Modulation of Acute Intestinal Inflammation by Dandelion Polysaccharides: An In-Depth Analysis of Antioxidative, Anti-Inflammatory Effects and Gut Microbiota Regulation
Abstract
1. Introduction
2. Results
2.1. Analysis of DPS
2.1.1. The Total Sugar Content of DPS
2.1.2. Molecular Weight of DPS
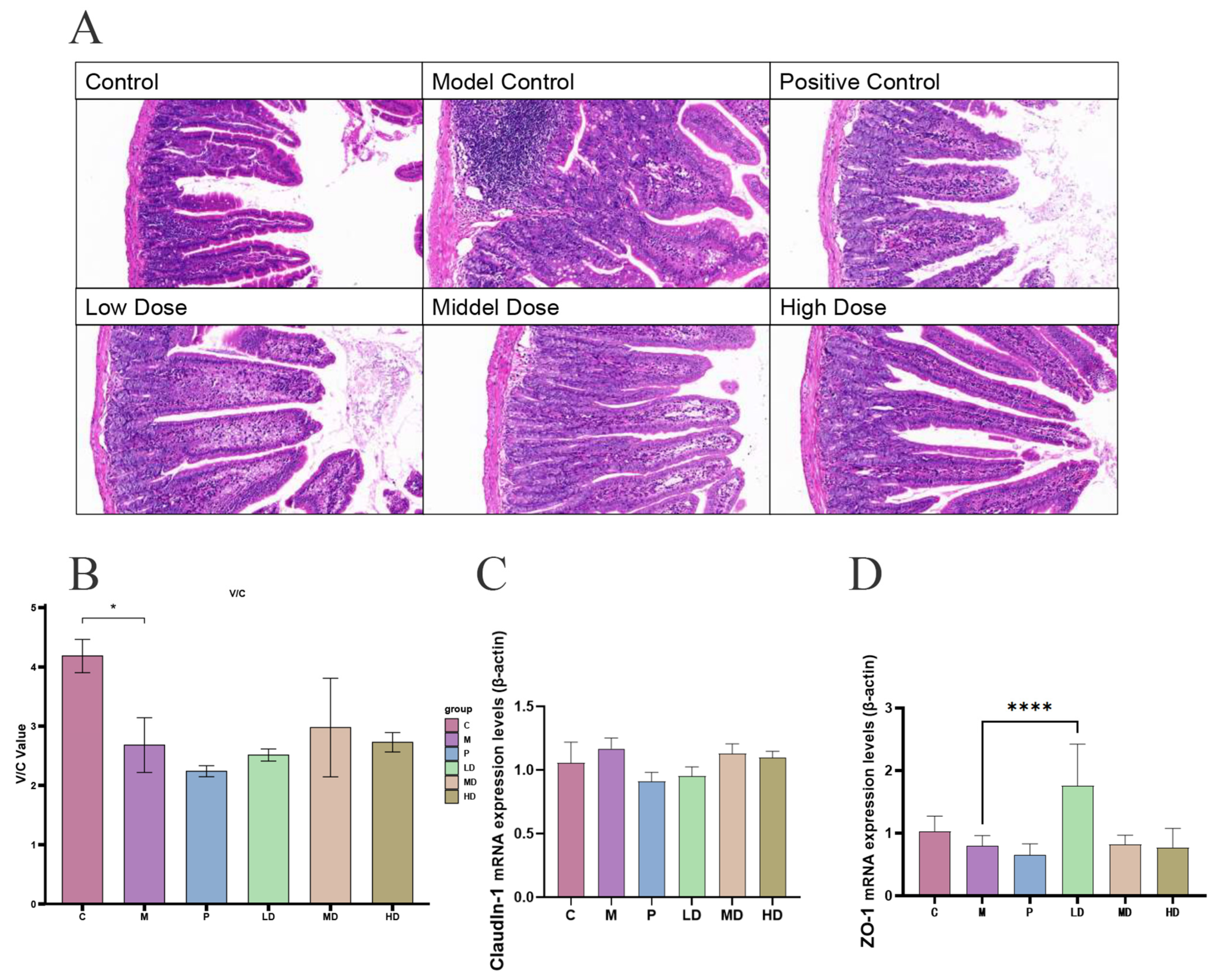
2.2. Effect of DPS on Intestinal Segment Morphology in Mice
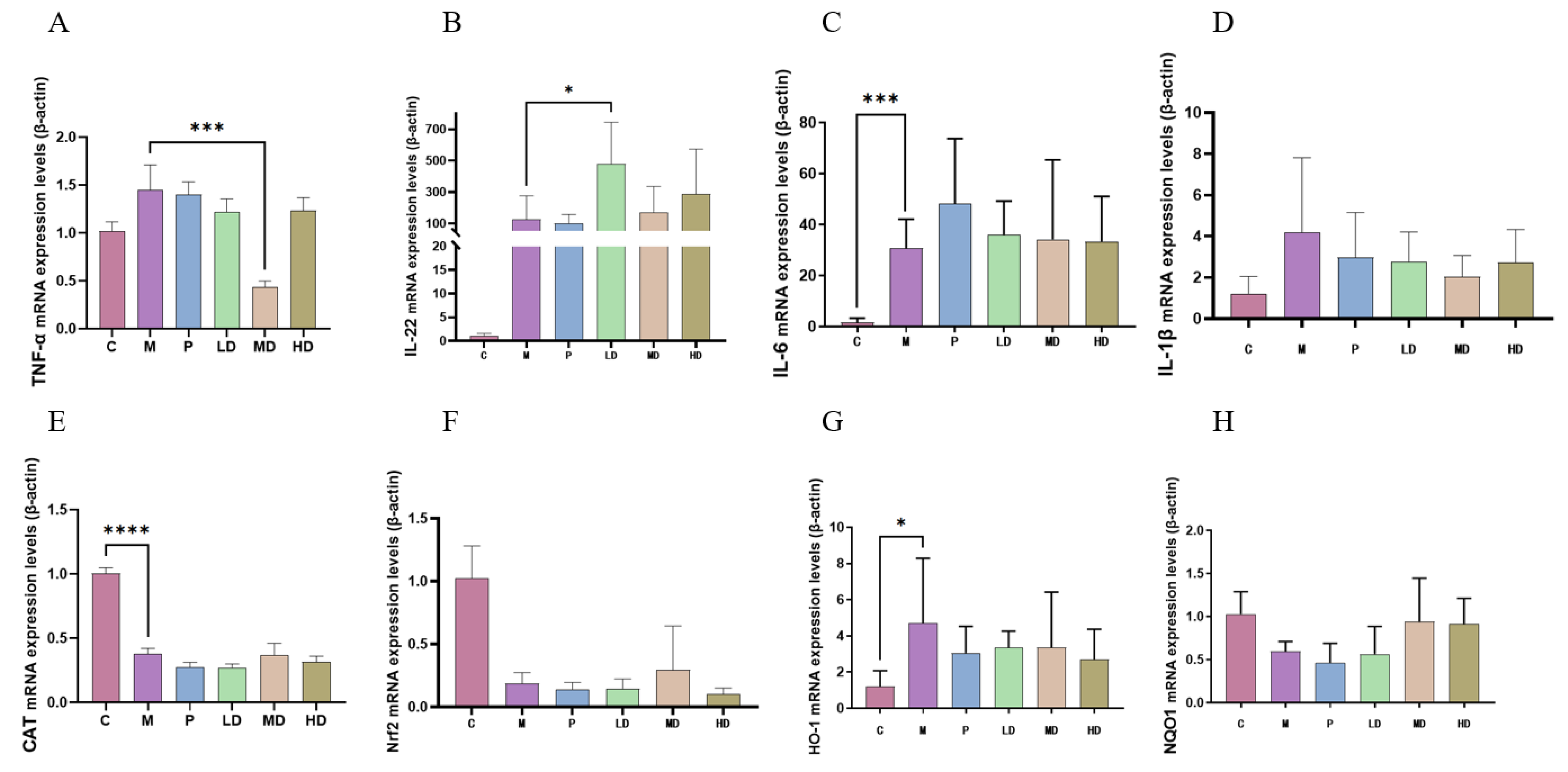
2.3. Anti-Inflammatory and Antioxidant Effects of DPS on Mice
2.4. DPS Regulates Intestinal Flora
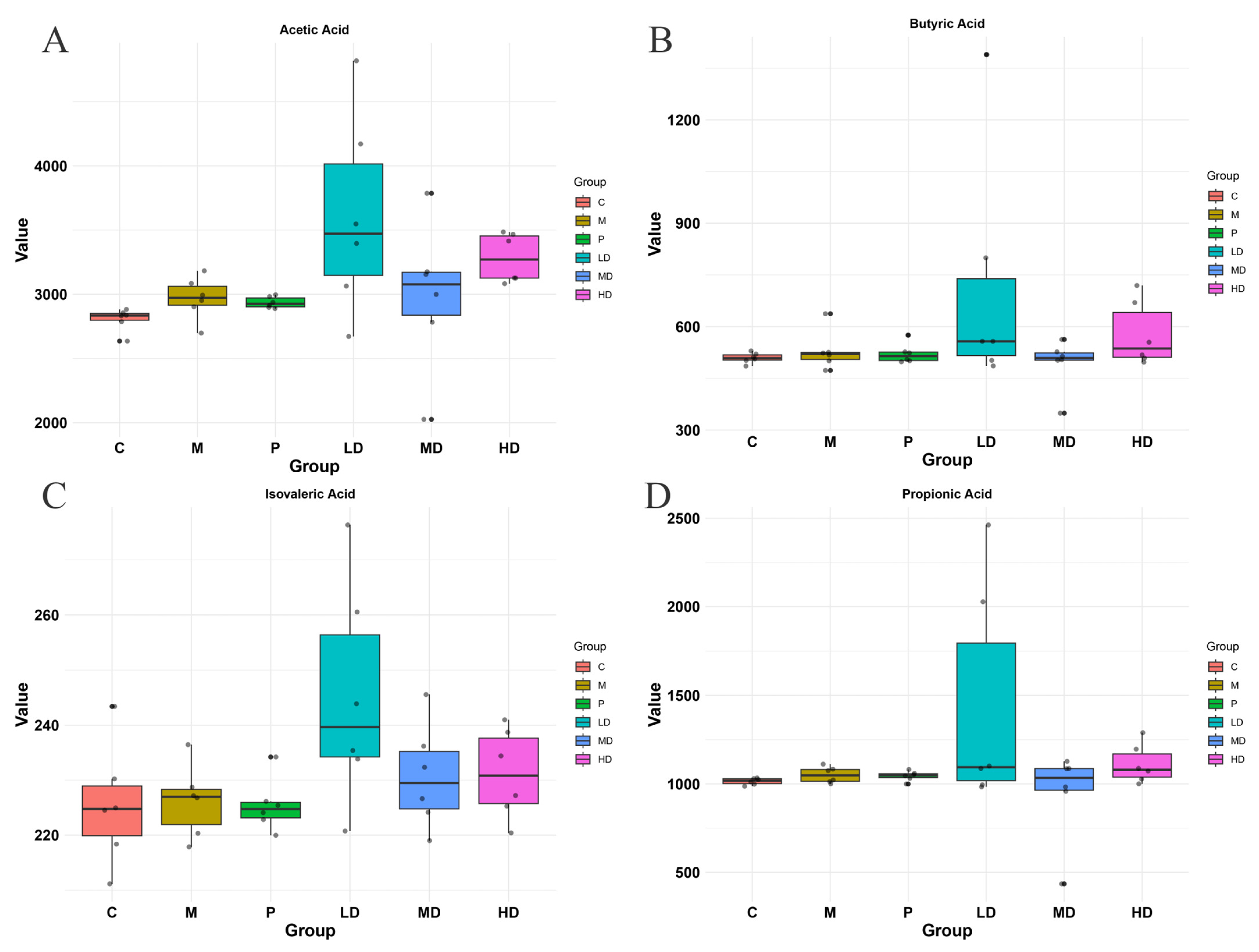
2.5. Effects of DPS on SCFAs in the Intestinal Contents of Mice
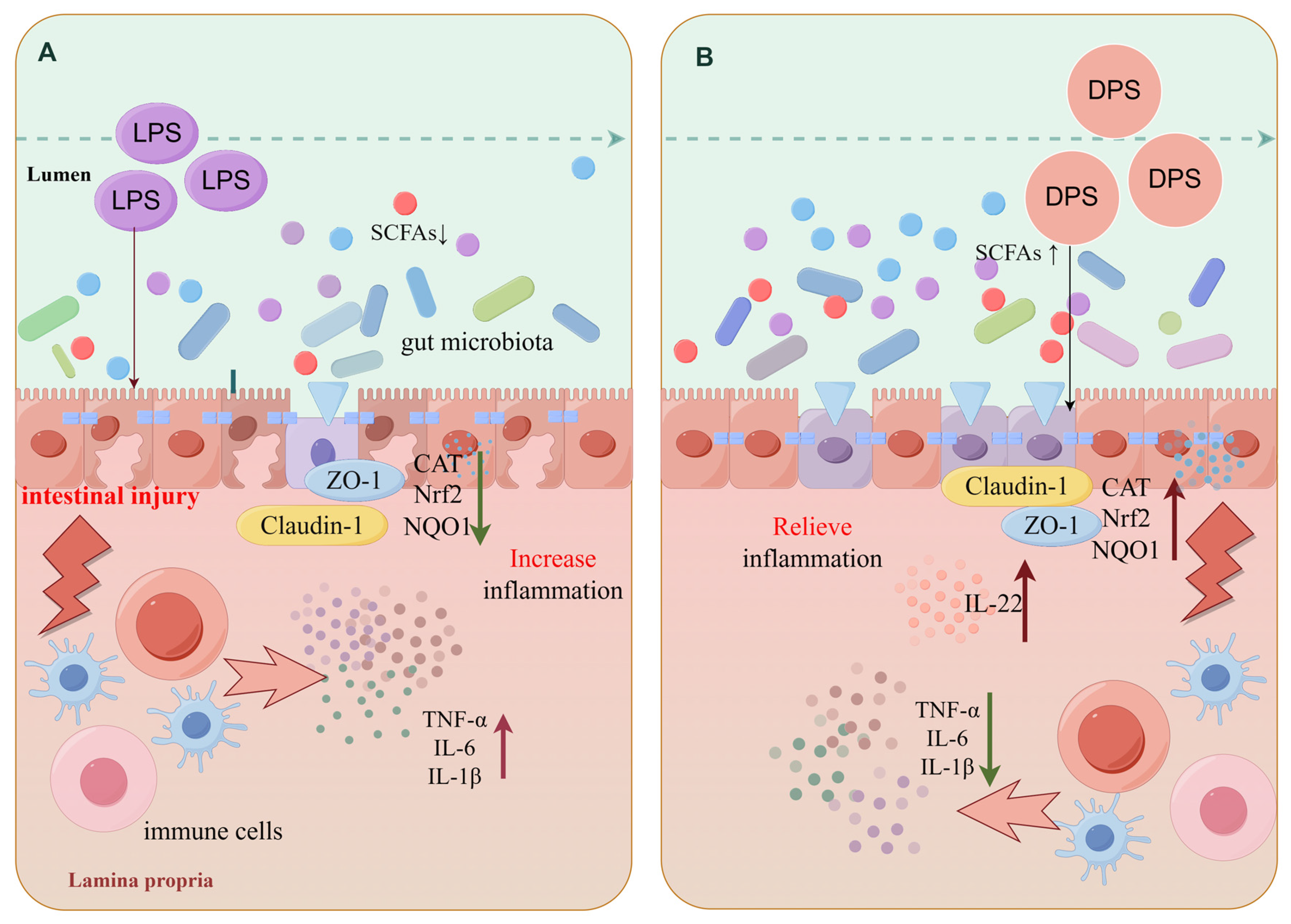
3. Discussion
4. Methods and Materials
4.1. Ethics Statement
4.2. Analysis of Polysaccharides
4.2.1. Total Sugar Content Determination
4.2.2. Molecular Weight Determination
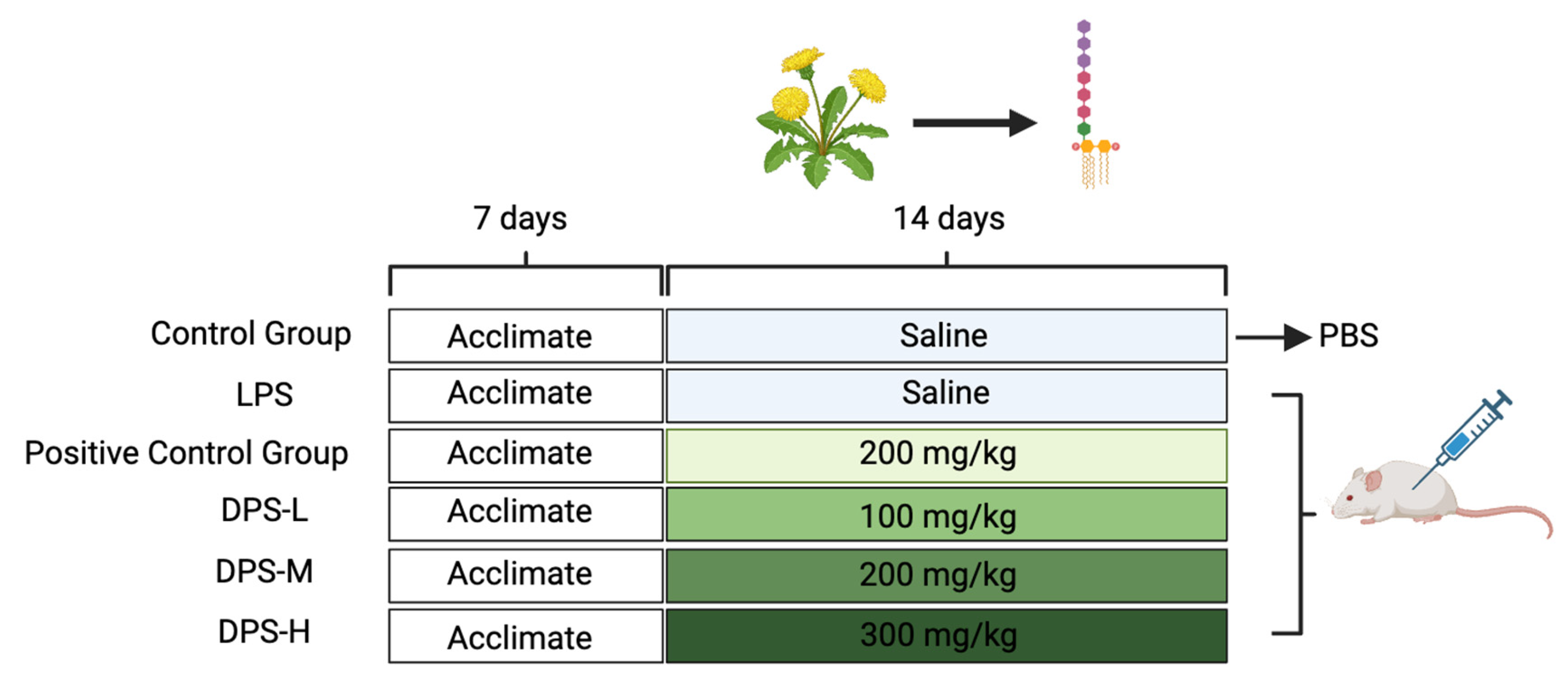
4.3. Animal Experiment and Sample Collection
4.4. Duodenal Morphological Observations
4.5. qPCR Assay and Detection of Antioxidant Activity Factors
4.6. Fecal Microbiota 16S rRNA Analysis
4.7. Determination of Short-Chain Fatty Acids in Intestinal Contents
4.8. Data Analysis and Mapping
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- van Baar, A.C.G.; Nieuwdorp, M.; Holleman, F.; Soeters, M.R.; Groen, A.K.; Bergman, J. The Duodenum harbors a Broad Untapped Therapeutic Potential. Gastroenterology 2018, 154, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Plessers, E.; Watteyn, A.; Wyns, H.; Pardon, B.; De