Phototoxic Reactions Inducted by Hydrochlorothiazide and Furosemide in Normal Skin Cells—In Vitro Studies on Melanocytes and Fibroblasts
Abstract
1. Introduction
2. Results
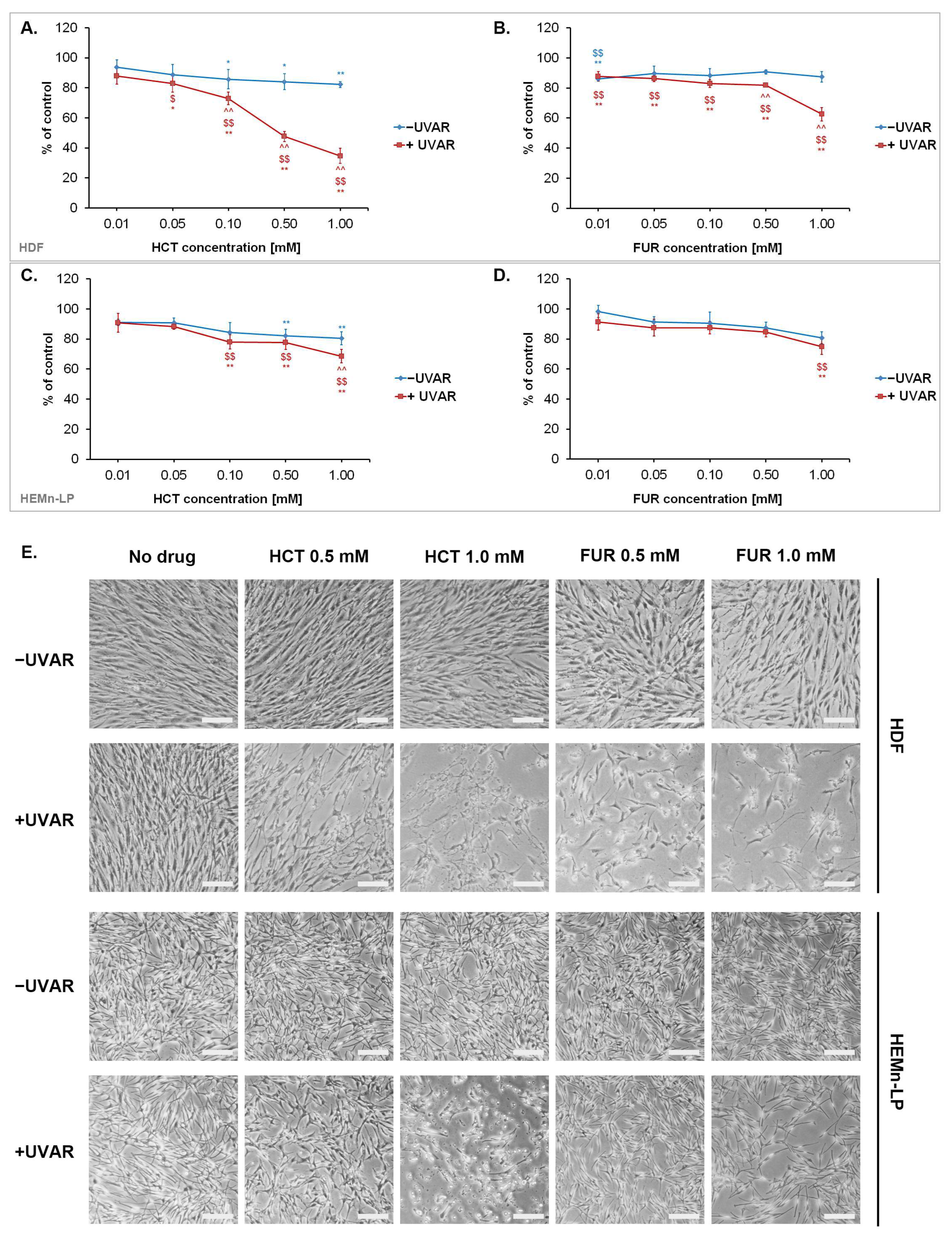
2.1. The Phototoxic Effect of Hydrochlorothiazide or Furosemide Decrease the Number of Metabolic Activity and Induce Morphological Changes of Normal Skin Cells
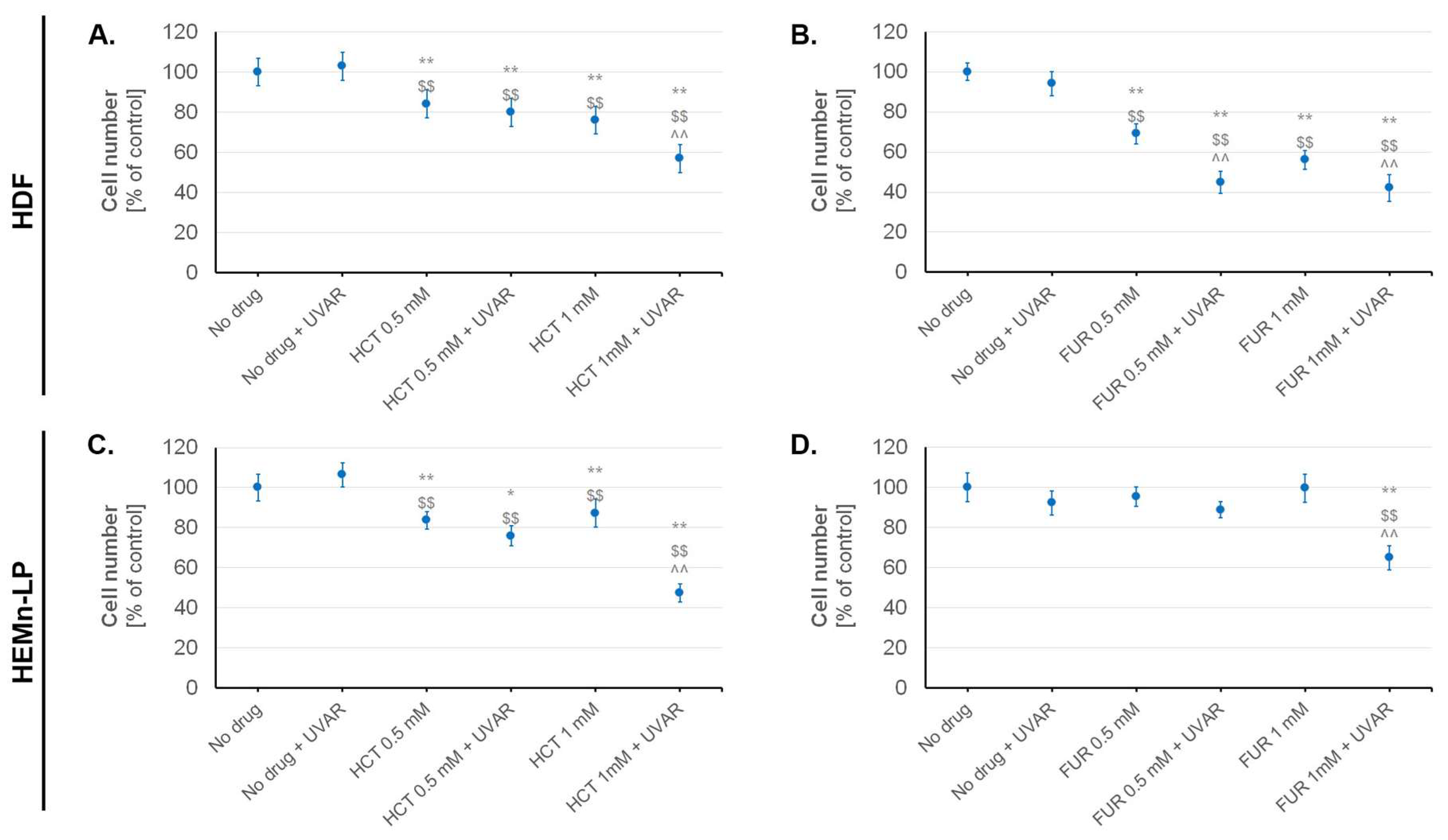
2.2. The Influence of Hydrochlorothiazide or Furosemide and/or UVA Irradiation on Cell Number and Viability
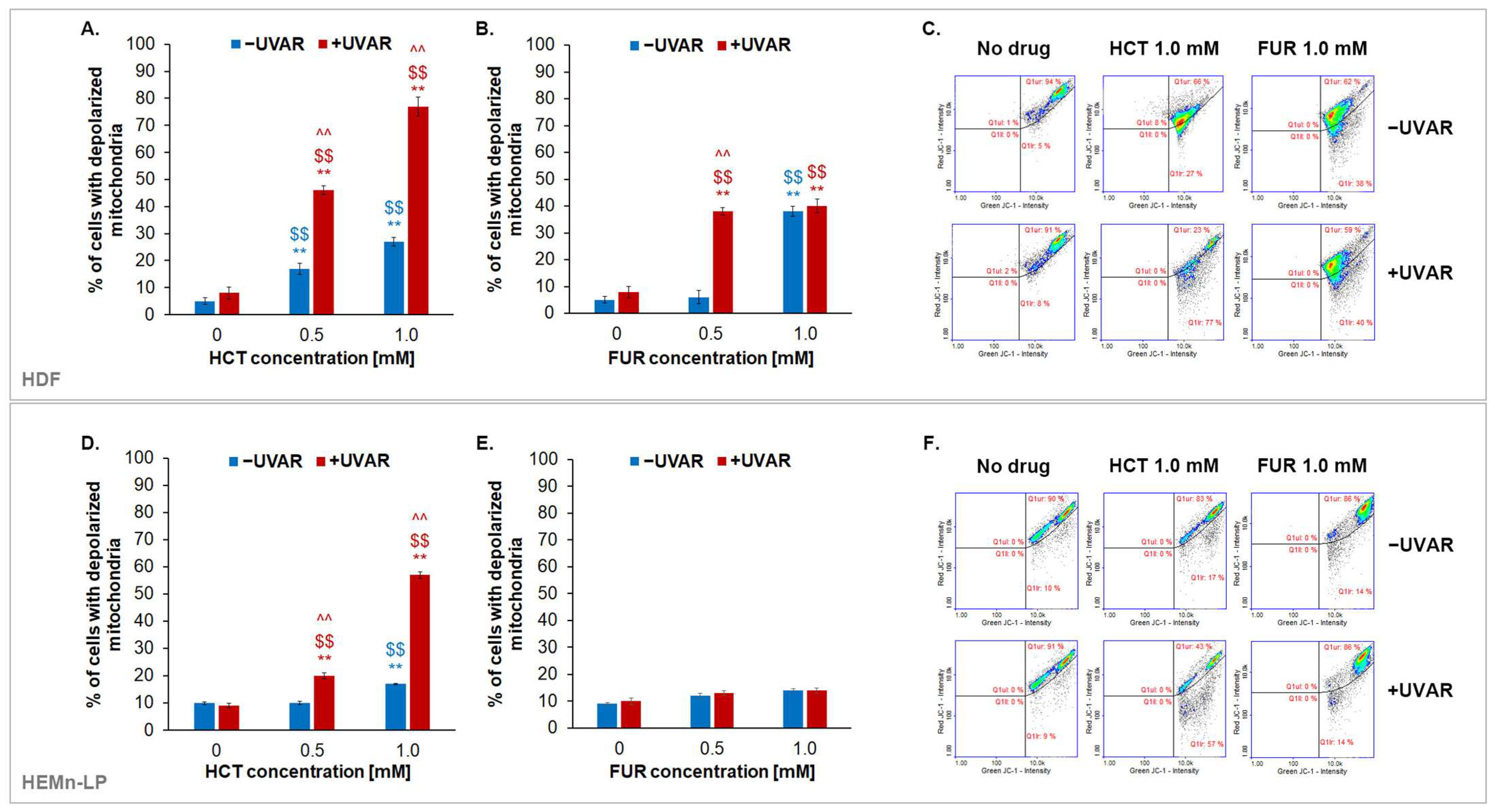
2.3. The Assessment of Changes in Transmembrane Mitochondrial Potential (TMP) in Melanocytes and Fibroblasts Exposed to UVA Irradiation and Hydrochlorothiazide or Furosemide
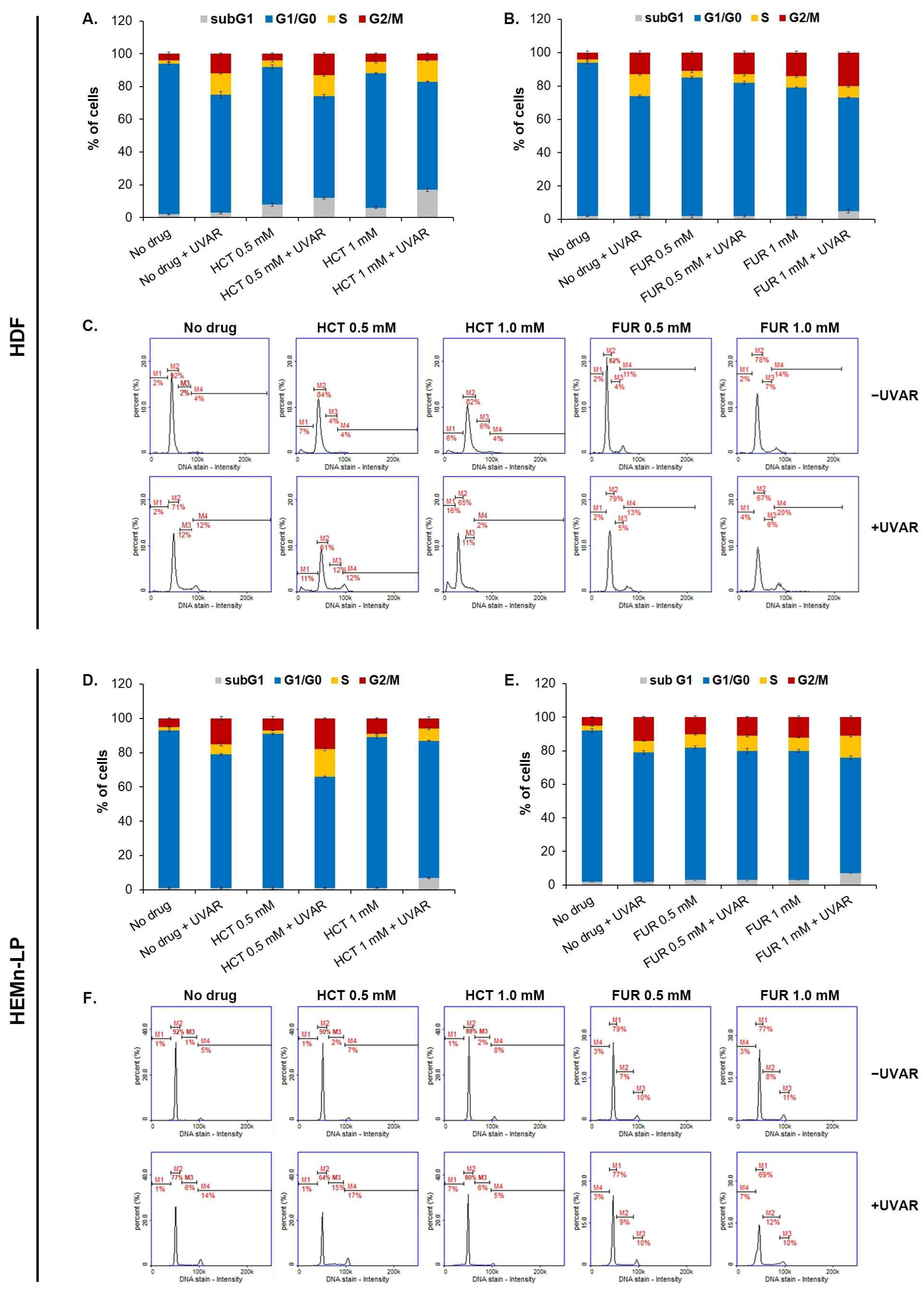
2.4. The Evaluation of the Changes in the Cell Cycle of Melanocytes and Fibroblasts Exposed to Furosemide or Hydrochlorothiazide and/or UVAR
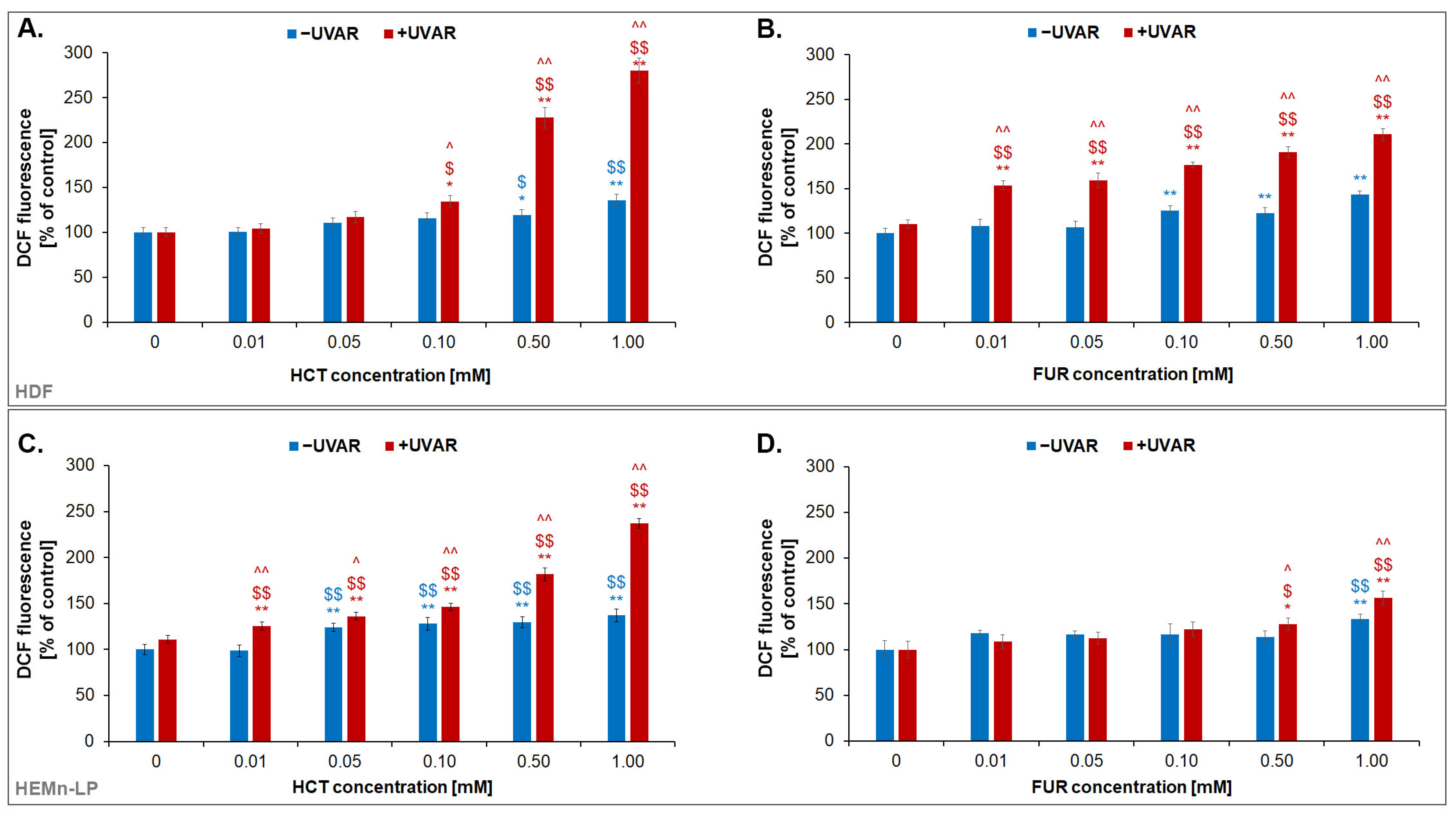
2.5. The Quantitative Analysis of the Effect of Hydrochlorothiazide or Furosemide and/or UVA Irradiation on Redox Homeostasis of Normal Skin Cells
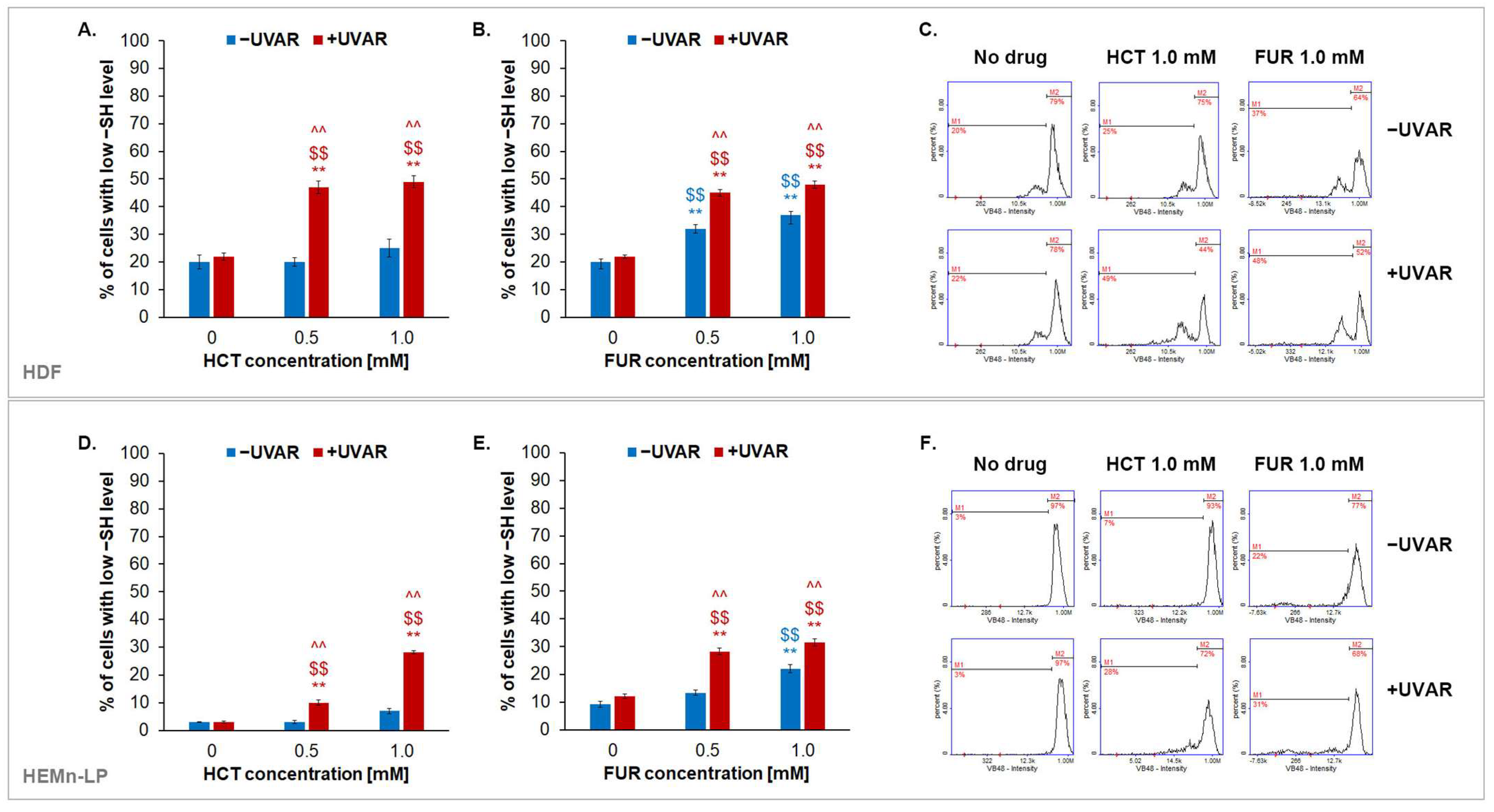
2.6. The Effect of Hydrochlorothiazide or Furosemide and/or UVA Irradiation on Intracellular Reduced Thiols Status in Melanocytes and Fibroblasts
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture, Treatment and Exposure to UVA Irradiation
4.3. The Assessment of Cells’ Metabolic Activity
4.4. The Analysis of Cell Number and Viability
4.5. Mitochondrial Potential Analysis
4.6. The Assessment of the Intracellular Content of Reduced Thiols
4.7. Cell Cycle Assessment
4.8. H2DCFDA Assay–Reactive Oxygen Species Quantitation
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cacabelos, R.; Naidoo, V.; Corzo, L.; Cacabelos, N.; Carril, J.C. Genophenotypic factors and pharmacogenomics in adverse drug reactions. Int. J. Mol. Sci. 2021, 22, 13302. [Google Scholar] [CrossRef] [PubMed]
