Platelet Proteome Reveals Novel Targets for Hypercoagulation in Pseudoexfoliation Syndrome
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics of Study Participants
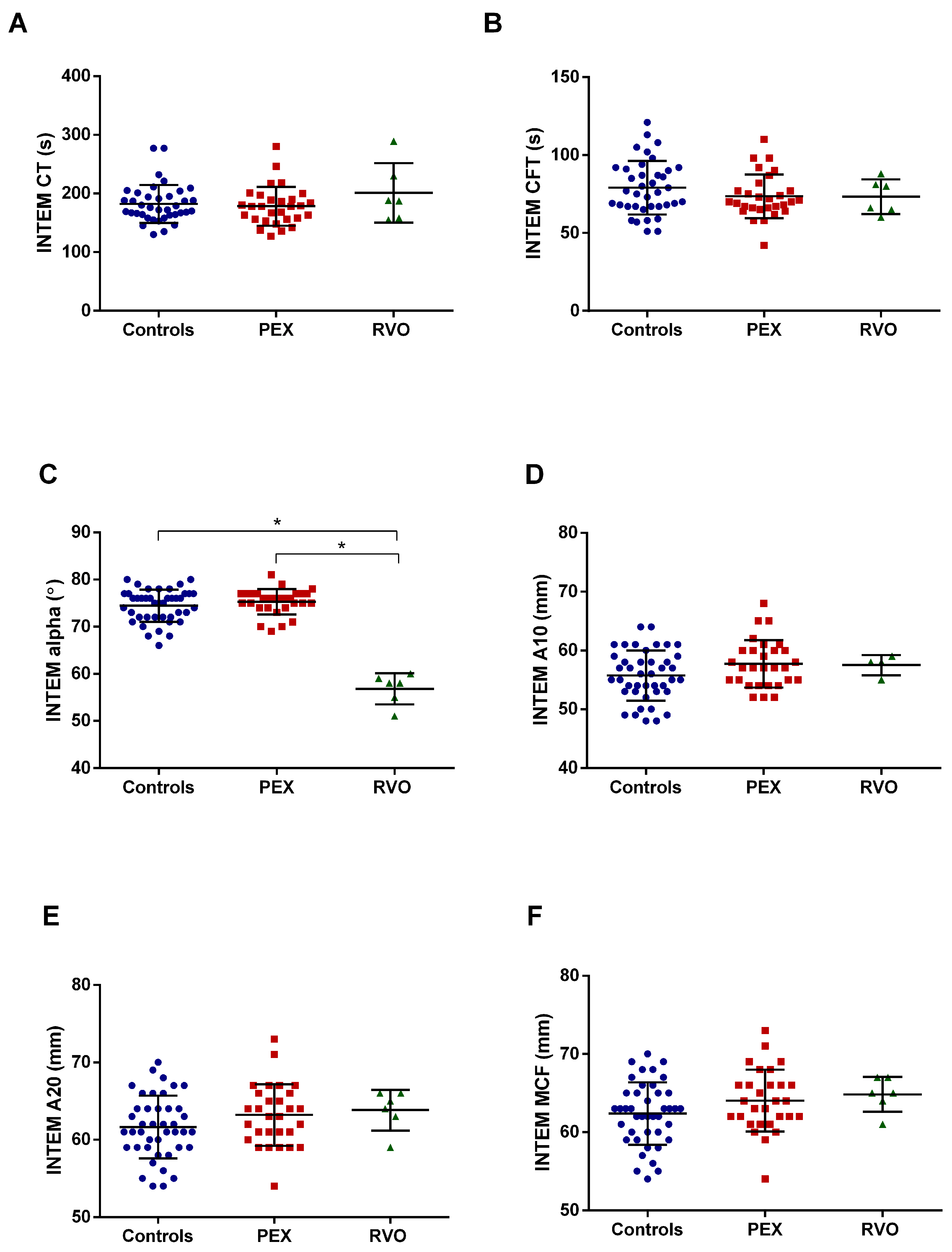
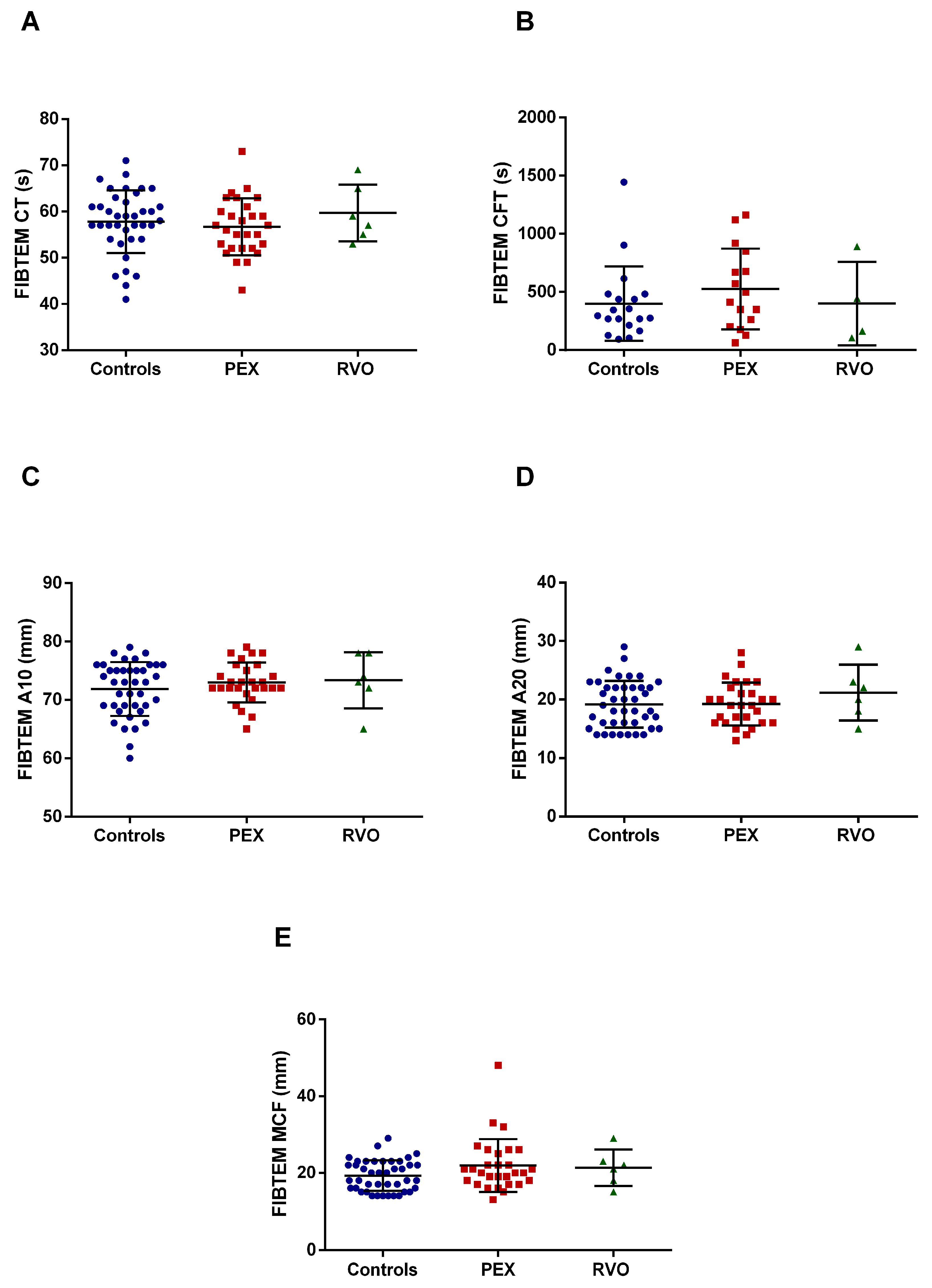
2.2. Coagulation Assessment by ROTEM
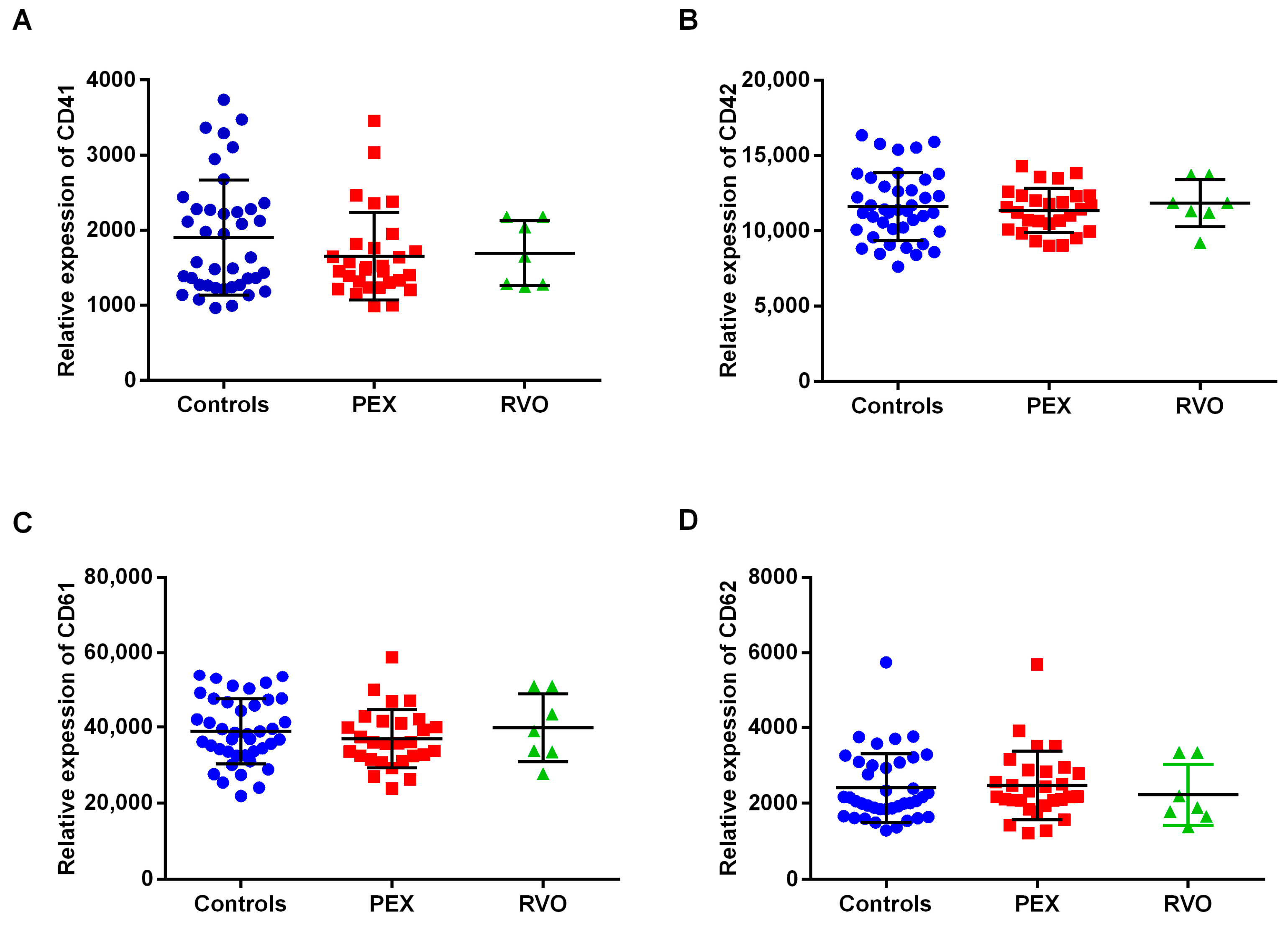
2.3. The Expression Levels of Platelet Markers
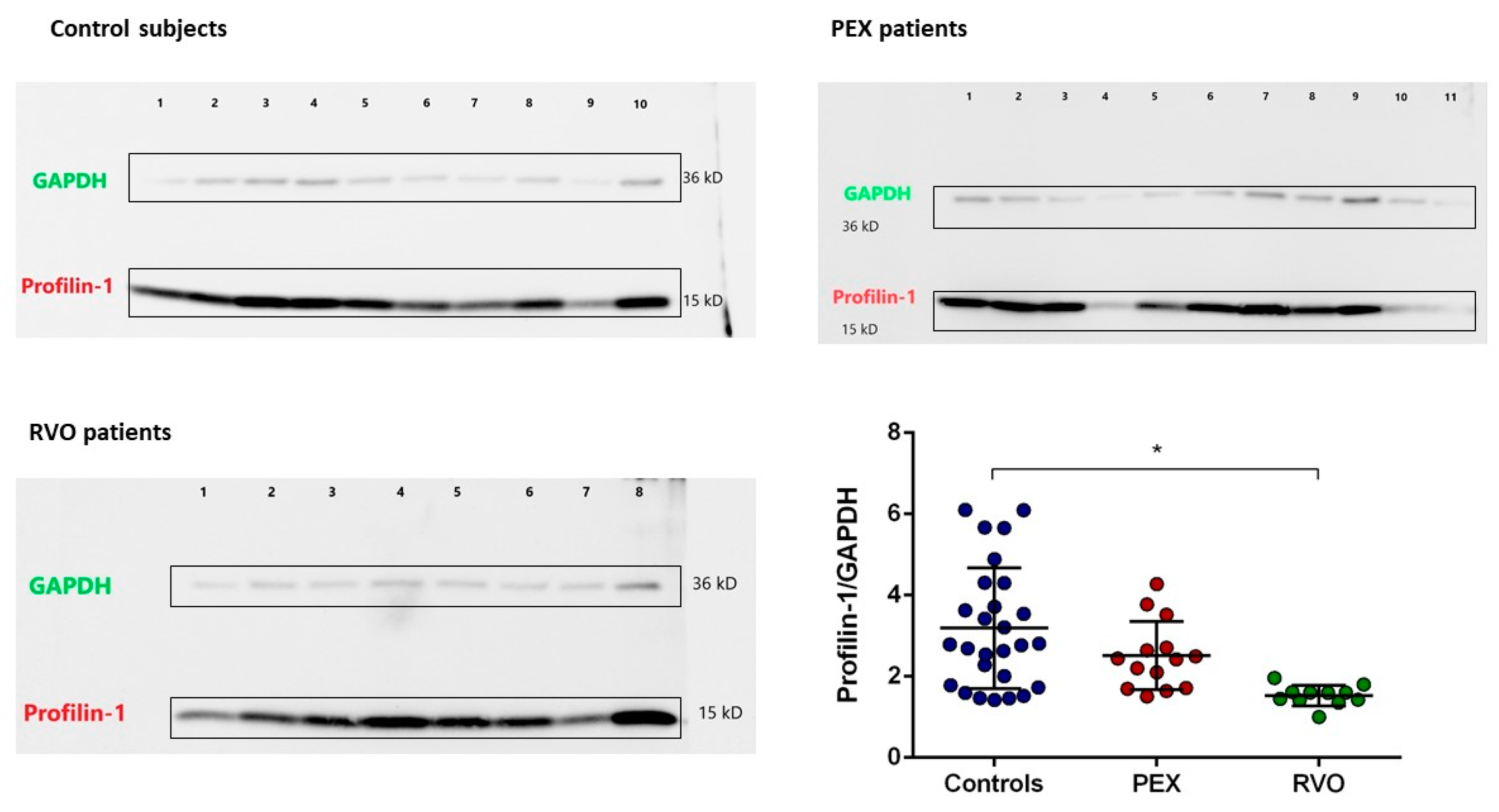
2.4. Proteomic Analysis
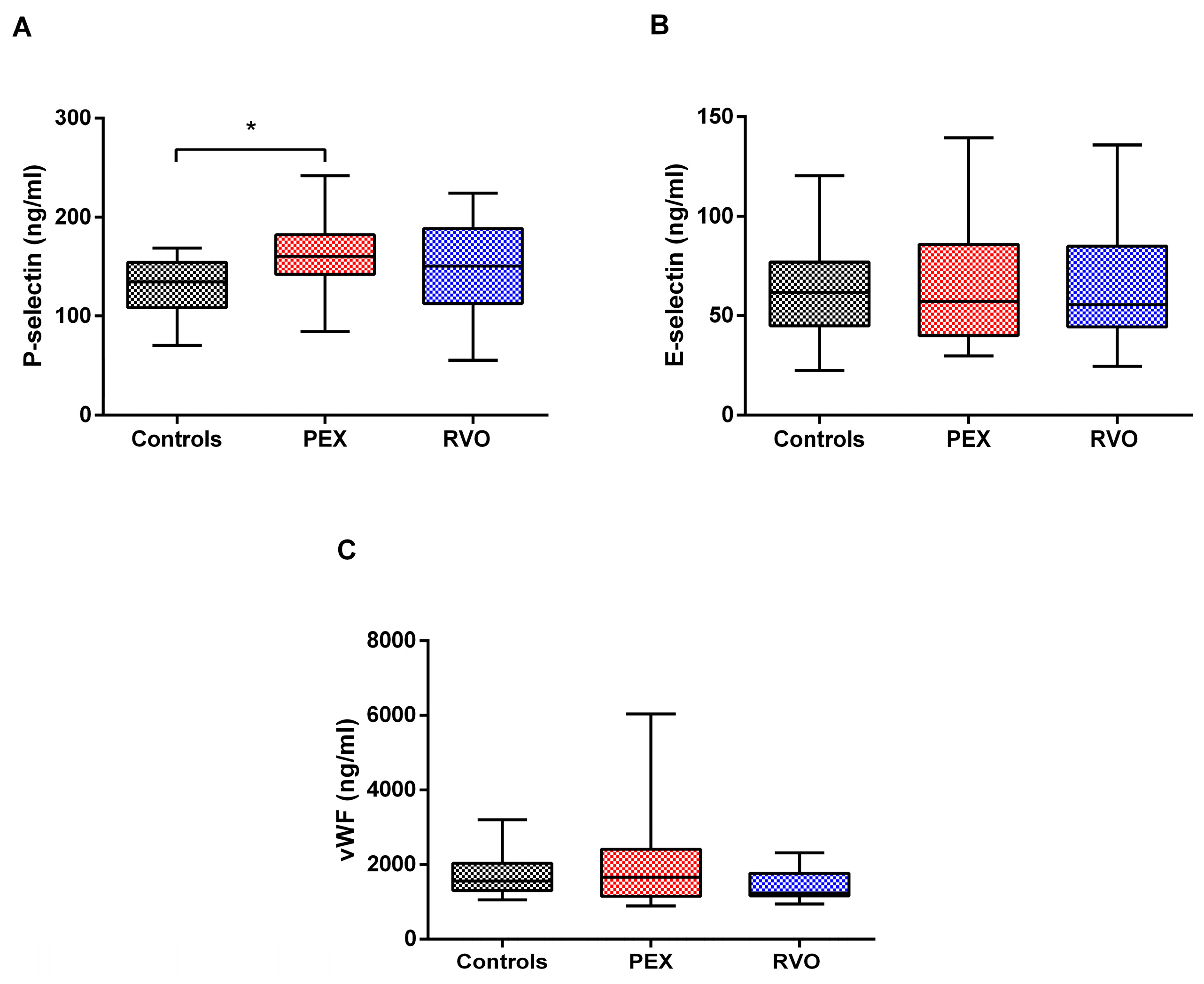
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
3. Discussion
4. Materials and Methods
4.1. Study Approval
4.2. Characteristics and Demographics of the Subjects
4.3. Blood Sampling
4.4. Rotational Thromboelastometry (ROTEM)
4.5. Platelet Separation and Flow Cytometry
4.6. Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Protein Extraction from Platelets
4.8. 2-D Fluorescence Difference Gel Electrophoresis (2DIGE)
4.9. Protein Identification by Mass Spectrometry
4.10. Validation Experiments by Western Blot
4.11. Statistical and Bioinformatics Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ritch, R. Exfoliation syndrome. Curr. Opin. Ophthalmol. 2001, 12, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Schlötzer-Schrehardt, U.; Naumann, G.O.H. Ocular and Systemic Pseudoexfoliation Syndrome. Am. J. Ophthalmol. 2006, 141, 921–937.e2. [Google Scholar] [CrossRef] [PubMed]
- Musch, D.C.; Shimizu, T.; Niziol, L.M.; Gillespie, B.W.; Cashwell, L.F.; Lichter, P.R. Clinical characteristics of newly diagnosed primary, pigmentary and pseudoexfoliative open-angle glaucoma in the Collaborative Initial Glaucoma Treatment Study. Br. J. Ophthalmol. 2012, 96, 1180–1184. [Google Scholar] [CrossRef] [PubMed]
- Schlötzer-Schrehardt, U. Neue pathogenetische Erkenntnisse zum Pseudoexfoliations-Syndrom/Glaukom. Ophthalmologe 2012, 109, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Andrikopoulos, G.K.; Alexopoulos, D.K.; Gartaganis, S.P. Pseudoexfoliation syndrome and cardiovascular diseases. World J. Cardiol. 