Backer, P.; Croubels, S. Study of the immunomodulatory properties of gamithromycin and dexamethasone in a lipopolysaccharide inflammation model in calves. Res. Vet. Sci. 2015, 103, 218–223. [Google Scholar] [CrossRef] [PubMed]
- De Wolde, S.D.; Hulskes, R.H.; Weenink, R.P.; Hollmann, M.W.; Van Hulst, R.A. The Effects of Hyperbaric Oxygenation on Oxidative Stress, Inflammation and Angiogenesis. Biomolecules 2021, 11, 1210. [Google Scholar] [CrossRef] [PubMed]
- Joffre, J.; Hellman, J. Oxidative Stress and Endothelial Dysfunction in Sepsis and Acute Inflammation. Antioxid. Redox Signal. 2021, 35, 1291–1307. [Google Scholar] [CrossRef]
- Liu, J.; Han, X.; Zhang, T.; Tian, K.; Li, Z.; Luo, F. Reactive oxygen species (ROS) scavenging biomaterials for anti-inflammatory diseases: From mechanism to therapy. J. Hematol. Oncol. 2023, 16, 116. [Google Scholar] [CrossRef] [PubMed]
- Moudgil, K.D.; Venkatesha, S.H. The Anti-Inflammatory and Immunomodulatory Activities of Natural Products to Control Autoimmune Inflammation. Int. J. Mol. Sci. 2022, 24, 95. [Google Scholar] [CrossRef] [PubMed]
- Scotti, L.; Scotti, M.T. Natural Products as Anti-Inflammatory Agents. Comb. Chem. High Throughput Screen. 2022, 25, 2315–2316. [Google Scholar] [CrossRef]
