Long-Term Cryopreservation May Cause Genomic Instability and the Premature Senescence of Cells
Abstract
1. Introduction
2. Results
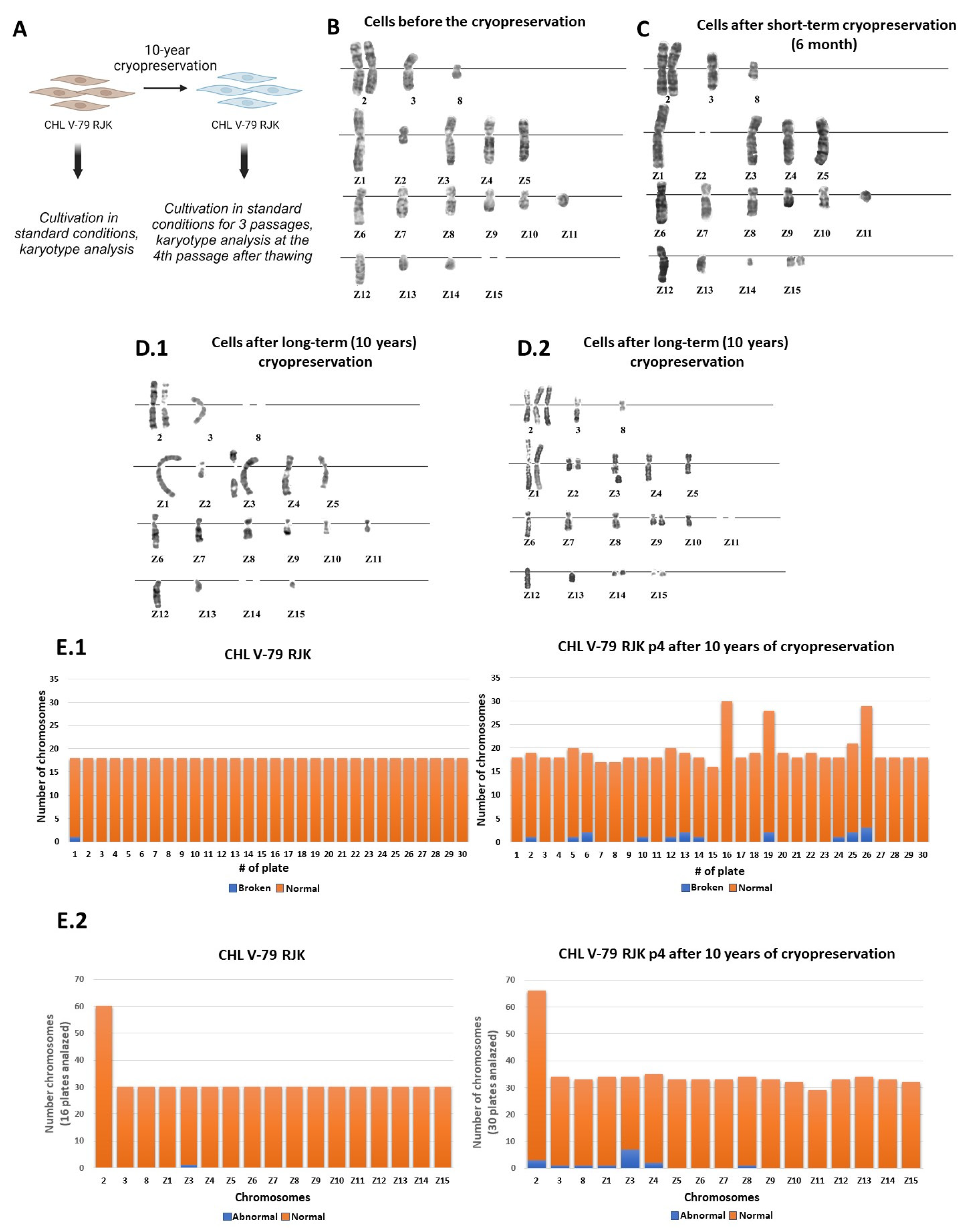
2.1. The Effect of Long-Term Cryopreservation on the Karyotype of CHL V-79 RJK Cells
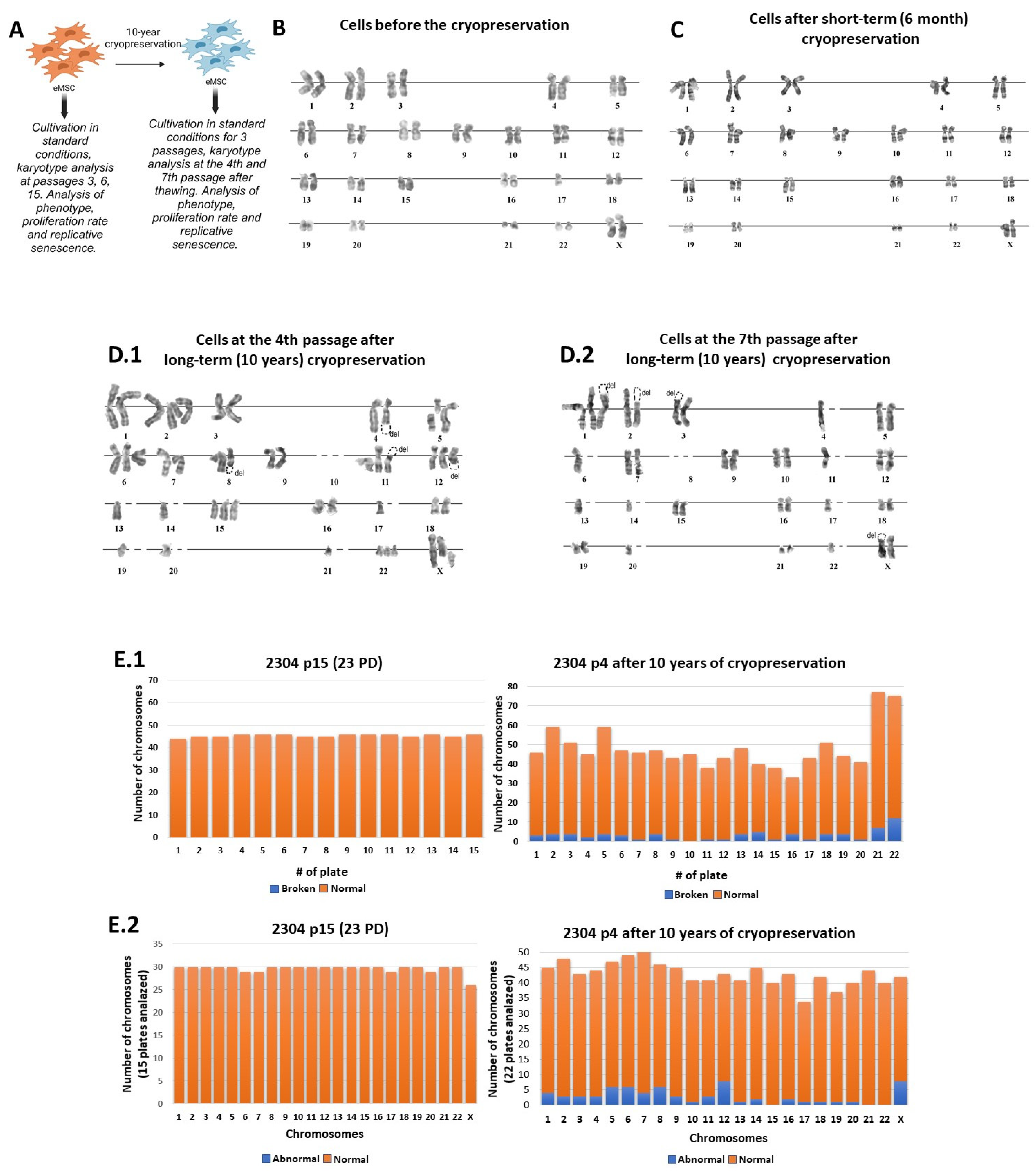
2.2. The Effect of Long-Term Cryopreservation on the Karyotype of Human Endometrial Mesenchymal Stem/Stromal Cells (eMSCs)
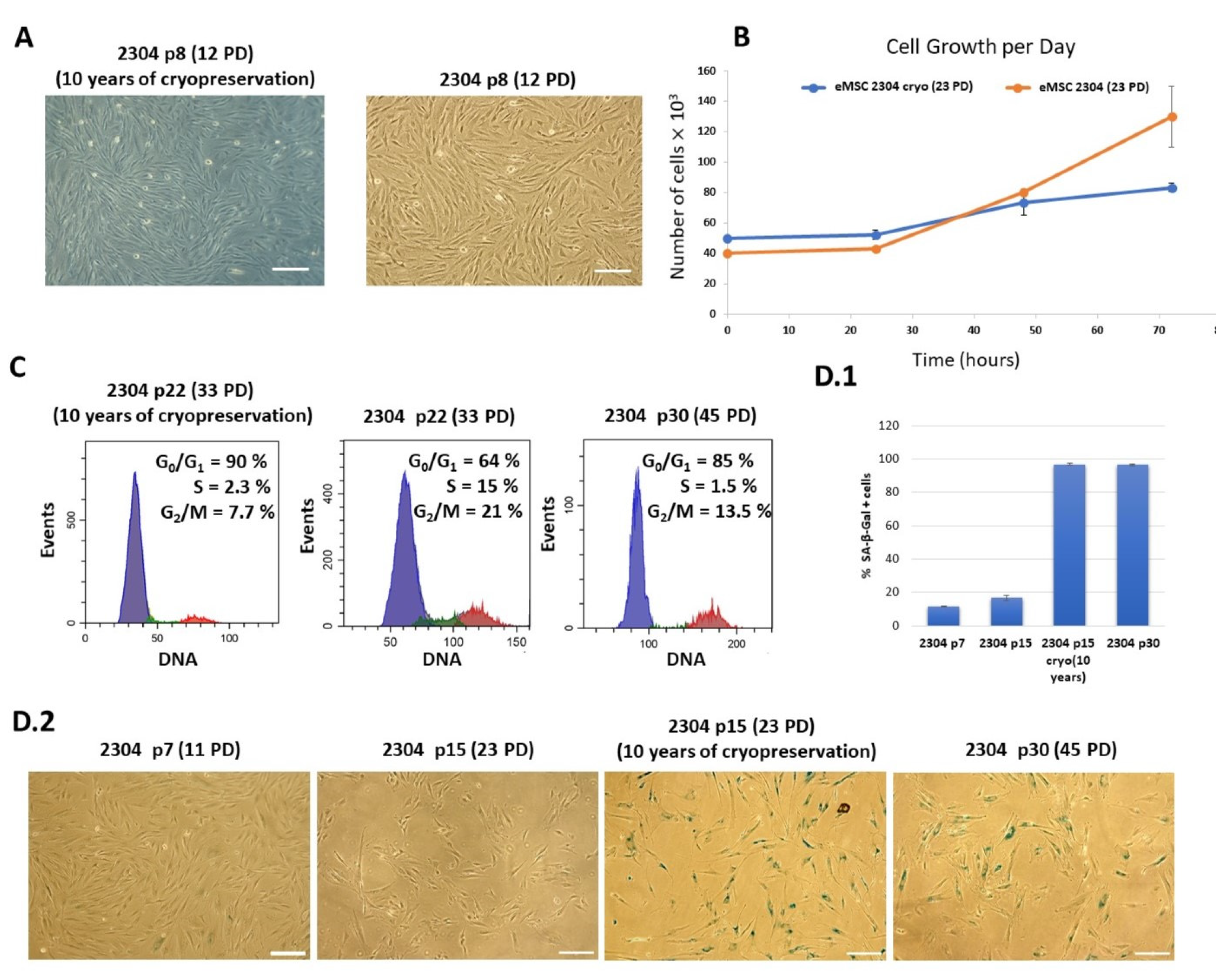
2.3. Phenotypic and Functional Characteristics of Thawed eMSCs Subjected to Long-Term Cryopreservation
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bahsoun, S.; Coopman, K.; Akam, E.C. The impact of cryopreservation on bone marrow-derived mesenchymal stem cells: A systematic review. J. Transl. Med. 2019, 17, 397. [Google Scholar] [CrossRef]
- Woods, E.J.; Perry, B.C.; Hockema, J.J.; Larson, L.; Zhou, D.; Goebel, W.S. Optimized cryopreservation method for human dental pulp-derived stem cells and their tissues of origin for banking and clinical use. Cryobiology 2009, 59, 150–157. [Google Scholar] [CrossRef]
- De Lima Prata, K.; De Santis, G.C.; Orellana, M.D.; Palma, P.V.B.; Brassesco, M.S.; Covas, D.T. Cryopreservation of umbilical cord mesenchymal cells in xenofree conditions. Cytotherapy 2012, 14, 694–700. [Google Scholar] [CrossRef]
- Polchow, B.; Kebbel, K.; Schmiedeknecht, G.; Reichardt, A.; Henrich, W.; Hetzer, R.; Lueders, C. Cryopreservation of human vascular umbilical cord cells under good manufacturing practice conditions for future cell banks. J. Transl. Med. 2012, 10, 98. [Google Scholar] [CrossRef]
