Abstract
Some parasites are known to influence brain proteins or induce changes in the functioning of the nervous system. In this study, our objective is to demonstrate how the two-dimensional gel technique is valuable for detecting differences in protein expression and providing detailed information on changes in the brain proteome during a parasitic infection. Subsequently, we seek to understand how the parasitic infection affects the protein composition in the brain and how this may be related to changes in brain function. By analyzing de novo-expressed proteins at 2, 4, and 8 weeks post-infection compared to the brains of the control mice, we observed that proteins expressed at 2 weeks are primarily associated with neuroprotection or the initial response of the mouse brain to the infection. At 8 weeks, parasitic infection can induce oxidative stress in the brain, potentially activating signaling pathways related to the response to cellular damage. Proteins expressed at 8 weeks exhibit a pattern indicating that, as the host fails to balance the Neuro-Immuno-Endocrine network of the organism, the brain begins to undergo an apoptotic process and consequently experiences brain damage.
1. Introduction
It is well established that certain parasites have the potential to occasionally influence brain proteins, triggering immune responses or nervous system function. One of the most well-known examples is Toxoplasma gondii, a parasite infecting mammals including humans, which has been associated with effects on behavior and brain function [1,2,3,4].
Taenia crassiceps (T. crassiceps) is a cestode parasite that primarily infects rodents and can exert influences on the host’s immune system and brain. The presence of T. crassiceps in the mouse’s body can lead to the modulation of the host’s immune system [5]. It has been observed that infection with T. crassiceps can induce immune responses in the brains of mice, which may lead to inflammation and changes in brain function [6,7]. Research on T. crassiceps infection in mice has investigated its correlation with behavioral changes, indicating that the presence of the parasite might influence mouse behavior. This finding could be pertinent to understanding how the parasite is transmitted to its definitive hosts, which are typically predators [8]. Human infection is believed to occur following the consumption of food or water contaminated with infectious eggs shed in the feces of carnivores. While all recognized cases involving muscles or subcutaneous tissue in humans have been associated with underlying immunosuppression, there are reported instances that do not seem to require a compromised immune system [9].
Two-dimensional gels, also known as 2D gels, are a powerful technique used in protein research to separate and analyze proteins in complex samples. These gels can be employed to identify differences in protein expression between samples and for the discovery of biomarkers, among other purposes. Two-dimensional gels have been employed in neuroproteomic studies to analyze the proteins present in the mouse brain, providing a better understanding of its composition and changes in response to various conditions, such as diseases or treatments. The results of these studies have provided essential information to advance the understanding of processes such as brain development, aging, neurological diseases, and the response to therapeutic treatments [10,11,12]. A limitation, as pointed out by some researchers when conducting analyses using proteomic methods, is that only the most abundant proteins are identified. Proteins that are expressed at low levels are often not detected. Thus, there is a risk that the approach does not account for all potentially relevant proteins. Although this is possible, it is unlikely given that the resolution level of the technique is very high.
Given that infection with T. crassiceps affects both the immune system and brain tissue, the observed changes in brain proteins are likely the result of a complex interaction between the direct effects of the parasite and the host’s immune response. The precise characterization of these effects and their relative contribution may require detailed studies in experimental models and advanced techniques, such as proteomics and mass spectrometry, or bioinformatics to identify specific proteins and their changes in response to the infection.
Research on the effects of T. crassiceps on the brain and the specific proteins involved in its interactions with the nervous system remains an active area of study. In this work, we aim to demonstrate how the 2D gel technique is valuable for detecting differences in protein expression and provide detailed information on changes in the brain proteome during a parasitic infection, thus subsequently understanding how parasitic infection affects protein composition in the brain and how this may be related to changes in brain function.
2. Results
Figure 1 provides a comprehensive overview of the changes in the expression of specific brain proteins in the infected group compared to the non-infected group. Notably, these differences exhibit variation concerning the duration infection. This figure highlights proteins that undergo modification in their expression, with a particular focus on those proteins that appear “de novo”, in relation to the control group, due to infection. In Figure 2, we present proteins expressed at specific times that are not found in the control brain at those times, indicating potential de novo synthesis in cells. At week 2, there are 12 such proteins, 2 proteins at week 4, and 12 proteins at week 8.
