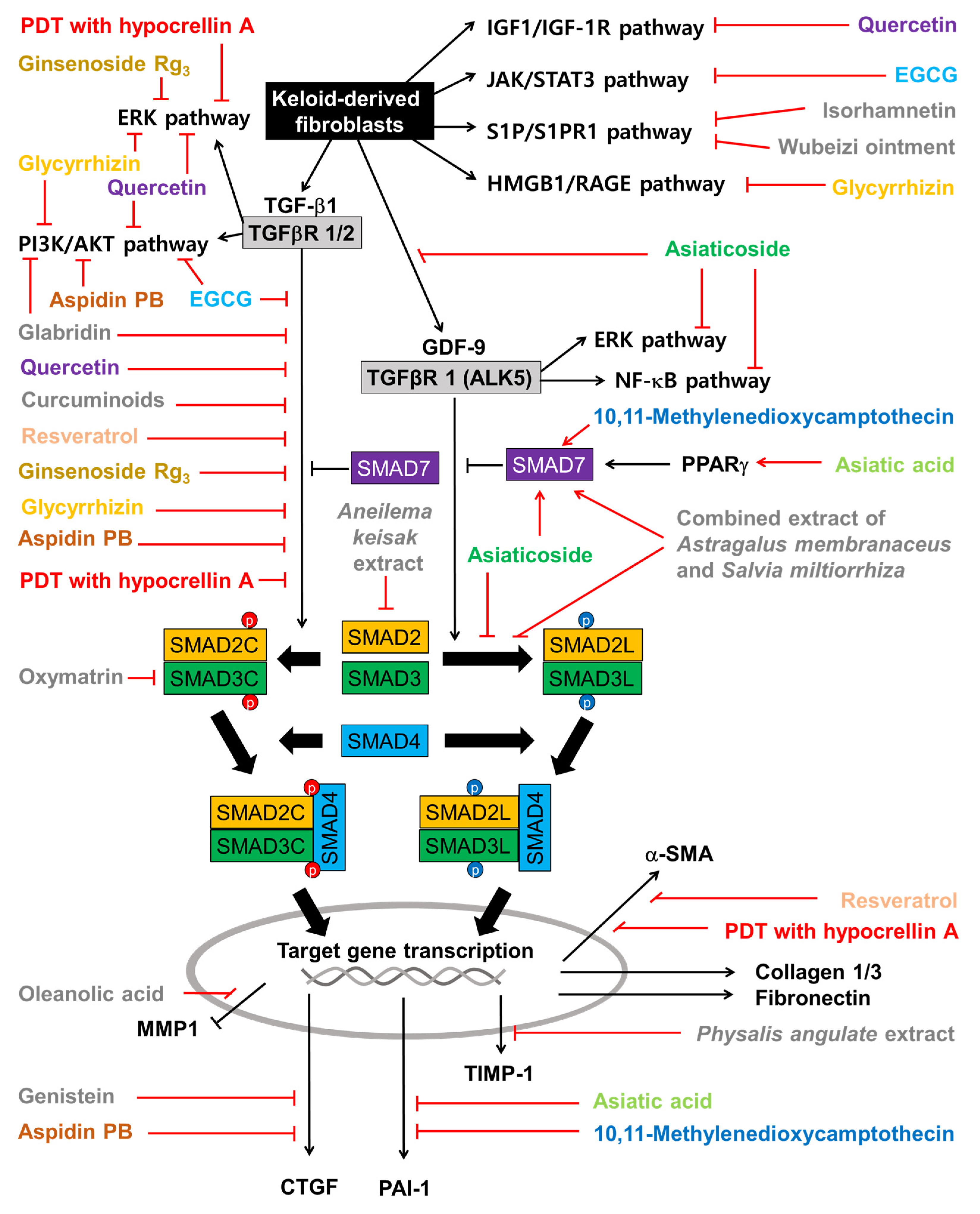
| Extracts | Therapeutic Targets | Plants | Phytochemical Information |
|---|---|---|---|
| Combined extract of Astragalus membranaceus and Salvia miltiorrhiza | Proliferation, Invasion, Collagen synthesis, SMAD pathway, PAI-1 [90] | Astragalus membranaceus | Contains flavonoids, such as quercetin, kaempferol, genisteine, dihydroxyflavone, and liquiricigenin, and terpenoids, such as astragaloside I, astragaloside II, astragaloside IV, isoastragaloside I, and isoastragalosides II [91,92]. |
| Salvia miltiorrhiza | Contains phenolic compounds, such as danshensu, salvianolic acid A, salvianolic acid B, protocatechuic aldehyde, rosmarinic acid, and caffeic acid, and terpenoids, such as tanshinone IIA, cryptotanshinone, dihydrotanshinone, isotanshinone I, isotanshinone IIA, and isocryptotanshinone [93]. | ||
| Aneilema keisak extract | Proliferation, Migration, Collagen synthesis, SMAD pathway, Cell cycle arrest, Senescence [94] | Aneilema keisak | Contains phenolic compounds, such as latifolicinin A, latifolicinin B, latifolicinin C, protocatechuic acid, hydroxytyrosol, oresbiusin A, kaempferol, epigallocatechin, rutin, ferulic acid, vanillic acid, chlorogenic acid, and p-coumaric acid [95]. |
| Wubeizi ointment | Proliferation, Cell cycle arrest, Apoptosis, AKT pathway, IGF-1, miR-21, PTEN [96,97,98,99] | Salvia miltiorrhiza | See above. |
| Galla Chinensis | Contains a variety of tannin compounds, such as 1,2,6-tri-O-galloyl-β-D-glucose and 1,2,3, 6-tetra-O-galloyl-β-D-glucose, and other phenolic compounds, such as methyl gallate, ethyl gallate, ellagic acid, myricetin-3-O-rhamnoside, and epigallocatechin [100]. | ||
| Lycium chinense | Contains rutin and chlorogenic acid [101]. It also contains various polysaccharides, carotenoids, alkaloids, and phenolic compounds [102]. | ||
| Physalis angulate extract | Cell viability, Collagen synthesis, TIMP-1 [103] | Physalis angulate | Contains phytosterols, such physalins A–I, physagulin A–G, withangulatin A, and withanolide T [104]. |
| Onion exract | Cell proliferation [105] | Allium cepa | Contains quercetin as the main active compound [105,106]. |
| Green tea extract | Collagen synthesis, AKT pathway [107] | Camellia sinensis | Contains (–)-epigallocatechin-3-gallate (EGCG) as the main active compound [107]. |
| Compounds | Proliferation/ Viability | Migration/ Invasion | Apoptosis | ECM Production | TGF-β Level | TGFβR Level | SMAD Pathway | AKT Pathway | ERK Pathway | Additional Targets | Literature |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Quercetin | √ | √ | √ | √ | √ | √ | √ | IGF1R | [87,108,109,110,111] | ||
| Kaempferol | √ | [111] | |||||||||
