Using 8-Hydroxy-2′-Deoxiguanosine (8-OHdG) as a Reliable Biomarker for Assessing Periodontal Disease Associated with Diabetes
Abstract
1. Introduction
2. Periodontal Disease and Diabetes: An Interconnected Relationship through Oxidative Stress
2.1. Diabetes Mellitus and Periodontal Disease
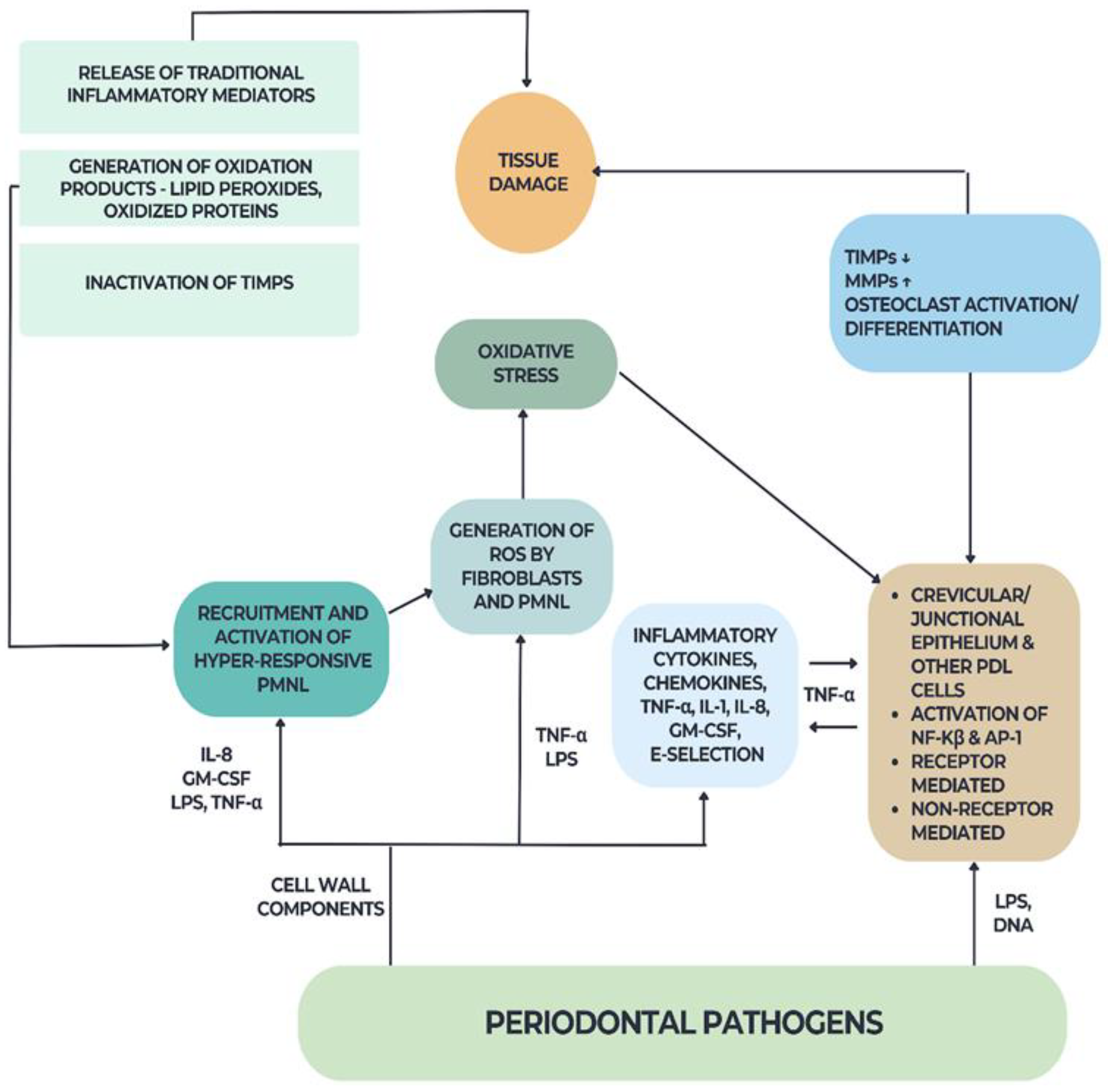
2.2. Oxidative Stress in Diabetes and Periodontal Disease
- ➢
- ROS or oxidative damage must always be present at the site of tissue injury.
- ➢
- The appearance of free radicals must coincide with the moment of tissue injury.
- ➢
- The direct application of ROS within a specific time frame and concentration should lead to similar oxidative tissue damage.
- ➢
- Preventing ROS formation or removing them from the site of injury should reduce tissue damage.
