The Gárdos Channel and Piezo1 Revisited: Comparison between Reticulocytes and Mature Red Blood Cells
Abstract
1. Introduction
2. Results
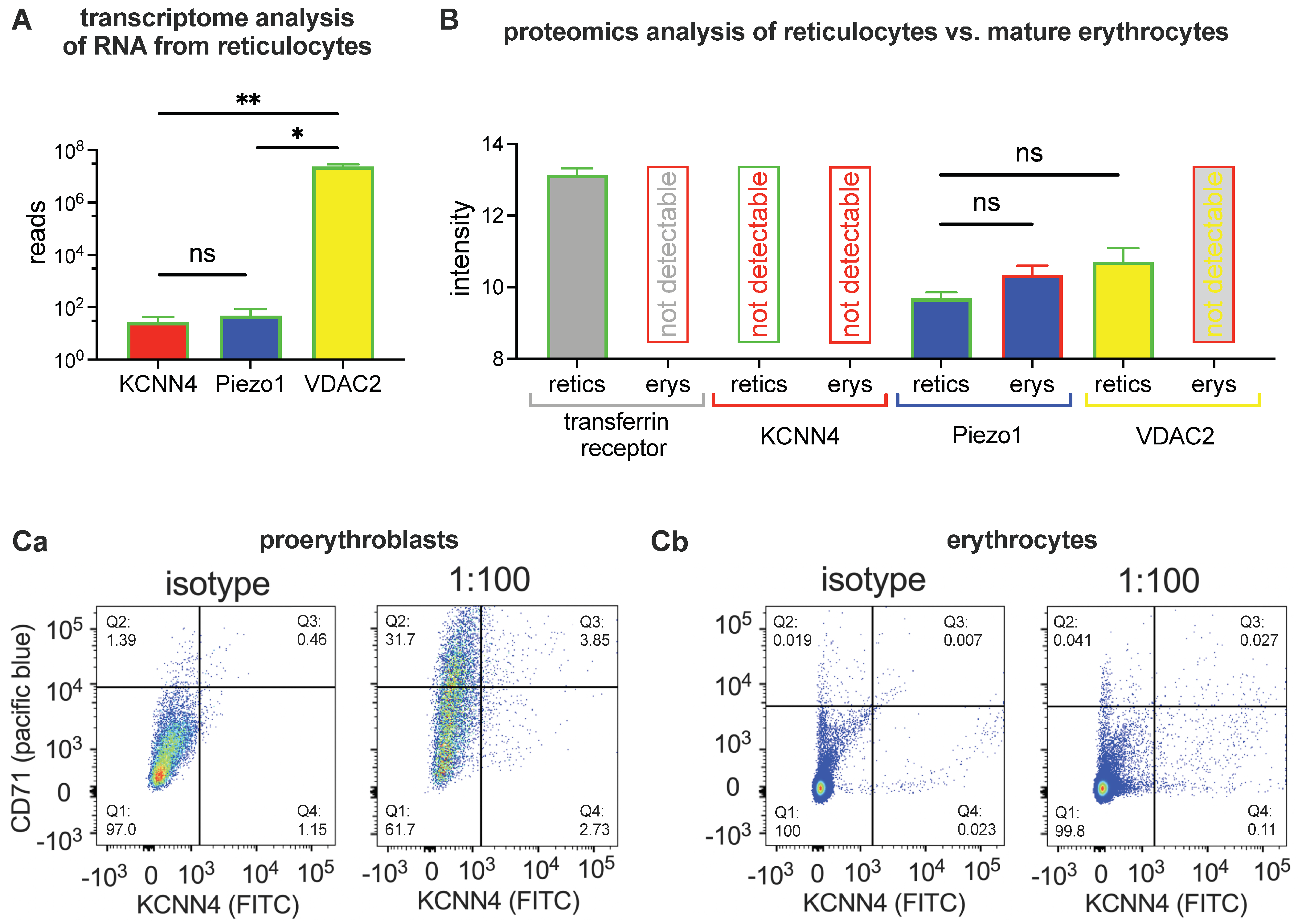
2.1. Biochemical Analysis
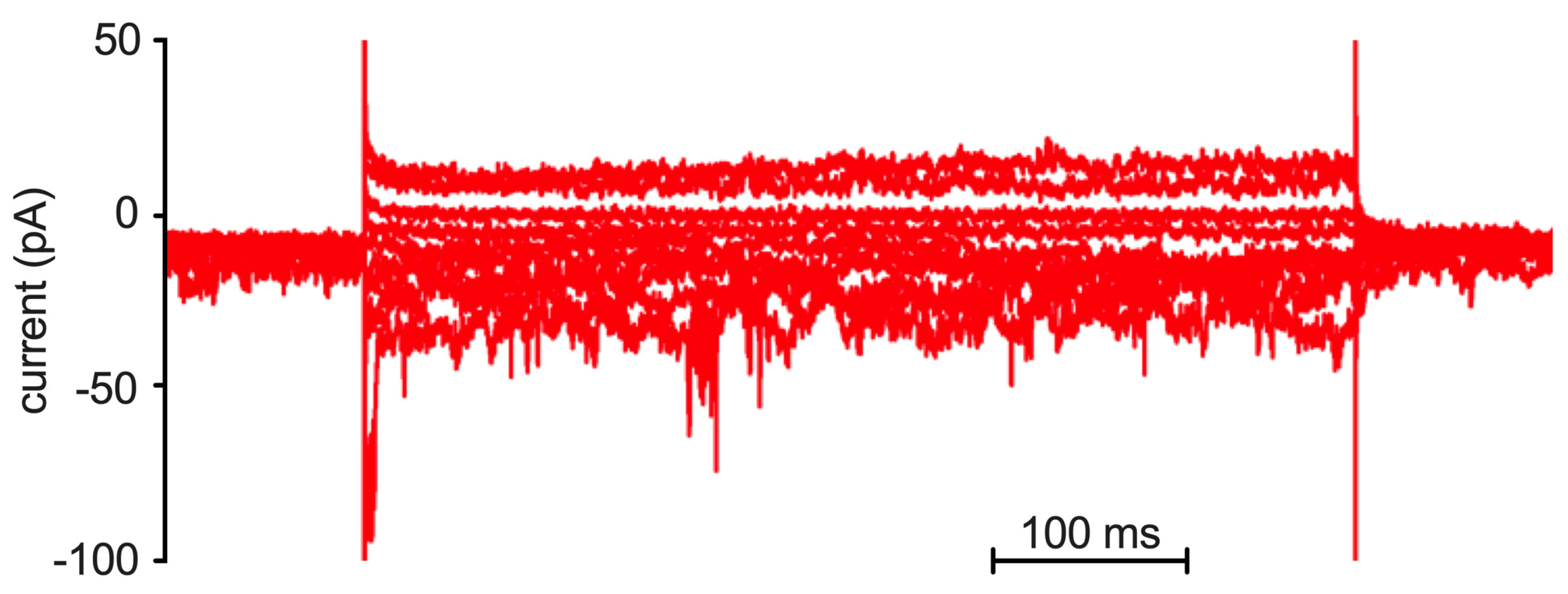
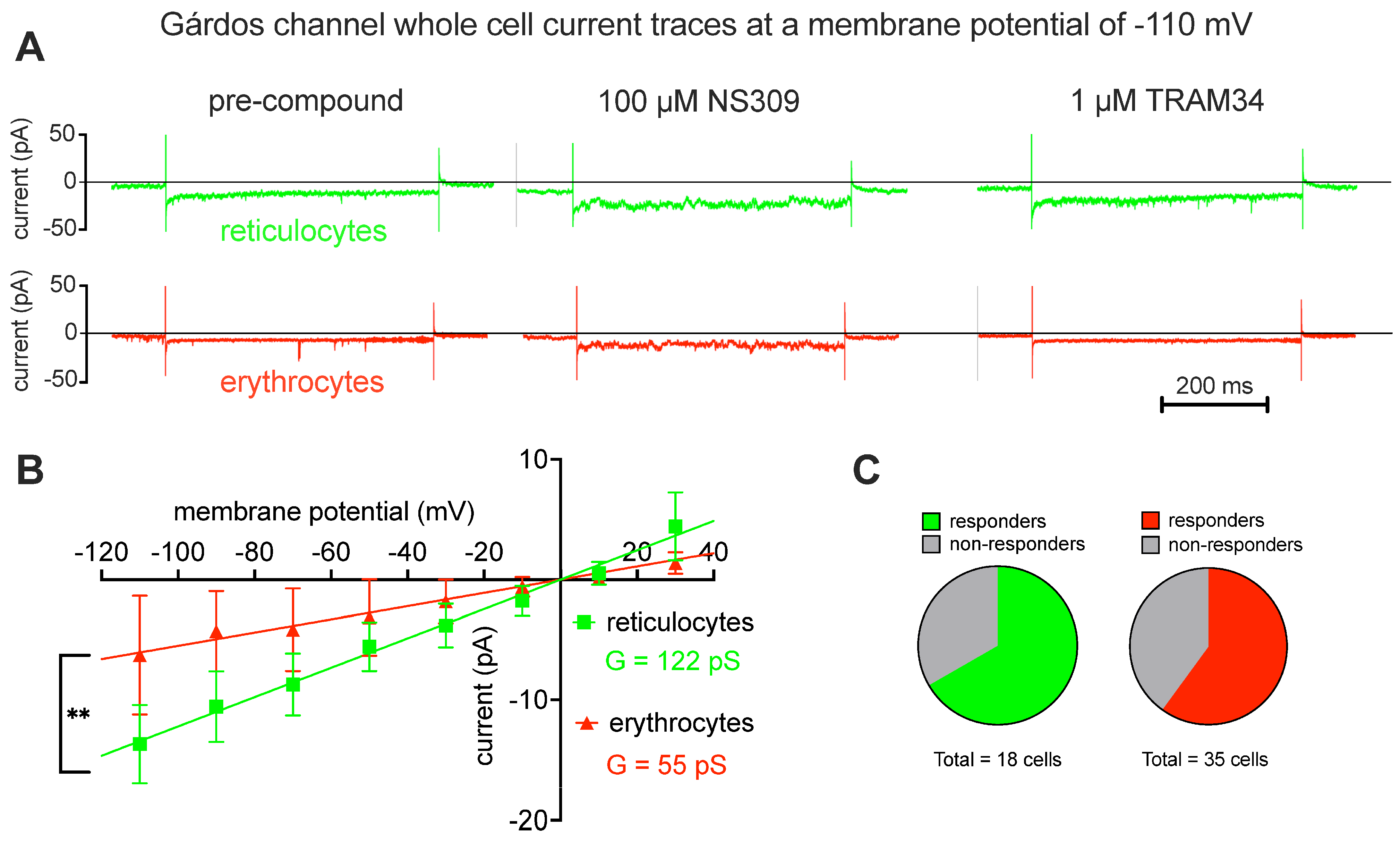
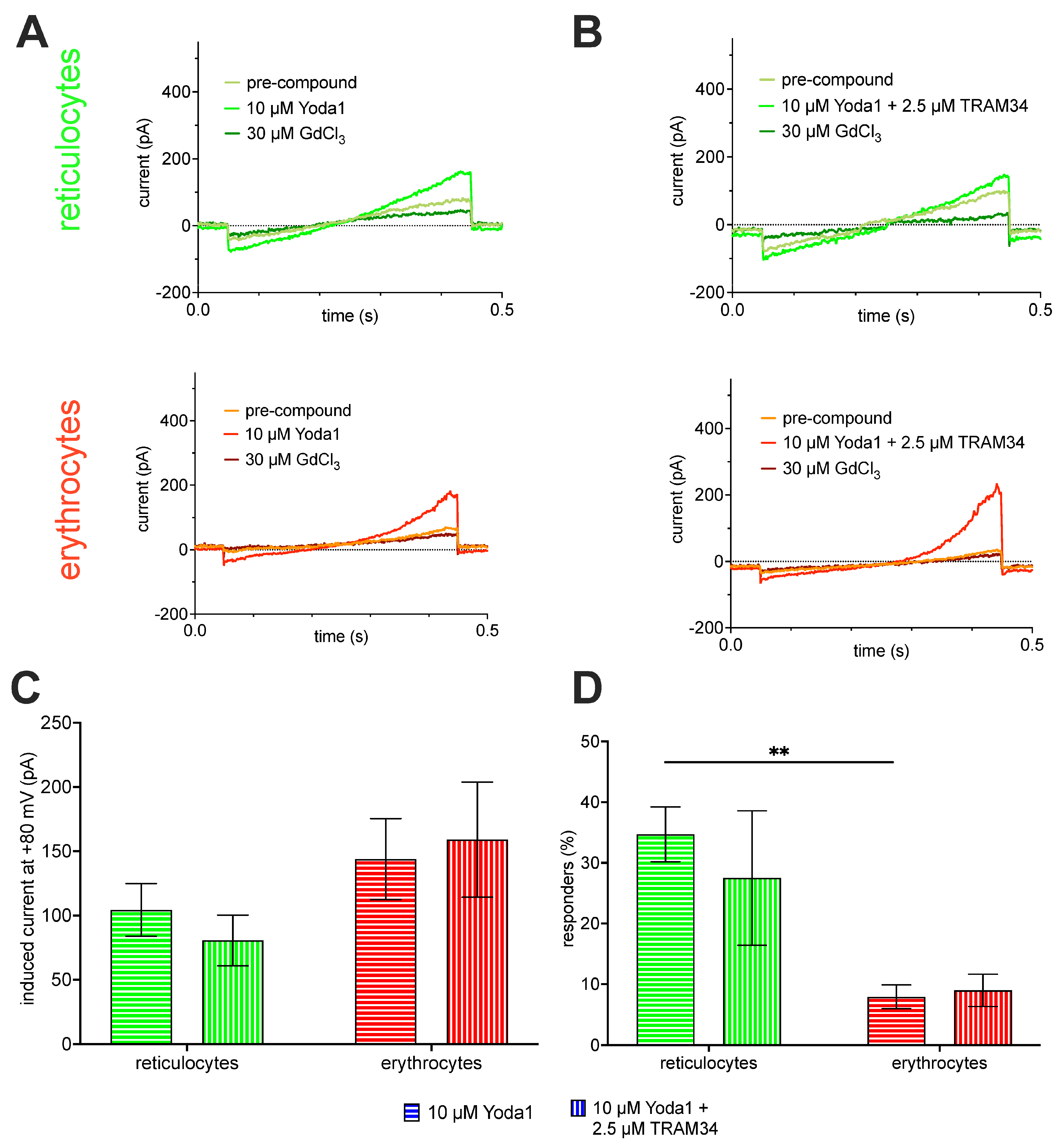
2.2. Patch Clamp Analysis
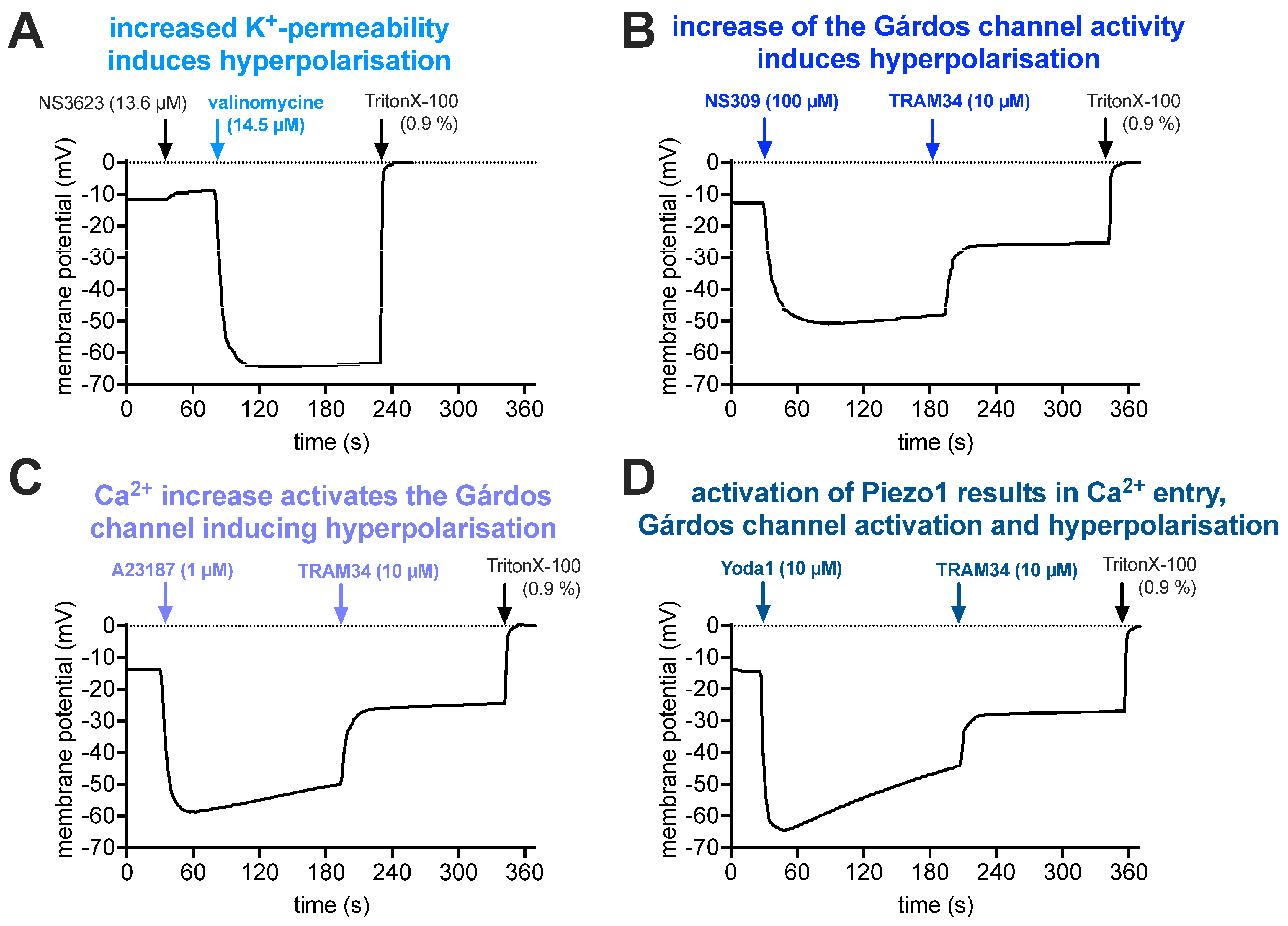
2.3. Membrane Potential Measurements
3. Discussion
3.1. The Copy Number of Ion Channels in Red Blood Cells—Context and Consequences
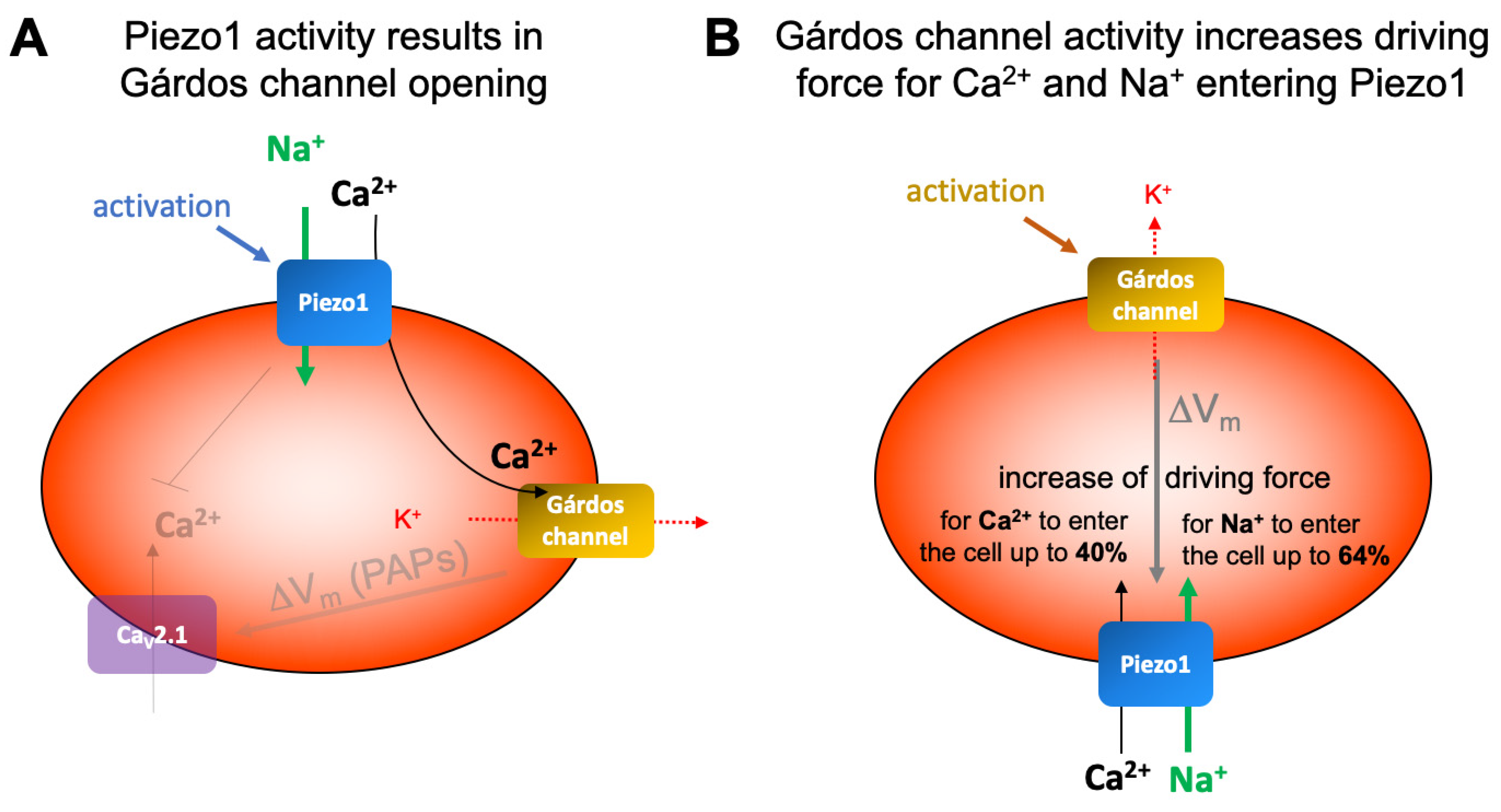
3.2. Ion Channel Interactions in Red Blood Cells
3.3. Ion Channel Differences between Reticulocytes and Mature Red Blood Cells
3.4. Physiological Function of Ion Channels in Red Blood Cells
4. Materials and Methods
4.1. Blood Collection
4.2. Transcriptome Analysis
4.3. Proteomic Analysis
4.4. PBMC Isolation and Culture
4.5. Flow Cytometry
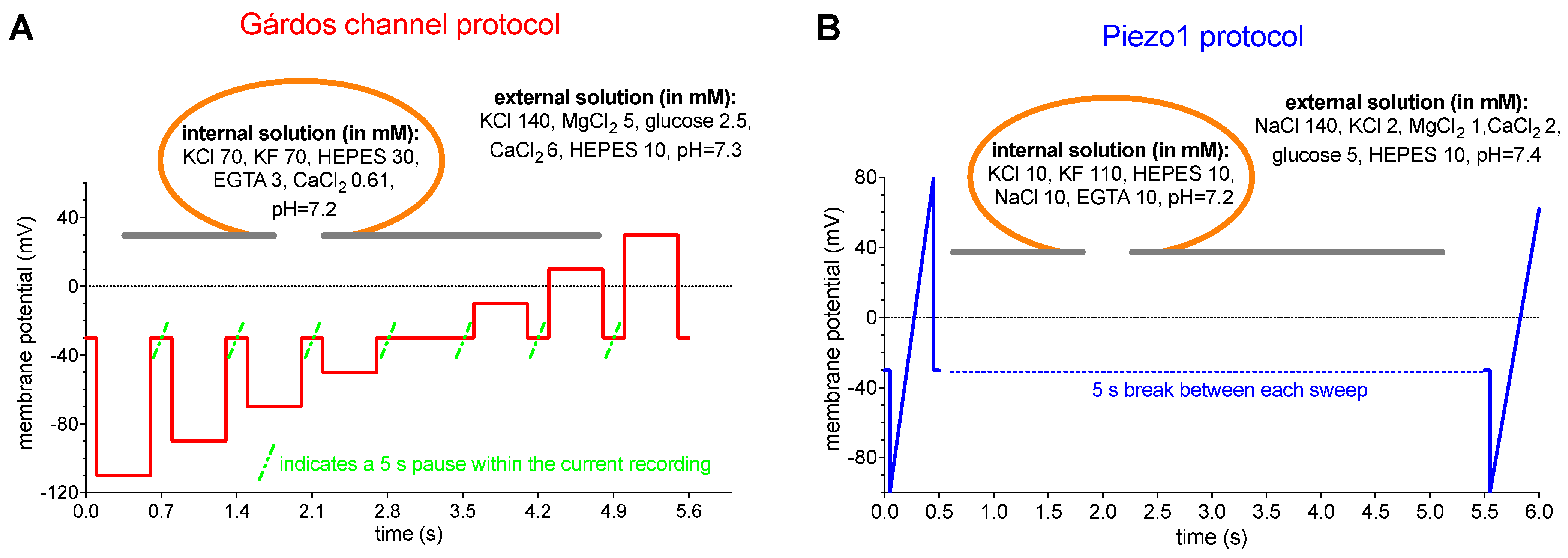
4.6. Patch Clamp Measurements
4.7. The Macey–Bennekou–Egée (MBE) Method
5. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Thomas, S.L.Y.; Bouyer, G.; Cueff, A.; Egée, S.; Glogowska, E.; Ollivaux, C. Ion Channels in Human Red Blood Cell Membrane: Actors or Relics? Blood Cells Mol. Dis. 2011, 46, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Kaestner, L.; Bernhardt, I. Ion Channels in the Human Red Blood Cell Membrane: Their Further Investigation and Physiological Relevance. Bioelectrochemistry 2002, 55, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Kaestner, L. Channelizing the Red Blood Cell: Molecular Biology Competes with Patch-Clamp. Front. Mol. Biosci. 2015, 2, 46. [Google Scholar] [CrossRef] [PubMed]
- Kaestner, L. Cation Channels in Erythrocytes—Historical and Future Perspective. Open Biol. J. 2011, 4, 27–34. [Google Scholar] [CrossRef]
- Petkova-Kirova, P.; Hertz, L.; Makhro, A.; Danielczok, J.; Huisjes, R.; Llaudet-Planas, E.; Mañú-Pereira, M.d.M.; Corrons, J.-L.V.; van Wijk, R.; Bogdanova, A.; et al. A Previously Unrecognized Ca2+-Inhibited Nonselective Cation Channel in Red Blood Cells. Hemasphere 2018, 2, e146. [Google Scholar] [CrossRef]
- Hertz, L.; Flormann, D.; Birnbaumer, L.; Wagner, C.; Laschke, M.W.; Kaestner, L. Evidence of in Vivo Exogen Protein Uptake by Red Blood Cells: A Putative Therapeutic Concept. Blood Adv. 2023, 7, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Andrews, D.A.; Low, P.S. Role of Red Blood Cells in Thrombosis. Curr. Opin. Hematol. 1999, 6, 76–82. [Google Scholar] [CrossRef]
- Bernhardt, I.; Wesseling, M.C.; Nguyen, D.B.; Kaestner, L. Red Blood Cells Actively Contribute to Blood Coagulation and Thrombus Formation. In Erythrocyte; Tombak, A., Ed.; IntechOpen: London, UK, 2019; ISBN 9781789842098. [Google Scholar]
- Hertz, L.; Huisjes, R.; Llaudet-Planas, E.; Petkova-Kirova, P.; Makhro, A.; Danielczok, J.G.; Egée, S.; Mañú-Pereira, M.D.M.; van Wijk, R.; Vives-Corrons, J.-L.; et al. Is Increased Intracellular Calcium in Red Blood Cells a Common Component in the Molecular Mechanism Causing Anemia? Front. Physiol. 2017, 8, 673. [Google Scholar] [CrossRef]
- Badens, C.; Guizouarn, H. Advances in Understanding the Pathogenesis of the Red Cell Volume Disorders. Br. J. Haematol. 2016, 174, 674–685. [Google Scholar] [CrossRef]
- Jansen, J.; Qiao, M.; Hertz, L.; Wang, X.; Fermo, E.; Zaninoni, A.; Colombatti, R.; Bernhardt, I.; Bianchi, P.; Kaestner, L. Mechanistic Ion Channel Interactions in Red Cells of Patients with Gárdos Channelopathy. Blood Adv. 2021, 5, 3303–3308. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Allegrini, B.; Rapetti-Mauss, R.; Picard, V.; Garçon, L.; Kohl, P.; Soriani, O.; Peyronnet, R.; Guizouarn, H. Hereditary Xerocytosis: Differential Behavior of PIEZO1 Mutations in the N-Terminal Extracellular Domain Between Red Blood Cells and HEK Cells. Front. Physiol. 2021, 12, 736585. [Google Scholar] [CrossRef] [PubMed]
