Abstract
In the last decade, many small molecules, usually characterized by heterocyclic scaffolds, have been designed and synthesized as tyrosine kinase inhibitors (TKIs). Among them, several compounds have been tested at preclinical and clinical levels to treat glioblastoma multiforme (GBM). GBM is the most common and aggressive type of cancer originating in the brain and has an unfavorable prognosis, with a median survival of 15–16 months and a 5-year survival rate of 5%. Despite recent advances in treating GBM, it represents an incurable disease associated with treatment resistance and high recurrence rates. For these reasons, there is an urgent need for the development of new pharmacological agents to fight this malignancy. In this review, we reported the compounds published in the last five years, which showed promising activity in GBM preclinical models acting as TKIs. We grouped the compounds based on the targeted kinase: first, we reported receptor TKIs and then, cytoplasmic and peculiar kinase inhibitors. For each small molecule, we included the chemical structure, and we schematized the interaction with the target for some representative compounds with the aim of elucidating the mechanism of action. Finally, we cited the most relevant clinical trials.
1. Introduction
Glioblastoma (GBM) is an intrinsic brain cancer originating from neuroglial stem or progenitor cell anomalies [1]. Under physiological conditions, neural progenitor cells (NPCs) give origin to central nervous system (CNS) glial and neuronal cell populations [2], while metabolic enzymes-related gene mutations afford pathological transformation in GBM cells [3]. GBM is characterized by a high degree of heterogeneity at the genetic and cellular level, thus it is difficult to treat. For this reason, the identification of new biomarkers constitutes a promising approach to improve diagnosis and to find cellular pathways to target [4]. According to the gliomas classification system, GBM is identified as grade IV lesion found in astrocytic cancer forms [5]. GBM development involves a total of 92 immune-related genes. Interestingly, the 14 most representative genes show an evident correlation with prognosis [6]. GBM has an overall incidence of approximately 240,000 new cases each year and its occurrence is closely tied to age, with the median age at diagnosis falling within the 65-year range, and affecting males approximately 1.7 times more frequently than females [7]. The disease is generally characterized by a poor prognosis with a 10-years survival rate of 0.71% and a median survival of 15 months [8,9]. The current standard of care includes maximal surgical resection, radiotherapy and temozolomide (TMZ) chemotherapy [10].
In addition to TMZ, there are currently three FDA-approved drugs for high-grade glioma (HGGs), i.e., bevacizumab (BVZ), lomustine and intravenous carmustine [11]. BVZ, approved in 2009 to treat recurrent GBM, is a therapeutic antibody binding and inhibiting vascular endothelial growth factor (VEGF). Since malignant gliomas are characterized by a strong neovascularization, BVZ is administered to prevent angiogenesis by VEGF inhibition. Lomustine, also named CCNU (from its chemical name chloroethyl-cyclohexyl-nitrosourea) is a non-specific alkylating agent approved in 1976 to treat HGGs. It is responsible to crosslink DNA and RNA in proliferating cells, thus triggering cell death in cancer cells. Similarly, carmustine, also known as BCNU (bis-chloroethyl-nitrosourea), is a non-specific alkylating agent approved by in 1977 to treat HGGs [11].
Among FDA-approved devices, only carmustine wafer implants and tumor treatment fields (TTFields) were approved for new diagnoses [11]. Carmustine wafer implants were approved for HGGs treatment in 1996 and consist of biodegradable polymer wafers, each containing 7.7 mg of carmustine. TTFields are portable devices applied to the shaved scalp, delivering low-intensity (1–3 V/cm) and intermediate-frequency (200 kHz) alternating electric fields, disrupting mitosis in cancer cells. When compared with the current standard of care, only TTFields improve overall survival (20.5 vs. 15.6 months) and progression-free survival at six months (56% vs. 37%) [11]. Moving towards strategies to sustain a longer response and increase the long-term survival rates, isotretinoin (13-cis retinoic acid) was studied. This compound is a synthetic retinoid inhibiting cell proliferation and migration. It was already evaluated for both sustainment therapy and relapsed disease in HGGs [12,13].
In the immunotherapy field, the use of chimeric antigen receptor (CAR) T cells and immune checkpoint inhibitors are emerging as valuable strategies to tackle GBM. CAR T cell therapy is a relatively recent approach in which T lymphocytes from a patient are genetically modified to target the tumor once reinfused into the patient’s body. For GBM, the antigen-recognition molecules that confer antigen specificity are, for example, epidermal growth factor receptor variant III (EGFRvIII), interleukin (IL)-13R2 and human epidermal growth factor receptor 2 (HER2) [14].
Immune checkpoint inhibitors enhance the body’s natural immune response against cancer cells. Some commonly targeted checkpoint proteins include programmed cell death protein 1 (PD-1), programmed cell death ligand 1 (PD-L1), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4). Several phase I trials are evaluating these approaches for GBM patients. For instance, phase I trial NCT02794883 investigated the safety of CTLA-4 and PD-L1 antibodies in recurrent GBM patients [15]. Also, Phase I trial NCT02311920 evaluated anti-CTLA-4 and/or anti- PD-1 [16] combined with TMZ for newly diagnosed GBM/gliosarcoma patients.
Protein kinases, one of the largest enzyme families in mammalians [17], exert their catalytic action by transferring the terminal phosphate group from nucleotide triphosphates to serine, tyrosine or threonine OH-groups. Indeed, protein kinases are divided in serine-threonine kinases (STK), tyrosine kinases (TKs) and non-specific TK. Kinases bind ATP in a pocket between the catalytic domain’s lobes, and the interaction is stabilized by multiple hydrogen bonds [18]. Kinases can be classified into transmembrane receptors and, intracellular kinases. In physiological conditions, these enzymes play a pivotal role in cell growth, proliferation, differentiation and apoptosis. These enzymes are overexpressed, hyperactivated or mutated in different types of cancers, included GBM [19]. Kinases represent pharmacologically attractive targets for anticancer treatment due to a high degree of conservation of their catalytic domain, low toxicity and oral availability [20]. Many kinase inhibitors, such as ATP-competitive types I and II, as well as allosteric types III and IV, have been developed in last decades [21].
GBM is characterized by several signaling pathways alterations. Among them, phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) regulatory pathway is one of the most important. In fact, aberrant AKT activation contributes to resistance towards first generation TKIs, such as the EGFR inhibitor gefitinib [22]. Interestingly, 67.3% of GBMs exhibits some kinases mutation or amplification, specifically for EGFR, PDGFR, FGFR2/3 and MET [23].
Many kinase inhibitors have been approved to treat several types of cancers but none of them still reached approval, alone or in combination, for GBM therapy. A schematic summary of the current and ongoing therapeutic approaches are reported in the Figure 1. In this review, we report the state-of-the-art of small molecule tyrosine kinase inhibitors (TKIs) tested in different GBM models, focusing on the last five years. The inhibitors are described based on the targeted kinases. Furthermore, the current clinical trials involving TKIs alone or in combination with other agents, are reported.
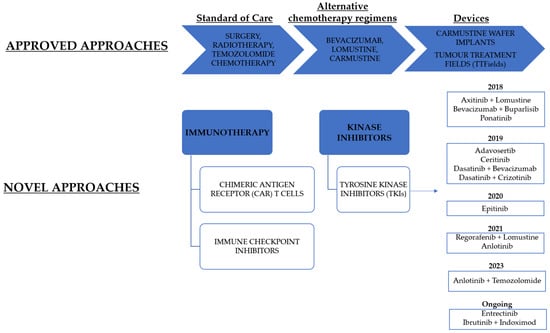
Figure 1.
A schematic representation of current and ongoing therapeutic approaches for GBM treatment. TKIs are classified based on the year of the clinical trial completion.
2. TK Inhibitors
2.1. ALK Inhibitors
Anaplastic lymphoma kinase (ALK) is a transmembrane receptor tyrosine kinase that plays a central role in neurogenesis [24,25]. Several cancer forms express aberrant ALK variants due to amplifications, point mutations, and chromosomal rearrangements [26,27,28]. Interestingly, ALK is overexpressed in glioblastoma (GBM) [29,30]. The analysis conducted by Karagkounis et al. on ALK expression in 51 GBM patients revealed its presence in most samples, although rearrangements appear to be relatively uncommon [29,31]. ALK receptor represents a starting signal able to stimulate various pathways (e.g., Ras/MAPK, mTOR/PI3K/Akt, phospholipase C-g) [31]. Since abnormal regulation of PI3K/Akt signaling has been found in U87 GBM cells [32], ALK inhibition represents a promising therapeutic option in GBM treatment. ALK inhibitors have been tested alone or in combination with other anticancer agents to treat GBM. Unfortunately, mutated ALK lacks sensitivity to monotherapies with ALK inhibitors and prevents their use as a first-line therapy [33].
Crizotinib, Alectinib and Ceritinib
Crizotinib 1 (Figure 2 and Figure 3) is an approved first generation ALK inhibitor (IC50 vs. EML4-ALK = 250–300 nM) [34] used to treat ALK-positive non-small cell lung cancer (NSCLC).
Crizotinib is a type I kinase inhibitor and binds ALK in its active conformation through the formation of different hydrogen bonds. In detail, the NH2 group and N1 of the pyridine ring form two hydrogen bonds with the backbone oxygen atom of Glu1197 and the backbone NH hydrogen atom of Met1199, respectively. In addition, two hydrogen bonds between the inhibitor and the catalytic pocket are mediated by two molecules of water; specifically, the pyrazole N2 nitrogen atom forms a hydrogen bond with a water molecule bound to Asp1203, and the NH group of the piperidine interacts with Ala1200 through another water molecule (Figure 2) [35].
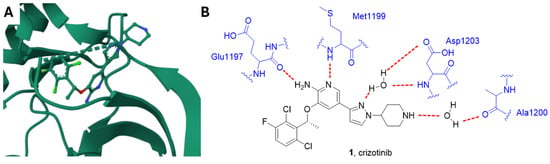
Figure 2.
(A) Crystal structure of ALK in complex with crizotinib (PDB: 2XP2) [36]. (B) Schematic representation of the main interactions between crizotinib and ALK with amino acid residues crucial to the interaction highlighted in blue. Hydrogen bonds are represented as red dashed lines.
During the first two years of administration, resistance phenomena were commonly observed, due to both ALK TK domain mutations and alternative signaling pathway activation. Thus, the development of more potent and structurally different inhibitors emerged as a primary need [34].
Recently, crizotinib in combination with TMZ and standard radiotherapy showed a tolerable safety profile and promising efficacy as a first-line therapy for GBM patients [37].
Alectinib 2 (Figure 3) is a second-generation ALK inhibitor, approved in 2015 for the advanced ALK-positive NSCLC patients who showed disease progression or were intolerant to crizotinib. It is a highly specific ALK blocker (IC50 = 0.83 nM) [38,39]. According to Sakamoto et al. [39], alectinib inhibited ALK L1196M, the pivotal mutation commonly conferring resistance to TKIs, and blocked EML4-ALK L1196M-driven cell growth. Clinically, it was also partially effective on NSCLC brain metastasis for 52% of examined patients in a phase I/II study [40]. Alectinib reduced proliferation and induced apoptosis in ALK expressing GMB cells, while cells depleted for ALK or showing a high expression of cMyc and activation of the ERK1/2 pathway led to a primary resistance against alectinib. The inhibition of these pathways using a cMyc inhibitor or a MEK inhibitor, overcame alectinib resistance. The combination of alectinib with radiotherapy induced a synergistic effect in inhibition of proliferation in non-resistant and alectinib resistant GBM cells [41].
Ceritinib 3 (Figure 3), another second-generation ALK inhibitor (IC50 = 0.15 nM versus ALK) was approved in 2014 for ALK-positive NSCLC treatment [42]. Interestingly, it is characterized by a high-ALK selectivity, about 20-fold more than crizotinib [42]. Moreover, a multicenter phase II trial showed a 45% intracranial response in NSCLC brain metastasis, based on response evaluation criteria in solid tumors [43].
Kawauchi et al. [44] demonstrated the anticancer activity of alectinib and ceritinib against U87MG, LN229, and GSC23 GBM cell lines. ALK inhibitors effectively induced GBM cell death and inhibited STAT3, causing caspase-dependent/independent cell death when administered alone. Furthermore, antitumor activity was also observed against a temozolomide osimertinib-U87MG cell line. Interestingly, oral administration of alectinib and ceritinib synergistically prolonged the survival of intracerebral GBM xenograft-harboring mice, when compared to controls.
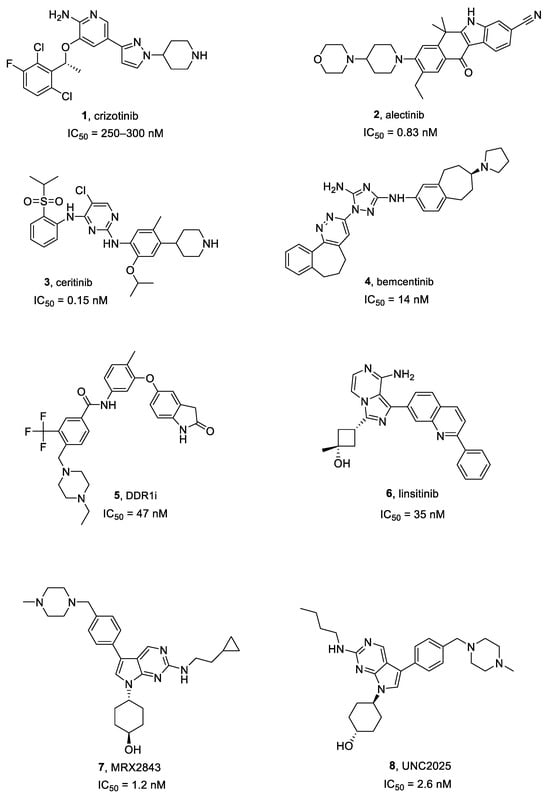
Figure 3.
Structures of ALK, AXL, DDR1, IGF1R, and MER inhibitors 1–8.
According to Goker Bagca et al. [45] alectinib/TMZ dual treatment exhibited a synergistic reduction of T98G GBM cells viability and decreased expression of PI3K/AKT and their associated genes. Interestingly, while alectinib induces G0/G1 cell cycle arrest, alectinib/TMZ activity promotes S-phase arrest.
2.2. AXL Inhibitors
AXL is a transmembrane TK receptor belonging to the Tyro3, Axl, and Mertk (TAM) kinase family and it can be activated by growth arrest-specific protein 6 (GAS6) and Pros1 (Protein S) [46,47]. AXL phosphorylation leads to PI3K and AKT activation. The Gas6/AXL/PI3K/Akt axis protects cells from apoptosis by S6K activation and BCL-2 phosphorylation [44]. AXL is widely expressed in GBM, and its biologically active form Phospho-AXL (P-AXL) is associated with poor prognosis [48]. The receptor activation leads to increased tumor proliferation, cell migration, and angiogenesis in both in vitro and in vivo GBM models [49,50,51].