2014, 6, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Gartaganis, S.P.; Georgakopoulos, C.D.; Mela, E.K.; Exarchou, A.; Ziouti, N.; Assouti, M.; Vynios, D.H. Matrix Metalloproteinases and Their Inhibitors in Exfoliation Syndrome. Ophthalmic Res. 2002, 34, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Zenkel, M.; Lewczuk, P.; Jünemann, A.; Kruse, F.; Naumann, G.O.H.; Schlötzer-Schrehardt, U. Proinflammatory cytokines are involved in the initiation of the abnormal matrix process in pseudoexfoliation syndrome/glaucoma. Am. J. Pathol. 2010, 176, 2868–2879. [Google Scholar] [CrossRef]
- Sharma, S.; Chataway, T.; Burdon, K.P.; Jonavicius, L.; Klebe, S.; Hewitt, A.W.; Mills, R.A.; Craig, J.E. Identification of LOXL1 protein and Apolipoprotein E as components of surgically isolated pseudoexfoliation material by direct mass spectrometry. Exp. Eye Res. 2009, 89, 479–485. [Google Scholar] [CrossRef]
- Sharma, S.; Martin, S.; Sykes, M.J.; Dave, A.; Hewitt, A.W.; Burdon, K.P.; Ronci, M.; Voelcker, N.H.; Craig, J.E. Biological effect of LOXL1 coding variants associated with pseudoexfoliation syndrome. Exp. Eye Res. 2016, 146, 212–223. [Google Scholar] [CrossRef]
- Thorleifsson, G.; Magnusson Kristinn, P.; Sulem, P.; Walters, G.B.; Gudbjartsson Daniel, F.; Stefansson, H.; Jonsson, T.; Jonasdottir, A.; Jonasdottir, A.; Stefansdottir, G.; et al. Common Sequence Variants in the LOXL1 Gene Confer Susceptibility to Exfoliation Glaucoma. Science 2007, 317, 1397–1400. [Google Scholar] [CrossRef]
- Praveen, M.R.; Shah, S.K.; Vasavada, A.R.; Diwan, R.P.; Shah, S.M.; Zumkhawala, B.R.; Thomas, R. Pseudoexfoliation as a risk factor for peripheral vascular disease: A case-control study. Eye 2011, 25, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Katsi, V.; Pavlidis, A.N.; Kallistratos, M.S.; Fitsios, A.; Bratsas, A.; Tousoulis, D.; Stefanadis, C.; Manolis, A.J.; Kallikazaros, I. Cardiovascular repercussions of the pseudoexfoliation syndrome. N. Am. J. Med. Sci. 2013, 5, 454–459. [Google Scholar] [PubMed]