3. 8-OHdG—An Overview
3.1. General Aspects
3.2. 8-OHdG in Pathology
3.2.1. 8-OHdG and Diabetes Mellitus
3.2.2. 8-OHdG and Chronic Versus Aggressive Periodontitis
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sies, H. Oxidative Stress: Oxidants and Antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Canakci, C.F.; Cicek, Y.; Canakci, V. Reactive Oxygen Species and Human Inflammatory Periodontal Diseases. Biochemistry 2005, 70, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Muthuraj, M.; Janakiram, S.; Chithresan, K. Is 8-OHdG a Reliable Marker in Periodontitis—The Sixth Complication of Diabetes Mellitus? Clin. Dent. 2021, 15, 12. [Google Scholar]
- Canakçi, C.F.; Canakçi, V.; Tatar, A.; Eltas, A.; Sezer, U.; Ciçek, Y.; Oztas, S. Increased Salivary Level of 8-Hydroxydeoxyguanosine Is a Marker of Premature Oxidative Mitochondrial DNA Damage in Gingival Tissue of Patients with Periodontitis. Arch. Immunol. Ther. Exp. 2009, 57, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, L.M.; Bacino, M.; Kapila, Y.L. Periodontal Disease: The Good, The Bad, and The Unknown. Front. Cell Infect. Microbiol. 2021, 11, 1210. [Google Scholar] [CrossRef] [PubMed]
- Luchian, I.; Goriuc, A.; Sandu, D.; Covasa, M. The Role of Matrix Metalloproteinases (MMP-8, MMP-9, MMP-13) in Periodontal and Peri-Implant Pathological Processes. Int. J. Mol. Sci. 2022, 23, 1806. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.A. Prevalence of Periodontal Disease, Its Association with Systemic Diseases and Prevention. Int. J. Health Sci. 2017, 11, 72–80. [Google Scholar]
- Nazir, M.; Al-Ansari, A.; Al-Khalifa, K.; Alhareky, M.; Gaffar, B.; Almas, K. Global Prevalence of Periodontal Disease and Lack of Its Surveillance. Sci. World J. 2020, 2020, 2146160. [Google Scholar] [CrossRef]
- McSorley, R. Does the Use of Antimicrobials in Different Periodontal Treatment Strategies Result in Better Treatment Outcomes?—A Radiographic Analysis. Evid. Based Dent. 2024, 1–2. [Google Scholar] [CrossRef]
- Haque, M.M.; Yerex, K.; Kelekis-Cholakis, A.; Duan, K. Advances in Novel Therapeutic Approaches for Periodontal Diseases. BMC Oral Health 2022, 22, 492. [Google Scholar] [CrossRef]
- Battino, M.; Bullon, P.; Wilson, M.; Newman, H. Oxidative Injury and Inflammatory Periodontal Diseases: The Challenge of Anti-Oxidants to Free Radicals and Reactive Oxygen Species. Crit. Rev. Oral Biol. Med. 1999, 10, 458–476. [Google Scholar] [CrossRef] [PubMed]
- Chiou, C.C.; Chang, P.Y.; Chan, E.C.; Wu, T.L.; Tsao, K.C.; Wu, J.T. Urinary 8-Hydroxydeoxyguanosine and Its Analogs as DNA Marker of Oxidative Stress: Development of an ELISA and Measurement in Both Bladder and Prostate Cancers. Clin. Chim. Acta 2003, 334, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.L.; Chiou, C.C.; Chang, P.Y.; Wu, J.T. Urinary 8-OHdG: A Marker of Oxidative Stress to DNA and a Risk Factor for Cancer, Atherosclerosis and Diabetics. Clin. Chim. Acta 2004, 339, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.C.; Matthews, J.B. The Role of Reactive Oxygen and Antioxidant Species in Periodontal Tissue Destruction. Periodontol. 2000 2007, 43, 160–232. [Google Scholar] [CrossRef] [PubMed]
- Varghese, J.; Bhat, V.; Chianeh, Y.R.; Kamath, V.; Al-Haj Husain, N.; Özcan, M. Salivary 8-Hydroxyguanosine Levels in Smokers and Non-Smokers with Chronic Periodontitis. Odontology 2020, 108, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Page, R.C. The Pathobiology of Periodontal Diseases May Affect Systemic Diseases: Inversion of a Paradigm. Ann. Periodontol. 1998, 3, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Lamster, I.B.; Lalla, E.; Borgnakke, W.S.; Taylor, G.W. The Relationship between Oral Health and Diabetes Mellitus. J. Am. Dent. Assoc. 2008, 139 (Suppl. S5), 19S–24S. [Google Scholar] [CrossRef]
- Page, R.C.; Kornman, K.S. The Pathogenesis of Human Periodontitis: An Introduction. Periodontol. 2000 1997, 14, 9–11. [Google Scholar] [CrossRef]
- Cesaratto, L.; Vascotto, C.; Calligaris, S.; Tell, G. The Importance of Redox State in Liver Damage. Ann. Hepatol. 2004, 3, 86–92. [Google Scholar] [CrossRef]
- Hyslop, P.A.; Hinshaw, D.B.; Scraufstatter, I.U.; Cochrane, C.G.; Kunz, S.; Vosbeck, K. Hydrogen Peroxide as a Potent Bacteriostatic Antibiotic: Implications for Host Defense. Free Radic. Biol. Med. 1995, 19, 31–37. [Google Scholar] [CrossRef]