- Hamill, O.P. Potassium Channel Currents in Human Red Blood Cells. J. Physiol. 1981, 319, 97P–98P. [Google Scholar]
- Hamill, O.P. Potassium and Chloride Channels in Red Blood Cells. In Single Channel Recording; Sakmann, B., Neher, E., Eds.; Plenum Press: New York, NY, USA, 1983; pp. 451–471. [Google Scholar]
- Gardos, G. The Permeability of Human Erythrocytes to Potassium. Acta Physiol. Hung. 1956, 10, 185–189. [Google Scholar] [PubMed]
- Gardos, G. The Function of Calcium in the Potassium Permeability of Human Erythrocytes. Biochim. Biophys. Acta 1958, 30, 653–654. [Google Scholar] [CrossRef] [PubMed]
- Dunn, P.M. The Action of Blocking Agents Applied to the Inner Face of Ca(2+)-Activated K+ Channels from Human Erythrocytes. J. Membr. Biol. 1998, 165, 133–143. [Google Scholar] [CrossRef]
- Grygorczyk, R.; Schwarz, W.; Passow, H. Ca2+-Activated K+ Channels in Human Red Cells. Comparison of Single-Channel Currents with Ion Fluxes. Biophys. J. 1984, 45, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Grygorczyk, R.; Schwarz, W. Ca2+-Activated K+ Permeability in Human Erythrocytes: Modulation of Single-Channel Events. Eur. Biophys. J. 1985, 12, 57–65. [Google Scholar] [CrossRef]
- Grygorczyk, R.; Schwarz, W. Properties of the Ca2+-Activated K+ Conductance of Human Red Cells as Revealed by the Patch-Clamp Technique. Cell Calcium 1983, 4, 499–510. [Google Scholar] [CrossRef]
- Schwarz, W.; Grygorczyk, R.; Hof, D. Recording Single-Channel Currents from Human Red Cells. Methods Enzym. 1989, 173, 112–121. [Google Scholar]
- Grygorczyk, R. Temperature Dependence of Ca2+-Activated K+ Currents in the Membrane of Human Erythrocytes. Biochim. Biophys. Acta 1987, 902, 159–168. [Google Scholar] [CrossRef]
- Leinders, T.; van Kleef, R.G.; Vijverberg, H.P. Distinct Metal Ion Binding Sites on Ca(2+)-Activated K+ Channels in inside-out Patches of Human Erythrocytes. Biochim. Biophys. Acta 1992, 1112, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Leinders, T.; Kleef, R.G.D.M.; Vijverberg, H.P.M. Single Ca2+-Activated K+ Channels in Human Erythrocytes: Ca2+ Dependence of Opening Frequency but Not of Open Lifetimes. Biochim. Biophys. Acta BBA—Biomembr. 1992, 1112, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.F.; Joiner, W.; Nehrke, K.; Potapova, O.; Foye, K.; Wickrema, A. The HSK4 (KCNN4) Isoform Is the Ca2+-Activated K+ Channel (Gardos Channel) in Human Red Blood Cells. Proc. Natl. Acad. Sci. USA 2003, 100, 7366–7371. [Google Scholar] [CrossRef] [PubMed]
- Von Lindern, M.; Egée, S.; Bianchi, P.; Kaestner, L. The Function of Ion Channels and Membrane Potential in Red Blood Cells: Toward a Systematic Analysis of the Erythroid Channelome. Front. Physiol. 2022, 13, 824478. [Google Scholar] [CrossRef] [PubMed]
- Rapetti-Mauss, R.; Lacoste, C.; Picard, V.; Guitton, C.; Lombard, E.; Loosveld, M.; Nivaggioni, V.; Dasilva, N.; Salgado, D.; Desvignes, J.-P.; et al. A Mutation in the Gardos Channel Is Associated with Hereditary Xerocytosis. Blood 2015, 126, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Rapetti-Mauss, R.; Picard, V.; Guitton, C.; Ghazal, K.; Proulle, V.; Badens, C.; Soriani, O.; Garçon, L.; Guizouarn, H. Red Blood Cell Gardos Channel (KCNN4): The Essential Determinant of Erythrocyte Dehydration in Hereditary Xerocytosis. Haematologica 2017, 102, e415–e418. [Google Scholar] [CrossRef]
