Long Noncoding RNA VLDLR-AS1 Levels in Serum Correlate with Combat-Related Chronic Mild Traumatic Brain Injury and Depression Symptoms in US Veterans
Abstract
1. Introduction
2. Results
2.1. Identifying lncRNAs Consistently Detected in Serum
2.2. LncRNAs Are Packaged in Exosomes Derived from the Brain
2.3. LncRNA VLDLR-AS1 Levels Are Lower in Participants with Repetitive mTBI
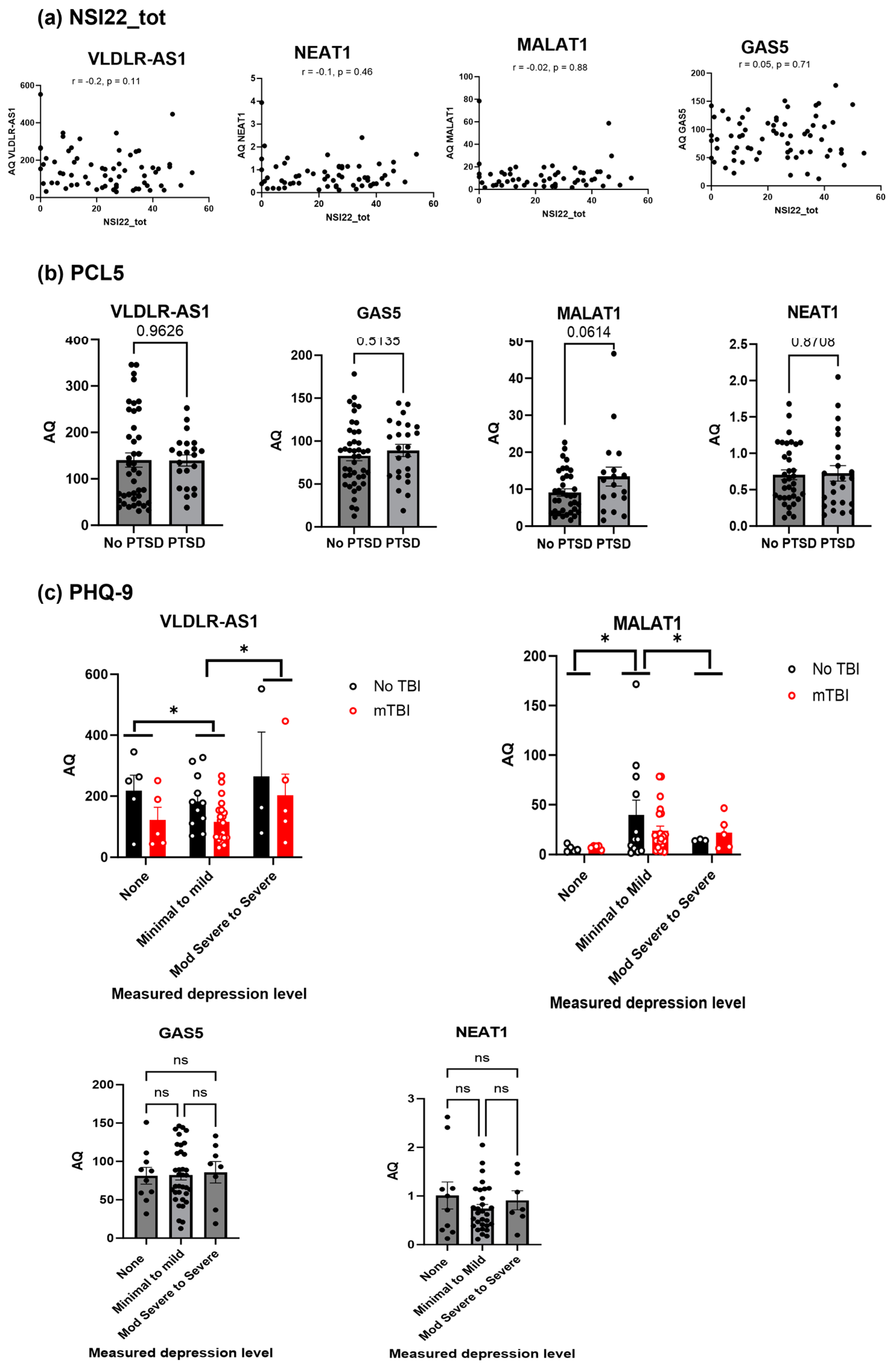
2.4. Secondary Analysis of the Clinical Data Shows VLDLR-AS1 and MALAT1 Levels Are Correlated with Depression
3. Discussion
Limitations
4. Materials and Methods
4.1. Study Samples
4.2. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
4.3. Droplet Digital Polymerase Chain Reaction (ddPCR)
4.4. CNS-Derived Exosome Purification
4.5. Neuropshycological Symptom Measures
4.6. Statistical Analyses Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dams-O’Connor, K.; Cuthbert, J.P.; Whyte, J.; Corrigan, J.D.; Faul, M.; Harrison-Felix, C. Traumatic brain injury among older adults at level I and II trauma centers. J. Neurotrauma 2013, 30, 2001–2013. [Google Scholar] [CrossRef]
- Leo, P.; McCrea, M. Epidemiology. In Translational Research in Traumatic Brain Injury; Laskowitz, D., Grant, G., Eds.; CRC Press/Taylor and Francis Group: Boca Raton, FL, USA, 2016. [Google Scholar]
- Vas, A.; Chapman, S.; Aslan, S.; Spence, J.; Keebler, M.; Rodriguez-Larrain, G.; Rodgers, B.; Jantz, T.; Martinez, D.; Rakic, J.; et al. Reasoning training in veteran and civilian traumatic brain injury with persistent mild impairment. Neuropsychol. Rehabil. 2016, 26, 502–531. [Google Scholar] [CrossRef]
- Kornblith, E.S.; Yaffe, K.; Langa, K.M.; Gardner, R.C. Prevalence of Lifetime History of Traumatic Brain Injury among Older Male Veterans Compared with Civilians: A Nationally Representative Study. J. Neurotrauma 2020, 37, 2680–2685. [Google Scholar] [CrossRef]
- Lindberg, M.A.; Martin, E.M.M.; Marion, D.W. Military Traumatic Brain Injury: The History, Impact, and Future. J. Neurotrauma 2022, 39, 1133–1145. [Google Scholar] [CrossRef]
- Eibner, C.; Schell, T.L.; Jaycox, L.H. Care of war veterans with mild traumatic brain injury. N. Engl. J. Med. 2009, 361, 537. [Google Scholar]
- Dismuke-Greer, C.; Hirsch, S.; Carlson, K.; Pogoda, T.; Nakase-Richardson, R.; Bhatnagar, S.; Eapen, B.; Troyanskaya, M.; Miles, S.; Nolen, T.; et al. Health Services Utilization, Health Care Costs, and Diagnoses by Mild Traumatic Brain Injury Exposure: A Chronic Effects of Neurotrauma Consortium Study. Arch. Phys. Med. Rehabil. 2020, 101, 1720–1730. [Google Scholar] [CrossRef]
- The Management of Concussion-mild Traumatic Brain Injury Working Group. VA/DoD Clinical Practice Guideline for Management of Concussion/Mild Traumatic Brain Injury. J. Rehabil. Res. Dev. 2009, 46, CP1–CP68. [Google Scholar] [CrossRef]
- O’Neil, M.E.; Carlson, K.; Storzbach, D.; Brenner, L.; Freeman, M.; Quinones, A.; Motu’apuaka, M.; Ensley, M.; Kansagara, D. Complications of Mild Traumatic Brain Injury in Veterans and Military Personnel: A Systematic Review; Department of Veterans Affairs Health Services Research & Development Service: Washington, DC, USA, 2013. [Google Scholar]
- Corrigan, F.; Wee, I.C.; Collins-Praino, L.E. Chronic motor performance following different traumatic brain injury severity—A systematic review. Front. Neurol. 2023, 14, 1180353. [Google Scholar] [CrossRef]
- Taylor, C.A.; Bell, J.M.; Breiding, M.J.; Xu, L. Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths—United States, 2007 and 2013. MMWR Surveill. Summ. 2017, 66, 1–16. [Google Scholar] [CrossRef]