- Fialkow, L.; Wang, Y.; Downey, G.P. Reactive Oxygen and Nitrogen Species as Signaling Molecules Regulating Neutrophil Function. Free Radic. Biol. Med. 2007, 42, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Free Radicals, Antioxidants, and Human Disease: Curiosity, Cause, or Consequence? Lancet 1994, 344, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Sculley, D.V.; Langley-Evans, S.C. Salivary Antioxidants and Periodontal Disease Status. Proc. Nutr. Soc. 2002, 61, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Jenkinson, H.F. Life Below the Gum Line: Pathogenic Mechanisms of Porphyromonas Gingivalis. Microbiol. Mol. Biol. Rev. 1998, 62, 1244–1263. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, C.S.; Kinane, D.F. Nutrition, Inflammation, and Periodontal Disease. Nutrition 2003, 19, 475–476. [Google Scholar] [CrossRef]
- Yasui, K.; Baba, A. Therapeutic Potential of Superoxide Dismutase (SOD) for Resolution of Inflammation. Inflamm. Res. 2006, 55, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Whiteman, M. Measuring Reactive Species and Oxidative Damage in Vivo and in Cell Culture: How Should You Do It and What Do the Results Mean? Br. J. Pharmacol. 2004, 142, 231–255. [Google Scholar] [CrossRef]
- Waddington, R.J.; Moseley, R.; Embery, G. Reactive Oxygen Species: A Potential Role in the Pathogenesis of Periodontal Diseases. Oral Dis. 2000, 6, 138–151. [Google Scholar] [CrossRef]
- Giannobile, W.V. C-Telopeptide Pyridinoline Cross-Links. Sensitive Indicators of Periodontal Tissue Destruction. Ann. N. Y. Acad. Sci. 1999, 878, 404–412. [Google Scholar] [CrossRef]
- Mahajan, A.; Singh, B.; Kashyap, D.; Kumar, A.; Mahajan, P. Interspecies Communication and Periodontal Disease. Sci. World J. 2013, 2013, e765434. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.K.; Slach, N.A. Impact of Tobacco Use on Periodontal Status. J. Dent. Educ. 2001, 65, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Preshaw, P.M.; Taylor, J.J. How Has Research into Cytokine Interactions and Their Role in Driving Immune Responses Impacted Our Understanding of Periodontitis? J. Clin. Periodontol. 2011, 38 (Suppl. S11), 60–84. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, C.K.; Chatterjee, I.B. Free Metal Ion-Independent Oxidative Damage of Collagen. Protection by Ascorbic Acid. J. Biol. Chem. 1994, 269, 30200–30205. [Google Scholar] [CrossRef] [PubMed]
- Garrett, I.R.; Boyce, B.F.; Oreffo, R.O.; Bonewald, L.; Poser, J.; Mundy, G.R. Oxygen-Derived Free Radicals Stimulate Osteoclastic Bone Resorption in Rodent Bone in Vitro and in Vivo. J. Clin. Investig. 1990, 85, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Bax, B.E.; Alam, A.S.; Banerji, B.; Bax, C.M.; Bevis, P.J.; Stevens, C.R.; Moonga, B.S.; Blake, D.R.; Zaidi, M. Stimulation of Osteoclastic Bone Resorption by Hydrogen Peroxide. Biochem. Biophys. Res. Commun. 1992, 183, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.J.; Schaeublin, M.; Jeker, H.; Fuller, K.; Chambers, T.J. The Role of Reactive Oxygen Intermediates in Osteoclastic Bone Resorption. Biochem. Biophys. Res. Commun. 1995, 207, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Moseley, R.; Waddington, R.J.; Embery, G.; Rees, S.G. The Modification of Alveolar Bone Proteoglycans by Reactive Oxygen Species in Vitro. Connect. Tissue Res. 1998, 37, 13–28. [Google Scholar] [CrossRef]
- Chu, L.; Dong, Z.; Xu, X.; Cochran, D.L.; Ebersole, J.L. Role of Glutathione Metabolism of Treponema Denticola in Bacterial Growth and Virulence Expression. Infect. Immun. 2002, 70, 1113–1120. [Google Scholar] [CrossRef]
- Carlsson, J.; Larsen, J.T.; Edlund, M.B. Peptostreptococcus Micros Has a Uniquely High Capacity to Form Hydrogen Sulfide from Glutathione. Oral Microbiol. Immunol. 1993, 8, 42–45. [Google Scholar] [CrossRef]
- Yaegaki, K.; Qian, W.; Murata, T.; Imai, T.; Sato, T.; Tanaka, T.; Kamoda, T. Oral Malodorous Compound Causes Apoptosis and Genomic DNA Damage in Human Gingival Fibroblasts. J. Periodontal Res. 2008, 43, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Akalin, F.A.; Işiksal, E.; Baltacioğlu, E.; Renda, N.; Karabulut, E. Superoxide Dismutase Activity in Gingiva in Type-2 Diabetes Mellitus Patients with Chronic Periodontitis. Arch. Oral Biol. 2008, 53, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.-Y.; Kim, Y.-G.; Park, J.-W.; Suh, J.-Y.; Lee, J.-M. The Expression of a Nitric Oxide Derivative, Tissue Inhibitors of Metalloproteinase-3, and Tissue Inhibitors of Metalloproteinase-4 in Chronic Periodontitis with Type 2 Diabetes Mellitus. J. Periodontal Implant. Sci. 2013, 43, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Pendyala, G.; Thomas, B.; Joshi, S.R. Evaluation of Total Antioxidant Capacity of Saliva in Type 2 Diabetic Patients with and without Periodontal Disease: A Case-Control Study. N. Am. J. Med. Sci. 2013, 5, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Monea, A.; Mezei, T.; Popsor, S.; Monea, M. Oxidative Stress: A Link between Diabetes Mellitus and Periodontal Disease. Int. J. Endocrinol. 2014, 2014, 917631. [Google Scholar] [CrossRef] [PubMed]