- Dayanir, V.; Topaloğlu, A.; Ozsunar, Y.; Keceli, M.; Okyay, P.; Harris, A. Orbital blood flow parameters in unilateral pseudoexfoliation syndrome. Int. Ophthalmol. 2009, 29, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Galassi, F.; Giambene, B.; Menchini, U. Ocular perfusion pressure and retrobulbar haemodynamics in pseudoexfoliative glaucoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 246, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Yüksel, N.; Karabaş, V.L.; Arslan, A.; Demirci, A.; Çağlar, Y. Ocular hemodynamics in pseudoexfoliation syndrome and pseudoexfoliation glaucoma. Ophthalmology 2001, 108, 1043–1049. [Google Scholar] [CrossRef]
- Citirik, M.; Acaroglu, G.; Batman, C.; Yildiran, L.; Zilelioglu, O. A possible link between the pseudoexfoliation syndrome and coronary artery disease. Eye 2007, 21, 11–15. [Google Scholar] [CrossRef][Green Version]
- Ritch, R.; Prata, T.S.; De Moraes, C.G.V.; Vessani, R.M.; Costa, V.P.; Konstas, A.G.P.; Liebmann, J.M.; Schlötzer-Schrehardt, U. Association of exfoliation syndrome and central retinal vein occlusion: An ultrastructural analysis. Acta Ophthalmol. 2010, 88, 91–95. [Google Scholar] [CrossRef]
- Saatçi, O.A.; Ferliel, S.T.; Ferliel, M.; Kaynak, S.; Ergin, M.H. Pseudoexfoliation and glaucoma in eyes with retinal vein occlusion. Int. Ophthalmol. 2004, 23, 75–78. [Google Scholar] [CrossRef]
- Cursiefen, C.; Hammer, T.; Küchle, M.; Naumann, G.O.H.; Schlötzer-Schrehardt, U. Pseudoexfoliation syndrome in eyes with ischemic central retinal vein occlusion. Acta Ophthalmol. Scand. 2001, 79, 476–478. [Google Scholar] [CrossRef]
- Goren Sahin, D.; Sahin, A.; Akay, O.M. Comparison of Rotational Thromboelastography Findings in Pseudoexfoliation Syndrome Patients and Healthy Controls. J. Glaucoma 2016, 25, 879–882. [Google Scholar] [CrossRef]
- Akay, O.M. The Double Hazard of Bleeding and Thrombosis in Hemostasis From a Clinical Point of View: A Global Assessment by Rotational Thromboelastometry (ROTEM). Clin. Appl. Thromb./Hemost. 2018, 24, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Botling Taube, A.; Konzer, A.; Alm, A.; Bergquist, J. Proteomic analysis of the aqueous humour in eyes with pseudoexfoliation syndrome. Br. J. Ophthalmol. 2019, 103, 1190. [Google Scholar] [CrossRef] [PubMed]