Bemcentinib
Bemcentinib 4 (Figure 3), also known as R428, is the most specific AXL inhibitor and entered clinical trials in 2014 (i.e., NCT02424617, NCT02922777). It is currently being tested in an early phase I clinical trial on recurrent forms of GBM (NCT03965494) [52,53]. It inhibits AXL with an IC50 of 14 nM [50].
Scherschinski et al. [54] tested the combination of bemcentinib and TMZ towards GBM to overcome TMZ resistance and to increase therapy effectiveness. Both naïve (SF126 and U118MG) and TMZ-resistant (SF126-TR and U118MG-TR) cell lines were treated and, whereas single bemcentinib or TMZ administration was ineffective, their combination resulted in a significant reduction of cell survival. Similar results were observed by exposing cells to a bemcentinib/radiation dual treatment vs. the radiation approach [54].
Furthermore, the combinatorial treatment of bemcentinib and the monoclonal antibody Nivolumab prolonged mice survival, suggesting that TKIs can be effective adjuvants to flank traditional therapies, such as PD-1 antibodies [55].
2.3. Discoidin Domain Receptor 1 (DDR1) Inhibitors
DDR1 is a receptor TK that is activated by collagens in the extracellular matrix. High levels of collagens have been identified in brain tumors highlighting DDR1 as a valuable target in GBM treatment [56].
DDR1 Inhibitors
Compound 5 (Figure 3), also known as DDR1-IN-1, is a selective and poorly studied DDR1 inhibitor [56], characterized by an IC50 of 47 nM [57]. According to Vehlow et al. [56], compound 5 significantly induced both radio-sensitization and chemo-sensitization to TMZ in GBM cell lines.
2.4. IGF1R Inhibitors
Insulin-like growth factor 1 receptor (IGF1R) is a ubiquitous receptor TK, activated by both IGF1 and IGF2. It controls several essential cell functions, and is involved in several chronic lung diseases, including cancer. The IGF1R signaling pathway can contribute to neoplastic cells proliferation, migration, survival and chemotherapy resistance. Thus, IGF1R has been evaluated as a pharmacological target for the treatment of several cancers, including NSCLC [58]. Martin et al. [59] observed that in vitro IGF1R nuclear localization increases GBM cell motility and metabolism, while in vivo, IGF1R can translocate to the nucleus, allowing both a higher proliferation rate and the earlier development of GBM.
Linsitinib
Linsitinib 6 (Figure 3), also named OSI-906, is a highly selective and bioavailable IGF1R inhibitor (IC50 = 35 nM) [60]. Two phase I clinical trials showed a total response rate of about 30% in advanced solid cancers, and some subjects have obtained enduring advantages from the IGF1R inhibition [61].
Linsitinib slightly decreased GBM cell viability, if compared with a multi-kinase inhibitor named BMS-754807, inducing G1 arrest [62].
2.5. MER Inhibitors
Membrane estrogen receptor (MER) TK is a major macrophage receptor associated with apoptotic cells clearance. Its expression is mainly detected in M2 macrophages [63]. Since cancer-associated macrophages often show an M2-like phenotype and participate in cancer progression, angiogenesis, and in tissue remodeling, it seems reasonable to investigate MER as a therapeutic target [63]. MER TK is overexpressed in GBM multiforme, being associated with increased invasiveness. Its depletion changes the glioma cells rounded morphology, also decreasing their invasive potential [64].
The effect of MER on glioma cell invasion is mediated by actomyosin contractility as the expression and phosphorylation of myosin light chain 2 are strongly associated with MER activity. Moreover, the DNA damage increased the upregulation and phosphorylation of MER preventing death cells. This effect is strongly decreased in case of kinase depletion or overexpression of its inactive mutant [64].
2.5.1. MRX2843
MRX2843 7 (Figure 3) is a dual inhibitor of MER (IC50 = 1.2 nM) and FLT3 and is active against FLT3 point mutations. It also showed therapeutic effectiveness in mouse xenograft models of AC220-resistant acute myeloid leukemia (AML) [65]. Furthermore, RX-2843 synergized with vincristine in the inhibition of acute lymphoblastic leukemia cell lines [66].
Su et al. [67] evaluated the effects of MRX2843 in several GBM cell lines, e.g., GSC407, GSC923, and U251, in macrophages, human brain microvascular endothelial cells (HBMECs), and in a syngeneic GL261 orthotopic GBM murine model. MRX-2843 inhibited cell growth and induced apoptosis in human GBM cells showing after 48 h of treatment EC50 values of 95.5, 288.1, and 217.7 nM in U251, GSC923, and GSC407, respectively. A reduction of colony formation was observed after 7–14 days when U251, GSC923, and GSC407 cells were treated with 7 at 100 or 500 nM for only 48 h. It also lowered Mer, AKT and ERK expression, essential for cell survival signaling. It induced an in vivo decrease in both vascular formation and levels of CD206+ glioma-associated microglia and macrophages [67]. The authors showed that MRX-2843 penetrates the blood–brain barrier (BBB) accumulating in the brain tumor tissue with levels about five times higher than the plasma, suggesting a better pharmacokinetic behavior compared to UNC2025 (see next paragraph) [67].
2.5.2. UNC2025
UNC2025 8 (Figure 3) is an ATP competitive class I MER inhibitor (IC50 = 2.6 nM) and showed a favorable pharmacokinetic (PK) profile in mice. Preclinical evaluations in NSCLC models exhibited a 50% reduction in cancer cell survival and colony formation abrogation at a 300 nM dose [68].
Wu et al. [69] studied both in vitro and in vivo UNC2025-induced Mer inhibition. In vitro tests were carried out in several cell lines, i.e., U87, U251, GSC11, GSC20 and EOC2. Among them, GSC11 were the more responsive to proliferation inhibition. Moreover, syngeneic orthotopic allograft mouse GBM models were randomized to receive vehicle (control), UNC2025, fractionated radiation or UNC2025/X-ray combination. Interestingly, while median survival rates were comparable with or without UNC2025, a significant growth delay was observed in UNC2025/X-ray treated mice, with complete responses in 19% of patients. Lastly, UNC2025 lowered CD206+ macrophages levels in mouse cancer models [69].
Analysis of PK profile of UNC2025 in in vivo mouse model of GBM showed a satisfactory BBB permeation and a brain accumulation 4-fold increase with respect to non-tumor brain tissues, demonstrating improved delivery probably due to the tumor microenvironment [69].
2.6. c-Met Inhibitors
Mesenchymal-epithelial transition factor (c-MET) is a hepatocyte growth factor (HGF) receptor regulating embryonal cells morphogenesis [70]. It was recently reported to be involved in cancer development regulation [71,72] and several studies have been made to develop c-MET-targeted agents to be employed in several malignancies [73,74]. Interestingly, c-MET expression inhibition was found to block glioma cell growth and migration [75].
2.6.1. Capmatinib
Capmatinib 9 (Figure 4) is a highly selective c-MET inhibitor (IC50 = 0.13 nM [76]), characterized by both in vitro and in vivo effectiveness vs. c-MET-activated preclinical cancer models [77]. It is being investigated as both a single and combinatorial therapeutic agent in several clinical trials [78].
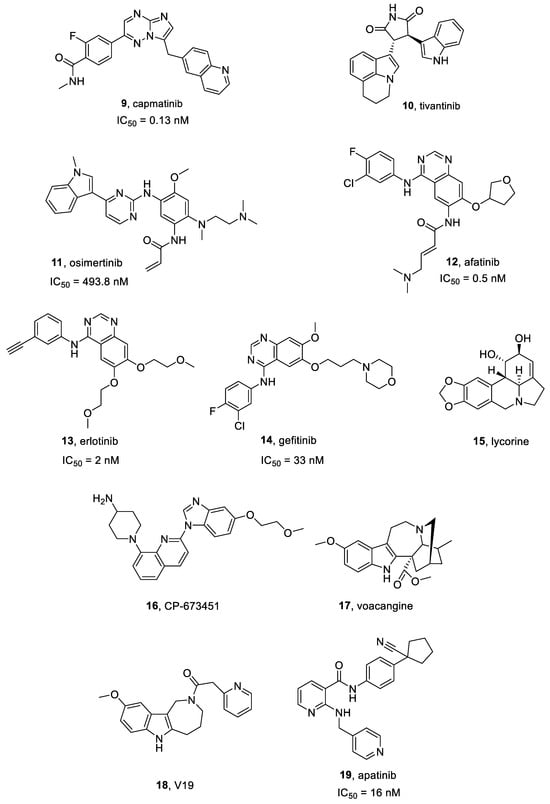
Figure 4.
Structures of c-Met, EGFR, PDGFR, and VEGFR inhibitors 9–19.
According to Baltschukat et al. [78], capmatinib was highly selective towards c-MET, as well as active against neoplastic models showing c-MET amplification, overexpression, exon 14 skipping mutations or HGF-mediated activation. Interestingly, in vitro sensitivity was observed in autocrine cell lines derived from GBM.
2.6.2. Tivantinib
Tivantinib 10 (Figure 4) is a highly specific small molecule c-MET inhibitor, characterized by antitumor activity in Microphthalmia transcription factor (MITF)-associated tumors [79] and possesses an IC50 = 300–400 nM in MKN45, EBC1, and A549 cells [80]. Interestingly, tivantinib also reduced proliferation of multiple myeloma cell lines downregulating c-MET signaling and inhibiting MAPK and PI3K pathways [81].
When tested at high concentration (1 μmol/L) [82], tivantinib inhibited U251 and T98MG GBM cells proliferation and colony formation, while a lower concentration (0.1 μmol/L) did not affect cell proliferation. High concentration of tivantinib in combination with the PI3K inhibitor LY294002 and the mTOR inhibitor rapamycin, strongly inhibited GBM cell proliferation [82].
2.7. EGFR Inhibitors
Epidermal growth factor receptor (EGFR) is formed by an extracellular binding domain, a transmembrane portion and intracellular domain endowed with the kinase activity. The receptor is activated by various ligands, including EGF and TGF-α. EGFR activation is induced by both homo- and heterodimerization on the cell surface, leading to the intracellular tyrosine kinase domain phosphorylation [83]. EGFR is overexpressed in both primary (about 60%) and secondary GBMs (about 10%) and is related to the most aggressive GBM forms [83]. Several comparison studies highlighted a significant difference in progression-free survival (PFS) between first and second-generation EGFR inhibitors. For instance, afatinib (second generation) was found to be superior to gefitinib (first generation) in terms of PFS (11 versus 10.9 months) in a phase 2B trial on lung cancer [84]. Third generation inhibitors (i.e., osimertinib) demonstrated a PFS superiority (18.9 versus 10.2 months) compared to second generation ones (i.e., erlotinib and gefitinib) [85].
2.7.1. Osimertinib
Osimertinib 11 (Figure 4) is a selective and irreversible third generation EGFR inhibitor. It is active on EGFR (IC50 = 493.8 nM) and different mutated forms, i.e., exon 19 deletion EGFR (IC50 = 12.92 nM), and L858R/T790M EGFR (IC50 = 11.44 nM) [86]. Osimertinib exhibited significant clinical activity in CNS metastases, due to its optimal permeability through the BBB, higher than other EGFR inhibitors. To date, osimertinib is the golden standard choice for EGFR-mutated NSCLC patients for its efficacy and safety profile [87].
According to Liu et al. [88], osimertinib showed a satisfactory preclinical activity in GBM in both in vitro and in vivo models by inhibiting the growth of six GBM cell lines in a dose-dependent manner. It induced cell cycle arrest, colony formation inhibition, migration and invasiveness of GBM cells. Moreover, osimertinib prolonged animal survival in an orthotopic xenograft murine model.
Osimertinib inhibited EGFRvIII TK with high potency (<100 nM) and its downstream pathway. Moreover, it blocked D317 GSC line growth within in vitro and in vivo models [89].
Analysis of the PK profile of osimertinib showed that the compound well penetrates the BBB in an in vivo mouse model of cancer achieving within three hours after the administration of a single 25 mg/kg oral dose, a brain concentration of 3,695 ± 425 nM, with a brain to plasma ratio >10 [89].
Osimertinib was showed to cause paraptosis in LN-229, U87MG, LN-18 and SF-539 cells. A time-dependent cytoplasmic vacuoles accumulation was observed in such cell lines, while cell membranes and nuclei mostly remained undamaged [90].
2.7.2. Afatinib
Afatinib 12 (Figure 4 and Figure 5) is an FDA-approved irreversible EGFR inhibitor (IC50 = 0.5 nM) [91], also acting on EGFRvIII mutation [92]. It also irreversibly binds HER2 and HER4.
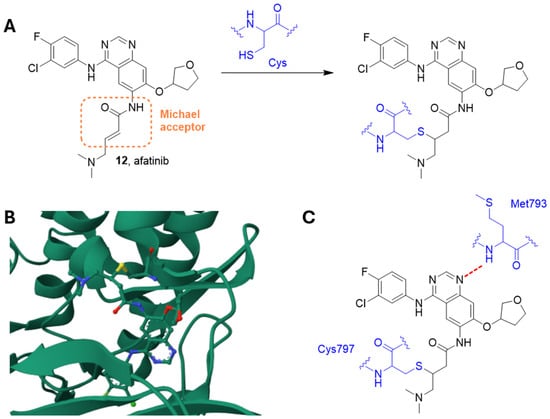
Figure 5.
(A) The irreversible reaction between afatinib and a residue of cysteine. (B) Crystal structure of AGFR in complex with afatinib (PDB: 4G5P). (C) Schematic representation of the main interactions between afatinib and EGFR, with amino acid residues crucial to the interaction highlighted in blue. Hydrogen bond is represented as a red dashed line [93].
Indeed, this compound contains an electrophilic group able to give Michael addition with conserved cysteine residues within the catalytic domains of these enzymes, i.e., EGFR Cys797 of EGFR, Cys805 of HER2, and Cys803 of HER4. Analysis of the crystal structure of wild-type EGFR (residues Gly696-Gly1022) in complex with afatinib showed the formation of the irreversible sulfur bridge together with a hydrogen bond between the amide nitrogen of Met793 at the hinge region and the quinazoline core of afatinib (Figure 5) [93].
Recent studies have proven a significant PFS increase in afatinib-treated NSCLC patients with EGFR mutations when compared to gemcitabine plus cisplatin or pemetrexed plus cisplatin treatments. The same parameter was improved in EGFR mutant NSCLC patients with brain metastases [94].