- Guvenir, H.; Arikoglu, T.; Vezir, E.; Misirlioglu, E.D. Clinical phenotypes of severe cutaneous drug hypersensitivity reactions. Curr. Pharm. Des. 2019, 25, 3840–3854. [Google Scholar] [CrossRef] [PubMed]
- Weidman-Evans, E.; Rhodes, A.; Ferrington, L. What is the relationship between photosensitizing drugs and skin cancer? JAAPA 2023, 36, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, S.; Worswick, S. Photosensitizing drug reactions. Clin. Dermatol. 2022, 40, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Henderson, M.; Jacobsen, G.; Lim, H.W. Comparison of photodermatoses in African-Americans and Caucasians: A follow-up study. Photodermatol. Photoimmunol. Photomed. 2014, 30, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Kutlubay, Z.; Sevim, A.; Engin, B.; Tuzun, Y. Photodermatoses, including phototoxic and photoallergic reactions (internal and external). Clin. Dermatol. 2014, 32, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Blakely, K.M.; Drucker, A.M.; Rosen, C.F. Drug-induced photosensitivity-an update: Culprit drugs, prevention and management. Drug Saf. 2019, 42, 827–847. [Google Scholar] [CrossRef]
- Kowalska, J.; Rok, J.; Rzepka, Z.; Wrześniok, D. Drug-induced photosensitivity—From light and chemistry to biological reactions and clinical symptoms. Pharmaceuticals 2021, 14, 723. [Google Scholar] [CrossRef]
- Harrison, T.J.; Chen, X.; Yasoshima, K.; Bauer, D. Phototoxicity-medicinal chemistry strategies for risk mitigation in drug discovery. J. Med. Chem. 2023, 66, 9345–9362. [Google Scholar] [CrossRef]
- Trouba, K.J.; Hamadeh, H.K.; Amin, R.P.; Germolec, D.R. Oxidative stress and its role in skin disease. Antioxid. Redox Signal 2002, 4, 665–673. [Google Scholar] [CrossRef]
- Kim, W.B.; Shelley, A.J.; Novice, K.; Joo, J.; Lim, H.W.; Glassman, S.J. Drug-induced phototoxicity: A systematic review. J. Am. Acad. Dermatol. 2018, 79, 1069–1075. [Google Scholar] [CrossRef]