- Hardenborg, E.; Botling-Taube, A.; Hanrieder, J.; Andersson, M.; Alm, A.; Bergquist, J. Protein content in aqueous humor from patients with pseudoexfoliation (PEX) investigated by capillary LC MALDI-TOF/TOF MS. Proteom.—Clin. Appl. 2009, 3, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Ovodenko, B.; Rostagno, A.; Neubert, T.A.; Shetty, V.; Thomas, S.; Yang, A.; Liebmann, J.; Ghiso, J.; Ritch, R. Proteomic Analysis of Exfoliation Deposits. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1447–1457. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Chataway, T.; Klebe, S.; Griggs, K.; Martin, S.; Chegeni, N.; Dave, A.; Zhou, T.; Ronci, M.; Voelcker, N.H.; et al. Novel protein constituents of pathological ocular pseudoexfoliation syndrome deposits identified with mass spectrometry. Mol. Vis. 2018, 24, 801–817. [Google Scholar] [PubMed]
- Bearer, E.L.; Prakash, J.M.; Li, Z. Actin dynamics in platelets. Int. Rev. Cytol. 2002, 217, 137–182. [Google Scholar] [PubMed]
- Stritt, S.; Birkholz, I.; Beck, S.; Sorrentino, S.; Sapra, K.T.; Viaud, J.; Heck, J.; Gaits-Iacovoni, F.; Schulze, H.; Du, X.; et al. Profilin 1–mediated cytoskeletal rearrangements regulate integrin function in mouse platelets. Blood Adv. 2018, 2, 1040–1045. [Google Scholar] [CrossRef]
- Stafiej, J.; Malukiewicz, G.; Lesiewska-Junk, H.; Rość, D.; Kaźmierczak, K. Endothelial Cell Markers in Patients with Pseudoexfoliation Syndrome. Sci. World J. 2012, 2012, 863949. [Google Scholar] [CrossRef]
- Ozgonul, C.; Sertoglu, E.; Mumcuoglu, T.; Ozge, G.; Gokce, G. Prediction of Pseudoexfoliation Syndrome and Pseudoexfoliation Glaucoma by Using Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio. Ocul. Immunol. Inflamm. 2016, 24, 665–670. [Google Scholar] [CrossRef]
- Türkcü, F.M.; Yüksel, H.; Şahin, A.; Cinar, Y.; HaticeYüksel Cingü, K.; Şahin, M.; Yildirim, A.; Çaça, I. Mean Platelet Volume in Pseudoexfoliation Syndrome and Glaucoma. Eur. J. Ophthalmol. 2013, 24, 71–75. [Google Scholar] [CrossRef]