From a PK point of view, afatinib achieves its maximum plasma concentration 2–5 h after oral administration. The contemporary assumption of food significantly reduces the blood levels of the drug. Afatinib possesses a half-life of 37 h and is excreted by feces (about 95%) and urine (about 5%) [91].
Vengoji et al. [92] demonstrated that afatinib and TMZ combination synergistically inhibited proliferation, invasiveness, motility and clonogenic survival of GBM cells, and induced their senescence. Afatinib lowered U87 GBM cells proliferation and motility by inhibiting EGFRvIII and focal adhesion kinase (FAK) signaling pathways, respectively. Noteworthy, afatinib/TMZ association decreased U87 GBM xenograft growth and progression when compared to single drug treatments. These findings encourage afatinib/TMZ use evaluation in EGFR/EGFRvIII GBM patients.
2.7.3. Erlotinib
Erlotinib 13 (Figure 4 and Figure 6), also known as CP-358774 and OSI-774, is a selective EGFR inhibitor, characterized by an IC50 value of 2 nM [95]. It blocks cancer cell proliferation and cell cycle and inhibits downstream EGFR signal transduction.
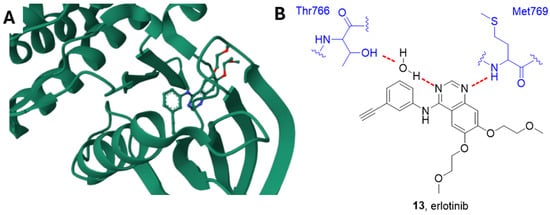
Figure 6.
(A) Crystal structure of EGFR in complex with erlotinib (PDB: 1M17). (B) Schematic representation of the main interactions between erlotinib and EGFR, with amino acid residues crucial to the interaction highlighted in blue. Hydrogen bonds are represented as red dashed lines [96].
Erlotinib is an ATP-competitive inhibitor, and the quinazoline scaffold accommodates into the catalytic pocket connecting the N- and C-lobes. According to the study carried out by Stamos et al., the N1 of the quinazoline forms a hydrogen bond with the Met769 amide nitrogen, while the N3 is involved in a water-mediated hydrogen bond with the OH Thr766 side chain (Figure 6) [96].
From a PK point of view, erlotinib showed good oral bioavailability (60% absorption), which can be increased to almost 100% if swallowed with food. Otherwise, the increase in the gastrointestinal pH reduced erlotinib absorption. About drug distribution, more than 90% of erlotinib is bound to albumin and alpha-1 acid glycoprotein. Erlotinib is mainly a CYP3A4 substrate, and about 83% of the administered drug was recovered from feces and 8% from urine, with a half-life of around 36 h [97]. Erlotinib was approved for NSCLC and pancreatic cancer treatments [97].
Amini et al. [98] reported that treating U373 GBM cells with PIK3R3-siRNA plus Erlotinib synergistically induced a decrease in cell survival. Such findings support the hypothesis that PIK3 pathway blockage could heighten the anticancer effects of erlotinib in GBM cells.
A combination of erlotinib and MLN0128 (a mTOR inhibitor) showed a synergistic anticancer activity inhibiting p-EGFR, MAPK and PI3K pathways in vitro. This combinatorial treatment also inhibited tumor growth in an orthotopic xenograft murine model, significantly prolonging GBM-bearing mice survival [99].
Moreover, it was demonstrated that treating U251MG cells with both erlotinib and the alkaloid oxymatrine significantly lowered p-EGFR, p-Akt and p-mTOR levels, and inhibited cancer cell proliferation, compared to erlotinib or oxymatrine monotherapies [100].
Mesbahi et al. [101] evaluated the erlotinib/arsenic trioxide combination on both U87-MG and A172 GBM cells. Their results showed a reduction of cell proliferation and metabolic activity. Interestingly, such combination induced G2/M cell cycle arrest, as well as an apoptotic cell death rate and significant increase in ROS (reactive oxygen species). These results suggest that EGFR inhibition could overcome GBM resistance to arsenic trioxide treatment.
2.7.4. Gefitinib
Gefitinib 14 (Figure 4), also known as ZD1839, is a selective ATP-competitive EGFR and HER-2 inhibitor, which displays an IC50 of 33 and 79 nM towards EGFR and HER-2, respectively [102,103]. It is the first EGFR-targeting anticancer molecule lunched in Japan, Australia and USA for NSCLC treatment. Gefitinib is prescribed as a monotherapy to treat both locally advanced or metastatic NSCLC after failure of docetaxel and platinum-based chemotherapies. In preclinical studies, gefitinib showed anticancer effects against several human cancer cell lines expressing EGFR (e.g., lung, ovarian, breast and colon cancers). In GBM murine xenograft models, gefitinib combined with others cytotoxic agents produced both cancer regression and tumor growth inhibition, improving the rate of survival. Gefitinib in combination with temozolomide in U87MG cell line induced cytotoxic effects with IC50 values of 11 μM [104].
2.7.5. Lycorine
Lycorine 15 (Figure 4) is a pyrrolo[3,2,1-de]phenanthridine ring-type alkaloid extracted from different Amaryllidaceae genera, and is characterized by several biological properties, such as anti-cancer, antiviral and anti-inflammatory effects. Lycorine exhibited cytotoxic effects on various malignancies, e.g., cervical cancer, leukemia, multiple myeloma, hepatocellular carcinoma, prostate cancer, bladder cancer, breast cancer and GBM [105].
Lycorine lowered U251 GBM cells proliferation, colony formation and migration by causing EGFR-mediated apoptosis. Moreover, it inhibited the cancer growth in different in vivo murine models, e.g., a U251-luc intracranially orthotopic xenograft, an EGFR knockdown U251 subcutaneous xenograft, and a patient-derived xenograft model [105].
2.8. PDGFR Inhibitors
Platelet-derived growth factor receptors (PDGFRs) are transmembrane receptor TKs. Their signaling is mediated by monomeric PDGFRs dimerization and intracellular tyrosine autophosphorylation. PDGFR pathway is crucial for cancer growth, angiogenesis and lymphangiogenesis. Aberrant PDGFR signaling has been observed in several malignancies, i.e., glial brain tumors, chronic myelomonocytic leukemia, prostate, lung/breast adenocarcinoma, HCC and NSCLC [106]. PDGFR and its ligand PDGF are co-expressed in GBM. Interestingly, PDGFR mutations or amplification have been identified as indicators of GBM subgroups originating from a PDGF receptor-responsive cell [107].
CP-673451
CP-673451 16 (Figure 4) is a potent PDGFR inhibitor, about 450-times more selective for PDGFRβ (IC50 = 1 nM in cells) than vs. other PDGFR subclasses [108,109].
Lane et al. [105] identified CP-673451 as an inducer of neurite-like outgrowth in three GBM cell lines (U87, U251 and LN229) and astrocytes, suggesting that the compound promotes cell differentiation into neural-like cells with a positive effect on GBM patients. Furthermore, CP-673451 improved anticancer TMZ activity in a U87 xenograft GBM murine model. Cancer volumes were significantly reduced by the combination treatment respect to both singular treatments [110].
The analysis of the PK profile of CP-673451 in a GBM rat model showed that a single oral dose of 50 mg/kg determined a plasma concentration above the EC50 for ~4 h [109].
2.9. VEGFR Inhibitors
Vascular endothelial growth factors receptors (VEGFRs) regulate both vasculogenesis and angiogenesis [111]. VEGFR family includes three members, i.e., VEGFR-1, VEGFR-2 and VEGFR-3. VEGFR-1 and VEGFR-2 play crucial roles in both physiological and pathological angiogenesis, including cancer angiogenesis. VEGFR-3 is involved in angiogenesis regulation in early embryogenesis, as well as functioning as lymphangiogenesis critical regulators [111]. In GBM, VEGFRs are highly upregulated, and the expression degree was associated with the malignancy grade. VEGFR signaling plays a key role in the development of the GBM immunosuppressive tumor microenvironment [112].
2.9.1. VGB
VGB is a peptide (sequence 2HNCIKPHQGQHICNDE-COOH) [113], specifically developed to bind both VEGFR1 and VEGFR2. Inhibitor design was based on residues contained in VEGFA, involved in the interaction with VEGFR1 and VEGFR2.
This peptide inhibits U87 GBM cells proliferation with a IC50 of 0.92 μM. Since, U87 GBM cells over-expressed VEGFR2, these data confirm the involvement of this receptor subtype in GBM [113].
2.9.2. Voacangine
Voacangine 17 (Figure 4) is an indole alkaloid isolated from Voacanga Africana and Tabernaemontana Catharinensis root barks [114]. It significantly suppresses VEGF-induced tube formation and chemoinvasive angiogenetic processes in vitro models. Furthermore, voacangine inhibits in vivo angiogenesis in chorioallantoic membrane at non-toxic concentrations. Moreover, decreased hypoxia inducible factor-1a and its target gene (VEGF) expression levels, in a dose-dependent manner [114].
Cho et al. [115] developed a set of voacangine analogues, specifically targeting and modulating VEGFR2 activity. Among these compounds, V19 18 (Figure 4) increased antiangiogenic activity against VEGF-induced VEGFR2 phosphorylation in a U87 GBM mouse xenograft model, without cytotoxic effects [115].
2.9.3. Apatinib
Apatinib 19 (Figure 4), also named Rivoceranib, is a small molecule TKI, approved in China for gastric cancer treatment. It was showed to block tumor angiogenesis by inhibiting VEGF signal transduction, particularly, binding VEGFR2 (IC50 = 16 nM) [116].
When tested in 15 patients (after chemoradiotherapy) suffering from recurrent grade IV GBM, apatinib exhibited a PFS of 2 months, as well as a median overall survival rate of 6.5 months with the most common side effects being thrombocytopenia, asthenia and hand–foot syndrome [117].
2.10. FAK Inhibitors
Focal adhesion kinase (FAK) is a non-receptor TK, which acts as an adaptor protein regulating adhesion signaling and cell migration. It can also promote cell survival responding to stress stimuli. FAK transduces signals ranging from cell adhesions to regulation of functions altered in cancer, like cell survival, migration, and invasion [118].
PF573228
PF573228 20 (Figure 7) is a small molecule inhibitor, selectively targeting FAK’s ATP-binding site (IC50 = 4 nM) [119] and effectively blocking its catalytic activity in several cell lines including neuroblastoma cells [120].
Figure 7.
Structures of FAK, JAK, LCK, SYK, Src, and DYRK1A inhibitors 20–31.
Nguemgo Kouam et al. [121] showed that PF573228 reduced the adhesion of U87 and U373 GBM cells. Interestingly, another study [122] showed that treating GBM cells with PF-573228 arrested proliferation and decreased cell size. It also lowered GBM neurosphere growth.
2.11. JAK Inhibitors
Janus kinases (JAKs) are non-receptor TKs that include four members, i.e., JAK1, JAK2, JAK3 and TYK2 [123]. JAK/STAT pathway is activated by many protein ligands including cytokines, growth factors, peptide hormones and interferons, and regulates several cellular processes like cell proliferation, growth, differentiation, and apoptosis. Persistent or dysregulated JAK/STAT3 signaling is involved in many diseases characterized by chronic inflammation and fibrosis, as well as cancer [124]. In GBM, the inhibition of JAK/STAT pathway is involved in the regulation of inflammatory response, frequently present in malignancies [125].
2.11.1. AG490
AG490 21 (Figure 7) is a TKI specific for JAK2, able to decrease STAT3 phosphorylation. Its therapeutic potential has been proved in brain hemorrhage, fibrosis and liver injury treatments [126].
Lebedev et al. [127] evaluated AG490 in neuroblastoma, GBM, breast and NSCLC cells. GBM cells resulted to be sensitive to the compound and the effect was increased when used in combination with doxorubicin.
2.11.2. Ruxolitinib
Ruxolitinib 22 (Figure 7) is a reversible class I JAK inhibitor that competes with ATP in the JAK kinase catalytic site (IC50 = 0.40 nM) and was approved for myelofibrosis treatment. Its effectiveness in myelofibrosis has been mainly associated to the reduction of the inflammation induced by constitutive JAK-STAT activation, as well as by a non-specific myelosuppression [128,129].
From a PK point of view, ruxolitinib achieves peak plasma concentrations within one hour after administration and possesses a half-life of 2.3 h [128].
Delen and Doganlar [130] showed that ruxolitinib inhibited JAK/STAT pathway in GBM spheroids in a dose-dependent manner.
Ruxolitinib was demonstrated to improve TMZ’s apoptotic effects on U87MG cells, BCSCs (brain cancer stem cells) and HBMECs (human brain microvascular endothelial cells). Interestingly, it regulates the WNT signaling pathway both as single agent and in combination with TMZ [131].
2.12. LCK Inhibitors
Lymphocyte-specific protein tyrosine kinase (LCK) is a non-receptor TK, member of the Src family. It is involved in T-cell receptor signaling, also playing a crucial role in mediating B-cell receptor signaling in chronic lymphocytic leukemia cells. Furthermore, its expression was detected in several neural tissues, including hippocampus, cerebellum, and retina [132].
LCK was expressed at a high level in primary central nervous system lymphoma patients but at a low level in GBM patients. However, LCK expression positively correlated with the levels of infiltrating B cells in diffuse large B-cell lymphoma and GBM [132].
LCK-I
A-770041 23 (Figure 7) is a highly selective small molecule LCK inhibitor (IC50 = 147 nM) [133].
According to Zepecki et al. [134], A-770041 mediated Lck inhibition and Crk-II phosphorylation, pseudopodia formation and migration of human glioma stem cells (hGSCs). A-770041 in vivo intraventricular administration, employing an orthotopic xenograft glioma model, significantly decreased the cancer size. Moreover, treating of hGSCs with A-770041 resulted in a significant inhibition of self-renewal and cancer-sphere formation.
2.13. SYK Inhibitors
Spleen tyrosine kinase (SYK) is a cytoplasmic enzyme involved in mediating antigen-associated signals in both innate and adaptive immune systems [135]. Its activity is necessary to B cells, mast cells and for platelet propagation/activation. After being recruited, SYK can undergo to autophosphorylation, as well as to phosphorylation by Src kinase. Upon activation, SYK triggers multiple signaling cascades, giving several inflammatory or immunological outcomes. Thus, SYK could be pharmacologically targeted to modulate and treat inflammatory and autoimmune diseases, as well as hematological cancers [130]. SYK was expressed in both human and murine glioma cell lines. Its inhibition blocked migration, proliferation and colony formation [136].