- Hofmann, G.A.; Weber, B. Drug-induced photosensitivity: Culprit drugs, potential mechanisms and clinical consequences. J. Dtsch. Dermatol. Ges. 2021, 19, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.E.; Fravel, M.A. Thiazide and the thiazide-like diuretics: Review of hydrochlorothiazide, chlorthalidone, and indapamide. Am. J. Hypertens. 2022, 35, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Bernal, S.; Alvarez-Perez, A.; Rodriguez-Pazos, L.; Gutierrez-Gonzalez, E.; Rodriguez-Granados, M.T.; Toribio, J. Photosensitivity due to thiazides. Actas. Dermosifiliogr. 2014, 105, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Haisma, M.S.; Greven, N.; Logendran, M.; Bos, J.; van der Vegt, B.; Horvath, B.; De Vos, S.; De Bock, G.H.; Hak, E.; Racz, E. Chronic use of hydrochlorothiazide and risk of skin cancer in Caucasian adults: A pharmlines initiative inception cohort study. Acta Derm. Venereol. 2023, 103, 3933. [Google Scholar] [CrossRef] [PubMed]
- Azoulay, L.; St-Jean, A.; Dahl, M.; Quail, J.; Aibibula, W.; Brophy, J.M.; Chan, A.W.; Bresee, L.; Carney, G.; Eltonsy, S.; et al. Hydrochlorothiazide use and risk of keratinocyte carcinoma and melanoma: A multisite population-based cohort study. J. Am. Acad. Dermatol. 2023, 89, 243–253. [Google Scholar] [CrossRef] [PubMed]
- d’Ischia, M.; Wakamatsu, K.; Cicoira, F.; Di Mauro, E.; Garcia-Borron, J.C.; Commo, S.; Galvan, I.; Ghanem, G.; Kenzo, K.; Meredith, P.; et al. Melanins and melanogenesis: From pigment cells to human health and technological applications. Pigment Cell Melanoma Res. 2015, 28, 20–44. [Google Scholar] [CrossRef]
- Burnier, M.; Bakris, G.; Williams, B. Redefining diuretics use in hypertension: Why select a thiazide-like diuretic? J. Hypertens. 2019, 37, 1574–1586. [Google Scholar] [CrossRef]
- Drucker, A.M.; Rosen, C.F. Drug-induced photosensitivity: Culprit drugs, management and prevention. Drug Saf. 2011, 34, 821–837. [Google Scholar] [CrossRef]
- Moore, D.E. Drug-induced cutaneous photosensitivity: Incidence, mechanism, prevention and management. Drug Saf. 2002, 25, 345–372. [Google Scholar] [CrossRef]