- Berndt, M.C.; Metharom, P.; Andrews, R.K. Primary haemostasis: Newer insights. Haemophilia 2014, 20 (Suppl. S4), 15–22. [Google Scholar] [CrossRef] [PubMed]
- Weyrich, A.S. Platelets: More than a sack of glue. Hematology 2014, 2014, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Kannan, M.; Ahmad, F.; Saxena, R. Platelet activation markers in evaluation of thrombotic risk factors in various clinical settings. Blood Rev. 2019, 37, 100583. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.R.; Charo, I.F.; Parise, L.V.; Fitzgerald, L.A. The Platelet Membrane Glycoprotein IIb-IIIa Complex. Blood 1988, 71, 831–843. [Google Scholar] [CrossRef] [PubMed]
- Blann, A.D.; Lip, G.Y.; Beevers, D.G.; McCollum, C.N. Soluble P-selectin in atherosclerosis: A comparison with endothelial cell and platelet markers. Thromb. Haemost. 1997, 77, 1077–1080. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, P.; Martini, F.; Riondino, S.; La Farina, F.; Magnapera, A.; Ciatti, F.; Guadagni, F. Soluble P-selectin as a marker of in vivo platelet activation. Clin. Chim. Acta 2009, 399, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, P.; Pulcinelli, F.M.; Lenti, L.; Gazzaniga, P.P. Is soluble P-selectin determination a more reliable marker of in vivo platelet activation than CD62P flow cytometric analysis? Thromb. Haemost. 1999, 81, 472–473. [Google Scholar] [CrossRef] [PubMed]
- Blann, A.D.; Nadar, S.K.; Lip, G.Y.H. The adhesion molecule P-selectin and cardiovascular disease. Eur. Heart J. 2003, 24, 2166–2179. [Google Scholar] [CrossRef]
- Ramacciotti, E.; Blackburn, S.; Hawley, A.E.; Vandy, F.; Ballard-Lipka, N.; Stabler, C.; Baker, N.; Guire, K.E.; Rectenwald, J.E.; Henke, P.K.; et al. Evaluation of Soluble P-Selectin as a Marker for the Diagnosis of Deep Venous Thrombosis. Clin. Appl. Thromb./Hemost. 2011, 17, 425–431. [Google Scholar] [CrossRef]