- Astrelina, T.A.; Gomzyakov, A.E.; Kobzeva, I.V.; Karpova, E.E.; Kruglova, A.Y.; Scorobogatova, E.V.; Balashov, D.N.; Knyazev, O.V.; Yakovleva, M.V. Evaluation of the quality and safety of cryopreserved human multipotent mesenchymal stromal cells derived from placenta for the clinical use. Genes Cells 2013, 8, 82–87. [Google Scholar] [CrossRef]
- Imaizumi, K.; Nishishita, N.; Muramatsu, M.; Yamamoto, T.; Takenaka, C.; Kawamata, S.; Kobayashi, K.; Nishikawa, S.I.; Akuta, T. A simple and highly effective method for slow-freezing human pluripotent stem cells using dimethyl sulfoxide, hydroxyethyl starch and ethylene glycol. PLoS ONE 2014, 9, e88696. [Google Scholar] [CrossRef] [PubMed]
- Moll, G.; Alm, J.J.; Davies, L.C.; Von Bahr, L.; Heldring, N.; Stenbeck-Funke, L.; Hamad, O.A.; Hinsch, R.; Ignatowicz, L.; Locke, M.; et al. Do cryopreserved mesenchymal stromal cells display impaired immunomodulatory and therapeutic properties? Stem Cells 2014, 32, 2430–2442. [Google Scholar] [CrossRef] [PubMed]
- Duarte, D.M.; Cornélio, D.A.; Corado, C.; Medeiros, V.K.S.; Da Costa Xavier De Araújo, L.A.; Cavalvanti, G.B.; De Medeiros, S.R.B. Chromosomal characterization of cryopreserved mesenchymal stem cells from the human subendothelium umbilical cord vein. Regen. Med. 2012, 7, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Antebi, B.; Asher, A.M.; Rodriguez, L.A.; Moore, R.K.; Mohammadipoor, A.; Cancio, L.C. Cryopreserved mesenchymal stem cells regain functional potency following a 24-h acclimation period. J. Transl. Med. 2019, 17, 297. [Google Scholar] [CrossRef] [PubMed]