- Shalaeva, E.V.; Messerli, F.H. What is resistant arterial hypertension? Blood Press 2023, 32, 2185457. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Dineva, C.; Uzunova, K.; Pavlova, V.; Filipowa, E.; Kalinov, K.; Vekov, T. Comparative efficacy and safety of chlorthalidone and hydrochlorothiazide-meta-analysis. J. Hum. Hypertens. 2019, 33, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Roush, G.; Kaur, R.; Ernst, M. Diuretics: A review and update. J. Cardiovasc. Pharmacol. Ther. 2014, 19, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Rouette, J.; Yin, H.; Pottegard, A.; Nirantharakumar, K.; Azoulay, L. Use of hydrochlorothiazide and risk of melanoma and nonmelanoma skin cancer. Drug Saf. 2021, 44, 245–254. [Google Scholar] [CrossRef]
- Lugović-Mihić, L.; Duvančić, T.; Ferček, I.; Vuković, P.; Japundžić, I.; Ćesić, D. Drug-induced photosensitivity—A continuing diagnostic challenge. Acta Clin. Croat. 2017, 56, 277–283. [Google Scholar] [CrossRef]
- Kaae, J.; Boyd, H.A.; Hansen, A.V.; Wulf, H.C.; Wohlfahrt, J.; Melbye, M. Photosensitizing medication use and risk of skin cancer. Cancer Epidemiol. Biomark. Prev. 2010, 11, 2942–2949. [Google Scholar] [CrossRef]
- Birck, M.G.; Moura, C.S.; Machado, M.A.A.; Liu, J.L.; Abrahamowicz, M.; Pilote, L.; Bernatsky, S. Skin cancer and hydrochlorothiazide: Novel population-based analyses considering personal risk factors including race/ethnicity. Hypertension 2023, 80, 2218–2225. [Google Scholar] [CrossRef]
- Götzinger, F.; Wilke, T.; Hardtstock, F.; Krieger, J.; Maywald, U.; Kunz, M.; Lauder, L.; Schulz, M.; Mahfoud, F.; Böhm, M. Association of hydrochlorothiazide treatment compared with alternative diuretics with overall and skin cancer risk: A propensity-matched cohort study. J. Hypertens. 2023, 41, 926–933. [Google Scholar] [CrossRef]
- Shin, D.; Lee, E.S.; Kim, J.; Guerra, L.; Naik, D.; Prida, X. Association between the use of thiazide diuretics and the risk of skin cancers: A meta-analysis of observational studies. J. Clin. Med. Res. 2019, 11, 247–255. [Google Scholar] [CrossRef]