- Yüksel, N.; Pirhan, D.; Altntas, Ö.; Çaglar, Y. Systemic High-sensitivity C-reactive Protein Level in Pseudoexfoliation Syndrome and Pseudoexfoliation Glaucoma. J. Glaucoma 2010, 19, 373–376. [Google Scholar] [CrossRef]
- Atalar, P.T.; Atalar, E.; Kilic, H.; Abbasoglu, O.E.; Ozer, N.; Aksoyek, S.; Ovunc, K.; Ozmen, F.; Gursel, E. Impaired Systemic Endothelial Function in Patients With Pseudoexfoliation Syndrome. Int. Heart J. 2006, 47, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, S.; Schlötzer-Schrehardt, U.; Martus, P.; Lang, W.; Naumann, G.O.H. Pseudoexfoliation syndrome and aneurysms of the abdominal aorta. Lancet 2001, 357, 359–360. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.; Bauters, A.; Braun, S.L.; Pötzsch, B.; von Pape, K.-W.; Kolde, H.-J.; Lakner, M. Multi-centre investigation on reference ranges for ROTEM thromboelastometry. Blood Coagul. Fibrinolysis 2005, 16, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Goren Sahin, D.; Bayraktutar, B.N.; Yıldız Taş, A.; Akay, O.M.; Ozkaya, A.; Yalcin, Ö.; Sahin, A. Can Rotational Thromboelastometry be a New Predictive Tool for Retinal Vein Occlusion Development? Curr. Eye Res. 2019, 44, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, J.H.; DeSisto, M. The cytoskeleton of the resting human blood platelet: Structure of the membrane skeleton and its attachment to actin filaments. J. Cell Biol. 1991, 112, 407–425. [Google Scholar] [CrossRef] [PubMed]
- Poulter, N.S.; Pollitt, A.Y.; Davies, A.; Malinova, D.; Nash, G.B.; Hannon, M.J.; Pikramenou, Z.; Rappoport, J.Z.; Hartwig, J.H.; Owen, D.M.; et al. Platelet actin nodules are podosome-like structures dependent on Wiskott–Aldrich syndrome protein and ARP2/3 complex. Nat. Commun. 2015, 6, 7254. [Google Scholar] [CrossRef] [PubMed]
- Seifert, J.; Rheinlaender, J.; Lang, F.; Gawaz, M.; Schäffer, T.E. Thrombin-induced cytoskeleton dynamics in spread human platelets observed with fast scanning ion conductance microscopy. Sci. Rep. 2017, 7, 4810. [Google Scholar] [CrossRef]
- Carlsson, L.; Nyström, L.E.; Sundkvist, I.; Markey, F.; Lindberg, U. Actin polymerizability is influenced by profilin, a low molecular weight protein in non-muscle cells. J. Mol. Biol. 1977, 115, 465–483. [Google Scholar] [CrossRef]