- Rapetti-Mauss, R.; Soriani, O.; Vinti, H.; Badens, C.; Guizouarn, H. Senicapoc: A Potent Candidate for the Treatment of a Subset of Hereditary Xerocytosis Caused by Mutations in the Gardos Channel. Haematologica 2016, 101, e431. [Google Scholar] [CrossRef] [PubMed]
- Fermo, E.; Bogdanova, A.; Petkova-Kirova, P.; Zaninoni, A.; Marcello, A.P.; Makhro, A.; Hanggi, P.; Hertz, L.; Danielczok, J.; Vercellati, C.; et al. “Gardos Channelopathy”: A Variant of Hereditary Stomatocytosis with Complex Molecular Regulation. Sci. Rep. 2017, 7, 1744. [Google Scholar] [CrossRef] [PubMed]
- Fermo, E.; Monedero-Alonso, D.; Petkova-Kirova, P.; Makhro, A.; Pérès, L.; Bouyer, G.; Marcello, A.P.; Longo, F.; Graziadei, G.; Barcellini, W.; et al. Gardos Channelopathy: Functional Analysis of a Novel KCNN4 Variant. Blood Adv. 2020, 4, 6336–6341. [Google Scholar] [CrossRef]
- Glogowska, E.; Lezon-Geyda, K.; Maksimova, Y.; Schulz, V.P.; Gallagher, P.G. Mutations in the Gardos Channel (KCNN4) Are Associated with Hereditary Xerocytosis. Blood 2015, 126, 1281–1284. [Google Scholar] [CrossRef]
- Andolfo, I.; Russo, R.; Manna, F.; Shmukler, B.E.; Gambale, A.; Vitiello, G.; Rosa, G.D.; Brugnara, C.; Alper, S.L.; Snyder, L.M.; et al. Novel Gardos Channel Mutations Linked to Dehydrated Hereditary Stomatocytosis (Xerocytosis). Am. J. Hematol. 2015, 90, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Wolff, D.; Cecchi, X.; Spalvins, A.; Canessa, M. Charybdotoxin Blocks with High Affinity the Ca-Activated K+ Channel of Hb A and Hb S Red Cells: Individual Differences in the Number of Channels. J. Membr. Biol. 1988, 106, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 Are Essential Components of Distinct Mechanically Activated Cation Channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Coste, B.; Xiao, B.; Santos, J.S.; Syeda, R.; Grandl, J.; Spencer, K.S.; Kim, S.E.; Schmidt, M.; Mathur, J.; Dubin, A.E.; et al. Piezo Proteins Are Pore-Forming Subunits of Mechanically Activated Channels. Nature 2012, 483, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Albuisson, J.; Murthy, S.E.; Bandell, M.; Coste, B.; Louis-dit-Picard, H.; Mathur, J.; Fénéant-Thibault, M.; Tertian, G.; de Jaureguiberry, J.-P.; Syfuss, P.-Y.; et al. Dehydrated Hereditary Stomatocytosis Linked to Gain-of-Function Mutations in Mechanically Activated PIEZO1 Ion Channels. Nat. Commun. 2013, 4, 1884. [Google Scholar] [CrossRef] [PubMed]
- Zarychanski, R.; Schulz, V.P.; Houston, B.L.; Maksimova, Y.; Houston, D.S.; Smith, B.; Rinehart, J.; Gallagher, P.G. Mutations in the Mechanotransduction Protein PIEZO1 Are Associated with Hereditary Xerocytosis. Blood 2012, 120, 1908–1915. [Google Scholar] [CrossRef]
- Bae, C.; Gnanasambandam, R.; Nicolai, C.; Sachs, F.; Gottlieb, P.A. Xerocytosis Is Caused by Mutations That Alter the Kinetics of the Mechanosensitive Channel PIEZO1. Proc. Natl. Acad. Sci. USA 2013, 110, E1162–E1168. [Google Scholar] [CrossRef]
- Syeda, R.; Xu, J.; Dubin, A.E.; Coste, B.; Mathur, J.; Huynh, T.; Matzen, J.; Lao, J.; Tully, D.C.; Engels, I.H.; et al. Chemical Activation of the Mechanotransduction Channel Piezo1. Elife 2015, 4, e07369. [Google Scholar] [CrossRef]
- Picard, V.; Guitton, C.; Thuret, I.; Rose, C.; Bendelac, L.; Ghazal, K.; Aguilar-Martinez, P.; Badens, C.; Barro, C.; Bénéteau, C.; et al. Clinical and Biological Features in PIEZO1-Hereditary Xerocytosis and Gardos Channelopathy: A Retrospective Series of 126 Patients. Haematologica 2019, 104, 1554–1564. [Google Scholar] [CrossRef]