- Seabury, S.A.; Gaudette, E.; Goldman, D.P.; Markowitz, A.J.; Brooks, J.; McCrea, M.A.; Okonkwo, D.O.; Manley, G.T.; The TRACK-TBI Investigators. Assessment of Follow-up Care After Emergency Department Presentation for Mild Traumatic Brain Injury and Concussion: Results from the TRACK-TBI Study. JAMA Netw. Open 2018, 1, e180210. [Google Scholar] [CrossRef]
- de Souza, N.L.; Esopenko, C.; Jia, Y.; Parrott, J.S.; Merkley, T.L.; Dennis, E.L.; Hillary, F.G.; Velez, C.; Cooper, D.B.; Kennedy, J.E.; et al. Discriminating Mild Traumatic Brain Injury and Posttraumatic Stress Disorder Using Latent Neuroimaging and Neuropsychological Profiles in Active-Duty Military Service Members. J. Head Trauma Rehabil. 2023, 38, E254–E266. [Google Scholar] [CrossRef]
- Nelson, L.D.; Temkin, N.R.; Barber, J.; Brett, B.L.; Okonkwo, D.O.; McCrea, M.A.; Giacino, J.T.; Bodien, Y.G.; Robertson, C.; Corrigan, J.D.; et al. Functional Recovery, Symptoms, and Quality of Life 1 to 5 Years After Traumatic Brain Injury. JAMA Netw. Open 2023, 6, e233660. [Google Scholar] [CrossRef]
- Agtarap, S.; Hungerford, L.D.; Ettenhofer, M.L. Identifying Unique Symptom Groups Following Mild Traumatic Brain Injury Using the Neurobehavioral Symptom Inventory and PTSD Checklist-5 in Military Personnel: A Bifactor Analysis. J. Head Trauma Rehabil. 2023, 38, E371–E383. [Google Scholar] [CrossRef]
- Maas, A.I.R.; Menon, D.K.; Manley, G.T.; Abrams, M.; Akerlund, C.; Andelic, N.; Aries, M.; Bashford, T.; Bell, M.J.; Bodien, Y.G.; et al. Traumatic brain injury: Progress and challenges in prevention, clinical care, and research. Lancet Neurol. 2022, 21, 1004–1060. [Google Scholar] [CrossRef]
- Feinberg, C.; Mayes, K.D.; Portman, E.; Carr, C.; Mannix, R. Non-invasive fluid biomarkers in the diagnosis of mild traumatic brain injury (mTBI): A systematic review. J. Neurol. Neurosurg. Psychiatry 2023, 95, 184–192. [Google Scholar] [CrossRef]
- Newcombe, V.; Richter, S.; Whitehouse, D.P.; Bloom, B.M.; Lecky, F. Fluid biomarkers and neuroimaging in mild traumatic brain injury: Current uses and potential future directions for clinical use in emergency medicine. Emerg. Med. J. 2023, 40, 671–677. [Google Scholar] [CrossRef]
- Vedaei, F.; Mashhadi, N.; Zabrecky, G.; Monti, D.; Navarreto, E.; Hriso, C.; Wintering, N.; Newberg, A.B.; Mohamed, F.B. Identification of chronic mild traumatic brain injury using resting state functional MRI and machine learning techniques. Front. Neurosci. 2022, 16, 1099560. [Google Scholar] [CrossRef]
- Agoston, D.V.; Helmy, A. Fluid-Based Protein Biomarkers in Traumatic Brain Injury: The View from the Bedside. Int. J. Mol. Sci. 2023, 24, 16267. [Google Scholar] [CrossRef]
- Wang, K.K.; Munoz Pareja, J.C.; Mondello, S.; Diaz-Arrastia, R.; Wellington, C.; Kenney, K.; Puccio, A.M.; Hutchison, J.; McKinnon, N.; Okonkwo, D.O.; et al. Blood-based traumatic brain injury biomarkers—Clinical utilities and regulatory pathways in the United States, Europe and Canada. Expert. Rev. Mol. Diagn. 2021, 21, 1303–1321. [Google Scholar] [CrossRef]
- Biberthaler, P.; Musaelyan, K.; Krieg, S.; Meyer, B.; Stimmer, H.; Zapf, J.; von Matthey, F.; Chandran, R.; Marino, J.A.; Beligere, G.; et al. Evaluation of Acute Glial Fibrillary Acidic Protein and Ubiquitin C-Terminal Hydrolase-L1 Plasma Levels in Traumatic Brain Injury Patients with and without Intracranial Lesions. Neurotrauma Rep. 2021, 2, 617–625. [Google Scholar] [CrossRef]
- Boucher, V.; Frenette, J.; Neveu, X.; Tardif, P.A.; Mercier, E.; Chauny, J.M.; Berthelot, S.; Archambault, P.; Lee, J.; Perry, J.J.; et al. Lack of association between four biomarkers and persistent post-concussion symptoms after a mild traumatic brain injury. J. Clin. Neurosci. 2023, 118, 34–43. [Google Scholar] [CrossRef]