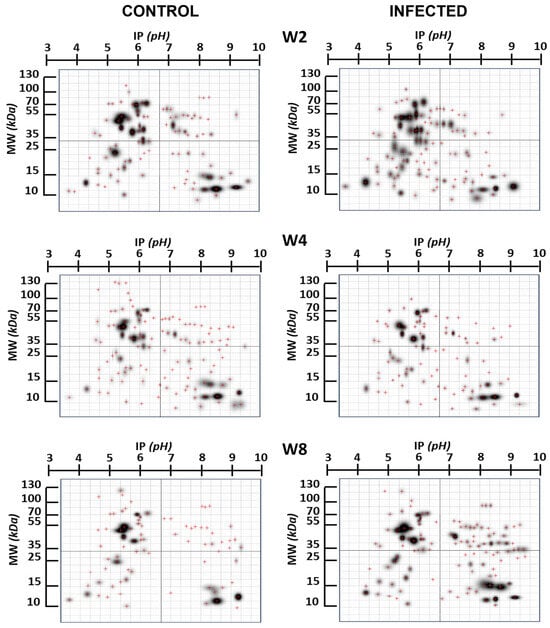
Figure 1.
Two-dimensional gels’ image of the brain proteins in infected and control mice at different infection time points. W2, W4, W8: weeks 2, 4, 8, respectively. The red crosses represent the center of the spot.
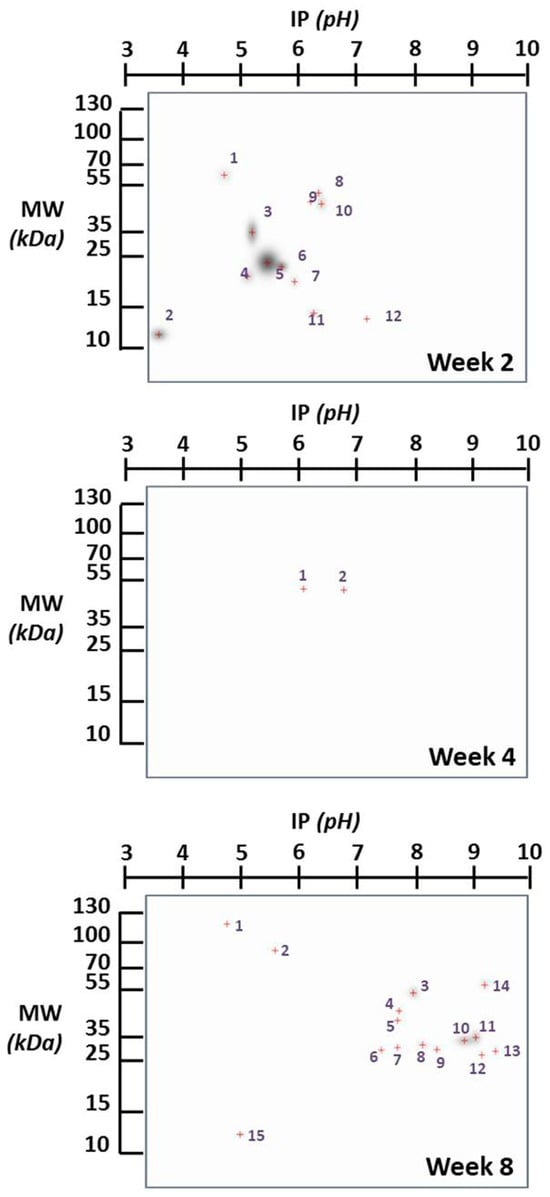
Figure 2.
Two-dimensional gels’ image of the brain proteins in infected and control mice at different infection time points. The proteins expressed at the corresponding time, which are not found in the control brain at that time (suggesting the possible de novo synthesis in cells), are exclusively presented. The spots are numbered to identify the proteins in Table 1. The red crosses represent the center of the spot.
The identification of proteins in Table 1 is based on comparisons between reports of mouse brain proteins with a matching molecular weight and isoelectric point and our experimental results. The identification draws from data representing the most comprehensive proteome coverage for mammalian brains to date, providing a foundation for future quantitative studies in brain proteomics using mouse models. The proteomic approach presented here may have broad applications for the rapid proteomic analysis of various mouse models of human brain diseases.

Table 1.