- Humbert, X.; Dolladille, C.; Chretien, B.; Sassier, M.; Fedrizzi, S.; Puddu, P.E.; Alexandre, J. Thiazides and nonmelanoma skin cancer: Is it a class effect? J. Am. Acad. Dermatol. 2020, 82, 25–26. [Google Scholar] [CrossRef]
- Morales, D.R.; Pacurariu, A.; Slattery, J.; Kurz, X. Association between hydrochlorothiazide exposure and different incident skin, lip and oral cavity cancers: A series of population-based nested case-control studies. Br. J. Clin. Pharmacol. 2020, 86, 1336–1345. [Google Scholar] [CrossRef]
- Adalsteinsson, J.A.; Muzumdar, S.; Waldman, R.; Hu, C.; Wu, R.; Ratner, D.; Ungar, J.; Silverberg, J.I.; Olafsdottir, G.H.; Kristjansson, A.K.; et al. Association between hydrochlorothiazide and the risk of in situ and invasive squamous cell skin carcinoma and basal cell carcinoma: A population-based case-control study. J. Am. Acad. Dermatol. 2021, 84, 669–675. [Google Scholar] [CrossRef]
- Daniels, B.; Pearson, S.A.; Vajdic, C.M.; Pottegard, A.; Buckley, N.A.; Zoega, H. Risk of squamous cell carcinoma of the lip and cutaneous melanoma in older Australians using hydrochlorothiazide: A population-based case-control study. Basic Clin. Pharmacol. Toxicol. 2020, 127, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Kreutz, R.; Algharably, E.A.H.; Douros, A. Reviewing the effects of thiazide and thiazide-like diuretics as photosensitizing drugs on the risk of skin cancer. J. Hypertens. 2019, 37, 1950–1958. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Sarna, T.; Płonka, P.M.; Raman, C.; Brożyna, A.A.; Slominski, A.T. Melanoma, melanin, and melanogenesis: The yin and yang relationship. Front. Oncol. 2022, 14, 842496. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. Photoprotection and skin pigmentation: Melanin-related molecules and some other new agents obtained from natural sources. Molecules 2020, 25, 1537. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Bookaman, M.M.; Wainer, I.; Patil, P.N. Relevance of drug-melanin interactions to ocular pharmacology and toxicology. J. Ocul. Pharmacol. 1994, 10, 217–239. [Google Scholar] [CrossRef]