- Polyanskaya, G.G.; Semenova, E.G.; Shubin, N.A. Cytological variations in the Indian muntjac skin fibroblast cell line as a result of cryoconservation. Tsytologiya 1990, 32, 256. [Google Scholar]
- Semenova, E.G. Unscheduled DNA synthesis in cultured cells after cryoconservation. Cryobiology 1988, 1, 17. [Google Scholar]
- Linkova, D.D.; Rubtsova, Y.P.; Egorikhina, M.N. Cryostorage of Mesenchymal Stem Cells and Biomedical Cell-Based Products. Cells 2022, 11, 2691. [Google Scholar] [CrossRef] [PubMed]
- Hornberger, K.; Yu, G.; McKenna, D.; Hubel, A. Cryopreservation of Hematopoietic Stem Cells: Emerging Assays, Cryoprotectant Agents, and Technology to Improve Outcomes. Transfus. Med. Hemother. 2019, 46, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Marquez-Curtis, L.A.; Janowska-Wieczorek, A.; McGann, L.E.; Elliott, J.A.W. Mesenchymal stromal cells derived from various tissues: Biological, clinical and cryopreservation aspects. Cryobiology 2015, 71, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Grinchuk, T.M.; Ignatova, T.N.; Sorokina, E.A.; Artsybasheva, I.V.; Panshina, Y.T. Chromosome polymorphism in mammalian cells with multidrug resistance. I. Karyotypical analysis of Chinese hamster cells resistant to ethidium bromide at the early passages of initial selection steps. Tsitologiya 1988, 30, 312–320. [Google Scholar]
- Shorokhova, M.A.; Grinchuk, T.M. Stability of the Human Endometrial Mesenchymal Stem Cells Karyotype In Vitro. Cell Tissue Biol. 2022, 16, 72–79. [Google Scholar] [CrossRef]