- Fan, M.; Zhang, X.; Song, H.; Zhang, Y. Dandelion (Taraxacum Genus): A Review of Chemical Constituents and Pharmacological Effects. Molecules 2023, 28, 5022. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Sun-Waterhouse, D. The potential of dandelion in the fight against gastrointestinal diseases: A review. J. Ethnopharmacol. 2022, 293, 115272. [Google Scholar] [CrossRef]
- Zeng, P.; Li, J.; Chen, Y.; Zhang, L. The structures and biological functions of polysaccharides from traditional Chinese herbs. Prog. Mol. Biol. Transl. Sci. 2019, 163, 423–444. [Google Scholar]
- Kayama, H.; Okumura, R.; Takeda, K. Interaction between the Microbiota, Epithelia, and Immune Cells in the Intestine. Annu. Rev. Immunol. 2020, 38, 23–48. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Z.; Zhang, J.; Hong, Z.; Bao, W.L.; Zhou, L.S.; Liu, Y.; Chen, D.F.; Lu, Y. Structural Characterization and Anti-inflammatory Activities of Anticomplementary Polysaccharides from Rhododendron principis. Planta Medica 2023, 89, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Guo, D.; Fang, L.; Sang, T.; Wu, J.; Guo, C.; Wang, Y.; Wang, Y.; Chen, C.; Chen, J.; et al. Ganoderma lucidum polysaccharide modulates gut microbiota and immune cell function to inhibit inflammation and tumorigenesis in colon. Carbohydr. Polym. 2021, 267, 118231. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.C.; Yu, W.W.; Zhou, H.C.; Lan, Z.C.; Wu, T.; Xiong, S.M.; Yan, L.; Liu, H.B. Lycium barbarum polysaccharides ameliorate LPS-induced inflammation of RAW264.7 cells and modify the behavioral score of peritonitis mice. J. Food Biochem. 2021, 45, e13889. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.; Zhang, L.Y.; He, W.; Li, W.Y.; Zeng, Z.; Qian, B.; Wang, C.; Song, J.L. Sinapic Acid Alleviated Inflammation-Induced Intestinal Epithelial Barrier Dysfunction in Lipopolysaccharide- (LPS-) Treated Caco-2 Cells. Mediat. Inflamm. 2021, 2021, 5514075. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Leon Coria, A.; Cornick, S.; Petri, B.; Mayengbam, S.; Jijon, H.B.; Moreau, F.; Shearer, J.; Chadee, K. Increased intestinal permeability exacerbates sepsis through reduced hepatic SCD-1 activity and dysregulated iron recycling. Nat. Commun. 2020, 11, 483. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.K.; Johansson, M.E.V. The role of goblet cells and mucus in intestinal homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 785–803. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, H. Curcumin Improved Intestinal Epithelial Barrier Integrity by Up-Regulating ZO-1/Occludin/Claudin-1 in Septic Rats. Evid.-Based Complement. Altern. Med. Ecam 2022, 2022, 2884522. [Google Scholar] [CrossRef]
- Zou, P.; Yang, F.; Ding, Y.; Zhang, D.; Liu, Y.; Zhang, J.; Wu, D.; Wang, Y. Lipopolysaccharide downregulates the expression of ZO-1 protein through the Akt pathway. BMC Infect. Dis. 2022, 22, 774. [Google Scholar] [CrossRef]