Probable identification of the proteins indicated in Figure 2. The proteins highlighted in black are brain proteins that closely match a high percentage with what is reported in the literature. The proteins highlighted in red are those that approximate a protein reported to one of the values of the experimental spot: either the molecular weight or the isoelectric point.
In Table 2, the proteins from Table 1 are displayed but as part of a functional group. From Table 2, it can be inferred that the identified proteins do not belong to a specific brain process but rather are ubiquitous proteins that support the idea of a generalized degenerative process in the brain rather than one confined to a particular region.

Table 2.
Association of the identified proteins with a functional group within metabolism.
3. Discussion
In the early stages of infection, it is common to observe a systemic TH1 response involving the production of cytokines, such as interferon-gamma (IFN-γ). The TH1 response is commonly associated with cellular immunity and the fight against intracellular infection. During this initial phase, the immune system may attempt to control and limit the spread of the parasite. The emergence of new brain proteins in mice infected with T. crassiceps may be the result of a combination of factors, including the direct action of the parasite and the host’s Neuro-Immuno-Endocrine network response. Often, it is challenging to completely separate the direct effects of the parasite from the immune responses triggered by the infection. The parasite T. crassiceps can have a direct impact on the mouse’s brain, either through the release of metabolic products, manipulation of the local immune response, or physical interaction with brain cells. This can influence brain proteins and other components of brain tissue [8].
Infection with T. crassiceps will also trigger an immune response from the host. This response may involve the activation of immune cells, the release of cytokines, and other inflammatory mediators in the brain. These changes in the brain environment can have a significant effect on the expression and activity of proteins in brain tissue, potentially influencing the expression of proteins related to inflammation and immune response. Cytokines such as gamma interferon (IFN-γ) and interleukin-6 (IL-6) are common activators of inflammatory signaling pathways in the mouse brain in response to parasitic infections [45].
The proteins listed in Table 1 reflect, or their expression is a consequence of, the series of chemical signals that converge during infection. At 2 weeks post-infection, the expressed proteins are associated with the physiology of brain cells, specifically the protection against anoxia, synaptic plasticity, detoxification, combating oxidative stress, addressing depression, neuronal damage, overcoming anxiety, and responding to inflammation [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33].
Intraperitoneal infection in the mouse cysticercosis model quickly shifts to the TH2 type or even a mixed profile of type 1/type 2 cytokines, which is permissive for parasite growth. The TH2 immune response is characterized by the production of cytokines such as interleukin-4 (IL-4), interleukin-5 (IL-5), and interleukin-13 (IL-13), and is associated with humoral immunity. This results in the unrestricted growth of the parasite, which, in experimental cases, can lead to the death of the animal, demonstrating little or no immunological resistance to parasitic growth.
On the other hand, during the infection, cells of the central nervous system (CNS) have the ability to produce inflammatory mediators such as chemokines, adhesion molecules, and cytokines [46]. These responses can lead to the significant infiltration of various leukocytes, culminating in pathogen-specific adaptive immune responses in the CNS. The direct recognition of microbial molecules by cells in nervous tissue and the subsequent innate immune response appear to be key elements in protecting the CNS [47]. The inflammatory response in the CNS plays a crucial reparative role and involves the participation of various immune cell types (macrophages, mast cells, T and B lymphocytes, dendritic cells) and resident CNS cells (microglia, astrocytes, neurons), as well as adhesion molecules, cytokines, and chemokines, among other protein components. During neuroinflammation, chemotaxis is a significant event in the recruitment of cells into the CNS.
The recruitment of lymphocytes involves the presence of chemokines and chemokine receptors, expression of adhesion molecules, interaction between lymphocytes and the blood–brain barrier (BBB) endothelium, and ultimately their passage through the BBB to reach the site of inflammation. The metabolic products released by the parasite, such as lipopolysaccharides or glycoproteins, or the cytokines and mediators of the parasite’s Neuroimmunendocrine network can intermingle with the constitutive signals of the brain, generating the regulation of protein expression mediated by cellular communication pathways. Apparently, by the eighth week, this process is uncontrolled and progressing. Under these conditions, the reparative effects of the inflammatory response are overwhelmed and can promote brain damage [46]. By week 8, the expressed proteins are associated with stress, combating oxidative stress, apoptosis, mobility, and learning. They play a role in regulating membranes and neurotransmission, especially at synapses and myelination. There is also the regulation of energetic homeostasis, and the aim is to control the neurodegeneration that begins to manifest itself [34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49]. It is possible that there may be protein expression differences at the level of small brain regions, and due to the strategy of processing the entire organ, we may not be able to detect them. The reasoning behind processing the entire brain instead of spatially expressing proteins was that, based on the results obtained, a group of proteins specifically associated with a region or a cell nucleus linked to a function could be identified. However, we did not find evidence of that. According to the results, the found proteins do not belong to a specific cerebral process but are ubiquitous proteins that support the idea of a generalized degenerative process in the brain rather than one confined to a particular region. Another possibility is that by processing the entire brain, the expression change occurring in a limited region of the brain may be “diluted”.