- Beylerli, O.; Tamrazov, R.; Gareev, I.; Ilyasova, T.; Shumadalova, A.; Bai, Y.; Yang, B. Role of exosomal ncRNAs in traumatic brain injury. Noncoding RNA Res. 2023, 8, 686–692. [Google Scholar] [CrossRef]
- Wapinski, O.; Chang, H.Y. Long noncoding RNAs and human disease. Trends Cell Biol. 2011, 21, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Dieter, C.; Lourenco, E.D.; Lemos, N.E. Association of long non-coding RNA and leukemia: A systematic review. Gene 2020, 735, 144405. [Google Scholar] [CrossRef]
- He, Z.; Yang, D.; Fan, X.; Zhang, M.; Li, Y.; Gu, X.; Yang, M. The Roles and Mechanisms of lncRNAs in Liver Fibrosis. Int. J. Mol. Sci. 2020, 21, 1482. [Google Scholar] [CrossRef]
- Moore, J.B., 4th; Uchida, S. Functional characterization of long noncoding RNAs. Curr. Opin. Cardiol. 2020, 35, 199–206. [Google Scholar] [CrossRef]
- Gao, S.; Fan, C.; Wang, Y.; Yang, W.; Jiang, H. LncRNA ENST00000440246.1 Promotes Alzheimer’s Disease Progression by Targeting PP2A. Biochem. Genet. 2023. [Google Scholar] [CrossRef]
- Ghamari, M.; Mohseni, M.M.; Taheri, M.; Neishabouri, S.M.; Shirvani-Farsani, Z. Abnormal expression of long non-coding RNAs RMRP, CTC-487M23.5, and DGCR5 in the peripheral blood of patients with Bipolar disorder. Metab. Brain Dis. 2023. [Google Scholar] [CrossRef]
- Greco, S.; Made, A.; Mutoli, M.; Zhang, L.; Piella, S.N.; Vausort, M.; Lumley, A.I.; Beltrami, A.P.; Srivastava, P.K.; Milani, V.; et al. HCG18, LEF1AS1 and lncCEACAM21 as biomarkers of disease severity in the peripheral blood mononuclear cells of COVID-19 patients. J. Transl. Med. 2023, 21, 758. [Google Scholar] [CrossRef]
- Qiu, T.; Xue, M.; Li, X.; Li, F.; Liu, S.; Yao, C.; Chen, W. Comparative evaluation of long non-coding RNA-based biomarkers in the urinary sediment and urinary exosomes for non-invasive diagnosis of bladder cancer. Mol. Omics 2022, 18, 938–947. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, J.; Bi, Q.; Wang, W. Exosomal lncRNA TUG1 derived from human urine-derived stem cells attenuates renal ischemia/reperfusion injury by interacting with SRSF1 to regulate ASCL4-mediated ferroptosis. Stem Cell Res. Ther. 2022, 13, 297. [Google Scholar] [CrossRef]
- Lopes, C.; Chaves, J.; Ortigao, R.; Dinis-Ribeiro, M.; Pereira, C. Gastric cancer detection by non-blood-based liquid biopsies: A systematic review looking into the last decade of research. United Eur. Gastroenterol. J. 2023, 11, 114–130. [Google Scholar] [CrossRef]
- Jin, H.; Du, W.; Huang, W.; Yan, J.; Tang, Q.; Chen, Y.; Zou, Z. lncRNA and breast cancer: Progress from identifying mechanisms to challenges and opportunities of clinical treatment. Mol. Ther. Nucleic Acids 2021, 25, 613–637. [Google Scholar] [CrossRef]
- Li, Z.; Cai, S.; Li, H.; Gu, J.; Tian, Y.; Cao, J.; Yu, D.; Tang, Z. Developing a lncRNA Signature to Predict the Radiotherapy Response of Lower-Grade Gliomas Using Co-expression and ceRNA Network Analysis. Front. Oncol. 2021, 11, 622880. [Google Scholar] [CrossRef] [PubMed]
- Kucukakcali, Z.; Colak, C.; Gozukara Bag, H.G.; Balikci Cicek, I.; Ozhan, O.; Yildiz, A.; Danis, N.; Koc, A.; Parlakpinar, H.; Akbulut, S. Modeling Based on Ensemble Learning Methods for Detection of Diagnostic Biomarkers from LncRNA Data in Rats Treated with Cis-Platinum-Induced Hepatotoxicity. Diagnostics 2023, 13, 1583. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhang, X.; Cai, Z.; Xi, Z.; Wang, F.; Wang, X.; Li, W.; Dai, P. Identification of novel lncRNA prognostic biomarkers and their associated ceRNAs in bladder urothelial carcinoma. J. Biochem. Mol. Toxicol. 2023, 37, e23441. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, J.; Yu, S.; Wang, Z.; He, X.; Su, Y.; Guo, T.; Sheng, H.; Chen, J.; Zheng, Q.; et al. Extracellular Vesicles Long RNA Sequencing Reveals Abundant mRNA, circRNA, and lncRNA in Human Blood as Potential Biomarkers for Cancer Diagnosis. Clin. Chem. 2019, 65, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Vanderploeg, R.D.; Cooper, D.B.; Belanger, H.G.; Donnell, A.J.; Kennedy, J.E.; Hopewell, C.A.; Scott, S.G. Screening for postdeployment conditions: Development and cross-validation of an embedded validity scale in the neurobehavioral symptom inventory. J. Head Trauma Rehabil. 2014, 29, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Belanger, H.G.; Silva, M.A.; Donnell, A.J.; McKenzie-Hartman, T.; Lamberty, G.J.; Vanderploeg, R.D. Utility of the Neurobehavioral Symptom Inventory As an Outcome Measure: A VA TBI Model Systems Study. J. Head Trauma Rehabil. 2017, 32, 46–54. [Google Scholar] [CrossRef]
- Silva, M.A. Review of the Neurobehavioral Symptom Inventory. Rehabil. Psychol. 2021, 66, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Soble, J.R.; Silva, M.A.; Vanderploeg, R.D.; Curtiss, G.; Belanger, H.G.; Donnell, A.J.; Scott, S.G. Normative Data for the Neurobehavioral Symptom Inventory (NSI) and post-concussion symptom profiles among TBI, PTSD, and nonclinical samples. Clin. Neuropsychol. 2014, 28, 614–632. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- You, Y.; Wang, W.; Zhu, W.; Xu, J. Identification of functional lncRNAs in atrial fibrillation based on RNA sequencing. BMC Cardiovasc. Disord. 2023, 23, 539. [Google Scholar] [CrossRef]
- Xiong, L.; Tang, M.; Xing, S.; Yang, X. The role of noncoding RNA and its diagnostic potential in intrahepatic cholestasis of pregnancy: A research update. Front. Genet. 2023, 14, 1239693. [Google Scholar] [CrossRef]
- Xie, Y.; Ye, J.; Luo, H. HOXC Cluster Antisense RNA 3, a Novel Long Non-Coding RNA as an Oncological Biomarker and Therapeutic Target in Human Malignancies. Onco Targets Ther. 2023, 16, 849–865. [Google Scholar] [CrossRef]
- Wang, L.; Xu, D. Regulation of long noncoding RNAs in the pathogenesis and clinical implications of pituitary adenomas. Immun. Inflamm. Dis. 2023, 11, e1047. [Google Scholar] [CrossRef]
- Cheng, C.; Liu, Z.; Liu, D.; Chen, H.; Wang, Y.; Sun, B. LncRNA CCAT1 participates in pancreatic ductal adenocarcinoma progression by forming a positive feedback loop with c-Myc. Carcinogenesis 2023. [Google Scholar] [CrossRef]
- Carter, G.; Miladinovic, B.; Patel, A.A.; Deland, L.; Mastorides, S.; Patel, N.A. Circulating long noncoding RNA GAS5 levels are correlated to prevalence of type 2 diabetes mellitus. BBA Clin. 2015, 4, 102–107. [Google Scholar] [CrossRef]
- Beylerli, O.; Gareev, I.; Sufianov, A.; Ilyasova, T.; Guang, Y. Long noncoding RNAs as promising biomarkers in cancer. Noncoding RNA Res. 2022, 7, 66–70. [Google Scholar] [CrossRef]
- Ransohoff, J.D.; Wei, Y.; Khavari, P.A. The functions and unique features of long intergenic non-coding RNA. Nat. Rev. Mol. Cell Biol. 2018, 19, 143–157. [Google Scholar] [CrossRef]
- Ang, C.E.; Trevino, A.E.; Chang, H.Y. Diverse lncRNA mechanisms in brain development and disease. Curr. Opin. Genet. Dev. 2020, 65, 42–46. [Google Scholar] [CrossRef]
- Samaddar, S.; Banerjee, S. Far from the nuclear crowd: Cytoplasmic lncRNA and their implications in synaptic plasticity and memory. Neurobiol. Learn. Mem. 2021, 185, 107522. [Google Scholar] [CrossRef]
- Srinivas, T.; Siqueira, E.; Guil, S. Techniques for investigating lncRNA transcript functions in neurodevelopment. Mol. Psychiatry 2023. [Google Scholar] [CrossRef] [PubMed]
- Glasgow, A.M.A.; De Santi, C.; Greene, C.M. Non-coding RNA in cystic fibrosis. Biochem. Soc. Trans. 2018, 46, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Magee, P.; Fassan, M.; Sahoo, S.; Leong, H.S.; Lee, D.; Sellers, R.; Brulle-Soumare, L.; Cairo, S.; Monteverde, T.; et al. A KRAS-responsive long non-coding RNA controls microRNA processing. Nat. Commun. 2021, 12, 2038. [Google Scholar] [CrossRef] [PubMed]