Bay61-3606, Piceatannol and NVP-QAB205
BAY61-3606 24 (Figure 7) is a selective SYK inhibitor (IC50 = 10 nM [137]), characterized by anti-inflammatory effects on pathological processes like acute kidney injury. It exerts its activity by suppressing inflammatory macrophage response [138].
Piceatannol 25 (Figure 7), otherwise called Pic, a resveratrol derivative, is a selective SYK micromolar inhibitor [139]. It inhibits phosphorylated SYK expression and suppressed both migration and invasiveness of CAL27 (tongue squamous carcinoma) cells in a wound healing assay evaluation [138].
NVP-QAB205 26 (Figure 7) is an effective SYK phosphorylation inhibitor (IC50 = 10 nM [140]) and demonstrated excellent activity in preventing human mast cell and basophil activation [141].
Moncayo et al. [136] tested the above cited small molecule inhibitors on four different cell lines: BS287 GBM-derived spheres, U87, SF767 and BS125 GBM cells showing cell proliferation inhibition in all lineages. Piceatannol was the best proliferation inhibitor for both BS287 and U87 cells, while BAY61-3606 reached the most satisfactory results on SF767 and BS125 cell lines, followed by NVP-QAB205. Compound 24 decreased in vivo GBM growth and invasiveness, also reducing B and CD11b+ cell mobility and infiltration [136].
2.14. Src Inhibitors
Src family is a class of non-receptor TKs, playing a pivotal role in several cancers [142]. SRC activity is involved in GBM through the regulation of networks that control inflammation and metabolism [143,144].
2.14.1. KX2-361
KX2-361 27 (Figure 7) is a novel, non-ATP competitive small molecule Src inhibitor with a satisfactory cytotoxic activity against several CNS cancer cell lines [145].
KX2-361 was investigated against human and murine glioma cells and also in a syngeneic orthotopic GBM murine model [146]. Interestingly, it reduced Src autophosphorylation, and disrupted microtubule structure in the GL261 murine GBM cell line.
2.14.2. Si306 and Analogue Compounds
In recent years, we obtained promising results in developing compounds active against GBM by inhibiting Src. Derivative Si306 28a (Figure 7 and Figure 8) is an ATP-competitive small molecule Src inhibitor (Ki = 0.13 μM) [147,148] which accommodates into the Src catalytic pocket forming two hydrogen bonds, one between the C4 amino group and the Thr338 OH side chain, and the other one between the N2 of the pyrazolopyrimidine and the NH of Met341 (Figure 8) [147].
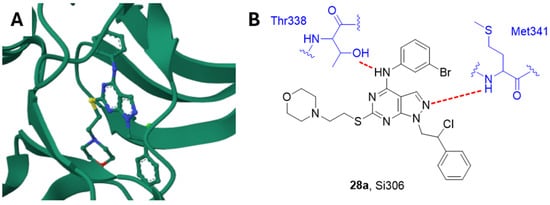
Figure 8.
(A) Crystal structure of Src in complex with Si306 (PDB: 4O2P). (B) Schematic representation of the main interactions between Si306 and Src, with amino acid residues crucial to the interaction highlighted in blue. Hydrogen bonds are represented as red dashed lines [147].
Furthermore, SI306 is able to induce apoptosis in patients derived invasive GBM cell lines [149] and in combination with radiotherapy significantly inhibited U87 GBM xenografts growth in nude mice, compared to both control and single treatment [150]. In an in vitro assay performed on human GBM cell lines (i.e., U87 and multidrug resistant U87-TxR cell lines), and three primary GBM cell cultures, Si306 and its prodrug (pro-Si306) 28b (Figure 7) showed a considerably degradation of the extracellular matrix emerging as potential GBM aggressiveness suppressors. In vivo, the two compounds exhibited an anti-invasive effect against U87 xenografts in zebrafish embryos [151]. The in vitro and in vivo PK profiles of Si306 and pro-Si306 were determined. Both molecules showed good metabolic stability, pro-SI306 showing an increasing water solubility compared to the parent compound. Furthermore, the prodrug showed comparable efficacy and a slightly increased median survival time of mice in an orthotopic GBM model with respect to Si306 [150]. Moreover, Si306 and its prodrug exhibited high pro-oxidative potential in patient-derived GBM cells determining an increase in ROS synthesis followed by double-strand DNA breaks, mitochondrial membrane potential disruption and senescence [152].
Lastly, Si306 and pro-Si306 autophagy-inducing ability was evaluated in both U87 and U87-TxR cells. Si306 and pro-Si306 significantly inhibited cell proliferation and triggered necrosis when administered in combination with the autophagy inhibitor bafilomycin A1 [153].
Compound Si388 29 (Figure 7) is another pyrazolo-pyrimidine Src inhibitor (Ki = 423 nM). It affected tumorigenicity and cell viability in 2D and 3D GBM cellular models (T98G, U251 and GBMSC83 cell lines), and it enhanced cancer cell sensitivity to ionizing radiation [154].
Compound 30 (Figure 7) showed a promising inhibitory activity against Src (Ki = 3.14 μM) and Abl (Ki = 0.44 μM) kinases, cell viability reduction towards U-87, LN-18, LN-229 and DBTRG GBM cell lines. Compound 30 in vitro ADME (absorption, distribution, metabolism and excretion) evaluation showed high metabolic stability and a satisfactory passive permeability across gastrointestinal and blood–brain barriers [155].
2.14.3. TAT-Cx43
TAT-Cx43 peptide (sequence TAT-AYFNGCSSPTAPLSPMSP) inhibits Src oncogenic activity, especially in GSCs, and decreases their survival, invasiveness and tumorigenicity. Furthermore, it boosts survival rate in animal models [156].
Furthermore, TAT-Cx43 decreased glucose uptake in human GSCs and reduced oxidative phosphorylation without a compensatory increase in glycolysis [157].
2.15. Dual-Specificity Tyrosine Phosphorylation-Regulated Kinase 1A (DYRK1A) Inhibitors
DYRKs belong to a group of proline-directed kinases and include five members, DYRK1A, DYRK1B, DYRK2, DYRK3 and DYRK4 [158]. Among them, DYRK1A is involved in GBM cell cycle arrest [159], as well as in promotion of cyclin B degradation in GSC cells, decreasing cyclin dependent kinase (CDK) activity and inducing cell cycle arrest [160].
VER-239353
VER-239353 31 (Figure 7) is a potent and selective DYRK1A/1B inhibitor (IC50 = 7 and 2.4 nM, respectively) with a partial activity towards DYRK2 (>30-fold selectivity) [161]. DYRK1A/B inhibition by VER-239353 in U87MG cells increased retinoblastoma protein (pRb) and cyclin D1, as well as cell cycle inhibitors p21 and p27. This led to an exit from G0 phase and a following arrest in G1. VER-239353 also reduced U87MG cell proliferation in both 2D and 3D culture. In vivo, VER-239353 induced proliferation stasis in a U87MG xenograft cancer model employing female nude mice [161].
2.16. MEK Inhibitors
Mitogen-activated protein kinases (MEKs) are a class of enzymes which can phosphorylate both tyrosine and serine/threonine residues. They are involved in human cancers through the activation of Ras/Raf/MEK/ERK transduction cascade [162]. In GBM, MEK/ERK pathway has been showed to enhance both cell migration and invasion [163].
2.16.1. Binimetinib
Binimetinib 32 (Figure 9) is a selective and allosteric inhibitor of MEK1/2. It was approved in several countries in combination with the selective B-RAF protein inhibitor encorafenib for patients with unresectable or metastatic melanoma. Binimetinib decreased pERK levels when tested in multiple cancer cell lines, ingluding neuroblastoma [164,165].
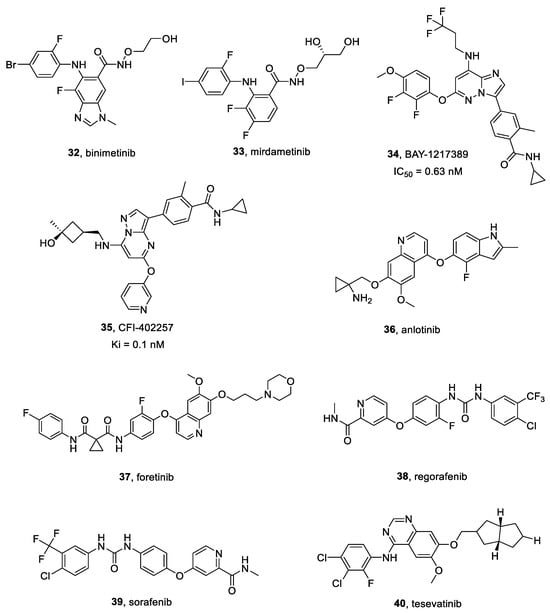
Figure 9.
Structures of MEK, TTK, and multitargeted inhibitors 32–40.
In vitro studies were performed by Bikhezar et al. [166], employing multicellular U87 human GBM spheroids. Binimetinib loaded nanocarriers inhibited spheroids growth, showing a synergistic effect when administered in combination with radiation both in vitro and in vivo (GBM murine xenografts) [167]. Furthermore, an additive effect was observed when it was combined with TMZ [167].
2.16.2. Mirdametinib
Mirdametinib 33 (Figure 9), also known as PD-0325901, is a selective MEK1/MEK2 inhibitor, characterized by an effective blood-brain barrier permeability, when compared to other MEK inhibitors. Clinical trial NCT04923126 (still in recruiting phase) was launched to evaluate its safety, preliminary efficacy and PK in patients suffering from pediatric low-grade glioma (pLGG) [168].
According to Houweling et al. [169], mirdametinib showed an in vitro radio sensitizing effect on GBM8 spheroids. Mice treated with radiation alone or combined with mirdametinib exhibited significantly better survival if compared to control.
2.17. TTK Inhibitors
Threonine tyrosine kinase (TTK), otherwise called MPS1 (monopolar spindle 1) kinase, is a multi-specific enzyme that phosphorylates tyrosine, serine and threonine residues [170]. TTK is essential for in vitro clonogenicity and in vivo tumor propagation in GSCs. TTK expression is high in GBM and is correlated with a poor prognosis in GBM patients [171].
BAY-1217389 and CFI-402257
BAY-1217389 34 (Figure 9) is a selective TTK inhibitor, competitively binding its ATP site (IC50 = 0.63 nM) [172]. It is characterized by a favorable PK profile, a high distribution volume and low blood clearance [173].
CFI-402257 35 (Figure 9) is a highly selective small molecule TTK inhibitor (Ki = 0.1 nM [174]), currently employed in a phase two clinical trial testing its safety and tolerability in breast cancer patients (NCT02792465). It can suppress HCC cell proliferation in a dose-dependent manner, as well as induce cell apoptosis [175].
Different concentrations of BAY-1217389 and CFI-402257 showed a significant growth suppression effect on both U87 and U251 GBM cells in a dose dependent manner [173]. BAY-1217389 and CFI-402257 significantly inhibited GBM cells colony formation. BAY-1217389 and CFI-402257 in combination with TMZ showed a higher cell viability decrease in both U251 and U87 cells than TMZ alone [173].
2.18. Multitarget Inhibitors
Targeting more than one kinase can lead to an effective increase due to a synergistic action and a reduction of the drug resistance. Several single kinase inhibitors resulted to be multi-target inhibitors due to the kinase ATP-binding sites homology. Moreover, the optimization of potent single kinases or the combination of selective agents led to development of many multi-target inhibitors [176].
2.18.1. Anlotinib
Anlotinib 36 (Figure 9) is small-molecule multitarget TKI, specific for VEGFRs, Kit, PDGFR-α and FGFRs [177]. Its IC50 towards VEGFR2 is 0.2 nM and it inhibited FGFR1 of 45.0% at 1 μM [177,178]. Moreover, it inhibited cancer angiogenesis, cell proliferation, cell migration, VEGFR-induced HUVEC cell proliferation, and VEGF/PDGF-BB/FGF-2-induced formation of capillary-like tubes in endothelial cell cultures. Furthermore, anlotinib suppressed HUVEC migration, tube formation and microvessel growth in vitro, and reduced vascular density in vivo.
Xu et al. [179] reported that anlotinib lowered the proliferation, migration and invasiveness of GBM cells (A172, U87, U251) in a dose-dependent manner. Moreover, anlotinib anti-GBM activity was increased by TMZ [179].
Evaluation of the PK profile in several solid tumors highlighted that anlotinib is quickly absorbed through the intestine with comparable brain and plasma distribution [180].
2.18.2. CR13626
CR13626 (chemical structure was not disclosed by Rottapharm Biotech company), a brand-new brain penetrant small molecule multitarget TKI, inhibited the in vitro activity of several kinases involved in GBM development, e.g., EGFR (IC50 = 3 µM) [174], VEGFR2, Fyn, Lck, Yes, RET and HGK.
Galimberti et al. evaluated in vivo CR13626 administration. Oral treatment in an orthotopic murine GBM xenograft model resulted in a time-dependent reduction of cancer growth, causing a significant increase in animal survival [181].
2.18.3. Foretinib
Foretinib 37 (Figure 9) is a multi-kinase inhibitor developed as an ATP-binding site competitor. Foretinib is an oral multikinase inhibitor targeting c-MET, RON, Axl, Tie-2, VEGFR, c-KIT, Flt-3, and PDGFR signaling pathways. As a potent c-MET inhibitor, foretinib acted on several c-MET-activated cell lines, reducing cancer growth in different animal models. Moreover, it inhibited the TAM family of RTKs, killing GBM cells [182].
Gortany et al. [183], reported that foretinib inhibited GBM cell proliferation through a G2/M cell cycle arrest and mitochondrial-mediated apoptosis. Moreover, foretinib lowered GBM cells invasiveness by downregulating MMP2, uPA and uPAR proteolytic cascade and epithelial-mesenchymal transition (EMT)-related genes [183].
2.18.4. Regorafenib
Regorafenib 38 (Figure 9) is a small molecule multi-target TKI active against VEGFR-1, VEGFR-2, VEGFR-3 with IC50 values of 13 nM, 4.2 nM and 46 nM, respectively [184]. It also inhibited the mutant oncogenic kinases KIT, RET and B-RAF, suppressing both cancer angiogenesis and cell proliferation.
Chiang et al. [185] observed that regorafenib significantly increased TMZ-induced apoptosis, also suppressing both invasiveness and migration potential in U87 and GBM8401 cells. Orthotopic xenograft experiments showed tumor size reduction, as well as prolonged survival in combinatorial administration, even with TMZ half-dose [185].
Regorafenib is a CYP3A4 substrate and is primarily metabolized in the liver to the corresponding N-oxide and demethylated N-oxide metabolites, which show similar kinase inhibitory activities. Although levels of regorafenib and its metabolites are lower in the cerebrospinal fluid than in plasma, it seems reasonable that sufficient CNS concentrations of these compounds are temporarily reached in glioma patients [186].