4. Materials and Methods
The study utilized female BALB/c strain mice which were housed in the animal facilities of the Faculty of Medicine at UNAM under controlled conditions of temperature (22 °C) in a pathogen-free environment, with a relative humidity of 50 to 60%, 12-h light-dark cycles, and free access to food and water.
Infection with the Taenia crassiceps cysticerci ORF strain and two-dimensional gel electrophoresis (2DE) were performed according to [48,49].
Proteomics Analysis
The 2DE gels were digitized using an HP Scanjet-G4050 scanner with a resolution of 300 DPI and analyzed using PDQuest™ 2DE software version 8.0 (Bio-Rad Laboratories, Inc., Hercules, CA, USA) to determine differences in the expression of proteins depending on the cysticercus infection time. Master images were created for each group from their 3 replicates. In other words, a Master image was obtained for protein extracts from each control group (2, 4, and 8 weeks) and from each of the infected mice (2, 4, and 8 weeks). The coordinates of each spot were calculated according to the isoelectric point markers of the 2DE standards (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Mouse Brain Protein Extraction
Brain tissue was quickly taken from cryopreserved vials and processed under cold conditions to prevent degradation. For each mouse in the group, a portion of the brain was taken, and tissue pooling was performed, from which proteins were extracted. Each brain tissue pool was manually homogenized with a teflon pestle on ice in 600 µL of 2D buffer (8 M Urea, 50 mM DTT, 2% CHAPS, 2% Ampholine pH 3–10 (Bio-Rad) in the presence of a protease inhibitor (Halt™ Protease Inhibitor Cocktail, Thermo Scientific, Waltham, MA, USA). It was sonicated in cold conditions at 15-s intervals, shaken for 2 h at 4 °C, and then centrifuged for 20 min at 12,000 rpm at 4 °C. The protein concentration in the supernatant was measured using the Bradford method.
Bioinformatic approach.
A literature search was conducted for articles reporting proteomic analyses and protein identification in the BALB/c mouse brain [12,50,51]. An analysis of metabolic pathways was conducted involving the proteins found using the Kyoto Encyclopedia of Gene and Genomes database (https://www.genome.jp/kegg/ (accessed on 13 January 2024)) and the UNIPROT database (https://www.uniprot.org (accessed on 13 January 2024)).
5. Conclusions
By analyzing de novo-expressed brain proteins at 2, 4, and 8 weeks post-infection compared to the brains of the control mice, we observed that the proteins expressed at 2 weeks are primarily associated with neuroprotection or the initial response of the mouse brain to infection. By 8 weeks, parasitic infection may induce oxidative stress in the brain, potentially activating signaling pathways related to the response to cellular damage. The proteins expressed at 8 weeks exhibit a pattern indicating that, when unable to balance the organism’s Neuroimmunendocrine network, the brain begins to undergo an apoptotic process, leading to consequential brain damage. This damage is manifested in previously reported behaviors, including sexual activity, aggression, social status, defense response, as well as the impairment of short-term memory. The characterization of the proteins reported in the study is at the level of the isoelectric point and molecular weight. It is evident that a deeper characterization at the sequence level of the proteins and their recognition by antibodies is required, and this is currently underway.
Author Contributions
Data curation, R.H.-Á.; Funding acquisition, A.L.; Investigation, A.L.; Methodology, M.D.-Z., R.H.-Á. and P.O.-S.; Project administration, P.O.-S.; Software, M.D.-Z.; Supervision, P.O.-S.; Validation, P.O.-S.; Visualization, M.D.-Z.; Writing—original draft, M.D.-Z.; Writing—review and editing, P.O.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by Dirección General de Asuntos del Personal Académico, UNAM (DGAPA-PAPIIT contract IN217419 and DGAPA-PAPIIT contract IN205422). The postdoctoral scholarship of Díaz-Zaragoza Mariana was supported by the Programa de Becas Posdoctorales of UNAM.