- Rok, J.; Rzepka, Z.; Respondek, M.; Beberok, A.; Wrześniok, D. Chlortetracycline and melanin biopolymer-the risk of accumulation and implications for phototoxicity: An in vitro study on normal human melanocytes. Chem. Biol. Interact. 2019, 303, 27–34. [Google Scholar] [CrossRef]
- Reinen, J.; van Sas, P.; van Huygevoort, T.; Rubio, L.; Scase, K.; Wenker, M. Development of a phototoxicity testing strategy for accurate photosafety evaluation of pharmaceuticals based on the assessment of possible melanin-binding effects. Int. J. Toxicol. 2018, 37, 296–307. [Google Scholar] [CrossRef]
- Beberok, A.; Buszman, E.; Wrześniok, D.; Otręba, M.; Trzcionka, J. Interaction between ciprofloxacin and melanin: The effect on proliferation and melanization in melanocytes. Eur. J. Pharmacol. 2011, 669, 32–37. [Google Scholar] [CrossRef]
- Selvaag, E.; Thune, P. Phototoxicity to sulphonamide-derived oral antidiabetics and diuretics: Investigations in hairless mice. Photodermatol. Photoimmunol. Photomed. 1997, 13, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Selvaag, E.; Petersen, A.B.; Gniadecki, R.; Thorn, T.; Wulf, H.C. Phototoxicity to diuretics and antidiabetics in the cultured keratinocyte cell line HaCaT: Evaluation by clonogenic assay and single cell gel electrophoresis Comet assay. Photodermatol. Photoimmunol. Photomed. 2002, 18, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Rimpela, A.K.; Reinisalo, M.; Hellinen, L.; Grazhdankin, E.; Kidron, H.; Urtti, A.; Del Amo, E.M. Implications of melanin binding in ocular drug delivery. Adv. Drug Deliv. Rev. 2018, 126, 23–43. [Google Scholar] [CrossRef]
- Karkoszka, M.; Rok, J.; Banach, K.; Kowalska, J.; Rzepka, Z.; Wrześniok, D. The assessment of meloxicam phototoxicity in human normal skin cells: In vitro studies on dermal fibroblasts and epidermal melanocytes. Molecules 2022, 27, 4215. [Google Scholar] [CrossRef]