- Dong, N.; Xu, X.; Xue, C.; Wang, C.; Li, X.; Shan, A.; Xu, L.; Li, D. Ethyl pyruvate protects against Salmonella intestinal infection in mice through down-regulation of pro-inflammatory factors and inhibition of TLR4/MAPK pathway. Int. Immunopharmacol. 2019, 71, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Song, J.; Xu, Y.; Liu, C.; Qian, W.; Bai, T.; Hou, X. Piezo1 regulates intestinal epithelial function by affecting the tight junction protein claudin-1 via the ROCK pathway. Life Sci. 2021, 275, 119254. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; He, L.; Hu, N.; Zhao, X.; Gong, L.; Wang, C.; Peng, C.; Li, Y. Aconiti Lateralis Radix Praeparata lipid-soluble alkaloids alleviates IL-1β-induced inflammation of human fibroblast-like synoviocytes in rheumatoid arthritis by inhibiting NF-κB and MAPKs signaling pathways and inducing apoptosis. Cytokine 2022, 151, 155809. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Guo, K.; Du, M.; Mao, X. Bovine α-lactalbumin-derived peptides attenuate TNF-α-induced insulin resistance and inflammation in 3T3-L1 adipocytes through inhibiting JNK and NF-κB signaling. Food Funct. 2022, 13, 2323–2335. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Tang, J.; Zhang, X.; He, Y.; Yu, Z.; Chen, S.; Xia, J.; Lin, J.; Ou, Q. CCN1 Promotes Inflammation by Inducing IL-6 Production via α6β1/PI3K/Akt/NF-κB Pathway in Autoimmune Hepatitis. Front. Immunol. 2022, 13, 810671. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, A. Healing of intestinal inflammation by IL-22. Inflamm. Bowel Dis. 2012, 18, 1777–1784. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, C.; Calderon, P.B. Catalase, a remarkable enzyme: Targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol. Chem. 2017, 398, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.; Siegel, D. The diverse functionality of NQO1 and its roles in redox control. Redox Biol. 2021, 41, 101950. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Elsheikh, N.A.H.; Li, C.; Yang, F.; Wang, G.; Li, L. HO-1 reduces heat stress-induced apoptosis in bovine granulosa cells by suppressing oxidative stress. Aging 2019, 11, 5535–5547. [Google Scholar] [CrossRef]
- Sha, W.; Zhao, B.; Wei, H.; Yang, Y.; Yin, H.; Gao, J.; Zhao, W.; Kong, W.; Ge, G.; Lei, T. Astragalus polysaccharide ameliorates vascular endothelial dysfunction by stimulating macrophage M2 polarization via potentiating Nrf2/HO-1 signaling pathway. Phytomed. Int. J. Phytother. Phytopharm. 2023, 112, 154667. [Google Scholar] [CrossRef]
- Wu, M.F.; Xi, Q.H.; Sheng, Y.; Wang, Y.M.; Wang, W.Y.; Chi, C.F.; Wang, B. Antioxidant Peptides from Monkfish Swim Bladders: Ameliorating NAFLD In Vitro by Suppressing Lipid Accumulation and Oxidative Stress via Regulating AMPK/Nrf2 Pathway. Mar. Drugs 2023, 21, 360. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Reviews. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Mlynarczyk-Bonikowska, B.; Kowalewski, C.; Krolak-Ulinska, A.; Marusza, W. Molecular Mechanisms of Drug Resistance in Staphylococcus aureus. Int. J. Mol. Sci. 2022, 23, 8088. [Google Scholar] [CrossRef]