- Southwick, F.S.; Young, C.L. The actin released from profilin--actin complexes is insufficient to account for the increase in F-actin in chemoattractant-stimulated polymorphonuclear leukocytes. J. Cell Biol. 1990, 110, 1965–1973. [Google Scholar] [CrossRef]
- Goldschmidt-Clermont, P.J.; Furman, M.I.; Wachsstock, D.; Safer, D.; Nachmias, V.T.; Pollard, T.D. The control of actin nucleotide exchange by thymosin beta 4 and profilin. A potential regulatory mechanism for actin polymerization in cells. Mol. Biol. Cell 1992, 3, 1015–1024. [Google Scholar] [CrossRef]
- Witke, W. The role of profilin complexes in cell motility and other cellular processes. Trends Cell Biol. 2004, 14, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Linden, M.D. Platelet Flow Cytometry. In Haemostasis: Methods and Protocols; Monagle, P., Ed.; Humana Press: Totowa, NJ, USA, 2013; pp. 241–262. [Google Scholar]
- Michelson, A.D.; Barnard, M.R.; Krueger, L.A.; Frelinger, A.L.; Furman, M.I. Evaluation of Platelet Function by Flow Cytometry. Methods 2000, 21, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Uslubas, I.; Kanli, A.; Kasap, M.; Akpinar, G.; Karabas, L. Effect of aflibercept on proliferative vitreoretinopathy: Proteomic analysis in an experimental animal model. Exp. Eye Res. 2021, 203, 108425. [Google Scholar] [CrossRef] [PubMed]

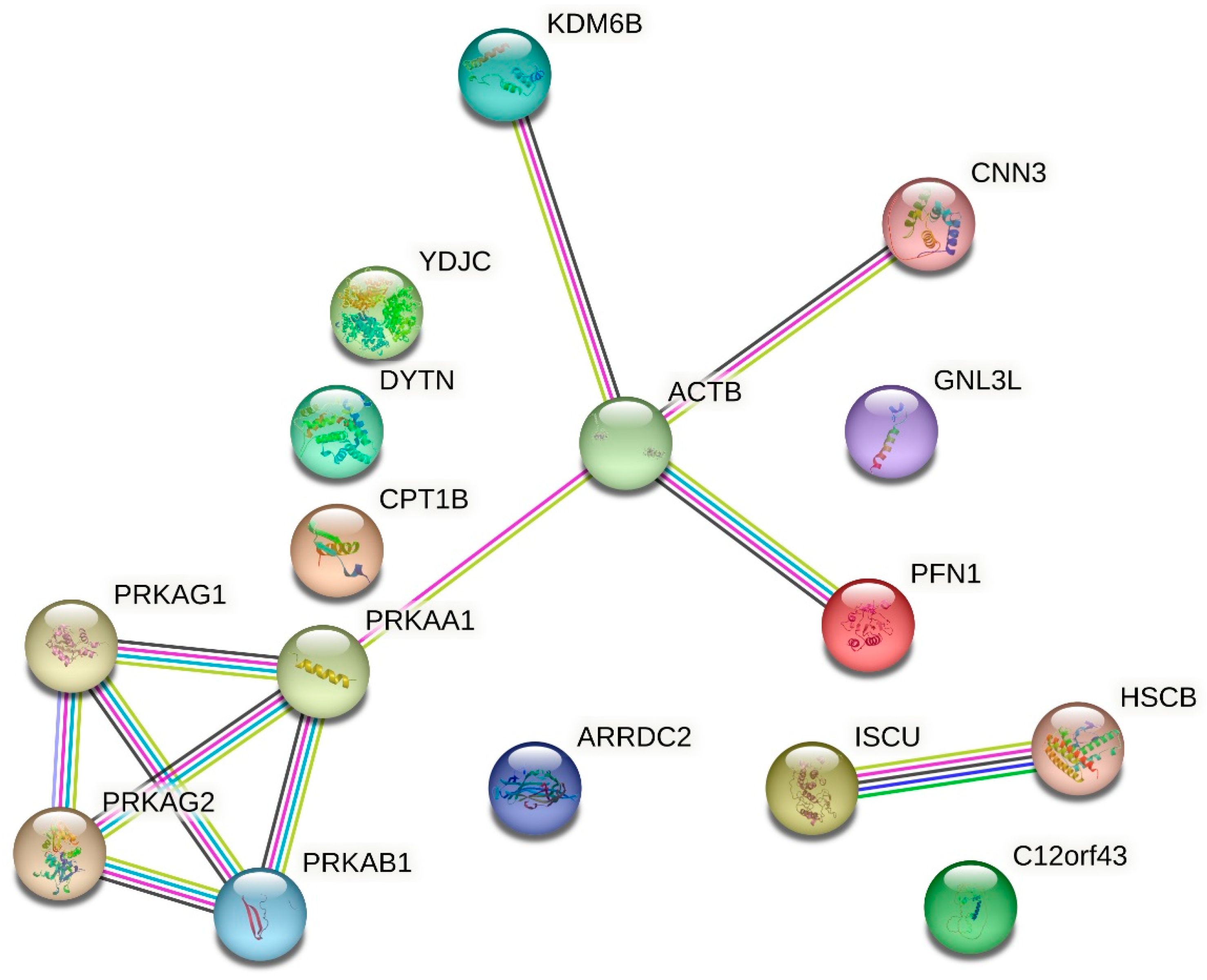
| UniProt No | Accession | Mass | Mascot Score | Protein |
|---|---|---|---|---|
| P07737 | PROF1 | 15,045 | 38 | Profilin-1 |
| Q9H1K1 | ISCU | 17,926 | 43 | Iron–sulfur cluster assembly enzyme ISCU, mitochondrial |
| A8MPS7 | YDJC | 34,444 | 31 | Carbohydrate deacetylase |
| Q7Z5P9 | MUC19 | 597,790 | 20 | Mucin-19 |
| O15054 | KDM6B | 180,299 | 20 | Lysine-specific demethylase 6B |
| Q96C57 | CL043 | 28,153 | 38 | Protein CUSTOS (C12orf43) |
| Q92523 | CPT1B | 87,744 | 26 | Carnitine O-palmitoyltransferase 1, muscle isoform |
| Q9NVN8 | GNL3L | 65,532 | 28 | Guanine nucleotide-binding protein-like 3-like protein |