- Ma, S.; Cahalan, S.; LaMonte, G.; Grubaugh, N.D.; Zeng, W.; Murthy, S.E.; Paytas, E.; Gamini, R.; Lukacs, V.; Whitwam, T.; et al. Common PIEZO1 Allele in African Populations Causes RBC Dehydration and Attenuates Plasmodium Infection. Cell 2018, 173, 443–455. [Google Scholar] [CrossRef]
- Shmukler, B.E.; Vandorpe, D.H.; Rivera, A.; Auerbach, M.; Brugnara, C.; Alper, S.L. Dehydrated Stomatocytic Anemia Due to the Heterozygous Mutation R2456H in the Mechanosensitive Cation Channel PIEZO1: A Case Report. Blood Cells Mol. Dis. 2014, 52, 53–54. [Google Scholar] [CrossRef] [PubMed]
- Andolfo, I.; Russo, R.; Rosato, B.E.; Manna, F.; Gambale, A.; Brugnara, C.; Iolascon, A. Genotype-Phenotype Correlation and Risk Stratification in a Cohort of 123 Hereditary Stomatocytosis Patients. Am. J. Hematol. 2018, 93, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Rotordam, G.M.; Fermo, E.; Becker, N.; Barcellini, W.; Brüggemann, A.; Fertig, N.; Egée, S.; Rapedius, M.; Bianchi, P.; Kaestner, L. A Novel Gain-of-Function Mutation of Piezo1 Is Functionally Affirmed in Red Blood Cells by High-Throughput Patch Clamp. Haematologica 2019, 104, e179. [Google Scholar] [CrossRef] [PubMed]
- Kaestner, L.; Christophersen, P.; Bernhardt, I.; Bennekou, P. The Non-Selective Voltage-Activated Cation Channel in the Human Red Blood Cell Membrane: Reconciliation between Two Conflicting Reports and Further Characterisation. Bioelectrochemistry 2000, 52, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Kaestner, L.; Egée, S. Commentary: Voltage Gating of Mechanosensitive PIEZO Channels. Front. Physiol. 2018, 9, 1565. [Google Scholar] [CrossRef] [PubMed]
- Cahalan, S.M.; Lukacs, V.; Ranade, S.S.; Chien, S.; Bandell, M.; Patapoutian, A. Piezo1 Links Mechanical Forces to Red Blood Cell Volume. Elife 2015, 4, e07370. [Google Scholar] [CrossRef] [PubMed]
- Danielczok, J.G.; Terriac, E.; Hertz, L.; Petkova-Kirova, P.; Lautenschläger, F.; Laschke, M.W.; Kaestner, L. Red Blood Cell Passage of Small Capillaries Is Associated with Transient Ca2+-Mediated Adaptations. Front. Physiol. 2017, 8, 979. [Google Scholar] [CrossRef]
- Faucherre, A.; Kissa, K.; Nargeot, J.; Mangoni, M.E.; Jopling, C. Piezo1 Plays a Role in Erythrocyte Volume Homeostasis. Haematologica 2013, 99, 70–75. [Google Scholar] [CrossRef]
- Kihm, A.; Kaestner, L.; Wagner, C.; Quint, S. Classification of Red Blood Cell Shapes in Flow Using Outlier Tolerant Machine Learning. PLoS Comput. Biol. 2018, 14, e1006278. [Google Scholar] [CrossRef]
- Wang, J.; Wagner-Britz, L.; Bogdanova, A.; Ruppenthal, S.; Wiesen, K.; Kaiser, E.; Tian, Q.; Krause, E.; Bernhardt, I.; Lipp, P.; et al. Morphologically Homogeneous Red Blood Cells Present a Heterogeneous Response to Hormonal Stimulation. PLoS ONE 2013, 8, e67697. [Google Scholar] [CrossRef]
- Bogdanova, A.; Kaestner, L.; Simionato, G.; Wickrema, A.; Makhro, A. Heterogeneity of Red Blood Cells: Causes and Consequences. Front. Physiol. 2020, 11, 392. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Kaestner, L.; Bogdanova, A.Y.; Minetti, G.; Rudloff, S.; Lundby, C.; Makhro, A.; Seiler, E.; Cromvoirt, A.; Fenk, S.; et al. Absence of Neocytolysis in Humans Returning from a 3-week High-altitude Sojourn. Acta Physiol. 2021, 232, e13647. [Google Scholar] [CrossRef] [PubMed]
- Kaestner, L.; Champigneulle, B.; Stauffer, É.; Furian, M.; Abreu, J.D.; John, T.; Nader, E.; Scheller, A.; Pichon, A.; Connes, P.; et al. Neocytolysis after Return from High Altitude (5100 m): Further Evidence for Absentia. Acta Physiol. 2023, 238, e14002. [Google Scholar] [CrossRef] [PubMed]