- Arana, C.; Moreno-Fernández, A.M.; Gómez-Moreno, G.; Morales-Portillo, C.; Serrano-Olmedo, I.; de la Cuesta Mayor, M.C.; Martín Hernández, T. Increased Salivary Oxidative Stress Parameters in Patients with Type 2 Diabetes: Relation with Periodontal Disease. Endocrinol. Diabetes Nutr. 2017, 64, 258–264. [Google Scholar] [CrossRef]
- Muthuraj, M.S.A.; Janakiram, S.; Chithresan, K.; Maradi, A.P.; Maddur, P.K.; Rangaraju, R. Effect of Scaling and Root Planing on Levels of 8-Hydroxydeoxyguanosine in Gingival Crevicular Fluid of Chronic Periodontitis Patients with and without Type II Diabetes Mellitus. J. Indian. Soc. Periodontol. 2017, 21, 201–206. [Google Scholar] [CrossRef]
- Koregol, A.C.; Kalburgi, N.B.; Kanniappa Sadasivan, S.; Warad, S.; Kamat Wagh, A.; Thomas, T.; Sinha, P. 8-Isoprostane in Chronic Periodontitis and Type II Diabetes: Exploring the Link. J. Dent. Res. Dent. Clin. Dent. Prospect. 2018, 12, 252–257. [Google Scholar] [CrossRef]
- Vincent, R.R.; Appukuttan, D.; Victor, D.J.; Balasundaram, A. Oxidative Stress in Chronic Periodontitis Patients with Type II Diabetes Mellitus. Eur. J. Dent. 2018, 12, 225–231. [Google Scholar] [CrossRef]
- Shee, F.; Pralhad, S.; Natarajan, S.; Manaktala, N.; Arun, S.; Marathe, A. Cellular and Biochemical Changes in Different Categories of Periodontitis: A Patient-Based Study. J. Int. Soc. Prev. Community Dent. 2020, 10, 341–349. [Google Scholar] [CrossRef]
- Shashikumar, P.; Nisha, S.; Das, D.; Debanth, K.; Kanthal, L.K.; Pattanayak, S. Effect of Morinda citrifolia L. Mouthwash on Periodontal Health in Type 2 Diabetes Mellitus Patients∔A Randomized Controlled Trial. Int. J. Nutr. Pharmacol. Neurol. Dis. 2022, 12, 7–13. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Hirose, H.; Saito, I.; Nishikai, K.; Saruta, T. Adiponectin, an Adipocyte-Derived Protein, Predicts Future Insulin Resistance: Two-Year Follow-up Study in Japanese Population. J. Clin. Endocrinol. Metab. 2004, 89, 87–90. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q. Periodontitis Aggravated Pancreatic β-Cell Dysfunction in Diabetic Mice through Interleukin-12 Regulation on Klotho. J. Diabetes Investig. 2016, 7, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Stanisic, D.; Obradovic, R.; Vujovic, S.; Jovanovic, M.; Zivkovic, V. The Connection of Periodontal Disease and Diabetes Mellitus: The Role of Matrix Metalloproteinases and Oxidative Stress. Exp. Appl. Biomed. Res. 2019; ahead of print. [Google Scholar] [CrossRef]
- Tomofuji, T.; Irie, K.; Sanbe, T.; Azuma, T.; Ekuni, D.; Tamaki, N.; Yamamoto, T.; Morita, M. Periodontitis and Increase in Circulating Oxidative Stress. JPN Dent. Sci. Rev. 2009, 45, 46–51. [Google Scholar] [CrossRef]
- McCord, J.M. Oxygen-Derived Radicals: A Link between Reperfusion Injury and Inflammation. Fed. Proc. 1987, 46, 2402–2406. [Google Scholar]
- Jena, N.R.; Mishra, P.C. Formation of Ring-Opened and Rearranged Products of Guanine: Mechanisms and Biological Significance. Free Radic. Biol. Med. 2012, 53, 81–94. [Google Scholar] [CrossRef]
- Yakes, F.M.; Van Houten, B. Mitochondrial DNA Damage Is More Extensive and Persists Longer than Nuclear DNA Damage in Human Cells Following Oxidative Stress. Proc. Natl. Acad. Sci. USA 1997, 94, 514–519. [Google Scholar] [CrossRef]
- Mustafa, M.F.; Fakurazi, S.; Abdullah, M.A.; Maniam, S. Pathogenic Mitochondria DNA Mutations: Current Detection Tools and Interventions. Genes 2020, 11, 192. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Ye, K.; Picard, M.; Gu, Z. Independent Impacts of Aging on Mitochondrial DNA Quantity and Quality in Humans. BMC Genom. 2017, 18, 890. [Google Scholar] [CrossRef]
- Takane, M.; Sugano, N.; Ezawa, T.; Uchiyama, T.; Ito, K. A Marker of Oxidative Stress in Saliva: Association with Periodontally-Involved Teeth of a Hopeless Prognosis. J. Oral Sci. 2005, 47, 53–57. [Google Scholar] [CrossRef]
- Rai, B.; Kharb, S.; Jain, R.; Anand, S.C. Biomarkers of Periodontitis in Oral Fluids. J. Oral Sci. 2008, 50, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Di Minno, A.; Turnu, L.; Porro, B.; Squellerio, I.; Cavalca, V.; Tremoli, E.; Di Minno, M.N.D. 8-Hydroxy-2-Deoxyguanosine Levels and Cardiovascular Disease: A Systematic Review and Meta-Analysis of the Literature. Antioxid. Redox Signal 2016, 24, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, K.S.; Butuner, B.D.; Roggenbuck, D. Detection of 8-OHdG as a Diagnostic Biomarker. J. Lab. Precis. Med. 2018, 3, 95. [Google Scholar] [CrossRef]