Institutional Review Board Statement
The Institutional Ethics Committee approved all animal protocols (permit No. 2015-175) that were carried out in strict adherence to the Official Mexican Standard for the Production, Care, and Use of Laboratory Animals (NOM-062-ZOO-1999) and the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health, USA.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
We thank Alicia Ochoa Sanchez for her valuable technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Adamo, S.A. Modulating the Modulators: Parasites, Neuromodulators and Host Behavioral Change. Brain Behav. Evol. 2002, 60, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.P.; Libersat, F. Parasite manipulation of host behavior. Curr. Biol. 2019, 29, R45–R47. [Google Scholar] [CrossRef] [PubMed]
- Libersat, F.; Kaiser, M.; Emanuel, S. Mind Control: How Parasites Manipulate Cognitive Functions in Their Insect Hosts. Front. Psychol. 2018, 9, 572. [Google Scholar] [CrossRef] [PubMed]
- Berdoy, M.; Webster, J.P.; Macdonald, D.W. Fatal attraction in rats infected with Toxoplasma gondii. Proc. R. Soc. Lond. B Biol. Sci. 2000, 267, 1591–1594. [Google Scholar] [CrossRef] [PubMed]
- Peón, A.N.; Espinoza-Jiménez, A.; Terrazas, L.I. Immunoregulation by Taenia crassiceps and Its Antigens. BioMed Res. Int. 2012, 2013, 498583. [Google Scholar] [CrossRef]
- Gourbal, B.; Lacroix, A.; Gabrion, C. Behavioural dominance and Taenia crassiceps parasitism in BALB/c male mice. Parasitol. Res. 2002, 88, 912–917. [Google Scholar] [CrossRef]
- Morales-Montor, J.; Arrieta, I.; Del Castillo, L.I.; Rodríguez-Dorantes, M.; Cerbón, M.A.; Larralde, C. Remote sensing of intraperitoneal parasitism by the host’s brain: Regional changes of c-fos gene expression in the brain of feminized cysticercotic male mice. Parasitology 2004, 128 Pt 3, 343–351. [Google Scholar] [CrossRef]
- Morales-Montor, J.; Picazo, O.; Besedovsky, H.; Hernández-Bello, R.; López-Griego, L.; Becerril-Villanueva, E.; Moreno, J.; Pavón, L.; Nava-Castro, K.; Camacho-Arroyo, I. Helminth Infection Alters Mood and Short-Term Memory as well as Levels of Neurotransmitters and Cytokines in the Mouse Hippocampus. Neuroimmunomodulation 2014, 21, 195–205. [Google Scholar] [CrossRef]
- Ntoukas, V.; Tappe, D.; Pfütze, D.; Simon, M.; Holzmann, T. Cerebellar Cysticercosis Caused by Larval Taenia crassiceps Tapeworm in Immunocompetent Woman, Germany. Emerg. Infect. Dis. 2013, 19, 2008–2011. [Google Scholar] [CrossRef]
- Tsugita, A.; Kawakami, T.; Uchida, T.; Sakai, T.; Kamo, M.; Matsui, T.; Watanabe, Y.; Morimasa, T.; Hosokawa, K.; Toda, T. Proteome analysis of mouse brain: Two-dimensional electrophoresis profiles of tissue proteins during the course of aging. Electrophoresis 2000, 21, 1853–1871. [Google Scholar] [CrossRef]
- Eckerskorn, C.; Jungblut, P.; Mewes, W.; Klose, J.; Lottspeich, F. Identification of mouse brain proteins after two-dimensional electrophoresis and electroblotting by microsequence analysis and amino acid composition analysis. Electrophoresis 1988, 9, 830–838. [Google Scholar] [CrossRef]
- Gauss, C.; Kalkum, M.; Löwe, M.; Lehrach, H.; Klose, J. Analysis of the mouse proteome. (I) Brain proteins: Separation by two-dimensional electrophoresis and identification by mass spectrometry and genetic variation. Electrophoresis 1999, 20, 575–600. [Google Scholar] [CrossRef]