- Bouyer, G.; Cueff, A.; Egée, S.; Kmiecik, J.; Maksimova, Y.; Glogowska, E.; Gallagher, P.G.; Thomas, S.L.Y. Erythrocyte Peripheral Type Benzodiazepine Receptor/Voltage-Dependent Anion Channels Are Upregulated by Plasmodium Falciparum. Blood 2011, 118, 2305–2312. [Google Scholar] [CrossRef] [PubMed]
- Gnanasambandam, R.; Gottlieb, P.A.; Sachs, F. The Kinetics and the Permeation Properties of Piezo Channels. Curr. Top. Membr. 2017, 79, 275–307. [Google Scholar] [CrossRef] [PubMed]
- Baunbaek, M.; Bennekou, P. Evidence for a Random Entry of Ca2+ into Human Red Cells. Bioelectrochemistry 2008, 73, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.D.; Starkey, B.J. Simpler Flame Photometric Determination of Erythrocyte Sodium and Potassium: The Reference Range for Apparently Healthy Adults. Clin. Chem. 1977, 23, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Bogdanova, A.; Makhro, A.; Wang, J.; Lipp, P.; Kaestner, L. Calcium in Red Blood Cells-a Perilous Balance. Int. J. Mol. Sci. 2013, 14, 9848–9872. [Google Scholar] [CrossRef]
- Nguyen, D.B.; Wagner-Britz, L.; Maia, S.; Steffen, P.; Wagner, C.; Kaestner, L.; Bernhardt, I. Regulation of Phosphatidylserine Exposure in Red Blood Cells. Cell Physiol. Biochem. 2011, 28, 847–856. [Google Scholar] [CrossRef]
- Shmukler, B.E.; Rivera, A.; Bhargava, P.; Nishimura, K.; Hsu, A.; Kim, E.H.; Trudel, M.; Rust, M.B.; Hubner, C.A.; Brugnara, C.; et al. Combined Genetic Disruption of K-Cl Cotransporters and Gardos Channel KCNN4 Rescues Erythrocyte Dehydration in the SAD Mouse Model of Sickle Cell Disease. Blood Cells Mol. Dis. 2019, 79, 102346. [Google Scholar] [CrossRef]
- Philp, A.R.; Riquelme, T.T.; Millar-Büchner, P.; González, R.; Sepúlveda, F.V.; Cid, L.P.; Flores, C.A. Kcnn4 Is a Modifier Gene of Intestinal Cystic Fibrosis Preventing Lethality in the Cftr-F508del Mouse. Sci. Rep. 2018, 8, 9320. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Andrews, D.A.; Low, P.S. Lysophosphatidic Acid Opens a Ca++ Channel in Human Erythrocytes. Blood 2000, 95, 2420–2425. [Google Scholar] [CrossRef] [PubMed]
- Wagner-Britz, L.; Wang, J.; Kaestner, L.; Bernhardt, I. Protein Kinase Ca and P-Type Ca 2+ Channel Ca V 2.1 in Red Blood Cell Calcium Signalling. Cell Physiol. Biochem. 2013, 31, 883–891. [Google Scholar] [CrossRef]
- Wang, J.; Hertz, L.; Ruppenthal, S.; Nemer, W.E.; Connes, P.; Goede, J.S.; Bogdanova, A.; Birnbaumer, L.; Kaestner, L. Lysophosphatidic Acid-Activated Calcium Signaling Is Elevated in Red Cells from Sickle Cell Disease Patients. Cells 2021, 10, 456. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Tong, Q.; Cheung, J.Y.; Wozney, J.; Conrad, K.; Mazack, V.; Zhang, W.; Stahl, R.; Barber, D.L.; Miller, B.A. Interaction of TRPC2 and TRPC6 in Erythropoietin Modulation of Calcium Influx. J. Biol. Chem. 2004, 279, 10514–10522. [Google Scholar] [CrossRef]
- Foller, M.; Kasinathan, R.S.; Koka, S.; Lang, C.; Shumilina, E.V.; Birnbaumer, L.; Lang, F.; Huber, S.M. TRPC6 Contributes to the Ca(2+) Leak of Human Erythrocytes. Cell Physiol. Biochem. 2008, 21, 183–192. [Google Scholar] [CrossRef]
- Kaestner, L.; Minetti, G. The Potential of Erythrocytes as Cellular Aging Models. Cell Death Differ. 2017, 24, 1475–1477. [Google Scholar] [CrossRef] [PubMed]
- Makhro, A.; Hanggi, P.; Goede, J.S.; Wang, J.; Brüggemann, A.; Gassmann, M.; Schmugge, M.; Kaestner, L.; Speer, O.; Bogdanova, A. N-Methyl D-Aspartate (NMDA) Receptors in Human Erythroid Precursor Cells and in Circulating Red Blood Cells Contribute to the Intracellular Calcium Regulation. AJP Cell Physiol. 2013, 305, C1123–C1138. [Google Scholar] [CrossRef]