2.18.5. Sorafenib
Sorafenib 39 (Figure 9) is a potent multi-target kinase inhibitor, that suppressed cancer cells proliferation by inhibiting the activity of RAF-1, B-RAF and RAS/RAF/MEK/ERK signaling pathways [187]. It also inhibited angiogenesis through targeting c-Kit, FLT-3, PDGFR-β, VEGFR-2, VEGFR-3 and other TKs.
Kim et al. [188] confirmed the combinatorial effectiveness of sorafenib plus TTFields, in GBM. Sorafenib prevented cancer expansion in murine GBM xenografts decreasing STAT3 levels and increasing sensitivity towards TTFields. Furthermore, sorafenib plus TTFields significantly inhibited tumor growth. Combinatorial treatment more effectively reduced STAT3 expression in vivo than in U87 and U373 cells [188].
Zajak et al. performed a study aimed at evaluating the synergistic effects [181] of Sorafenib plus LY294002 in T98G cells. Simultaneous treatment with both compounds was more effective in inducing apoptosis than single applications. Effectiveness was associated with both mitochondrial membrane potential decreasing and Cas3/9 activation. Raf and PI3K expression was also inhibited [189].
2.18.6. Tesevatinib
Tesevatinib 40 (Figure 9) is an effective multi-TKI (IC50 = 0.3 nM for EGFR), employed in a phase II clinical trial for autosomal dominant polycystic kidney disease treatment (NCT01559363). Tesevatinib exhibited a satisfactory inhibition profile for Src kinase, also decreasing activity of both EGFR and cAMP pathways [190].
Kizilbash et al. [191] evaluated tesevatinib in vitro cytotoxicity on patient derived GBM12 and GBM6 cells. Tesevatinib efficiently reduced cell viability and inhibited EGFR signaling in vitro. They also carried out in vivo experiments by employing murine models bearing either intracranial or flank GBMs. However, its effectiveness, when compared to vehicle in intracranial and flank GBM models, was found to be modest and due to partial EGFR signaling inhibition [191].
The same authors showed that tesevatinib efficacy in EGFR-amplified patient-derived xenograft GBM models is higher in vitro than in vivo, despite a significant distribution in the brain with respect to the plasma, probably because of drug-tissue binding and compensatory signaling [191].
3. Challenges and Opportunities for Drug Delivery of Small Molecules Acting as Tyrosine Kinase Inhibitors
The effectiveness of chemotherapy drugs for the treatment of malignant brain tumors is mainly limited by the BBB presence. Thus, the drug efficacy depends on the capacity to cross adequately the BBB reaching a therapeutic level. The main failure of TKIs in the treatment of GBM is due to their poor BBB permeability and the lack of tumor specificity [192]. Although BBB may be chemically or physically disrupted to enhance permeability through methods such as bradykinin analogues-induced tight junction alterations, osmotic disruption using mannitol and focused ultrasound techniques [193], several drug delivery strategies are being studied to overcome the weakness of TKIs and improve their BBB permeability and tumor specificity [192].
Nanocarriers, such as liposomes, polymeric nanoparticles/micelles, albumin nanoparticles, inorganic nanocarriers and lipid nanocapsules, are currently studied to better reach GBM brain sites [192].
Liposomes, composed of concentric single or multiple lipid bilayers with an aqueous core, can contain both hydrophilic and lipophilic molecules. They are morphologically like cellular membranes, biocompatible and non-immunogenic [194]. Such a technology has been employed to deliver doxorubicin and erlotinib, using transferrin to target transferrin receptors overexpressed on brain endothelial and GBM cells, as well as a cell-penetrating peptide to enhance intracellular uptake of carriers [195].
Polymeric nanoparticles, used to specifically deliver drugs to various malignancies, can be characterized by several compositions of natural polymer nanomaterials (e.g., cellulose, chitosan, gelatine, alginate and hyaluronic acid) or synthetic polymers (e.g., polycaprolactone, polyvinyl alcohol, poly-lactide-co-glycolic acid, polyethyleneimine, and polylactic acid) [196]. For instance, optimized imatinib mesylate loaded poly-lactide-co-glycolic acid nanoparticles were functionalized with Pluronic® P84, to overcome the drug P-glycoprotein efflux mediated that is increased in GBM cells [197].
Micelles are formed via amphiphilic block copolymers self-associating in aqueous solution [198]. They are largely employed as drug carriers due to their properties such as narrow size distribution, thermodynamic stability, and suitability to carry hydrophobic molecules [198,199]. Interestingly, Greish et al. [200] encapsulated both dasatinib and crizotinib in poly (styrene-co-maleic acid) micelles affording a more selective distribution and a reduced systemic toxicity in GBM.
Albumin is a plasma protein characterized by both a great safety profile and enhanced permeability and retention effect-induced high accumulation in solid cancers. Such a set of features makes albumin an ideal tool to develop drug delivery systems for malignancies targeting [201]. Yang et al. [202] formulated human albumin-based nanoparticles to deliver both ibrutinib and hydroxychloroquine in a glioma animal model, demonstrating nanoparticles accumulation at the tumor site after intravenous injection.
Inorganic nanocarriers drug delivery vehicles, such as gold, silica, graphene, and carbon nanotubes have been employed due to their versatile physicochemical properties (i.e., easy availability/functionalization, accumulation in cancer cells without recognition by P-glycoprotein) [203]. Moore et al. [204] used carbon nanotubes with multiple polymer coatings to enhance both the therapeutic efficacy and the release kinetics of dasatinib, although carrier toxicity is still questioned.
Lastly, lipid nanocapsules are being studied as drug carriers due to their many advantages, such as high stability, high drug loading, and the opportunity for easy production scale-up [205]. For instance, Clavreul et al. [206] overcame the limitations of free drug in GBM treatment loading sorafenib into these nanocapsules.
4. Conclusions
GBM is both the most aggressive and common type of malignancy originating in the brain. It is characterized by a very poor prognosis, with a median value at diagnosis of 65-years-old, and a male sexual predisposition. Many kinase inhibitors are being tested for the treatment of GBM but none of these compounds have been approved, alone or in combination to treat this tumor. Several inhibitors are currently in clinical trials, as reported in Table 1. Although numerous compounds have demonstrated noteworthy in vivo efficacy, the heterogeneity of the disease supports the employment of combinatorial therapeutic regimens. In particular, the complexity of glioblastoma biology, coupled with the onset of resistance mechanisms, underscores the urgency to develop personalized approaches. The combination of immunotherapy with selected TKIs, guided by patient categorization based on specific kinase overexpression, could unlock the full therapeutic capabilities of TKIs and reshape the glioblastoma treatment landscape.

Table 1.
Clinical Trials involving tyrosine kinase inhibitors.
Furthermore, the need for compounds to cross the BBB emphasizes the demand for innovative TKI delivery strategies. In this context, nanotechnology and targeted drug delivery systems emerge as promising approaches for optimizing the pharmacokinetics and biodistribution of TKIs in GBM therapy with good chances of translating these findings into impactful clinical applications.
Author Contributions
All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We thank AIRC Foundation for Cancer Research in Italy (Fondazione AIRC per la Ricerca sul Cancro, AIRC), Grant IG-2019, project code 23725, PI Silvia Schenone.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| A172 | human glioblastoma cells |
| A549 | lung carcinoma cells |
| AC220 | quizartinib |
| ALK | anaplastic lymphoma kinase |
| Akt | protein kinase B |
| BCL-2 | B-cell lymphoma 2 |
| BS-125 | human glioblastoma cell line |
| BS-287 | human glioblastoma cell line |
| Crk-II | CT10 regulator of kinase |
| CXCL | C-X-C motif chemokinel |
| DBTRG | human glioblastoma cell line |
| EBC1 | lung squamous carcinoma cells |
| EGF | epidermal growth factor |
| EML4 | echinoderm microtubule-associated protein-like 4 |
| EOC2 | mouse microglial cell lines |
| ERK | extracellular signal-regulated kinase |
| FGFR | fibroblast growth factor receptor |
| FLT3 | Fms Related Receptor Tyrosine Kinase 3 |
| Fyn | Src family tyrosine kinase |
| GBM6 | human glioblastoma multiforme primary tissue cell line |
| GBM12 | TMZ-resistant GBM cell line |
| GBM8401 | human glioblastoma multiforme cell line |
| GBMSC83 | glioblastoma stem cell line |
| GL261 | glioma stem cell line |
| GSC11 | glioma stem cell line |
| GSC20 | glioma stem cell line |
| GSC23 | glioma stem cell line |
| GSC407 | glioma stem cell line |
| GSC923 | glioma stem cell line |
| GSCD317 | glioma stem cell line |
| HCC | hepatocellular carcinoma |
| HER | human epidermal growth factor receptor |
| HGK | hepatocyte progenitor kinase-like/germinal center kinase-like kinase |
| HUVECs | human umbilical vein endothelial cells |
| IGF | Insulin-like growth factor |
| JAK | Janus kinase |
| Kit | human tyrosine kinase receptor |
| LN-18 | epithelial-like cell line |
| LN-229 | epithelial-like cell line |
| MAPK | mitogen activated protein kinases |
| MCT | mast cell tumor |
| MDA-MB-231 | epithelial, human breast cancer cell line |
| MKN45 | gastric adenocarcinoma cells |
| MMP2 | matrix metalloproteinase-2 |
| mTOR | mammalian target of rapamycin |
| NF-κB | nuclear factor κB |
| PDA | pancreatic ductal adenocarcinoma cells |
| PDGF | platelet-derived growth factor |
| PI3K | phosphoinositide 3-kinase |
| PIK3R3 | phosphoinositide-3-kinase regulatory subunit 3 |
| Raf | rapidly accelerated fibrosarcoma |
| Ras | rat sarcoma |
| RET | rearranged during transfection tyrosine kinase receptor |
| RON | receptor tyrosine kinase |
| RT-PCR | Real time polymerase chain reaction |
| S6K | p70 ribosomal S6 kinase |
| siRNA | small interference RNA |
| SF-126-TR | high-grade human glioma cells temozolomide resistant |
| SF-539 | human glioblastoma cell line |
| SF-767 | human glioblastoma cell line |
| STAT3 | signal transducer and activator of transcription 3 |
| Tie-2 | Angiopoietin-1 receptor tyrosine kinase |
| TGF-α | transforming growth factor-α |
| T98G | fibroblast-like cells |
| T98MG | human glioblastoma cancer cells |
| TGF-α | transforming growth factor alpha |
| U118MG-TR | U118 malignant glioma cells temozolomide resistant |
| U251 | human glioblastoma cancer cells |
| U251-luc | transformed U251 human glioblastoma cancer cells |
| U373 | uppsala human glioblastoma astrocytoma cell line |
| U87MG | uppsala 87 malignant glioma cell line |
| U87TxR | multidrug resistant uppsala 87 glioma cell line |
| uPA | urokinase-type plasminogen activator |
| uPAR | urokinase-type plasminogen activator receptor |
| VEGF | vascular endothelial growth factor |
| Yes | human non-receptor tyrosine kinase |
References
- Le Rhun, E.; Preusser, M.; Roth, P.; Reardon, D.A.; van den Bent, M.; Wen, P.; Reifenberger, G.; Weller, M. Molecular targeted therapy of glioblastoma. Cancer Treat. Rev. 2019, 80, 101896. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cerdeño, V.; Noctor, S.C. Neural Progenitor Cell Terminology. Front. Neuroanat. 2018, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Q.; Yang, H.; Mao, Y. The Oncogenesis of Glial Cells in Diffuse Gliomas and Clinical Opportunities. Neurosci. Bull. 2023, 39, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Friedmann-Morvinski, D. Glioblastoma heterogeneity and cancer cell plasticity. Crit. Rev. Oncog. 2014, 19, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Holland, E.C.; Cairncross, J.G. Glioma classification: A molecular reappraisal. Am. J. Pathol. 2001, 159, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Fan, X.; Zhao, C.; Zhao, Z.; Hu, L.; Wang, D.; Wang, R.; Fang, Z. Molecular subtyping of glioblastoma based on immune-related genes for prognosis. Sci. Rep. 2020, 10, 15495. [Google Scholar] [CrossRef] [PubMed]