- Balestrino, M.; Lensman, M.; Parodi, M.; Perasso, L.; Rebaudo, R.; Melani, R.; Polenov, S.; Cupello, A. Role of creatine and phosphocreatine in neuronal protection from anoxic and ischemic damage. Amino Acids 2002, 23, 221–229. [Google Scholar] [CrossRef]
- Suzuki, T.; Mitake, S.; Okumura-Noji, K.; Shimizu, H.; Tada, T.; Fujii, T. Excitable membranes and synaptic transmission: Postsynaptic mechanisms.: Localization of α-internexin in the postsynaptic density of the rat brain. Brain Res. 1997, 765, 74–80. [Google Scholar] [CrossRef]
- Kuhla, B.; Boeck, K.; Lüth, H.-J.; Schmidt, A.; Weigle, B.; Schmitz, M.; Ogunlade, V.; Münch, G.; Arendt, T. Age-dependent changes of glyoxalase I expression in human brain. Neurobiol. Aging 2006, 27, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Bu, J.; Yao, X.; Liu, C.; Shen, H.; Li, X.; Li, H.; Chen, G. Phosphorylation at S153 as a Functional Switch of Phosphatidylethanolamine Binding Protein 1 in Cerebral Ischemia-Reperfusion Injury in Rats. Front. Mol. Neurosci. 2017, 10, 358. [Google Scholar] [CrossRef]
- Matoba, K.; Dohi, E.; Choi, E.Y.; Kano, S.-I. Glutathione S-transferases Control astrocyte activation and neuronal health during neuroinflammation. Front. Mol. Biosci. 2023, 9, 1080140. [Google Scholar] [CrossRef]
- Bezek, S.; Biberthaler, P.; Martinez-Espina, I.; Bogner-Flatz, V. Pathophysiology and clinical implementation of traumatic brain injury biomarkers: Neuron-specific enolase. In Biomarkers for Traumatic Brain Injury; Elsevier: Amsterdam, The Netherlands, 2020; pp. 169–182. [Google Scholar] [CrossRef]
- Hua, L.V.; Green, M.; Warsh, J.J.; Li, P.P. Molecular cloning of a novel isoform of diphosphoinositol polyphosphate phosphohydrolase: A potential target of lithium therapy. Neuropsychopharmacology 2001, 24, 640–651. [Google Scholar] [CrossRef] [PubMed]
- Austin, R.; Chander, P.; Zimmerman, A.J.; Overby, M.; Digilio, L.; Yap, C.C.; Linsenbardt, D.N.; Müller, H.K.; Weick, J.P. Global loss of Neuron-specific gene 1 causes alterations in motor coordination, increased anxiety, and diurnal hyperactivity in male mice. Genes Brain Behav. 2022, 21, e12816. [Google Scholar] [CrossRef]
- Kopajtich, R.; Nicholls, T.J.; Rorbach, J.; Metodiev, M.D.; Freisinger, P.; Mandel, H.; Vanlander, A.; Ghezzi, D.; Carrozzo, R.; Taylor, R.W.; et al. Mutations in GTPBP3 Cause a Mitochondrial Translation Defect Associated with Hypertrophic Cardiomyopathy, Lactic Acidosis, and Encephalopathy. Am. J. Hum. Genet. 2014, 95, 708–720. [Google Scholar] [CrossRef]
- Chebbok, E. Basic Leucine Zipper and W2 Domaincontaining Protein 2 (BZW2): A Novel Cardiac WNT Component. Ph.D. Thesis, Georg-August University Göttingen, Göttingen, Germany, September 2015. [Google Scholar] [CrossRef]
- Chapman, G.; Shanmugalingam, U.; Smith, P.D. The Role of Neuronal Pentraxin 2 (NP2) in Regulating Glutamatergic Signaling and Neuropathology. Front. Cell. Neurosci. 2020, 13, 575. [Google Scholar] [CrossRef]
- Peddada, N.; Sagar, A.; Ashish; Garg, R. Plasma gelsolin: A general prognostic marker of health. Med. Hypotheses 2012, 78, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-T.; Yu, J.; Grass, D.; de Beer, F.C.; Kindy, M.S. Inflammation-Dependent Cerebral Deposition of Serum Amyloid A Protein in a Mouse Model of Amyloidosis. J. Neurosci. 2002, 22, 5900–5909. [Google Scholar] [CrossRef] [PubMed]