- Dai, L.; Watanabe, M.; Qureshi, A.R.; Mukai, H.; Machowska, A.; Heimbürger, O.; Barany, P.; Lindholm, B.; Stenvinkel, P. Serum 8-Hydroxydeoxyguanosine, a Marker of Oxidative DNA Damage, Is Associated with Mortality Independent of Inflammation in Chronic Kidney Disease. Eur. J. Intern. Med. 2019, 68, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Long, J.D.; Matson, W.R.; Juhl, A.R.; Leavitt, B.R.; Paulsen, J.S.; The PREDICT-HD Investigators and Coordinators of the Huntington Study Group. 8OHdG as a Marker for Huntington Disease Progression. Neurobiol. Dis. 2012, 46, 625. [Google Scholar] [CrossRef]
- Gmitterová, K.; Heinemann, U.; Gawinecka, J.; Varges, D.; Ciesielczyk, B.; Valkovic, P.; Benetin, J.; Zerr, I. 8-OHdG in Cerebrospinal Fluid as a Marker of Oxidative Stress in Various Neurodegenerative Diseases. Neurodegener. Dis. 2009, 6, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Li, X.; Wang, R.; Yu, J.; Ye, M.; Mao, L.; Zhang, S.; Zheng, S. Association between Oxidative DNA Damage and Risk of Colorectal Cancer: Sensitive Determination of Urinary 8-Hydroxy-2′-Deoxyguanosine by UPLC-MS/MS Analysis. Sci. Rep. 2016, 6, 32581. [Google Scholar] [CrossRef] [PubMed]
- Qing, X.; Shi, D.; Lv, X.; Wang, B.; Chen, S.; Shao, Z. Prognostic Significance of 8-Hydroxy-2′-Deoxyguanosine in Solid Tumors: A Meta-Analysis. BMC Cancer 2019, 19, 997. [Google Scholar] [CrossRef]
- Szendroedi, J.; Phielix, E.; Roden, M. The Role of Mitochondria in Insulin Resistance and Type 2 Diabetes Mellitus. Nat. Rev. Endocrinol. 2011, 8, 92–103. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2015; ISBN 978-0-19-180213-3. [Google Scholar]
- Ding, X.-W.; Robinson, M.; Li, R.; Aldhowayan, H.; Geetha, T.; Babu, J.R. Mitochondrial Dysfunction and Beneficial Effects of Mitochondria-Targeted Small Peptide SS-31 in Diabetes Mellitus and Alzheimer’s Disease. Pharmacol. Res. 2021, 171, 105783. [Google Scholar] [CrossRef]
- Leguisamo, N.M.; Lehnen, A.M.; Machado, U.F.; Okamoto, M.M.; Markoski, M.M.; Pinto, G.H.; Schaan, B.D. GLUT4 Content Decreases along with Insulin Resistance and High Levels of Inflammatory Markers in Rats with Metabolic Syndrome. Cardiovasc. Diabetol. 2012, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Kowaltowski, A.J.; de Souza-Pinto, N.C.; Castilho, R.F.; Vercesi, A.E. Mitochondria and Reactive Oxygen Species. Free Radic. Biol. Med. 2009, 47, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, K.-A.; Luchian, I.; Goriuc, A.; Antoci, L.-M.; Ciobanu, C.-G.; Popescu, R.; Vlad, C.-E.; Blaj, M.; Foia, L.G. Mitochondrial Dysfunction, Oxidative Stress, and Therapeutic Strategies in Diabetes, Obesity, and Cardiovascular Disease. Antioxidants 2023, 12, 658. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Kang, Y.J. Oxidative Stress and Diabetic Cardiomyopathy: A Brief Review. Cardiovasc. Toxicol. 2001, 1, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cai, L. Diabetes/Obesity-Related Inflammation, Cardiac Cell Death and Cardiomyopathy. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2006, 31, 814–818. [Google Scholar] [PubMed]
- Wei, W.; Liu, Q.; Tan, Y.; Liu, L.; Li, X.; Cai, L. Oxidative Stress, Diabetes, and Diabetic Complications. Hemoglobin 2009, 33, 370–377. [Google Scholar] [CrossRef]
- Cooke, M.S.; Lunec, J.; Evans, M.D. Progress in the Analysis of Urinary Oxidative DNA Damage. Free Radic. Biol. Med. 2002, 33, 1601–1614. [Google Scholar] [CrossRef]
- Suzuki, S.; Hinokio, Y.; Komatu, K.; Ohtomo, M.; Onoda, M.; Hirai, S.; Hirai, M.; Hirai, A.; Chiba, M.; Kasuga, S.; et al. Oxidative Damage to Mitochondrial DNA and Its Relationship to Diabetic Complications. Diabetes Res. Clin. Pract. 1999, 45, 161–168. [Google Scholar] [CrossRef]
- Negishi, H.; Ikeda, K.; Kuga, S.; Noguchi, T.; Kanda, T.; Njelekela, M.; Liu, L.; Miki, T.; Nara, Y.; Sato, T.; et al. The Relation of Oxidative DNA Damage to Hypertension and Other Cardiovascular Risk Factors in Tanzania. J. Hypertens. 2001, 19, 529–533. [Google Scholar] [CrossRef]
- Leinonen, J.; Lehtimäki, T.; Toyokuni, S.; Okada, K.; Tanaka, T.; Hiai, H.; Ochi, H.; Laippala, P.; Rantalaiho, V.; Wirta, O.; et al. New Biomarker Evidence of Oxidative DNA Damage in Patients with Non-Insulin-Dependent Diabetes Mellitus. FEBS Lett. 1997, 417, 150–152. [Google Scholar] [CrossRef]