- Grochans, S.; Cybulska, A.M.; Simińska, D.; Korbecki, J.; Kojder, K.; Chlubek, D.; Baranowska-Bosiacka, I. Epidemiology of Glioblastoma multiforme-literature review. Cancers 2022, 14, 2412. [Google Scholar] [CrossRef]
- Tykocki, T.; Eltayeb, M. Ten-year survival in glioblastoma. A systematic review. J. Clin. Neurosci. 2018, 54, 7–13. [Google Scholar] [CrossRef]
- Fernandes, C.; Costa, A.; Osório, L.; Costa Lago, R.; Linhares, P.; Carvalho, B.; Caeiro, C. Current Standards of Care in Glioblastoma Therapy. In Glioblastoma; De Vleeschouwer, S., Ed.; Codon Publications: Brisbane, Australia, 2017. [Google Scholar] [CrossRef]
- Minniti, G.; Niyazi, M.; Alongi, F.; Navarria, P.; Belka, C. Current status and recent advances in reirradiation of glioblastoma. Radiat. Oncol. 2021, 16, 36. [Google Scholar] [CrossRef]
- Fisher, J.P.; Adamson, D.C. Current FDA-Approved Therapies for High-Grade Malignant Gliomas. Biomedicines 2021, 9, 324. [Google Scholar] [CrossRef]
- Wismeth, C.; Hau, P.; Fabel, K.; Baumgart, U.; Hirschmann, B.; Koch, H.; Jauch, T.; Grauer, O.; Drechsel, L.; Brawanski, A.; et al. Maintenance therapy with 13-cis retinoid acid in high-grade glioma at complete response after first-line multimodal therapy—A phase-II study. J. Neurooncol. 2004, 68, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.E.; Choi, S.S.; Rogers, J.E.; Lei, X.; De Groot, J.F. Isotretinoin maintenance therapy for glioblastoma: A retrospective review. J. Oncol. Pharm. Pract. 2014, 20, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Zheng, Y.; Hong, W.; Chen, X.; Li, H.; Huang, B.; Huang, Z.; Tang, H.; Geng, W. Recent Advances in Immune Cell Therapy for Glioblastoma. Front. Immunol. 2020, 11, 544563. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Wang, Y.; Sun, Y.; Zhang, C.; Ma, S.; Zhang, D.; Li, D.; Jia, W. CTLA4-Mediated Immunosuppression in Glioblastoma is Associated with the Infiltration of Macrophages in the Tumor Microenvironment. J. Inflamm. Res. 2021, 14, 7315–7329. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, S.; Quezado, M.; Garren, N.; Boris, L.; Siegel, C.; Lopes Abath Neto, O.; Theeler, B.J.; Park, D.M.; Nduom, E.; Zaghloul, K.A.; et al. Clinical decision making in the era of immunotherapy for high grade-glioma: Report of four cases. BMC Cancer 2018, 18, 239. [Google Scholar] [CrossRef]
- Duong-Ly, K.C.; Peterson, J.R. The human kinome and kinase inhibition. Curr. Protoc. Pharmacol. 2013. [Google Scholar] [CrossRef]
- Arter, C.; Trask, L.; Ward, S.; Yeoh, S.; Bayliss, R. Structural features of the protein kinase domain and targeted binding by small-molecule inhibitors. J. Biol. Chem. 2022, 298, 102247. [Google Scholar] [CrossRef]
- Du, Z.; Lovly, C.M. Mechanisms of receptor tyrosine kinase activation in cancer. Mol. Cancer 2018, 17, 58. [Google Scholar] [CrossRef]
- Taylor, S.S.; Kornev, A.P. Protein kinases: Evolution of dynamic regulatory proteins. Trends Biochem. Sci. 2011, 36, 65–77. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Lagarón, N.O.; McGowan, E.M.; Parmar, I.; Jha, A.; Hubbard, B.P.; Rupasinghe, H.P.V. Kinase-targeted cancer therapies: Progress, challenges and future directions. Mol. Cancer 2018, 17, 48. [Google Scholar] [CrossRef]
- Li, X.; Wu, C.; Chen, N.; Gu, H.; Yen, A.; Cao, L.; Wang, E.L. PI3K/Akt/mTOR signaling pathway and targeted therapy for glioblastoma. Oncotarget 2016, 7, 33440–33450. [Google Scholar] [CrossRef]
- Liu, H.; Qiu, W.; Sun, T.; Wang, L.; Du, C.; Hu, Y.; Liu, W.; Feng, F.; Chen, Y.; Sun, H. Therapeutic strategies of glioblastoma (GBM): The current advances in the molecular targets and bioactive small molecule compounds. Acta Pharm. Sin. B 2022, 12, 1781–1804. [Google Scholar] [CrossRef] [PubMed]
- Cazes, A.; Lopez-Delisle, L.; Tsarovina, K.; Pierre-Eugène, C.; De Preter, K.; Peuchmaur, M.; Nicolas, A.; Provost, C.; Louis-Brennetot, C.; Daveau, R.; et al. Activated Alk triggers prolonged neurogenesis and Ret upregulation providing a therapeutic target in ALK-mutated neuroblastoma. Oncotarget 2014, 5, 2688–2702. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Cheng, M.; Zhang, Q.; Wasik, M.; Kelsh, R.; Winkler, C. Anaplastic lymphoma kinase is required for neurogenesis in the developing central nervous system of zebrafish. PLoS ONE 2013, 8, e63757. [Google Scholar] [CrossRef] [PubMed]
- Chiarle, R.; Voena, C.; Ambrogio, C.; Piva, R.; Inghirami, G. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat. Rev. Cancer 2008, 8, 11–23. [Google Scholar] [CrossRef]
- Bagci, O.; Tumer, S.; Olgun, N.; Altungoz, O. Copy number status and mutation analyses of anaplastic lymphoma kinase (ALK) gene in 90 sporadic neuroblastoma tumors. Cancer Lett 2012, 317, 72–77. [Google Scholar] [CrossRef]
- Salido, M.; Pijuan, L.; Martínez-Avilés, L.; Galván, A.B.; Cañadas, I.; Rovira, A.; Zanui, M.; Martínez, A.; Longarón, R.; Sole, F.; et al. Increased ALK gene copy number and amplification are frequent in non-small cell lung cancer. J. Thorac. Oncol. 2011, 6, 21–27. [Google Scholar] [CrossRef]
- Karagkounis, G.; Stranjalis, G.; Argyrakos, T.; Pantelaion, V.; Mastoris, K.; Rontogianni, D.; Komaitis, S.; Kalamatianos, T.; Sakas, D.; Tiniakos, D. Anaplastic lymphoma kinase expression and gene alterations in glioblastoma: Correlations with clinical outcome. J. Clin. Pathol. 2017, 70, 593–599. [Google Scholar] [CrossRef]
- Ferguson, S.D.; Xiu, J.; Weathers, S.P.; Zhou, S.; Kesari, S.; Weiss, S.E.; Verhaak, R.G.; Hohl, R.J.; Barger, G.R.; Reddy, S.K.; et al. GBM-associated mutations and altered protein expression are more common in young patients. Oncotarget 2016, 7, 69466–69478. [Google Scholar] [CrossRef]
- Huang, H. Anaplastic Lymphoma Kinase (ALK) Receptor Tyrosine Kinase: A Catalytic Receptor with Many Faces. Int. J. Mol. Sci. 2018, 19, 3448. [Google Scholar] [CrossRef]
- Yi, G.Z.; Xiang, W.; Feng, W.Y.; Chen, Z.Y.; Li, Y.M.; Deng, S.Z.; Guo, M.L.; Zhao, L.; Sun, X.G.; He, M.Y.; et al. Identification of Key Candidate Proteins and Pathways Associated with Temozolomide Resistance in Glioblastoma Based on Subcellular Proteomics and Bioinformatical Analysis. Biomed. Res. Int. 2018, 2018, 5238760. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, A.; Maione, P.; Gridelli, C. Evolution in the treatment landscape of non-small cell lung cancer with ALK gene alterations: From the first- to third-generation of ALK inhibitors. Expert. Opin. Emerg. Drugs 2018, 23, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Savooji, J.; Liu, D. Second- and third-generation ALK inhibitors for non-small cell lung cancer. J. Hematol. Oncol. 2016, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Cheon, S.Y.; Kwon, S. Molecular anatomy of the EML4-ALK fusion protein for the development of novel anticancer drugs. Int. J. Mol. Sci. 2023, 24, 5821. [Google Scholar] [CrossRef]
- Cui, J.J.; Tran-Dubé, M.; Shen, H.; Nambu, M.; Kung, P.P.; Pairish, M.; Jia, L.; Meng, J.; Funk, L.; Botrous, I.; et al. Structure based drug design of crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK). J. Med. Chem. 2011, 54, 6342–6363. [Google Scholar] [CrossRef]
- Martínez-García, M.; Velasco, G.; Pineda, E.; Gil-Gil, M.; Alameda, F.; Capellades, J.; Martín-Soberón, M.C.; López-Valero, I.; Ambel, E.T.; Foro, P.; et al. Safety and Efficacy of Crizotinib in Combination with Temozolomide and Radiotherapy in Patients with Newly Diagnosed Glioblastoma: Phase Ib GEINO 1402 Trial. Cancers 2022, 14, 2393. [Google Scholar] [CrossRef]
- Larkins, E.; Blumenthal, G.M.; Chen, H.; He, K.; Agarwal, R.; Gieser, G.; Stephens, O.; Zahalka, E.; Ringgold, K.; Helms, W.; et al. FDA Approval: Alectinib for the Treatment of Metastatic, ALK-Positive Non-Small Cell Lung Cancer Following Crizotinib. Clin. Cancer Res. 2016, 22, 5171–5176. [Google Scholar] [CrossRef]
- Sakamoto, H.; Tsukaguchi, T.; Hiroshima, S.; Kodama, T.; Kobayashi, T.; Fukami, T.A.; Oikawa, N.; Tsukuda, T.; Ishii, N.; Aoki, Y. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell 2011, 19, 679–690. [Google Scholar] [CrossRef]
- Gadgeel, S.M.; Gandhi, L.; Riely, G.J.; Chiappori, A.A.; West, H.L.; Azada, M.C.; Morcos, P.N.; Lee, R.-M.; Linta Garcia, L.; Yu, L.; et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): Results from the dose-finding portion of a phase ½ study. Lancet Oncol. 2014, 15, 1119–1128. [Google Scholar] [CrossRef]
- Berberich, A.; Schmitt, L.M.; Pusch, S.; Hielscher, T.; Rübmann, P.; Hucke, N.; Latzer, P.; Heßling, B.; Lemke, D.; Kessler, T.; et al. cMyc and ERK activity are associated with resistance to ALK inhibitory treatment in glioblastoma. J. Neurooncol. 2020, 146, 9–23. [Google Scholar] [CrossRef]
- Friboulet, L.; Li, N.; Katayama, R.; Lee, C.C.; Gainor, J.F.; Crystal, A.S.; Michellys, P.Y.; Awad, M.M.; Yanagitani, N.; Kim, S.; et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov. 2014, 4, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Crinò, L.; Ahn, M.J.; De Marinis, F.; Groen, H.J.; Wakelee, H.; Hida, T.; Mok, T.; Spigel, D.; Felip, E.; Nishio, M.; et al. Multicenter phase II study of whole-body and intracranial activity with ceritinib in patients with alk-rearranged non-small-cell lung cancer previously treated with chemotherapy and crizotinib: Results from ASCEND-2. J. Clin. Oncol. 2016, 34, 2866–2873. [Google Scholar] [CrossRef] [PubMed]
- Kawauchi, D.; Takahashi, M.; Satomi, K.; Yamamuro, S.; Kobayashi, T.; Uchida, E.; Honda-Kitahara, M.; Narita, Y.; Iwadate, Y.; Ichimura, K.; et al. The ALK inhibitors, alectinib and ceritinib, induce ALK-independent and STAT3-dependent glioblastoma cell death. Cancer Sci. 2021, 112, 2442–2453. [Google Scholar] [CrossRef]
- Goker Bagca, B.; Ozates, N.P.; Asik, A.; Caglar, H.O.; Gunduz, C.; Biray Avci, C. Temozolomide treatment combined with AZD3463 shows synergistic effect in glioblastoma cells. Biochem. Biophys. Res. Commun. 2020, 533, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Siemann, D.W. Gas6/Axl signaling pathway in the tumor immune microenvironment. Cancers 2020, 12, 1850. [Google Scholar] [CrossRef] [PubMed]
- Korshunov, V.A. Axl-dependent signalling: A clinical update. Clin. Sci. (Lond.) 2012, 122, 361–368. [Google Scholar] [CrossRef]
- Onken, J.; Vajkoczy, P.; Torka, R.; Hempt, C.; Patsouris, V.; Heppner, F.L.; Radke, J. Phospho-AXL is widely expressed in glioblastoma and associated with significant shorter overall survival. Oncotarget 2017, 8, 50403–50414. [Google Scholar] [CrossRef]
- Scaltriti, M.; Elkabets, M.; Baselga, J. Molecular Pathways: AXL, a Membrane Receptor Mediator of Resistance to Therapy. Clin. Cancer Res. 2016, 22, 1313–1317. [Google Scholar] [CrossRef]
- Myers, S.H.; Brunton, V.G.; Unciti-Broceta, A. AXL inhibitors in cancer: A medicinal chemistry perspective. J. Med. Chem. 2016, 59, 3593–3608. [Google Scholar] [CrossRef]
- Gay, C.M.; Balaji, K.; Byers, L.A. Giving AXL the axe: Targeting AXL in human malignancy. Br. J. Cancer 2017, 116, 415–423. [Google Scholar] [CrossRef]
- Chen, F.; Song, Q.; Yu, Q. Axl inhibitor R428 induces apoptosis of cancer cells by blocking lysosomal acidification and recycling independent of Axl inhibition. Am. J. Cancer Res. 2018, 8, 1466–1482. [Google Scholar] [PubMed]
- Sun, Y.; Bailey, C.P.; Sadighi, Z.; Zaky, W.; Chandra, J. Pediatric high-grade glioma: Aberrant epigenetics and kinase signaling define emerging therapeutic opportunities. J. Neurooncol. 2020, 150, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Scherschinski, L.; Prem, M.; Kremenetskaia, I.; Tinhofer, I.; Vajkoczy, P.; Karbe, A.G.; Onken, J.S. Regulation of the receptor tyrosine kinase AXL in response to therapy and its role in therapy resistance in glioblastoma. Int. J. Mol. Sci. 2022, 23, 982. [Google Scholar] [CrossRef] [PubMed]
- Sadahiro, H.; Kang, K.D.; Gibson, J.T.; Minata, M.; Yu, H.; Shi, J.; Chhipa, R.; Chen, Z.; Lu, S.; Simoni, Y.; et al. Activation of the receptor tyrosine kinase AXL regulates the immune microenvironment in glioblastoma. Cancer Res. 2018, 78, 3002–3013. [Google Scholar] [CrossRef] [PubMed]