- Kowalska, J.; Banach, K.; Rok, J.; Beberok, A.; Rzepka, Z.; Wrześniok, D. Molecular and biochemical basis of fluoroquinolones-induced phototoxicity-the study of antioxidant system in human melanocytes exposed to UV-A radiation. IJMS 2020, 21, 9714. [Google Scholar] [CrossRef] [PubMed]
- Bigagli, E.; Cinci, L.; D’Amrbosio, M.; Nardini, P.; Portelli, F.; Colucci, R.; Lodovici, M.; Mugelli, A.; Luceri, C. Hydrochlorothiazide use and risk of nonmelanoma skin cancers: A biological plausibility study. Oxidative Med. Cell. Longev. 2021, 6655542. [Google Scholar] [CrossRef]
- Kowalska, J.; Banach, K.; Rzepka, Z.; Rok, J.; Karkoszka, M.; Wrześniok, D. Changes in the oxidation-reduction state of human dermal fibroblasts as an effect of lomefloxacin phototoxic action. Cells 2022, 11, 1971. [Google Scholar] [CrossRef]
- Di Bartolomeo, L.; Irrera, N.; Campo, G.M.; Borgia, F.; Motolese, A.; Vaccaro, F.; Squadrito, F.; Altavilla, D.; Condorelli, A.G.; Moto-lese, A.; et al. Drug-induced photosensitivity: Clinical types of phototoxicity and photoallergy and pathogenetic mechanisms. Front. Allergy 2022, 3, 876695. [Google Scholar] [CrossRef]
- Vargas, F.; Martinez, V.I.; Sequera, J.; Mendez, H.; Rojas, J.; Fraile, G.; Velasquez, M.; Medina, R. Photodegradation and phototoxicity studies of furosemide. Involvement of singlet oxygen in the photoinduced hemolysis and lipid peroxidation. J. Photochem. Photobiol. B 1998, 42, 19–25. [Google Scholar] [CrossRef]
- Kim, K.; Park, H.; Lim, K.M. Phototoxicity: Its mechanism and animal alternative test methods. Toxicol. Res. 2015, 31, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Hinton, A.N.; Goldminz, A.M. Feeling the burn: Phototoxicity and photoallergy. Dermatol. Clin. 2020, 38, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, R.P.; Chandel, N.S. Mitochondria as signaling organelles control mammalian stem cell fate. Cell Stem Cell 2021, 28, 394–408. [Google Scholar] [CrossRef]
- Sarsour, E.H.; Kumar, M.G.; Chaudhuri, L.; Kalen, A.L.; Goswami, P.C. Redox control of the cell cycle in health and disease. Antioxid. Redox Signal 2009, 11, 2985–3011. [Google Scholar] [CrossRef]