- Kucherenko, Y.V.; Wagner-Britz, L.; Bernhardt, I.; Lang, F. Effect of Chloride Channel Inhibitors on Cytosolic Ca2+ Levels and Ca2+-Activated K+ (Gardos) Channel Activity in Human Red Blood Cells. J. Membr. Biol. 2013, 246, 315–326. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Guo, Y.R.; Miyagi, A.; Levring, J.; MacKinnon, R.; Scheuring, S. Force-Induced Conformational Changes in PIEZO1. Nature 2019, 573, 230–234. [Google Scholar] [CrossRef]
- Kaestner, L.; Bogdanova, A.; Egee, S. Calcium Channels and Calcium-Regulated Channels in Human Red Blood Cells. Adv. Exp. Med. Biol. 2020, 1131, 625–648. [Google Scholar] [CrossRef] [PubMed]
- Kaestner, L.; Wang, X.; Hertz, L.; Bernhardt, I. Voltage-Activated Ion Channels in Non-Excitable Cells-A Viewpoint Regarding Their Physiological Justification. Front. Physiol. 2018, 9, 450. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, P.G. Disorders of Red Cell Volume Regulation. Curr. Opin. Hematol. 2013, 20, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.; Paschen, K.; Spieckermann, P.G.; Westberg, C.v. Bestimmung Des Ionisierten Calciums Im Serum Mit Einer Ionenselektiven Durchflußelektrode: Methodik Und Normalwerte. Klin. Wochenschr. 1972, 50, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Liappis, N. Sodium-, Potassium- and Chloride-Concentrations in the Serum of Infants, Children and Adults. Monatsschrift Fur Kinderheilkd. 1972, 120, 138–142. [Google Scholar]
- Schoeppe, W.; Brecht, H.M. Frequency of Decreased and Increased Potassium Concentration in Plasma and Erythrocytes. Med. Klin. 1969, 64, 736–739. [Google Scholar] [PubMed]
- Tiffert, T.; Bookchin, R.M.; Lew, V.L. Calcium Homeostasis in Normal and Abnormal Human Red Cells. In Red Cell Membrane Transport in Health and Disease; Bernhardt, I., Ellory, C., Eds.; Springer: Heidelberg, Germany, 2003; pp. 373–405. [Google Scholar]
- Smith, E.K. Observations on the Measurement and Regulation of the Sodium Content of Human Erythrocytes. Clin. Sci. 1972, 42, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Rizzuto, V.; Mencattini, A.; Álvarez-González, B.; Giuseppe, D.D.; Martinelli, E.; Beneitez-Pastor, D.; Mañú-Pereira, M.d.M.; Lopez-Martinez, M.J.; Samitier, J. Combining Microfluidics with Machine Learning Algorithms for RBC Classification in Rare Hereditary Hemolytic Anemia. Sci. Rep. 2021, 11, 13553. [Google Scholar] [CrossRef]
- Egee, S.; Kaestner, L. The Transient Receptor Potential Vanilloid Type 2 (TRPV2) Channel—A New Druggable Ca2+ Pathway in Red Cells, Implications for Red Cell Ion Homeostasis. Front. Physiol. 2021, 12, 677573. [Google Scholar] [CrossRef]
- Belkacemi, A.; Fecher-Trost, C.; Tinschert, R.; Flormann, D.; Malihpour, M.; Wagner, C.; Meyer, M.R.; Beck, A.; Flockerzi, V. The TRPV2 Channel Mediates Ca2+ Influx and the Δ9-THC-Dependent Decrease in Osmotic Fragility in Red Blood Cells. Haematologica 2021, 106, 2246–2250. [Google Scholar] [CrossRef]
- Chari-Bitron, A.; Shahar, A. Changes in Rat Erythrocyte Membrane Induced ByΔ1-Tetrahydrocannabinol, Scanning Electron Microscope Study. Experientia 1979, 35, 365–366. [Google Scholar] [CrossRef] [PubMed]
- Flormann, D.; Qiao, M.; Murciano, N.; Iacono, G.; Darras, A.; Hof, S.; Recktenwald, S.M.; Rotordam, M.G.; Becker, N.; Geisel, J.; et al. Transient Receptor Potential Channel Vanilloid Type 2 in Red Cells of Cannabis Consumer. Am. J. Hematol. 2022, 97, E180–E183. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E. Biphasic Loss of Red Cell Enzyme Activity during in Vivo Aging. Prog. Clin. Biol. Res. 1985, 195, 317–333. [Google Scholar] [PubMed]