- Watson, W.; Srivastava, M.; Leighton, X.; Glasman, M.; Faraday, M.; Fossam, L.; Pollard, H.; Verma, A. Annexin 7 mobilizes calcium from endoplasmic reticulum stores in brain. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2004, 1742, 151–160. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Petroff, O.A.C. Book Review: GABA and Glutamate in the Human Brain. Neuroscientist 2002, 8, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Okui, M.; Ito, F.; Ogita, K.; Kuramoto, N.; Kudoh, J.; Shimizu, N.; Ide, T. Expression of APG-2 protein, a member of the heat shock protein 110 family, in developing rat brain. Neurochem. Int. 2000, 36, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A. Ubiquitin carboxyl-terminal hydrolase L-1 in brain: Focus on its oxidative/nitrosative modification and role in brains of subjects with Alzheimer disease and mild cognitive impairment. Free Radic. Biol. Med. 2021, 177, 278–286. [Google Scholar] [CrossRef]
- Huang, C.; Chen, M.; Pang, D.; Bi, D.; Zou, Y.; Xia, X.; Yang, W.; Luo, L.; Deng, R.; Tan, H.; et al. Developmental and Activity-Dependent Expression of LanCL1 Confers Antioxidant Activity Required for Neuronal Survival. Dev. Cell 2014, 30, 479–487. [Google Scholar] [CrossRef]
- Horibata, Y.; Elpeleg, O.; Eran, A.; Hirabayashi, Y.; Savitzki, D.; Tal, G.; Mandel, H.; Sugimoto, H. EPT1 (selenoprotein I) is critical for the neural development and maintenance of plasmalogen in humans. J. Lipid Res. 2018, 59, 1015–1026. [Google Scholar] [CrossRef]
- Ellis, J.M.; Wong, G.W.; Wolfgang, M.J. Acyl Coenzyme A Thioesterase 7 Regulates Neuronal Fatty Acid Metabolism to Prevent Neurotoxicity. Mol. Cell. Biol. 2013, 33, 1869–1882. [Google Scholar] [CrossRef]
- Murshid, A.; Srivastava, A.; Kumar, R.; Presley, J.F. Characterization of the localization and function of NECAP 1 in neurons. J. Neurochem. 2006, 98, 1746–1762. [Google Scholar] [CrossRef] [PubMed]
- Kleopa, K.A.; Orthmann-Murphy, J.; Sargiannidou, I. Gap Junction Disorders of Myelinating Cells. Rev. Neurosci. 2010, 21, 397–419. [Google Scholar] [CrossRef] [PubMed]
- Bartesaghi, S.; Betts-Henderson, J.; Cain, K.; Dinsdale, D.; Zhou, X.; Karlsson, A.; Salomoni, P.; Nicotera, P. Loss of thymidine kinase 2 alters neuronal bioenergetics and leads to neurodegeneration. Hum. Mol. Genet. 2010, 19, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Tatton, W.G.; Chalmers-Redman, R.M.E.; Elstner, M.; Leesch, W.; Jagodzinski, F.B.; Stupak, D.P.; Sugrue, M.M.; Tatton, N.A. Glyceraldehyde-3-phosphate dehydrogenase in neurodegeneration and apoptosis signaling. In Advances in Research on Neurodegeneration; Springer: Vienna, Austria, 2000; pp. 77–100. [Google Scholar] [CrossRef]
- Liu, G.; Ruan, Y.; Zhang, J.; Wang, X.; Wu, W.; He, P.; Wang, J.; Xiong, J.; Cheng, Y.; Liu, L.; et al. ABHD11 Is Critical for Embryonic Stem Cell Expansion, Differentiation and Lipid Metabolic Homeostasis. Front. Cell Dev. Biol. 2020, 8, 570. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Cheng, N.; Xu, X.; Yang, Z.; Hoffman, R.M.; Zhu, J. AMMECR1 Inhibits Apoptosis and Promotes Cell-cycle Progression and Proliferation of the A549 Human Lung Cancer Cell Line. Anticancer Res. 2019, 39, 4637–4642. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.T.; Balogh, S.A.; Davydov, I.V.; Kashina, A.S.; Yoon, J.K.; Xie, Y.; Gaur, A.; Hyde, L.; Denenberg, V.H.; Varshavsky, A. Altered activity, social behavior, and spatial memory in mice lacking the NTAN1p amidase and the asparagine branch of the N-end rule pathway. Mol. Cell. Biol. 2000, 20, 4135–4148. [Google Scholar] [CrossRef] [PubMed]