- Rok, J.; Karkoszka, M.; Rzepka, Z.; Respondek, M.; Banach, K.; Beberok, A.; Wrześniok, D. Cytotoxic and proapoptotic effect of doxycycline—An in vitro study on the human skin melanoma cells. Toxicol. Vitr. 2020, 65, 104790. [Google Scholar] [CrossRef]
| Relative Cell Viability (% of Control) | ||
|---|---|---|
| HDF | HEMn-LP | |
| No drug | 100.0 | 100.0 |
| No drug + UVAR | 99.9 | 101.4 |
| HCT 0.5 mM | 99.7 | 101.7 |
| HCT 0.5 mM + UVAR | 99.6 | 102.5 |
| HCT 1 mM | 91.8 | 98.4 |
| HCT 1 mM + UVAR | 58.6 | 90.5 |
| FUR 0.5 mM | 100.5 | 96.0 |
| FUR 0.5 mM + UVAR | 98.9 | 88.5 |
| FUR 1 mM | 98.0 | 94.3 |
| FUR 1 mM + UVAR | 104.5 | 82.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karkoszka, M.; Rok, J.; Rzepka, Z.; Banach, K.; Kowalska, J.; Wrześniok, D. Phototoxic Reactions Inducted by Hydrochlorothiazide and Furosemide in Normal Skin Cells—In Vitro Studies on Melanocytes and Fibroblasts. Int. J. Mol. Sci. 2024, 25, 1432. https://doi.org/10.3390/ijms25031432
Karkoszka M, Rok J, Rzepka Z, Banach K, Kowalska J, Wrześniok D. Phototoxic Reactions Inducted by Hydrochlorothiazide and Furosemide in Normal Skin Cells—In Vitro Studies on Melanocytes and Fibroblasts. International Journal of Molecular Sciences. 2024; 25(3):1432. https://doi.org/10.3390/ijms25031432
Chicago/Turabian StyleKarkoszka, Marta, Jakub Rok, Zuzanna Rzepka, Klaudia Banach, Justyna Kowalska, and Dorota Wrześniok. 2024. "Phototoxic Reactions Inducted by Hydrochlorothiazide and Furosemide in Normal Skin Cells—In Vitro Studies on Melanocytes and Fibroblasts" International Journal of Molecular Sciences 25, no. 3: 1432. https://doi.org/10.3390/ijms25031432
APA StyleKarkoszka, M., Rok, J., Rzepka, Z., Banach, K., Kowalska, J., & Wrześniok, D. (2024). Phototoxic Reactions Inducted by Hydrochlorothiazide and Furosemide in Normal Skin Cells—In Vitro Studies on Melanocytes and Fibroblasts. International Journal of Molecular Sciences, 25(3), 1432. https://doi.org/10.3390/ijms25031432