- Chasis, J.A.; Prenant, M.; Leung, A.; Mohandas, N. Membrane Assembly and Remodeling during Reticulocyte Maturation. Blood 1989, 74, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Tiffert, T.; Daw, N.; Etzion, Z.; Bookchin, R.M.; Lew, V.L. Age Decline in the Activity of the Ca2+-Sensitive K+ Channel of Human Red Blood Cells. J. Gen. Physiol. 2007, 129, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Aglialoro, F.; Abay, A.; Yagci, N.; Rab, M.A.E.; Kaestner, L.; van Wijk, R.; von Lindern, M.; Akker, E. van den Mechanical Stress Induces Ca2+-Dependent Signal Transduction in Erythroblasts and Modulates Erythropoiesis. Int. J. Mol. Sci. 2021, 22, 955. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, V.; Chubinskiy-Nadezhdin, V. Regulation of PIEZO1 Channels by Lipids and the Structural Components of Extracellular Matrix/Cell Cytoskeleton. J. Cell. Physiol. 2023, 238, 918–930. [Google Scholar] [CrossRef]
- Lin, Y.; Buyan, A.; Corry, B. Computational Studies of Piezo1 Yield Insights into Key Lipid–Protein Interactions, Channel Activation, and Agonist Binding. Biophys. Rev. 2022, 14, 209–219. [Google Scholar] [CrossRef]
- Buyan, A.; Cox, C.D.; Barnoud, J.; Martinac, B.; Marrink, S.-J.; Corry, B. Understanding Piezo1’s Relationship with Lipids. Biophys. J. 2019, 116, 459a. [Google Scholar] [CrossRef]
- Minetti, G.; Kaestner, L.; Dorn, I. Terminal Maturation of Human Reticulocytes to Red Blood Cells by Extensive Remodelling and Progressive Liquid Ordering of Membrane Lipids. bioRxiv 2023. [Google Scholar] [CrossRef]
- Rashidi, Y.; Simionato, G.; Zhou, Q.; John, T.; Kihm, A.; Bendaoud, M.; Krüger, T.; Bernabeu, M.O.; Kaestner, L.; Laschke, M.W.; et al. Red Blood Cell Lingering Modulates Hematocrit Distribution in the Microcirculation. Biophys. J. 2023, 122, 1526–1537. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.L.; Povstyan, O.V.; Vecchis, D.D.; Macrae, F.; Lichtenstein, L.; Futers, T.S.; Parsonage, G.; Humphreys, N.E.; Adamson, A.; Kalli, A.C.; et al. RBCs Prevent Rapid PIEZO1 Inactivation and Expose Slow Deactivation as a Mechanism of Dehydrated Hereditary Stomatocytosis. Blood 2020, 136, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Murciano, N.; Rotordam, M.G.; Becker, N.; Ludlow, M.J.; Parsonage, G.; Darras, A.; Kaestner, L.; Beech, D.J.; George, M.; Fertig, N.; et al. A High-Throughput Electrophysiology Assay to Study the Response of PIEZO1 to Mechanical Stimulation. J. Gen. Physiol. 2023, 155, e202213132. [Google Scholar] [CrossRef]
- Klei, T.R.L.; Dalimot, J.J.; Beuger, B.M.; Veldthuis, M.; Ichou, F.A.; Verkuijlen, P.J.J.H.; Seignette, I.M.; Ligthart, P.C.; Kuijpers, T.W.; van Zwieten, R.; et al. The Gardos Effect Drives Erythrocyte Senescence and Leads to Lu/BCAM and CD44 Adhesion Molecule Activation. Blood Adv. 2020, 4, 6218–6229. [Google Scholar] [CrossRef] [PubMed]
- Pivkin, I.V.; Peng, Z.; Karniadakis, G.E.; Buffet, P.A.; Dao, M.; Suresh, S. Biomechanics of Red Blood Cells in Human Spleen and Consequences for Physiology and Disease. Proc. Natl. Acad. Sci. USA 2016, 113, 7804–7809. [Google Scholar] [CrossRef] [PubMed]
- Kaestner, L.; Tabellion, W.; Lipp, P.; Bernhardt, I. Prostaglandin E2 Activates Channel-Mediated Calcium Entry in Human Erythrocytes: An Indication for a Blood Clot Formation Supporting Process. Thromb. Haemost. 2004, 92, 1269–1272. [Google Scholar]
- Murciano, N.; Kaestner, L. The Putative Role of the Transient Receptor Potential Ion Channel of Vanilloid Type 2 in Red Blood Cell Storage Lesions. Transfus. Med. Hemotherapy 2023. [Google Scholar] [CrossRef]
- Beutler, E.; West, C.; Blume, K.G. The Removal of Leukocytes and Platelets from Whole Blood. J. Lab. Clin. Med. 1976, 88, 328–333. [Google Scholar]