- Zemelko, V.I.; Grinchuk, T.M.; Domnina, A.P.; Artzibasheva, I.V.; Zenin, V.V.; Kirsanov, A.A.; Bichevaia, N.K.; Korsak, V.S.; Nikolsky, N.N. Multipotent mesenchymal stem cells of desquamated endometrium: Isolation, characterization, and application as a feeder layer for maintenance of human embryonic stem cells. Cell Tissue Biol. 2012, 6, 1–11. [Google Scholar] [CrossRef]
- Oja, S.; Kaartinen, T.; Ahti, M.; Korhonen, M.; Laitinen, A.; Nystedt, J. The utilization of freezing steps in mesenchymal stromal cell (MSC) manufacturing: Potential impact on quality and cell functionality attributes. Front. Immunol. 2019, 10, 1627. [Google Scholar] [CrossRef]
- Heng, H.H.Q.; Liu, G.; Stevens, J.B.; Abdallah, B.Y.; Horne, S.D.; Ye, K.J.; Bremer, S.W.; Chowdhury, S.K.; Ye, C.J. Karyotype heterogeneity and unclassified chromosomal abnormalities. Cytogenet. Genome Res. 2013, 139, 144–157. [Google Scholar] [CrossRef]
- Passerini, V.; Ozeri-Galai, E.; De Pagter, M.S.; Donnelly, N.; Schmalbrock, S.; Kloosterman, W.P.; Kerem, B.; Storchová, Z. The presence of extra chromosomes leads to genomic instability. Nat. Commun. 2016, 7, 10754. [Google Scholar] [CrossRef]
- McGranahan, N.; Burrell, R.A.; Endesfelder, D.; Novelli, M.R.; Swanton, C. Cancer chromosomal instability: Therapeutic and diagnostic challenges. EMBO Rep. 2012, 13, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Rangel, N.; Forero-Castro, M.; Rondón-Lagos, M. New insights in the cytogenetic practice: Karyotypic chaos, non-clonal chromosomal alterations and chromosomal instability in human cancer and therapy response. Genes 2017, 8, 155. [Google Scholar] [CrossRef]