- Ratajczak, W.; Rył, A.; Mizerski, A.; Walczakiewicz, K.; Sipak, O.; Laszczyńska, M. Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs). Acta Biochim. Pol. 2019, 66, 1–12. [Google Scholar] [CrossRef]

| 1-1 Absorbance Value | 1-2 Absorbance Value | 1-3 Absorbance Value | Mean | Sample Size (mg/mL) | Total Sugar Content % | |
|---|---|---|---|---|---|---|
| DPS | 2.583 | 3.181 | 3.075 | 2.946 | 100 | 9.27 |
| RT (min) | lgMp | lgMw | lgMn | Mp | Mw | Mn | Peak Area Ratio % |
|---|---|---|---|---|---|---|---|
| 37.523 | 4.7 | 4.7 | 4.7 | 52,257 | 52,655 | 51,042 | 51.357 |
| 42.08 | 3.9 | 3.9 | 3.9 | 8030 | 8254 | 7951 | 48.643 |
| Standards | Manufacturers | Lot Number | Storage Condition | Purity | Expiry Date |
|---|---|---|---|---|---|
| Dextranstandards 1152 | Yuanye Bio-Technology (Shanghai China) | A16A8L41850 | Sealed storage | 99% | Two years |
| 5000 | Sigma (Saint Louis, MO, USA) | 102084138 | Sealed storage | ≥99% | Two years |
| 11600 | Sigma | 102136543 | Sealed storage | 99% | Two years |
| 23800 | Sigma | 102124529 | Sealed storage | ≥97% | Two years |
| 48600 | Sigma | 102104509 | Sealed storage | ≥99% | Two years |
| 80900 | Sigma | 102108375 | Sealed storage | >98% | Two years |
| 148000 | Sigma | 102089360 | Sealed storage | >98% | Two years |
| 273000 | Sigma | 102110878 | Sealed storage | 98% | Two years |
| 409800 | Sigma | 102124507 | Sealed storage | >98% | Two years |