- Aliperti, V.; Skonieczna, J.; Cerase, A. Long Non-Coding RNA (lncRNA) Roles in Cell Biology, Neurodevelopment and Neurological Disorders. Noncoding RNA 2021, 7, 36. [Google Scholar] [CrossRef]
- Takahashi, K.; Yan, I.K.; Wood, J.; Haga, H.; Patel, T. Involvement of extracellular vesicle long noncoding RNA (linc-VLDLR) in tumor cell responses to chemotherapy. Mol. Cancer Res. 2014, 12, 1377–1387. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Guo, J.; Zhang, H.; Cao, B.; Xu, G.; Zhang, Z.; Tong, J. Four Prognosis-Associated lncRNAs Serve as Biomarkers in Ovarian Cancer. Front. Genet. 2021, 12, 672674. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, T.; Wang, B.; Li, L.; Ye, D.; Yu, S. Identification and functional analysis of a potential key lncRNA involved in fat loss of cancer cachexia. J. Cell Biochem. 2018, 119, 1679–1688. [Google Scholar] [CrossRef]
- Rouillard, A.D.; Hurle, M.R.; Agarwal, P. Systematic interrogation of diverse Omic data reveals interpretable, robust, and generalizable transcriptomic features of clinically successful therapeutic targets. PLoS Comput. Biol. 2018, 14, e1006142. [Google Scholar] [CrossRef]
- Rouillard, A.D.; Gundersen, G.W.; Fernandez, N.F.; Wang, Z.; Monteiro, C.D.; McDermott, M.G.; Ma’ayan, A. The harmonizome: A collection of processed datasets gathered to serve and mine knowledge about genes and proteins. Database 2016, 2016, baw100. [Google Scholar] [CrossRef]
- Ros, G.; Pegoraro, S.; De Angelis, P.; Sgarra, R.; Zucchelli, S.; Gustincich, S.; Manfioletti, G. HMGA2 Antisense Long Non-coding RNAs as New Players in the Regulation of HMGA2 Expression and Pancreatic Cancer Promotion. Front. Oncol. 2019, 9, 1526. [Google Scholar] [CrossRef]
- Shi, Y.; Parag, S.; Patel, R.; Lui, A.; Murr, M.; Cai, J.; Patel, N.A. Stabilization of lncRNA GAS5 by a Small Molecule and Its Implications in Diabetic Adipocytes. Cell Chem. Biol. 2019, 26, 319–330.e6. [Google Scholar] [CrossRef]
- Powell, J.M.; Ferraro, J.V.; Dikmen, S.S.; Temkin, N.R.; Bell, K.R. Accuracy of mild traumatic brain injury diagnosis. Arch. Phys. Med. Rehabil. 2008, 89, 1550–1555. [Google Scholar] [CrossRef] [PubMed]
- Pape, T.L.B.; Smith, B.; Babcock-Parziale, J.; Evans, C.T.; Herrold, A.A.; Maieritsch, K.P.; High, W.M., Jr. Diagnostic Accuracy of the Veteran Affairs’ Traumatic Brain Injury Screen. Arch. Phys. Med. Rehabil. 2018, 99, 1370–1382. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.H.; Powell-Cope, G.; Belanger, H.G. The Veterans Health Administration’s Traumatic Brain Injury Screen and Evaluation: Service Delivery Insights. Mil. Med. 2018, 183, e494–e501. [Google Scholar] [CrossRef]
- Kohler, M.J.; Hendrickx, M.D.; Powell-Jones, A.; Bryan-Hancock, C. A Systematic Review of Cognitive Functioning After Traumatic Brain Injury in Individuals Aged 10–30 Years. Cogn. Behav. Neurol. 2020, 33, 233–252. [Google Scholar] [CrossRef]
- Kenney, K.; Qu, B.X.; Lai, C.; Devoto, C.; Motamedi, V.; Walker, W.C.; Levin, H.S.; Nolen, T.; Wilde, E.A.; Diaz-Arrastia, R.; et al. Higher exosomal phosphorylated tau and total tau among veterans with combat-related repetitive chronic mild traumatic brain injury. Brain Inj. 2018, 32, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.S.; Carter, G.; El Bassit, G.; Patel, A.A.; Cooper, D.R.; Murr, M.; Patel, N.A. Adipose-derived stem cells from lean and obese humans show depot specific differences in their stem cell markers, exosome contents and senescence: Role of protein kinase C delta (PKCdelta) in adipose stem cell niche. Stem Cell Investig. 2016, 3, 2. [Google Scholar]