- Vehlow, A.; Klapproth, E.; Jin, S.; Hannen, R.; Hauswald, M.; Bartsch, J.W.; Nimsky, C.; Temme, A.; Leitinger, B.; Cordes, N. Interaction of discoidin domain receptor 1 with a 14-3-3-beclin-1-Akt1 complex modulates glioblastoma therapy sensitivity. Cell Rep. 2019, 26, 3672–3683.e7. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Tan, L.; Weisberg, E.L.; Liu, F.; Canning, P.; Choi, H.G.; Ezell, S.A.; Wu, H.; Zhao, Z.; Wang, J.; et al. Discovery of a potent and selective DDR1 receptor tyrosine kinase inhibitor. ACS Chem. Biol. 2013, 8, 2145–2150. [Google Scholar] [CrossRef]
- Alfaro-Arnedo, E.; López, I.P.; Piñeiro-Hermida, S.; Canalejo, M.; Gotera, C.; Sola, J.J.; Roncero, A.; Peces-Barba, G.; Ruíz-Martínez, C.; Pichel, J.G. IGF1R acts as a cancer-promoting factor in the tumor microenvironment facilitating lung metastasis implantation and progression. Oncogene 2022, 41, 3625–3639. [Google Scholar] [CrossRef]
- Martin, A.; Fernandez, M.C.; Cattaneo, E.R.; Schuster, C.D.; Venara, M.; Clément, F.; Berenstein, A.; Lombardi, M.G.; Bergadá, I.; Gutierrez, M.; et al. Type 1 insulin-like growth factor receptor nuclear localization in high-grade glioma cells enhances motility, metabolism, and in vivo tumorigenesis. Front. Endocrinol. (Lausanne) 2022, 13, 849279. [Google Scholar] [CrossRef]
- Davis, S.L.; Eckhardt, S.G.; Diamond, J.R.; Messersmith, W.A.; Dasari, A.; Weekes, C.D.; Lieu, C.H.; Kane, M.; Choon Tan, A.; Pitts, T.M.; et al. A phase i dose-escalation study of linsitinib (OSI-906), a small-molecule dual insulin-like growth factor-1 receptor/insulin receptor kinase inhibitor, in combination with irinotecan in patients with advanced cancer. Oncologist 2018, 23, 1409-e140. [Google Scholar] [CrossRef]
- Wu, J.; Chen, K.; Zhang, F.; Jin, J.; Zhang, N.; Li, D.; Ying, L.; Chen, W.; Yu, H.; Mao, W.; et al. Overcoming Linsitinib intrinsic resistance through inhibition of nuclear factor-κB signaling in esophageal squamous cell carcinoma. Cancer Med. 2017, 6, 1353–1361. [Google Scholar] [CrossRef]
- Fuentes-Baile, M.; Ventero, M.P.; Encinar, J.A.; García-Morales, P.; Poveda-Deltell, M.; Pérez-Valenciano, E.; Barberá, V.M.; Gallego-Plazas, J.; Rodríguez-Lescure, Á.; Martín-Nieto, J.; et al. Differential effects of IGF-1R small molecule tyrosine kinase inhibitors BMS-754807 and OSI-906 on human cancer cell lines. Cancers 2020, 12, 3717. [Google Scholar] [CrossRef] [PubMed]
- Pipitone, R.M.; Calvaruso, V.; Di Marco, L.; Di Salvo, F.; Gaggianesi, M.; Lupo, G.; Zito, R.; La Mantia, C.; Ramazzotti, M.; Petta, S.; et al. Mer Tyrosine Kinase (MERTK) modulates liver fibrosis progression and hepatocellular carcinoma development. Front. Immunol. 2022, 13, 926236. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Moncayo, G.; Morin, P.; Xue, G.; Grzmil, M.; Lino, M.M.; Clément-Schatlo, V.; Frank, S.; Merlo, A.; Hemmings, B.A. Mer receptor tyrosine kinase promotes invasion and survival in glioblastoma multiforme. Oncogene 2013, 32, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Minson, K.A.; Smith, C.C.; Lee-Sherick, A.B.; DeRyckere, D.; Lasater, E.; Hill, A.A.; Wang, X.; Frye, S.V.; Earp, H.S.; Shah, N.P.; et al. MRX2843, a novel dual MerTK-FLT3 Inhibitor with activity against resistance-conferring FLT3 mutations in acute myeloid leukemia. Blood 2014, 124, 3757. [Google Scholar] [CrossRef]
- Kelvin, J.M.; Chimenti, M.L.; Zhang, D.Y.; Williams, E.K.; Moore, S.G.; Humber, G.M.; Baxter, T.A.; Birnbaum, L.A.; Qui, M.; Zecca, H.; et al. Development of constitutively synergistic nanoformulations to enhance chemosensitivity in T-cell leukemia. J. Control Release 2023, 361, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.T.; Butler, M.; Zhang, M.; Zhang, W.; Song, H.; Hwang, L.; Tran, A.D.; Bash, R.E.; Schorzman, A.N.; Pang, Y.; et al. MerTK inhibition decreases immune suppressive glioblastoma-associated macrophages and neoangiogenesis in glioblastoma microenvironment. Neurooncol. Adv. 2020, 2, vdaa065. [Google Scholar] [CrossRef] [PubMed]
- Sufit, A.; Lee-Sherick, A.B.; DeRyckere, D.; Rupji, M.; Dwivedi, B.; Varella-Garcia, M.; Pierce, A.M.; Kowalski, J.; Wang, X.; Frye, S.V.; et al. MERTK inhibition induces polyploidy and promotes cell death and cellular senescence in glioblastoma multiforme. PLoS ONE 2016, 11, e0165107. [Google Scholar] [CrossRef]
- Wu, J.; Frady, L.N.; Bash, R.E.; Cohen, S.M.; Schorzman, A.N.; Su, Y.T.; Irvin, D.M.; Zamboni, W.C.; Wang, X.; Frye, S.V.; et al. MerTK as a therapeutic target in glioblastoma. Neuro-Oncology 2018, 20, 92–102. [Google Scholar] [CrossRef]
- Bladt, F.; Riethmacher, D.; Isenmann, S.; Aguzzi, A.; Birchmeier, C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature 1995, 376, 768–771. [Google Scholar] [CrossRef]
- Sennino, B.; Ishiguro-Oonuma, T.; Wei, Y.; Naylor, R.M.; Williamson, C.W.; Bhagwandin, V.; Tabruyn, S.P.; You, W.K.; Chapman, H.A.; Christensen, J.G.; et al. Suppression of tumor invasion and metastasis by concurrent inhibition of c-Met and VEGF signaling in pancreatic neuroendocrine tumors. Cancer Discov. 2012, 2, 270–287. [Google Scholar] [CrossRef]
- Han, Y.; Luo, Y.; Zhao, J.; Li, M.; Jiang, Y. Overexpression of c-Met increases the tumor invasion of human prostate LNCaP cancer cells in vitro and in vivo. Oncol. Lett. 2014, 8, 1618–1624. [Google Scholar] [CrossRef] [PubMed]
- Granito, A.; Guidetti, E.; Gramantieri, L. c-MET receptor tyrosine kinase as a molecular target in advanced hepatocellular carcinoma. J. Hepatocell. Carcinoma 2015, 2, 29–38. [Google Scholar] [CrossRef]
- Park, H.; Kim, D.; Kim, E.; Sa, J.K.; Lee, H.W.; Yu, S.; Oh, J.; Kim, S.H.; Yoon, Y.; Nam, D.H. Tumor inhibitory effect of IRCR201, a novel cross-reactive c-met antibody targeting the PSI domain. Int. J. Mol. Sci. 2017, 18, 1968. [Google Scholar] [CrossRef]
- Miekus, K.; Kijowski, J.; Sekuła, M.; Majka, M. 17AEP-GA, an HSP90 antagonist, is a potent inhibitor of glioblastoma cell proliferation, survival, migration and invasion. Oncol. Rep. 2012, 28, 1903–1909. [Google Scholar] [CrossRef] [PubMed]
- Schuler, M.; Berardi, R.; Lim, W.T.; de Jonge, M.; Bauer, T.M.; Azaro, A.; Gottfried, M.; Han, J.Y.; Lee, D.H.; Wollner, M.; et al. Molecular correlates of response to capmatinib in advanced non-small-cell lung cancer: Clinical and biomarker results from a phase I trial. Ann. Oncol. 2020, 31, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Q.; Yang, G.; Marando, C.; Koblish, H.K.; Hall, L.M.; Fridman, J.S.; Behshad, E.; Wynn, R.; Li, Y.; et al. A novel kinase inhibitor, INCB28060, blocks c-MET-dependent signaling, neoplastic activities, and cross-talk with EGFR and HER-3. Clin. Cancer Res. 2011, 17, 7127–7138. [Google Scholar] [CrossRef] [PubMed]
- Baltschukat, S.; Engstler, B.S.; Huang, A.; Hao, H.X.; Tam, A.; Wang, H.Q.; Liang, J.; DiMare, M.T.; Bhang, H.C.; Wang, Y.; et al. Capmatinib (INC280) is active against models of non-small cell lung cancer and other cancer types with defined mechanisms of MET activation. Clin. Cancer Res. 2019, 25, 3164–3175. [Google Scholar] [CrossRef]
- Wagner, A.J.; Goldberg, J.M.; Dubois, S.G.; Choy, E.; Rosen, L.; Pappo, A.; Geller, J.; Judson, I.; Hogg, D.; Senzer, N.; et al. Tivantinib (ARQ 197), a selective inhibitor of MET, in patients with microphthalmia transcription factor-associated tumors: Results of a multicenter phase 2 trial. Cancer 2012, 118, 5894–5902. [Google Scholar] [CrossRef]
- Katayama, R.; Aoyama, A.; Yamori, T.; Qi, J.; Oh-hara, T.; Song, Y.; Engelman, J.A.; Fujita, N. Cytotoxic activity of tivantinib (ARQ 197) is not due solely to c-MET inhibition. Cancer Res 2013, 73, 3087–3096. [Google Scholar] [CrossRef]
- Giannoni, P.; Daniela de Totero, D. The HGF/c-MET axis as a potential target to overcome survival signals and improve therapeutic efficacy in multiple myeloma. Cancer Drug Resist. 2021, 4, 923–933. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Z.; Zhang, L.; Liu, G. Tivantinib hampers the proliferation of glioblastoma cells via PI3K/Akt/mammalian target of rapamycin (mTOR) signaling. Med. Sci. Monit. 2019, 25, 7383–7390. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zong, H.; Ma, C.; Ming, X.; Shang, M.; Li, K.; He, X.; Du, H.; Cao, L. Epidermal growth factor receptor in glioblastoma. Oncol. Lett. 2017, 14, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Tan, E.H.; O’Byrne, K.; Zhang, L.; Boyer, M.; Mok, T.; Hirsh, V.; Yang, J.C.; Lee, K.H.; Lu, S.; et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): A phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016, 17, 577–589. [Google Scholar] [CrossRef]
- Soria, J.C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.M.; Pawara, R.; Surana, S.J. Chapter 2—Approved and clinical trial third-generation EGFR Inhibitors. In Third Generation EGFR Inhibitors; Elsevier: Amsterdam, The Netherlands, 2019; pp. 25–43. [Google Scholar] [CrossRef]
- Fu, K.; Xie, F.; Wang, F.; Fu, L. Therapeutic strategies for EGFR-mutated non-small cell lung cancer patients with osimertinib resistance. J. Hematol. Oncol. 2022, 15, 173. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, X.; Shi, L.; Shan, Q.; Cao, Q.; Yue, C.; Li, H.; Li, S.; Wang, J.; Gao, S.; et al. The third-generation EGFR inhibitor AZD9291 overcomes primary resistance by continuously blocking ERK signaling in glioblastoma. J. Exp. Clin. Cancer Res. 2019, 38, 219. [Google Scholar] [CrossRef] [PubMed]
- Chagoya, G.; Kwatra, S.G.; Nanni, C.W.; Roberts, C.M.; Phillips, S.M.; Nullmeyergh, S.; Gilmore, S.P.; Spasojevic, I.; Corcoran, D.L.; Young, C.C.; et al. Efficacy of osimertinib against EGFRvIII+ glioblastoma. Oncotarget 2020, 11, 2074–2082. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Shi, J.; Shen, D.; Zhai, X.; Liang, D.; Wang, J.; Xie, C.; Xia, Z.; Cui, J.; Liu, F.; et al. Osimertinib induces paraptosis and TRIP13 confers resistance in glioblastoma cells. Cell Death Discov. 2023, 9, 333. [Google Scholar] [CrossRef]
- Wecker, H.; Waller, C.F. Afatinib. Recent Results Cancer Res. 2018, 211, 199–215. [Google Scholar] [CrossRef]
- Vengoji, R.; Macha, M.A.; Nimmakayala, R.K.; Rachagani, S.; Siddiqui, J.A.; Mallya, K.; Gorantla, S.; Jain, M.; Ponnusamy, M.P.; Batra, S.K.; et al. Afatinib and Temozolomide combination inhibits tumorigenesis by targeting EGFRvIII-cMet signaling in glioblastoma cells. J. Exp. Clin. Cancer Res. 2019, 38, 266. [Google Scholar] [CrossRef]
- Solca, F.; Dahl, G.; Zoephel, A.; Bader, G.; Sanderson, M.; Klein, C.; Kraemer, O.; Himmelsbach, F.; Haaksma, E.; Adolf, G.R. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J. Pharmacol. Exp. Ther. 2012, 343, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Dungo, R.T.; Keating, G.M. Afatinib: First global approval. Drugs 2013, 73, 1503–1515. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M. Erlotinib: Preclinical investigations. Oncology (Williston Park) 2003, 17, 11–16. [Google Scholar]
- Stamos, J.; Sliwkowski, M.X.; Eigenbrot, C. Structure of the epidermal growth factor receptor kinase domain alone and in complex with a 4-anilinoquinazoline inhibitor. J. Biol. Chem. 2002, 277, 46265–46272. [Google Scholar] [CrossRef] [PubMed]
- Abdelgalil, A.A.; Al-Kahtani, H.M.; Al-Jenoobi, F.I. Erlotinib. Profiles Drug Subst. Excip. Relat. Methodol. 2020, 45, 93–117. [Google Scholar] [CrossRef]
- Amini, R.; Karami, H.; Bayat, M. Combination Therapy with PIK3R3-siRNA and EGFR-TKI Erlotinib Synergistically Suppresses Glioblastoma Cell Growth In Vitro. Asian Pac. J. Cancer Prev. 2021, 22, 3993–4000. [Google Scholar] [CrossRef]
- Sidorov, M.; Dighe, P.; Woo, R.W.L.; Rodriguez-Brotons, A.; Chen, M.; Ice, R.J.; Vaquero, E.; Jian, D.; Desprez, P.Y.; Nosrati, M.; et al. Dual targeting of EGFR and MTOR pathways inhibits glioblastoma growth by modulating the tumor microenvironment. Cells 2023, 12, 547. [Google Scholar] [CrossRef]
- Dai, Z.; Wang, L.; Wang, X.; Zhao, B.; Zhao, W.; Bhardwaj, S.S.; Ye, J.; Yin, Z.; Zhang, J.; Zhao, S. Oxymatrine induces cell cycle arrest and apoptosis and suppresses the invasion of human glioblastoma cells through the EGFR/PI3K/Akt/mTOR signaling pathway and STAT3. Oncol. Rep. 2018, 40, 867–876. [Google Scholar] [CrossRef]
- Mesbahi, Y.; Zekri, A.; Ahmadian, S.; Alimoghaddam, K.; Ghavamzadeh, A.; Ghaffari, S.H. Targeting of EGFR increase anti-cancer effects of arsenic trioxide: Promising treatment for glioblastoma multiform. Eur. J. Pharmacol. 2018, 820, 274–285. [Google Scholar] [CrossRef]