- Balog, J.; Mehta, S.L.; Vemuganti, R. Mitochondrial fission and fusion in secondary brain damage after CNS insults. J. Cereb. Blood Flow Metab. 2016, 36, 2022–2033. [Google Scholar] [CrossRef]
- Westergard, L.; Christensen, H.M.; Harris, D.A. The cellular prion protein (PrPC): Its physiological function and role in disease. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2007, 1772, 629–644. [Google Scholar] [CrossRef]
- Schrader, M.; Reuber, B.E.; Morrell, J.C.; Jimenez-Sanchez, G.; Obie, C.; Stroh, T.A.; Valle, D.; Schroer, T.A.; Gould, S.J. Expression of PEX11β Mediates Peroxisome Proliferation in the Absence of Extracellular Stimuli. J. Biol. Chem. 1998, 273, 29607–29614. [Google Scholar] [CrossRef]
- Amorim, I.S.; Lach, G.; Gkogkas, C.G. The Role of the Eukaryotic Translation Initiation Factor 4E (eIF4E) in Neuropsychiatric Disorders. Front. Genet. 2018, 9, 561. [Google Scholar] [CrossRef]
- Tabe, S.; Hikiji, H.; Ariyoshi, W.; Hashidate-Yoshida, T.; Shindou, H.; Shimizu, T.; Okinaga, T.; Seta, Y.; Tominaga, K.; Nishihara, T. Lysophosphatidylcholine acyltransferase 4 is involved in chondrogenic differentiation of ATDC5 cells. Sci. Rep. 2017, 7, 16701. [Google Scholar] [CrossRef]
- López-Griego, L.; Nava-Castro, K.E.; López-Salazar, V.; Hernández-Cervantes, R.; Guzmán, N.T.; Muñiz-Hernández, S.; Hernández-Bello, R.; Besedovsky, H.O.; Pavón, L.; Villanueva, L.E.B.; et al. Gender-Associated Differential Expression of Cytokines in Specific Areas of the Brain During Helminth Infection. J. Interf. Cytokine Res. 2015, 35, 116–125. [Google Scholar] [CrossRef]
- Chavarria, A.; Alcocer-Varela, J. Is damage in central nervous system due to inflammation? Autoimmun. Rev. 2004, 3, 251–260. [Google Scholar] [CrossRef]
- Mishra, B.B.; Mishra, P.K.; Teale, J.M. Expression and distribution of Toll-like receptors in the brain during murine neurocysticercosis. J. Neuroimmunol. 2006, 181, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, L.; Díaz-Zaragoza, M.; Hernández, M.; Navarro, L.; Hernández-Ávila, R.; Encarnación-Guevara, S.; Ostoa-Saloma, P.; Landa, A. Differential Protein Expression of Taenia crassiceps ORF Strain in the Murine Cysticercosis Model Using Resistant (C57BL/6) Mice. Pathogens 2023, 12, 678. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Zaragoza, M.; Jiménez, L.; Hernández, M.; Hernández-Ávila, R.; Navarro, L.; Ochoa-Sánchez, A.; Encarnación-Guevara, S.; Ostoa-Saloma, P.; Landa, A. Protein expression profile of Taenia crassiceps cysticerci related to Th1- and Th2-type responses in the mouse cysticercosis model. Acta Trop. 2020, 212, 105696. [Google Scholar] [CrossRef] [PubMed]
- Taraslia, V.K.; Kouskoukis, A.; Anagnostopoulos, A.K.; Stravopodis, D.; Margaritis, L.H.; Tsangaris, G.T. Proteomic Analysis of Normal Murine Brain Parts. Cancer Genom. Proteom. 2013, 10, 125–154. [Google Scholar]
- Sharma, K.; Schmitt, S.; Bergner, C.G.; Tyanova, S.; Kannaiyan, N.; Manrique-Hoyos, N.; Kongi, K.; Cantuti, L.; Hanisch, U.-K.; Philips, M.-A.; et al. Cell type- and brain region-resolved mouse brain proteome. Nat. Neurosci. 2015, 18, 1819–1831. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).