- Meng, X.; Ichim, T.E.; Zhong, J.; Rogers, A.; Yin, Z.; Jackson, J.; Wang, H.; Ge, W.; Bogin, V.; Chan, K.W.; et al. Endometrial regenerative cells: A novel stem cell population. J. Transl. Med. 2007, 5, 57. [Google Scholar] [CrossRef]
- Patel, S.A.; Sherman, L.; Munoz, J.; Rameshwar, P. Immunological properties of mesenchymal stem cells and clinical implications. Arch. Immunol. Ther. Exp. 2008, 56, 1–8. [Google Scholar] [CrossRef]
- Hiyama, E.; Hiyama, K. Telomere and telomerase in stem cells. Br. J. Cancer 2007, 96, 1020–1024. [Google Scholar] [CrossRef]
- Shilina, M.; Kovaleva, Z.; Nikolsky, N.; Grinchuk, T. Genetic stability of human mesenchymal stem cells exposed to X-rays or heat shock in culture. In Proceedings of the RAD Conference Proceedings, Budva, Montenegro, 11–16 June 2017; Volume 2. [Google Scholar]
- Ray, M.; Mohandas, T. Proposed banding nomenclature for the chinese hamster chromosomes (Cricetulus griseus). Cytogenet. Cell Genet. 1976, 16, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Simons, A.; Shaffer, L.G.; Hastings, R.J. Cytogenetic nomenclature: Changes in the ISCN 2013 compared to the 2009 edition. Cytogenet. Genome Res. 2013, 141, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Elso, C.M.; Roberts, L.J.; Smyth, G.K.; Thomson, R.J.; Baldwin, T.M.; Foote, S.J.; Handman, E. Leishmaniasis host response loci (lmr13) modify disease severity through a Th1/Th2-independent pathway. Genes Immun. 2004, 2004. 5, 93–100. [Google Scholar] [CrossRef]