- Al-Aubaidy, H.A.; Jelinek, H.F. 8-Hydroxy-2-Deoxy-Guanosine Identifies Oxidative DNA Damage in a Rural Prediabetes Cohort. Redox Rep. 2010, 15, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Dinçer, Y.; Akçay, T.; Alademir, Z.; Ilkova, H. Assessment of DNA Base Oxidation and Glutathione Level in Patients with Type 2 Diabetes. Mutat. Res. 2002, 505, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.W.; Yao, Q.H.; Weng, Q.F.; Su, B.L.; Zhang, X.; Xiong, J.H. Study of Urinary 8-Hydroxydeoxyguanosine as a Biomarker of Oxidative DNA Damage in Diabetic Nephropathy Patients. J. Pharm. Biomed. Anal. 2004, 36, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Sugano, N.; Yokoyama, K.; Oshikawa, M.; Kumagai, K.; Takane, M.; Tanaka, H.; Ito, K. Detection of Streptococcus Anginosus and 8-Hydroxydeoxyguanosine in Saliva. J. Oral Sci. 2003, 45, 181–184. [Google Scholar] [CrossRef][Green Version]
- Sezer, U.; Ciçek, Y.; Canakçi, C.F. Increased Salivary Levels of 8-Hydroxydeoxyguanosine May Be a Marker for Disease Activity for Periodontitis. Dis. Markers 2012, 32, 165–172. [Google Scholar] [CrossRef]
- Sawamoto, Y.; Sugano, N.; Tanaka, H.; Ito, K. Detection of Periodontopathic Bacteria and an Oxidative Stress Marker in Saliva from Periodontitis Patients. Oral Microbiol. Immunol. 2005, 20, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, C.; Pan, Y. The Influences of Periodontal Status and Periodontal Pathogen Quantity on Salivary 8-Hydroxydeoxyguanosine and Interleukin-17 Levels. J. Periodontol. 2016, 87, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Dede, F.Ö.; Ozden, F.O.; Avcı, B. 8-Hydroxy-Deoxyguanosine Levels in Gingival Crevicular Fluid and Saliva in Patients with Chronic Periodontitis after Initial Periodontal Treatment. J. Periodontol. 2013, 84, 821–828. [Google Scholar] [CrossRef]
- Hendek, M.K.; Erdemir, E.O.; Kisa, U.; Ozcan, G. Effect of Initial Periodontal Therapy on Oxidative Stress Markers in Gingival Crevicular Fluid, Saliva, and Serum in Smokers and Non-Smokers with Chronic Periodontitis. J. Periodontol. 2015, 86, 273–282. [Google Scholar] [CrossRef]
- Konopka, T.; Król, K.; Kopeć, W.; Gerber, H. Total Antioxidant Status and 8-Hydroxy-2′-Deoxyguanosine Levels in Gingival and Peripheral Blood of Periodontitis Patients. Arch. Immunol. Ther. Exp. 2007, 55, 417–422. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kim, K.-R.; Kim, H.-N. The Potential Impact of Salivary IL-1 on the Diagnosis of Periodontal Disease: A Pilot Study. Healthcare 2021, 9, 729. [Google Scholar] [CrossRef] [PubMed]
- Grigorian, M.; Caraiane, A.; Nucă, C.; Marius, R.; Petcu, A.; Balaban, D.P.; Badea, V. Oxidative Stress Marker in the Aggressive Periodontal Disease—Salivary 8-Hidroxydeoxyguanosine. Rom. J. Oral Rehabil. 2015, 7, 33–37. [Google Scholar]
- Zamora-Perez, A.L.; Ortiz-García, Y.M.; Lazalde-Ramos, B.P.; Guerrero-Velázquez, C.; Gómez-Meda, B.C.; Ramírez-Aguilar, M.Á.; Zúñiga-González, G.M. Increased Micronuclei and Nuclear Abnormalities in Buccal Mucosa and Oxidative Damage in Saliva from Patients with Chronic and Aggressive Periodontal Diseases. J. Periodontal Res. 2015, 50, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Popa, C.G.; Luchian, I.; Ioanid, N.; Goriuc, A.; Martu, I.; Bosinceanu, D.; Martu, M.A.; Tirca, T.; Martu, S. ELISA Evaluation of RANKL Levels in Gingival Fluid in Patients with Periodontitis and Occlusal Trauma. Rev. Chim. 2018, 69, 1578–1580. [Google Scholar] [CrossRef]
- Spiridon, I.; Darie-Nita, R.; Kozlowski, M.; Nechita, A.; Ursu, R. Influence of Accelerated Weathering on the Performance of Polylactic Acid Based Materials. Cellul. Chem. Technol. 2016, 50, 5–6. [Google Scholar]
- Önder, C.; Kurgan, Ş.; Altıngöz, S.M.; Bağış, N.; Uyanık, M.; Serdar, M.A.; Kantarcı, A.; Günhan, M. Impact of Non-Surgical Periodontal Therapy on Saliva and Serum Levels of Markers of Oxidative Stress. Clin. Oral Investig. 2017, 21, 1961–1969. [Google Scholar] [CrossRef]
- Kurgan, Ş.; Önder, C.; Altıngöz, S.M.; Bağış, N.; Uyanık, M.; Serdar, M.A.; Kantarcı, A. High Sensitivity Detection of Salivary 8-Hydroxy Deoxyguanosine Levels in Patients with Chronic Periodontitis. J. Periodontal Res. 2015, 50, 766–774. [Google Scholar] [CrossRef]
- Kemer Doğan, E.S.; Kırzıoğlu, F.Y.; Doğan, B.; Fentoğlu, Ö.; Kale, B. The Effect of Menopause on the Relationship between Hyperlipidemia and Periodontal Disease via Salivary 8-Hydroxy-2′-Deoxyguanosine and Myeloperoxidase Levels. Acta Odontol. Scand. 2018, 76, 92–97. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Ngo, L.Q.; Promsudthi, A.; Surarit, R. Salivary Oxidative Stress Biomarkers in Chronic Periodontitis and Acute Coronary Syndrome. Clin. Oral Investig. 2017, 21, 2345–2353. [Google Scholar] [CrossRef]