- Yang, N.; Li, S.; Li, G.; Zhang, S.; Tang, X.; Ni, S.; Jian, X.; Xu, C.; Zhu, J.; Lu, M. The role of extracellular vesicles in mediating progression, metastasis and potential treatment of hepatocellular carcinoma. Oncotarget 2017, 8, 3683–3695. [Google Scholar] [CrossRef]
- Ren, D.; Chen, W.; Cao, K.; Wang, Z.; Zheng, P. Expression Profiles of Long Non-coding RNA and Messenger RNA in Human Traumatic Brain Injury. Mol. Ther. Nucleic Acids 2020, 22, 99–113. [Google Scholar] [CrossRef]
- Meng, J.; Ding, T.; Chen, Y.; Long, T.; Xu, Q.; Lian, W.; Liu, W. LncRNA-Meg3 promotes Nlrp3-mediated microglial inflammation by targeting miR-7a-5p. Int. Immunopharmacol. 2021, 90, 107141. [Google Scholar] [CrossRef]
- Li, Z.; Han, K.; Zhang, D.; Chen, J.; Xu, Z.; Hou, L. The role of long noncoding RNA in traumatic brain injury. Neuropsychiatr. Dis. Treat. 2019, 15, 1671–1677. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Gou, Y.; Jiang, X.; Wang, S.; Wang, R.; Liang, C.; Yang, G.; Wang, T.; Yu, A.; Zhu, G. Long Non-coding RNAs in Traumatic Brain Injury Accelerated Fracture Healing. Front. Surg. 2021, 8, 663377. [Google Scholar] [CrossRef]
- Cheng, S.; Zhang, Y.; Chen, S.; Zhou, Y. LncRNA HOTAIR Participates in Microglia Activation and Inflammatory Factor Release by Regulating the Ubiquitination of MYD88 in Traumatic Brain Injury. J. Mol. Neurosci. 2021, 71, 169–177. [Google Scholar] [CrossRef]
- Li, S.; Qiu, N.; Ni, A.; Hamblin, M.H.; Yin, K.J. Role of regulatory non-coding RNAs in traumatic brain injury. Neurochem. Int. 2023, 172, 105643. [Google Scholar] [CrossRef]
- Smith, C.M.; Steitz, J.A. Classification of gas5 as a multi-small-nucleolar-RNA (snoRNA) host gene and a member of the 5′-terminal oligopyrimidine gene family reveals common features of snoRNA host genes. Mol. Cell. Biochem. 1998, 18, 6897–6909. [Google Scholar] [CrossRef]
- Coccia, E.M.; Cicala, C.; Charlesworth, A.; Ciccarelli, C.; Rossi, G.B.; Philipson, L.; Sorrentino, V. Regulation and expression of a growth arrest-specific gene (gas5) during growth, differentiation, and development. Mol. Cell. Biochem. 1992, 12, 3514–3521. [Google Scholar]
- Ferguson, S.; McCartan, R.; Browning, M.; Hahn-Townsend, C.; Gratkowski, A.; Morin, A.; Abdullah, L.; Ait-Ghezala, G.; Ojo, J.; Sullivan, K.; et al. Impact of gulf war toxic exposures after mild traumatic brain injury. Acta Neuropathol. Commun. 2022, 10, 147. [Google Scholar] [CrossRef]
- Kryza-Lacombe, M.; Santiago, R.; Hwang, A.; Raptentsetsang, S.; Maruyama, B.A.; Chen, J.; Cassar, M.; Abrams, G.; Novakovic-Agopian, T.; Mukherjee, P. Resting-State Connectivity Changes After Goal-Oriented Attentional Self-Regulation Training in Veterans With Mild Traumatic Brain Injury: Preliminary Findings from a Randomized Controlled Trial. Neurotrauma Rep. 2023, 4, 420–432. [Google Scholar] [CrossRef]
- Martindale, S.L.; Vujanovic, A.A.; Ord, A.S.; Cary, A.; Rowland, J.A. Distress tolerance mitigates effects of posttraumatic stress, traumatic brain injury, and blast exposure on psychiatric and health outcomes. Rehabil. Psychol. 2023, 68, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Pickett, T.C.; Walker, W.C.; Lippa, S.M.; Lange, R.T.; Brickell, T.A.; Dittmer, T.A.; Smith, J.M.; Cifu, D.X.; French, L.M. Cross-Walk Comparison of the DVBIC-TBICoE and LIMBIC-CENC Combat-Related Concussion Prospective Longitudinal Study Datasets. Arch. Phys. Med. Rehabil. 2023, 104, 1072–1080.e1. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.C.; Clark, S.W.; Eppich, K.; Wilde, E.A.; Martin, A.M.; Allen, C.M.; Cortez, M.M.; Pugh, M.J.; Walton, S.R.; Kenney, K. Headache among combat-exposed veterans and service members and its relation to mild traumatic brain injury history and other factors: A LIMBIC-CENC study. Front. Neurol. 