| 667800 | Sigma | 102104510 | Sealed storage | >98% | Two years |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Li, X.; Shi, P.; Li, P.; Fu, Y.; Tan, G.; Zhou, J.; Zeng, J.; Huang, P. Modulation of Acute Intestinal Inflammation by Dandelion Polysaccharides: An In-Depth Analysis of Antioxidative, Anti-Inflammatory Effects and Gut Microbiota Regulation. Int. J. Mol. Sci. 2024, 25, 1429. https://doi.org/10.3390/ijms25031429
Li Z, Li X, Shi P, Li P, Fu Y, Tan G, Zhou J, Zeng J, Huang P. Modulation of Acute Intestinal Inflammation by Dandelion Polysaccharides: An In-Depth Analysis of Antioxidative, Anti-Inflammatory Effects and Gut Microbiota Regulation. International Journal of Molecular Sciences. 2024; 25(3):1429. https://doi.org/10.3390/ijms25031429
Chicago/Turabian StyleLi, Zhu, Xinyao Li, Panpan Shi, Pingping Li, Yue Fu, Guifeng Tan, Junjuan Zhou, Jianguo Zeng, and Peng Huang. 2024. "Modulation of Acute Intestinal Inflammation by Dandelion Polysaccharides: An In-Depth Analysis of Antioxidative, Anti-Inflammatory Effects and Gut Microbiota Regulation" International Journal of Molecular Sciences 25, no. 3: 1429. https://doi.org/10.3390/ijms25031429
APA StyleLi, Z., Li, X., Shi, P., Li, P., Fu, Y., Tan, G., Zhou, J., Zeng, J., & Huang, P. (2024). Modulation of Acute Intestinal Inflammation by Dandelion Polysaccharides: An In-Depth Analysis of Antioxidative, Anti-Inflammatory Effects and Gut Microbiota Regulation. International Journal of Molecular Sciences, 25(3), 1429. https://doi.org/10.3390/ijms25031429