- Shin, M.-S.; Shin, H.-S.; Ahn, Y.-B.; Kim, H.-D. Association between Periodontitis and Salivary 8-Hydroxydeoxyguanosine among Korean Rural Adults. Community Dent. Oral Epidemiol. 2016, 44, 381–389. [Google Scholar] [CrossRef]
- Paredes-Sánchez, E.; Montiel-Company, J.M.; Iranzo-Cortés, J.E.; Almerich-Torres, T.; Bellot-Arcís, C.; Almerich-Silla, J.M. Meta-Analysis of the Use of 8-OHdG in Saliva as a Marker of Periodontal Disease. Dis. Markers 2018, 2018, 7916578. [Google Scholar] [CrossRef] [PubMed]
- Altıngöz, S.M.; Kurgan, Ş.; Önder, C.; Serdar, M.A.; Ünlütürk, U.; Uyanık, M.; Başkal, N.; Tatakis, D.N.; Günhan, M. Salivary and Serum Oxidative Stress Biomarkers and Advanced Glycation End Products in Periodontitis Patients with or without Diabetes: A Cross-Sectional Study. J. Periodontol. 2021, 92, 1274–1285. [Google Scholar] [CrossRef] [PubMed]
- Villa-Correa, Y.A.; Isaza-Guzmán, D.M.; Tobón-Arroyave, S.I. Prognostic Value of 8-Hydroxy-2′-Deoxyguanosine and Human Neutrophil Elastase/A1-Proteinase Inhibitor Complex as Salivary Biomarkers of Oxidative Stress in Chronic Periodontitis. J. Periodontol. 2015, 86, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Almerich-Silla, J.M.; Montiel-Company, J.M.; Pastor, S.; Serrano, F.; Puig-Silla, M.; Dasí, F. Oxidative Stress Parameters in Saliva and Its Association with Periodontal Disease and Types of Bacteria. Dis. Markers 2015, 2015, 653537. [Google Scholar] [CrossRef]


| Author, Year | Design of the Study | Oral Oxidative Stress Biomarker | Results | Conclusion |
|---|---|---|---|---|
| Akalın et al., 2008 [42] | Cross-sectional | Gingival: Superoxide dismutase | HbA1c, glucose, and triglyceride levels were higher in diabetic groups; There were correlations between periodontal parameters and superoxide dismutase values. | Superoxide dismutase values increased in diabetic patients. |
| Jung et al., 2013 [43] | Cross-sectional | Gingival: inducible nitric oxide synthase | Inducible nitric oxide synthases and tissue inhibitors of metalloproteinase were significantly higher in diabetic and periodontitis patients as compared to healthy group. | Inducible nitric oxide synthase values increased in diabetes and chronic periodontitis group. |
| Pendyala et al., 2013 [44] | Cross-sectional | Saliva: Total antioxidant capacity | Total antioxidant capacity is proportional to the inflammation. | Total antioxidant was lower in diabetic patients with periodontal disease. |
| Monea et al., 2014 [45] | Cross-sectional | Biopsy specimen (dental-periodontal unit in the posterior region of dental arches): Malondialdehyde Glutathione | Histological alterations in diabetic patients were present in gingival mucosa (epithelium and lamina propria). | Malondialdehyde levels was higher in diabetic tissues. Glutathione values was significantly lower in diabetic tissues. |
| Arana et al., 2017 [46] | Cross-sectional | Saliva: glutathione peroxidase, glutathione reductase, reduced glutathione, oxidized glutathione. | The diabetic patients with good metabolic control showed a significant increase in glutathione peroxidase and glutathione reductase activity. | Elevated levels of oxidative stress in the saliva of individuals with diabetes are correlated with poorer metabolic control, and a deteriorated state of periodontal health. |
| Muthuraj et al., 2017 [47] | Prospective study | Gingival crevicular fluid: 8-OHdG | Levels of 8-OHdG and HbA1c in periodontal patients with diabetes showed a greater reduction after scaling and root planing. | 8-OHdG values increased in diabetic and periodontitis patients. |
| Koregol et al., 2018 [48] | Cross-sectional | Saliva: 8-isoprostane | A statistically significant difference was observed in the concentrations of 8-isoprostane among the various groups. | 8-isoprostane values decreased in diabetic and periodontitis group. |
| Vincent et al., 2018 [49] | Cross-sectional | Gingival crevicular fluid: Total antioxidant capacity | The clinical parameters (plaque index, gingival index, probing pocket depth) showed the statistically significant difference between the groups. | Total antioxidant capacity was higher in healthy group. |
| Shee et al., 2020 [50] | Cross-sectional | Saliva: Malondialdehyde | Malondialdehyde levels showed an exponential rise with the inclusion of diabetes and TBSCH, while no such increase was noted in relation to periodontitis. | Malondialdehyde values increased in diabetic and periodontitis group. |
| Shashikumar et al., 2022 [51] | Prospective study | Saliva: Total antioxidant capacity | Total antioxidant level was lower in diabetic and periodontitis group after 3 months therapy with Morinda citrifolia L. mouthwash. | Total antioxidant level was lower in diabetic and periodontitis group. |