2023, 14, 1242871. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.A.; Lurie, J.K. Principal components analysis of the Neurobehavioral Symptom Inventory in a nonclinical civilian sample. Appl. Neuropsychol. Adult 2017, 24, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Esterov, D.; Lennon, R.J.; Bergquist, T.; Brown, A. Predictors of neurobehavioral symptom reporting in a community based sample with mild traumatic brain injury. NeuroRehabilitation 2020, 47, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Cole, W.R.; Cecchini, A.S.; Remigio-Baker, R.A.; Gregory, E.; Bailie, J.M.; Ettenhofer, M.L.; McCulloch, K.L. “Return to duty” as an outcome metric in military concussion research: Problems, pitfalls, and potential solutions. Clin. Neuropsychol. 2020, 34, 1156–1174. [Google Scholar] [CrossRef] [PubMed]
- Lind, P.A.; Zhu, G.; Montgomery, G.W.; Madden, P.A.; Heath, A.C.; Martin, N.G.; Slutske, W.S. Genome-wide association study of a quantitative disordered gambling trait. Addict. Biol. 2013, 18, 511–522. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.; Stone, S.S.; Liu, N.; Gong, K.; Ren, C.; Sun, K.; Zhang, C.; Shao, G. The Role of the lncRNA MALAT1 in Neuroprotection against Hypoxic/Ischemic Injury. Biomolecules 2022, 12, 146. [Google Scholar] [CrossRef]
- Patel, N.A.; Moss, L.D.; Lee, J.Y.; Tajiri, N.; Acosta, S.; Hudson, C.; Parag, S.; Cooper, D.R.; Borlongan, C.V.; Bickford, P.C. Long noncoding RNA MALAT1 in exosomes drives regenerative function and modulates inflammation-linked networks following traumatic brain injury. J. Neuroinflamm. 2018, 15, 204. [Google Scholar] [CrossRef]
- Qiu, J.J.; Lin, X.J.; Tang, X.Y.; Zheng, T.T.; Lin, Y.Y.; Hua, K.Q. Exosomal Metastasis-Associated Lung Adenocarcinoma Transcript 1 Promotes Angiogenesis and Predicts Poor Prognosis in Epithelial Ovarian Cancer. Int. J. Biol. Sci. 2018, 14, 1960–1973. [Google Scholar] [CrossRef]
- Pan, Y.; Tong, S.; Cui, R.; Fan, J.; Liu, C.; Lin, Y.; Tang, J.; Xie, H.; Lin, P.; Zheng, T.; et al. Long Non-Coding MALAT1 Functions as a Competing Endogenous RNA to Regulate Vimentin Expression by Sponging miR-30a-5p in Hepatocellular Carcinoma. Cell Physiol. Biochem. 2018, 50, 108–120. [Google Scholar] [CrossRef]
- Huang, J.; Zheng, Y.; Xiao, X.; Liu, C.; Lin, J.; Zheng, S.; Yang, B.; Ou, Q. A Circulating Long Noncoding RNA Panel Serves as a Diagnostic Marker for Hepatocellular Carcinoma. Dis. Markers 2020, 2020, 5417598. [Google Scholar] [CrossRef]
- Hu, Y.; Zheng, L.; Zhang, J.; Shen, Y.; Zhang, X.; Lin, L. LncRNA-MALAT1 is a promising biomarker for prognostic evaluation of tongue squamous cell carcinoma. Eur. Arch. Otorhinolaryngol. 2020, 277, 3155–3160. [Google Scholar] [CrossRef]
- de Aguiar Neto, F.S.; Rosa, J.L.G. Depression biomarkers using non-invasive EEG: A review. Neurosci. Biobehav. Rev. 2019, 105, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Harsanyi, S.; Kupcova, I.; Danisovic, L.; Klein, M. Selected Biomarkers of Depression: What Are the Effects of Cytokines and Inflammation? Int. J. Mol. Sci. 2022, 24, 578. [Google Scholar] [CrossRef] [PubMed]
- Arriaga-Canon, C.; Contreras-Espinosa, L.; Aguilar-Villanueva, S.; Bargallo-Rocha, E.; Garcia-Gordillo, J.A.; Cabrera-Galeana, P.; Castro-Hernandez, C.; Jimenez-Trejo, F.; Herrera, L.A. The Clinical Utility of lncRNAs and Their Application as Molecular Biomarkers in Breast Cancer. Int. J. Mol. Sci. 2023, 24, 7426. [Google Scholar] [CrossRef] [PubMed]