| Cell Surface Markers | Percent of Cells Expressing Marker (%) | |
|---|---|---|
| Continuous Culture | Cryopreserved Culture | |
| MSC 2304 (12 PD) | MSC 2304 (17 PD) | |
| CD13 | 99.1 ± 1 | 99.7 ± 1 |
| CD44 | 99.6 ± 2 | 99.9 ± 1 |
| CD73 | 99.7 ± 0.3 | 96.2 ± 2.4 |
| CD90 | 95.2 ± 2 | 98.4 ± 1 |
| CD105 | 98.2 ± 3 | 83.1 ± 4 |
| CD34 | 0.37 ± 1 | 1.13 ± 0.4 |
| CD45 | 2.40 ± 3 | 7.10 ± 2 |
| HLA-DR (class II) | 0.08 ± 1 | 0.56 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shorokhova, M.; Pugovkina, N.; Zemelko, V.; Lyublinskaya, O.; Grinchuk, T. Long-Term Cryopreservation May Cause Genomic Instability and the Premature Senescence of Cells. Int. J. Mol. Sci. 2024, 25, 1467. https://doi.org/10.3390/ijms25031467
Shorokhova M, Pugovkina N, Zemelko V, Lyublinskaya O, Grinchuk T. Long-Term Cryopreservation May Cause Genomic Instability and the Premature Senescence of Cells. International Journal of Molecular Sciences. 2024; 25(3):1467. https://doi.org/10.3390/ijms25031467
Chicago/Turabian StyleShorokhova, Mariia, Natalia Pugovkina, Victoria Zemelko, Olga Lyublinskaya, and Tatiana Grinchuk. 2024. "Long-Term Cryopreservation May Cause Genomic Instability and the Premature Senescence of Cells" International Journal of Molecular Sciences 25, no. 3: 1467. https://doi.org/10.3390/ijms25031467
APA StyleShorokhova, M., Pugovkina, N., Zemelko, V., Lyublinskaya, O., & Grinchuk, T. (2024). Long-Term Cryopreservation May Cause Genomic Instability and the Premature Senescence of Cells. International Journal of Molecular Sciences, 25(3), 1467. https://doi.org/10.3390/ijms25031467