| Author, Year | Patients/Method of 8-OHdG Detection | Results | Clinical Importance |
|---|---|---|---|
| Altıngöz SM et al., 2021 [104] | periodontitis patients +/− diabetes 8-OHdG ELISA | the combination of 8-OHdG for diabetes patients yielded highest AUCs | salivary 8-OHdG levels were significantly higher in periodontitis compared to controls |
| Kemer Doğan ES et al., 2017 [100] | menopausal groups and premenopausal 8-OHdG ELISA | 8-OHdG levels were higher in menopausal groups than in premenopausal ones | salivary 8-OHdG levels may be used as an indicator of the relationship between periodontal disease |
| Nguyen TT et al., 2017 [101] | patients with acute coronary syndrome (ACS) 8-OHdG ELISA | salivary levels of 8-OHdG, were significantly higher in the chronic periodontitis ACS, ACS, and CP | salivary 8-OHdG had a strong potential to be utilized in chronic periodontitis investigation |
| Önder C et al., 2017 [98] | healthy individuals and diagnosed with CP. saliva and serum 8-OHdG ELISA | 8-OHdG levels in the saliva were significantly higher in the CP group in comparison with the control group | salivary 8-OHdG levels may be used as indicators for severity of periodontal disease |
| Shin MS et al., 2016 [102] | residents participated in both dental and medical examinations 8-OHdG ELISA | TZhe association of salivary 8-OHdG with severe periodontitis increased from OR of 2.40 to OR of 4.10 for drinking and 3.14 for smoking | the association between salivary level of 8-OHdG and severe periodontitis was significant salivary 8-OHdG could be a useful marker for severe periodontitis. |
| Kurgan Ş et al., 2015 [99] | healthy patients with CP and healthy individuals salivary 8-OHdG ELISA liquid chromatography with tandem mass spectrometry analysis 8-OHdG | levels of 8-OHdG were significantly higher in the chronic periodontitis group compared to the control group pretreatment values of LC-MS/MS 8-OHdG also showed a positive correlation with 8-OHdG measured by ELISA | salivary levels of 8-OHdG measured by LC-MS/MS and ELISA were significantly higher in the chronic periodontitis group compared to the control group. 8-OHdG correlates well with the clinical parameters of periodontal disease. LC-MS/MS could be used to determine the lower concentrations of 8-OHdG that are not in the detection range of the ELISA. |
| Villa-Correa YA et al., 2015 [105] | patients with untreated chronic periodontitis and healthy controls salivary levels of 8-OHdG ELISA | 8-OHdG salivary levels were significantly increased in the CP group in comparison to healthy group | increased salivary levels of 8-OHdG may be strong/independent prognostic indicators of the amount and extent of oxidative stress-induced periodontal breakdown |
| Almerich-Silla JM et al., 2015 [106] | individuals with periodontal problems 8-OHdG ELISA porphyromona gingivalis, aggregatibacter actinomycetemcomitans, treponema denticola, and tannerella forsythia was detected by PCR | oxidative stress levels were significantly higher in the periodontal disease group | the 8-OHdG levels were increased in the presence of all bacterial types. determination of these levels and periodontal bacteria could be a potent tool for controlling periodontal disease development. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goriuc, A.; Cojocaru, K.-A.; Luchian, I.; Ursu, R.-G.; Butnaru, O.; Foia, L. Using 8-Hydroxy-2′-Deoxiguanosine (8-OHdG) as a Reliable Biomarker for Assessing Periodontal Disease Associated with Diabetes. Int. J. Mol. Sci. 2024, 25, 1425. https://doi.org/10.3390/ijms25031425
Goriuc A, Cojocaru K-A, Luchian I, Ursu R-G, Butnaru O, Foia L. Using 8-Hydroxy-2′-Deoxiguanosine (8-OHdG) as a Reliable Biomarker for Assessing Periodontal Disease Associated with Diabetes. International Journal of Molecular Sciences. 2024; 25(3):1425. https://doi.org/10.3390/ijms25031425
Chicago/Turabian StyleGoriuc, Ancuta, Karina-Alexandra Cojocaru, Ionut Luchian, Ramona-Garbriela Ursu, Oana Butnaru, and Liliana Foia. 2024. "Using 8-Hydroxy-2′-Deoxiguanosine (8-OHdG) as a Reliable Biomarker for Assessing Periodontal Disease Associated with Diabetes" International Journal of Molecular Sciences 25, no. 3: 1425. https://doi.org/10.3390/ijms25031425
APA StyleGoriuc, A., Cojocaru, K.-A., Luchian, I., Ursu, R.-G., Butnaru, O., & Foia, L. (2024). Using 8-Hydroxy-2′-Deoxiguanosine (8-OHdG) as a Reliable Biomarker for Assessing Periodontal Disease Associated with Diabetes. International Journal of Molecular Sciences, 25(3), 1425. https://doi.org/10.3390/ijms25031425