- Carter, G.; Apostolatos, A.; Patel, R.; Mathur, A.; Cooper, D.; Murr, M.; Patel, N.A. Dysregulated Alternative Splicing Pattern of PKC during Differentiation of Human Preadipocytes Represents Distinct Differences between Lean and Obese Adipocytes. ISRN Obes. 2013, 2013, 9. [Google Scholar]
- Coulter, S.J. Mitigation of the effect of variability in digital PCR assays through use of duplexed reference assays for normalization. Biotechniques 2018, 65, 86–91. [Google Scholar] [CrossRef]
- King, P.R.; Donnelly, K.T.; Donnelly, J.P.; Dunnam, M.; Warner, G.; Kittleson, C.J.; Bradshaw, C.B.; Alt, M.; Meier, S.T. Psychometric study of the Neurobehavioral Symptom Inventory. J. Rehabil. Res. Dev. 2012, 49, 879–888. [Google Scholar] [CrossRef]
- Blevins, C.A.; Weathers, F.W.; Davis, M.T.; Witte, T.K.; Domino, J.L. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): Development and Initial Psychometric Evaluation. J. Trauma Stress 2015, 28, 489–498. [Google Scholar] [CrossRef]
- Budczies, J.; Klauschen, F.; Sinn, B.V.; Gyorffy, B.; Schmitt, W.D.; Darb-Esfahani, S.; Denkert, C. Cutoff Finder: A comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS ONE 2012, 7, e51862. [Google Scholar] [CrossRef] [PubMed]



| Characteristic | Study Group | |
|---|---|---|
| No TBI (No. = 24) | mTBI (No. = 43) | |
| Age, mean (SD) (year) | 41.0 (12.7) | 41.0 (12.7) |
| Male, No. (%) | 19 (79.2) | 34 (79.1) |
| Racial background, No. (%) | ||
| White | 16 (66.6) | 33 (76.7) |
| Black | 8 (33.3) | 10 (23.3) |
| Education, No. (%) | ||
| High school graduate or GED | 3 (12.5) | 2 (4.7) |
| Some college or technical training | 9 (37.5) | 22 (51.2) |
| College graduate or higher | 12 (50.0) | 19 (44.2) |
| Number of TBI, mean (SD) | 0 | 2.8 (2.1) |
| Number of blast TBI, mean (SD) | 0 | 0.8 (1.0) |
| Number of general TBI, mean (SD) | 0 | 2.0 (1.6) |
| Years since first TBI, mean (SD) | 20.2 (12.2) | |
| Years since last TBI, mean (SD) | 9.6 (8.5) | |
| PHQ-9 total, mean (SD) | 4.0 (4.6) | 8.0 (5.6) |
| PCL-5 total, mean (SD) | 14.2 (15.4) | 26.5 (16.5) |
| NSI, mean (SD) | ||
| NSI total | 13.3 (13.1) | 28.1 (13.3) |
| Somatic | 3.0 (4.2) | 7.4 (4.1) |
| Affective | 5.8 (5.0) | 9.9 (5.1) |
| Cognitive | 2.8 (2.8) | 6.1 (3.7) |
| Vestibular | 1.0 (1.6) | 2.9 (2.2) |
| Number obese (BMI over 30), No. (%) | 11 (46.0) | 19 (44.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, R.S.; Krause-Hauch, M.; Kenney, K.; Miles, S.; Nakase-Richardson, R.; Patel, N.A. Long Noncoding RNA VLDLR-AS1 Levels in Serum Correlate with Combat-Related Chronic Mild Traumatic Brain Injury and Depression Symptoms in US Veterans. Int. J. Mol. Sci. 2024, 25, 1473. https://doi.org/10.3390/ijms25031473
Patel RS, Krause-Hauch M, Kenney K, Miles S, Nakase-Richardson R, Patel NA. Long Noncoding RNA VLDLR-AS1 Levels in Serum Correlate with Combat-Related Chronic Mild Traumatic Brain Injury and Depression Symptoms in US Veterans. International Journal of Molecular Sciences. 2024; 25(3):1473. https://doi.org/10.3390/ijms25031473
Chicago/Turabian StylePatel, Rekha S., Meredith Krause-Hauch, Kimbra Kenney, Shannon Miles, Risa Nakase-Richardson, and Niketa A. Patel. 2024. "Long Noncoding RNA VLDLR-AS1 Levels in Serum Correlate with Combat-Related Chronic Mild Traumatic Brain Injury and Depression Symptoms in US Veterans" International Journal of Molecular Sciences 25, no. 3: 1473. https://doi.org/10.3390/ijms25031473
APA StylePatel, R. S., Krause-Hauch, M., Kenney, K., Miles, S., Nakase-Richardson, R., & Patel, N. A. (2024). Long Noncoding RNA VLDLR-AS1 Levels in Serum Correlate with Combat-Related Chronic Mild Traumatic Brain Injury and Depression Symptoms in US Veterans. International Journal of Molecular Sciences, 25(3), 1473. https://doi.org/10.3390/ijms25031473