| (–)-Epigallocatechin -3-gallate | √ | √ | √ | √ | STAT3 | [59,85,107] | |||||
| Genistein | CTGF | [112] | |||||||||
| Luteolin | √ | √ | FRAT1 | [113] | |||||||
| Glabridin | √ | √ | √ | √ | √ | [114] | |||||
| Isorhamnetin | √ | √ | √ | √ | S1PR1 | [74] | |||||
| Protocatechuic acid | √ | √ | [111] | ||||||||
| Gallic acid | √ | √ | [111] | ||||||||
| p-Coumaric acid | √ | [111] | |||||||||
| Ferulic acid | √ | [111] | |||||||||
| Chlorogenic acid | √ | √ | [111] | ||||||||
| Curcumin | √ | √ | √ | √ | [111,115] | ||||||
| Demethoxycurcumin | √ | √ | √ | [115] | |||||||
| Bisdemethoxycurcumin | √ | √ | √ | [115] | |||||||
| Resveratrol | √ | √ | √ | √ | √ | HSP47, α-SMA | [116] | ||||
| Asiaticoside | √ | √ | √ | √ | √ | √ | p38, GDF-9 | [117] | |||
| Asiatic acid | √ | √ | PAI-1, PPARγ | [82,118] | |||||||
| Ginsenoside Rg3 | √ | √ | √ | √ | √ | Angiogenesis | [119] | ||||
| Tagitinin C | √ | √ | [120] | ||||||||
| Ingenol-mebutate | √ | √ | miR-34a | [121] | |||||||
| Glycyrrhizin | √ | √ | √ | √ | √ | √ | √ | NF-κB, HMGB1, Autophagy | [70] | ||
| Oleanolic acid | √ | √ | √ | MMP1 | [122] | ||||||
| Camptothecin | √ | [123] | |||||||||
| 10,11-Methylenedioxy camptothecin | √ | √ | √ | PAI-1 | [124] | ||||||
| Oxymatrine | √ | √ | [125] | ||||||||
| Vincristine | √ | [24] | |||||||||
| Paclitaxel | √ | √ | √ | √ | √ | [126] | |||||
| Aspidin PB | √ | √ | √ | CTGF | [127] | ||||||
| Tanshinone IIA | √ | √ | Survivin | [128] | |||||||
| Selenium- polysaccharide | √ | √ | PARP | [129] | |||||||
| Photodynamic therapy with Hypocrellin A | √ | √ | √ | √ | √ | √ | √ | Autophagy, α-SMA | [130] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boo, Y.C. Insights into How Plant-Derived Extracts and Compounds Can Help in the Prevention and Treatment of Keloid Disease: Established and Emerging Therapeutic Targets. Int. J. Mol. Sci. 2024, 25, 1235. https://doi.org/10.3390/ijms25021235
Boo YC. Insights into How Plant-Derived Extracts and Compounds Can Help in the Prevention and Treatment of Keloid Disease: Established and Emerging Therapeutic Targets. International Journal of Molecular Sciences. 2024; 25(2):1235. https://doi.org/10.3390/ijms25021235
Chicago/Turabian StyleBoo, Yong Chool. 2024. "Insights into How Plant-Derived Extracts and Compounds Can Help in the Prevention and Treatment of Keloid Disease: Established and Emerging Therapeutic Targets" International Journal of Molecular Sciences 25, no. 2: 1235. https://doi.org/10.3390/ijms25021235
APA StyleBoo, Y. C. (2024). Insights into How Plant-Derived Extracts and Compounds Can Help in the Prevention and Treatment of Keloid Disease: Established and Emerging Therapeutic Targets. International Journal of Molecular Sciences, 25(2), 1235. https://doi.org/10.3390/ijms25021235