- Achilli, C.; Ciana, A.; Balduini, C.; Risso, A.; Minetti, G. Application of Gelatin Zymography for Evaluating Low Levels of Contaminating Neutrophils in Red Blood Cell Samples. Anal. Biochem. 2011, 409, 296–297. [Google Scholar] [CrossRef]
- Heshusius, S.; Heideveld, E.; Burger, P.; Thiel-Valkhof, M.; Sellink, E.; Varga, E.; Ovchynnikova, E.; Visser, A.; Martens, J.H.A.; von Lindern, M.; et al. Large-Scale in Vitro Production of Red Blood Cells from Human Peripheral Blood Mononuclear Cells. Blood Adv. 2019, 3, 3337–3350. [Google Scholar] [CrossRef]
- Macey, R.I.; Adorante, J.S.; Orme, F.W. Erythrocyte Membrane Potentials Determined by Hydrogen Ion Distribution. Biochim. Et. Biophys. Acta BBA—Biomembr. 1978, 512, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Qi, X.; Han, K.; Wang, S.; Hu, G.; Li, X. Computational Investigation of Flow Dynamics and Mechanical Retention of Age-Associated Red Blood Cells in the Spleen. Phys. Rev. Fluids 2023, 8, 063103. [Google Scholar] [CrossRef]
- Nardo-Marino, A.; Glenthøj, A.; Brewin, J.N.; Petersen, J.; Braunstein, T.H.; Kurtzhals, J.A.L.; Williams, T.N.; Rees, D.C. Decreased Red Blood Cell Deformability Contributes to Loss of Splenic Filtration Function and Variations in Spleen Size in Children with Sickle Cell Anaemia. Blood 2022, 140, 1639–1640. [Google Scholar] [CrossRef]
- Kaestner, L.; Bernhardt, I. Further Characterisation of the Non-Selective Cation Channel in the Human Red Blood Cell Membrane. 2001; p. 9. [Google Scholar]
- Steffen, P.; Jung, A.; Nguyen, D.B.; Müller, T.; Bernhardt, I.; Kaestner, L.; Wagner, C. Stimulation of Human Red Blood Cells Leads to Ca2+-Mediated Intercellular Adhesion. Cell Calcium 2011, 50, 54–61. [Google Scholar] [CrossRef]
| Ion | Ca2+ | Na+ | K+ |
|---|---|---|---|
| extracellular concentration | 1.2 mM [76] | 140 mM [77] | 4 mM [78] |
| intracellular concentration | 60 nM [79] | 7.35 mM [80] | 102 mM [59] |
| chemical potential * | −132 mV | −79 mV | 86 mV |
| electrochemical potential at resting membrane potential | −144 mV | −91 mV | 74 mV |
| electrochemical potential when Gárdos channel is open | −202 mV | −149 mV | 16 mV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petkova-Kirova, P.; Murciano, N.; Iacono, G.; Jansen, J.; Simionato, G.; Qiao, M.; van der Zwaan, C.; Rotordam, M.G.; John, T.; Hertz, L.; et al. The Gárdos Channel and Piezo1 Revisited: Comparison between Reticulocytes and Mature Red Blood Cells. Int. J. Mol. Sci. 2024, 25, 1416. https://doi.org/10.3390/ijms25031416
Petkova-Kirova P, Murciano N, Iacono G, Jansen J, Simionato G, Qiao M, van der Zwaan C, Rotordam MG, John T, Hertz L, et al. The Gárdos Channel and Piezo1 Revisited: Comparison between Reticulocytes and Mature Red Blood Cells. International Journal of Molecular Sciences. 2024; 25(3):1416. https://doi.org/10.3390/ijms25031416
Chicago/Turabian StylePetkova-Kirova, Polina, Nicoletta Murciano, Giulia Iacono, Julia Jansen, Greta Simionato, Min Qiao, Carmen van der Zwaan, Maria Giustina Rotordam, Thomas John, Laura Hertz, and et al. 2024. "The Gárdos Channel and Piezo1 Revisited: Comparison between Reticulocytes and Mature Red Blood Cells" International Journal of Molecular Sciences 25, no. 3: 1416. https://doi.org/10.3390/ijms25031416
APA StylePetkova-Kirova, P., Murciano, N., Iacono, G., Jansen, J., Simionato, G., Qiao, M., van der Zwaan, C., Rotordam, M. G., John, T., Hertz, L., Hoogendijk, A. J., Becker, N., Wagner, C., von Lindern, M., Egee, S., van den Akker, E., & Kaestner, L. (2024). The Gárdos Channel and Piezo1 Revisited: Comparison between Reticulocytes and Mature Red Blood Cells. International Journal of Molecular Sciences, 25(3), 1416. https://doi.org/10.3390/ijms25031416