| Q96N23 | CFA54 | 92,263 | 27 | Cilia- and flagella-associated protein 54 |
| A2CJ06 | DYTN | 65,279 | 22 | Dystrotelin |
| Q8TBH0 | ARRD2 | 44,351 | 21 | Arrestin domain-containing protein 2 |
| Q15417 | CNN3 | 36,391 | 32 | Calponin-3 |
| Q9Y478 | AAKB1 (PRKAB1) | 30,363 | 32 | 5’-AMP-activated protein kinase subunit beta-1 |
| Controls n = 42 | PEX n = 29 | RVO n = 11 | |
|---|---|---|---|
| Gender (f/m) | 18/24 | 14/15 | 6/5 |
| Age (year) | 63 ± 7 | 70 ± 4 | 58 ± 2 |
| Platelet count (×103/µL) | 257.58 ± 53.39 | 268.40 ± 80.8 | 259.71 ± 36 |
| Mean platelet volume (MPV) (fL) | 10.37 ± 0.96 | 10.18 ± 0.96 | 10.36 ± 0.81 |
| Neutrophil/LymphocyteRatio (NLR) | 2.38 ± 0.76 | 2.60 ± 1.04 | 2.51 ± 0.93 |
| Visual acuity (log MAR) | 20/22 ± 2 | 20/22 ± 2 | 20/120 ± 5 * |
| Intraocular pressure (mmHg) | 14.95 ± 1.81 | 16.24 ± 2.0 | 14.35 ± 3.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ugurel, E.; Narimanfar, G.; Cilek, N.; Kesim, C.; Altan, C.; Sahin, A.; Yalcin, O. Platelet Proteome Reveals Novel Targets for Hypercoagulation in Pseudoexfoliation Syndrome. Int. J. Mol. Sci. 2024, 25, 1403. https://doi.org/10.3390/ijms25031403
Ugurel E, Narimanfar G, Cilek N, Kesim C, Altan C, Sahin A, Yalcin O. Platelet Proteome Reveals Novel Targets for Hypercoagulation in Pseudoexfoliation Syndrome. International Journal of Molecular Sciences. 2024; 25(3):1403. https://doi.org/10.3390/ijms25031403
Chicago/Turabian StyleUgurel, Elif, Ghazal Narimanfar, Neslihan Cilek, Cem Kesim, Cigdem Altan, Afsun Sahin, and Ozlem Yalcin. 2024. "Platelet Proteome Reveals Novel Targets for Hypercoagulation in Pseudoexfoliation Syndrome" International Journal of Molecular Sciences 25, no. 3: 1403. https://doi.org/10.3390/ijms25031403
APA StyleUgurel, E., Narimanfar, G., Cilek, N., Kesim, C., Altan, C., Sahin, A., & Yalcin, O. (2024). Platelet Proteome Reveals Novel Targets for Hypercoagulation in Pseudoexfoliation Syndrome. International Journal of Molecular Sciences, 25(3), 1403. https://doi.org/10.3390/ijms25031403