- Knight, L.A.; Di Nicolantonio, F.; Whitehouse, P.; Mercer, S.; Sharma, S.; Glaysher, S.; Johnson, P.; Cree, I.A. The in vitro effect of gefitinib (‘Iressa’) alone and in combination with cytotoxic chemotherapy on human solid tumours. BMC Cancer 2004, 4, 83. [Google Scholar] [CrossRef]
- Abdel-Aziz, A.A.; El-Azab, A.S.; AlSaif, N.A.; Obaidullah, A.J.; Al-Obaid, A.M.; Al-Suwaidan, I.A. Synthesis, potential antitumor activity, cell cycle analysis, and multitarget mechanisms of novel hydrazones incorporating a 4-methylsulfonylbenzene scaffold: A molecular docking study. J. Enzyme Inhib. Med. Chem. 2021, 36, 1521–1539. [Google Scholar] [CrossRef] [PubMed]
- Karami, A.; Hossienpour, M.; Mohammadi Noori, E.; Rahpyma, M.; Najafi, K.; Kiani, A. Synergistic effect of gefitinib and temozolomide on U87MG glioblastoma angiogenesis. Nutr. Cancer 2022, 74, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, T.; Cheng, Z.; Zhu, N.; Wang, H.; Lin, L.; Wang, Z.; Yi, H.; Hu, M. Lycorine inhibits glioblastoma multiforme growth through EGFR suppression. J. Exp. Clin. Cancer Res. 2018, 37, 157. [Google Scholar] [CrossRef] [PubMed]
- Kanaan, R.; Strange, C. Use of multitarget tyrosine kinase inhibitors to attenuate platelet-derived growth factor signalling in lung disease. Eur. Respir. Rev. 2017, 26, 170061. [Google Scholar] [CrossRef] [PubMed]
- Westermark, B. Platelet-derived growth factor in glioblastoma-driver or biomarker. Ups J. Med. Sci. 2014, 119, 298–305. [Google Scholar] [CrossRef]
- Xi, Y.; Chen, M.; Liu, X.; Lu, Z.; Ding, Y.; Li, D. CP-673451, a platelet-derived growth-factor receptor inhibitor, suppresses lung cancer cell proliferation and migration. OncoTargets Ther. 2014, 7, 1215–1221. [Google Scholar] [CrossRef]
- Roberts, W.G.; Whalen, P.M.; Soderstrom, E.; Moraski, G.; Lyssikatos, J.P.; Wang, H.F.; Cooper, B.; Baker, D.A.; Savage, D.; Dalvie, D.; et al. Antiangiogenic and antitumor activity of a selective PDGFR tyrosine kinase inhibitor, CP-673,451. Cancer Res. 2005, 65, 957–966. [Google Scholar] [CrossRef]
- Lane, R.; Cilibrasi, C.; Chen, J.; Shah, K.; Messuti, E.; Mazarakis, N.K.; Stebbing, J.; Critchley, G.; Song, E.; Simon, T.; et al. PDGF-R inhibition induces glioblastoma cell differentiation via DUSP1/p38MAPK signalling. Oncogene 2022, 41, 2749–2763. [Google Scholar] [CrossRef]
- Schenone, S.; Bondavalli, F.; Botta, M. Antiangiogenic agents: An update on small molecule VEGFR inhibitors. Curr. Med. Chem. 2007, 14, 2495–2516. [Google Scholar] [CrossRef]
- Tamura, R.; Morimoto, Y.; Kosugi, K.; Sato, M.; Oishi, Y.; Ueda, R.; Kikuchi, R.; Nagashima, H.; Hikichi, T.; Noji, S.; et al. Clinical and histopathological analyses of VEGF receptors peptide vaccine in patients with primary glioblastoma—A case series. BMC Cancer 2020, 20, 196. [Google Scholar] [CrossRef]
- Sadremomtaz, A.; Mansouri, K.; Alemzadeh, G.; Safa, M.; Rastaghi, A.E.; Asghari, S.M. Dual blockade of VEGFR1 and VEGFR2 by a novel peptide abrogates VEGF-driven angiogenesis, tumor growth, and metastasis through PI3K/AKT and MAPK/ERK1/2 pathway. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2688–2700. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Jung, H.J.; Kwon, H.J. A natural small molecule voacangine inhibits angiogenesis both in vitro and in vivo. Biochem. Biophys. Res. Commun. 2012, 417, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.M.; Kim, Y.; Jung, Y.; Ko, M.; Marko-Varga, G.; Kwon, H.J. Development of novel VEGFR2 Inhibitors originating from natural product analogues with antiangiogenic impact. J. Med. Chem. 2021, 64, 15858–15867. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Strickland, B.; Finis, L.; Kooijman, J.J.; Melis, J.J.T.M.; Zaman, G.J.R.; Van Tornout, J. Comparative biochemical kinase activity analysis identifies rivoceranib as a highly selective VEGFR2 inhibitor. Cancer Chemother. Pharmacol. 2023, 91, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhou, X.; Sheng, X.; Liang, X. Efficacy and Safety of Apatinib in Patients with Recurrent Glioblastoma. Drugs R&D 2023, 23, 239–244. [Google Scholar] [CrossRef]
- Dawson, J.C.; Serrels, A.; Stupack, D.G.; Schlaepfer, D.D.; Frame, M.C. Targeting FAK in anticancer combination therapies. Nat Rev Cancer 2021, 21, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Slack-Davis, J.K.; Martin, K.H.; Tilghman, R.W.; Iwanicki, M.; Ung, E.J.; Autry, C.; Luzzio, M.J.; Cooper, B.; Kath, J.C.; Roberts, W.G.; et al. Cellular characterization of a novel focal adhesion kinase inhibitor. J. Biol. Chem. 2007, 282, 14845–14852. [Google Scholar] [CrossRef]
- Megison, M.L.; Stewart, J.E.; Nabers, H.C.; Gillory, L.A.; Beierle, E.A. FAK inhibition decreases cell invasion, migration and metastasis in MYCN amplified neuroblastoma. Clin. Exp. Metastasis 2013, 30, 555–568. [Google Scholar] [CrossRef]
- Nguemgo Kouam, P.; Bühler, H.; Hero, T.; Adamietz, I.A. The increased adhesion of tumor cells to endothelial cells after irradiation can be reduced by FAK-inhibition. Radiat. Oncol. 2019, 14, 25. [Google Scholar] [CrossRef]
- Alza, L.; Nàger, M.; Visa, A.; Cantí, C.; Herreros, J. FAK Inhibition Induces Glioblastoma Cell Senescence-Like State through p62 and p27. Cancers 2020, 12, 1086. [Google Scholar] [CrossRef]
- Menet, C.J.; Rompaey, L.V.; Geney, R. Advances in the discovery of selective JAK inhibitors. Prog. Med. Chem. 2013, 52, 153–223. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, U.; Kasembeli, M.M.; Robinson, P.; Tweardy, D.J. Targeting Janus kinases and signal transducer and activator of transcription 3 to treat inflammation, fibrosis, and cancer: Rationale, progress, and caution. Pharmacol. Rev. 2020, 72, 486–526. [Google Scholar] [CrossRef] [PubMed]
- Park, A.K.; Kim, P.; Ballester, L.Y.; Esquenazi, Y.; Zhao, Z. Subtype-specific signaling pathways and genomic aberrations associated with prognosis of glioblastoma. Neuro-Oncology 2019, 21, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Sun, Y.; Hou, W.; Ma, L.; Tao, Y.; Li, D.; Xu, C.; Bao, J.; Fan, W. The JAK2/STAT3 pathway inhibitor, AG490, suppresses the abnormal behavior of keloid fibroblasts in vitro. Int. J. Mol. Med. 2020, 46, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, T.D.; Vagapova, E.R.; Astashkova, O.O.; Spirin, P.V.; Prassolov, V.S. Inhibition of non-receptor tyrosine kinase JAK2 reduces neuroblastoma cell growth and enhances the action of doxorubicin. Mol. Biol. 2020, 54, 256–261. [Google Scholar] [CrossRef]
- Ajayi, S.; Becker, H.; Reinhardt, H.; Engelhardt, M.; Zeiser, R.; von Bubnoff, N.; Wäsch, R. Ruxolitinib. Recent Results Cancer Res. 2018, 212, 119–132. [Google Scholar] [CrossRef]
- Appeldoorn, T.Y.J.; Munnink, T.H.O.; Morsink, L.M.; Hooge, M.N.L.; Touw, D.J. Pharmacokinetics and pharmacodynamics of ruxolitinib: A review. Clin. Pharmacokinet. 2023, 62, 559–571. [Google Scholar] [CrossRef]
- Delen, E.; Doğanlar, O. The dose dependent effects of ruxolitinib on the invasion and tumorigenesis in gliomas cells via inhibition of interferon gamma-depended JAK/STAT signaling pathway. J. Korean Neurosurg. Soc. 2020, 63, 444–454. [Google Scholar] [CrossRef]
- Goker Bagca, B.; Ozates, N.P.; Biray Avci, C. Ruxolitinib enhances cytotoxic and apoptotic effects of temozolomide on glioblastoma cells by regulating WNT signaling pathway-related genes. Med. Oncol. 2022, 40, 37. [Google Scholar] [CrossRef]
- Ge, L.; Xu, L.; Lu, S.; Yan, H. LCK expression is a potential biomarker for distinguishing primary central nervous system lymphoma from glioblastoma multiforme. FEBS Open Bio 2020, 10, 904–911. [Google Scholar] [CrossRef]
- Stachlewitz, R.F.; Hart, M.A.; Bettencourt, B.; Kebede, T.; Schwartz, A.; Ratnofsky, S.E.; Calderwood, D.J.; Waegell, W.O.; Hirst, G.C. A-770041, a novel and selective small-molecule inhibitor of Lck, prevents heart allograft rejection. J. Pharmacol. Exp. Ther. 2005, 315, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Zepecki, J.P.; Snyder, K.M.; Moreno, M.M.; Fajardo, E.; Fiser, A.; Ness, J.; Sarkar, A.; Toms, S.A.; Tapinos, N. Regulation of human glioma cell migration, tumor growth, and stemness gene expression using a Lck targeted inhibitor. Oncogene 2019, 38, 1734–1750. [Google Scholar] [CrossRef] [PubMed]
- Mansueto, M.S.; Reens, A.; Rakhilina, L.; Chi, A.; Pan, B.S.; Miller, J.R. A reevaluation of the spleen tyrosine kinase (SYK) activation mechanism. J. Biol. Chem. 2019, 294, 7658–7668. [Google Scholar] [CrossRef] [PubMed]
- Moncayo, G.; Grzmil, M.; Smirnova, T.; Zmarz, P.; Huber, R.M.; Hynx, D.; Kohler, H.; Wang, Y.; Hotz, H.R.; Hynes, N.E.; et al. SYK inhibition blocks proliferation and migration of glioma cells and modifies the tumor microenvironment. Neuro-Oncology 2018, 20, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, S.S. Kinase inhibitors for the treatment of inflammatory and autoimmune disorders. Purinergic Signal 2009, 5, 107–115. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Huang, Y.; Liu, Y.; Zhang, X.; Yue, P.; Ma, X.; Miao, Z.; Long, X.; Yang, Y.; Wan, X.; et al. BAY61-3606 attenuates neuroinflammation and neurofunctional damage by inhibiting microglial Mincle/Syk signaling response after traumatic brain injury. Int. J. Mol. Med. 2022, 49, 5. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Seo, S.G.; Heo, Y.S.; Yue, S.; Cheng, J.X.; Lee, K.W.; Kim, K.H. Piceatannol, natural polyphenolic stilbene, inhibits adipogenesis via modulation of mitotic clonal expansion and insulin receptor-dependent insulin signaling in early phase of differentiation. J. Biol. Chem. 2012, 287, 11566–11578. [Google Scholar] [CrossRef]
- MacGlashan, D.; Undem, B.J. Inducing an anergic state in mast cells and basophils without secretion. J. Allergy Clin. Immunol. 2008, 121, 1500–1506.e4. [Google Scholar] [CrossRef]
- Patou, J.; Holtappels, G.; Affleck, K.; van Cauwenberge, P.; Bachert, C. Syk-kinase inhibition prevents mast cell activation in nasal polyps. Rhinology 2011, 49, 100–106. [Google Scholar] [CrossRef]
- Caner, A.; Asik, E.; Ozpolat, B. SRC signaling in cancer and tumor microenvironment. Adv. Exp. Med. Biol. 2021, 1270, 57–71. [Google Scholar] [CrossRef]
- Musumeci, F.; Schenone, S.; Brullo, C.; Botta, M. An update on dual Src/Abl inhibitors. Future Med. Chem. 2012, 4, 799–822. [Google Scholar] [CrossRef] [PubMed]
- Cirotti, C.; Contadini, C.; Barilà, D. SRC kinase in glioblastoma news from an old acquaintance. Cancers 2020, 12, 1558. [Google Scholar] [CrossRef] [PubMed]
- Smolinski, M.P.; Bu, Y.; Clements, J.; Gelman, I.H.; Hegab, T.; Cutler, D.L.; Fang, J.W.S.; Fetterly, G.; Kwan, R.; Barnett, A.; et al. Discovery of Novel Dual Mechanism of Action Src Signaling and Tubulin Polymerization Inhibitors (KX2-391 and KX2-361). J. Med. Chem. 2018, 61, 4704–4719. [Google Scholar] [CrossRef]
- Ciesielski, M.J.; Bu, Y.; Munich, S.A.; Teegarden, P.; Smolinski, M.P.; Clements, J.L.; Lau, J.Y.N.; Hangauer, D.G.; Fenstermaker, R.A. KX2-361: A novel orally bioavailable small molecule dual Src/tubulin inhibitor that provides long term survival in a murine model of glioblastoma. J. Neurooncol. 2018, 140, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Tintori, C.; Fallacara, A.L.; Radi, M.; Zamperini, C.; Dreassi, E.; Crespan, E.; Maga, G.; Schenone, S.; Musumeci, F.; Brullo, C.; et al. Combining X-ray crystallography and molecular modeling toward the optimization of pyrazolo[3,4-d]pyrimidines as potent c-Src inhibitors active in vivo against neuroblastoma. J. Med. Chem. 2015, 58, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Rango, E.; Pastorino, F.; Brignole, C.; Mancini, A.; Poggialini, F.; Di Maria, S.; Zamperini, C.; Iovenitti, G.; Fallacara, A.L.; Sabetta, S.; et al. The pyrazolo[3,4-d]pyrimidine derivative Si306 encapsulated into anti-GD2-immunoliposomes as therapeutic treatment of neuroblastoma. Biomedicines 2022, 10, 659. [Google Scholar] [CrossRef] [PubMed]
- Greco, C.; Taresco, V.; Pearce, A.K.; Vasey, C.E.; Smith, S.; Rahman, R.; Alexander, C.; Cavanagh, R.J.; Musumeci, F.; Schenone, S. Development of pyrazolo[3,4-d]pyrimidine kinase inhibitors as potential clinical candidates for glioblastoma multiforme. ACS Med. Chem. Lett. 2020, 11, 657–663. [Google Scholar] [CrossRef]
- Vignaroli, G.; Iovenitti, G.; Zamperini, C.; Coniglio, F.; Calandro, P.; Molinari, A.; Fallacara, A.L.; Sartucci, A.; Calgani, A.; Colecchia, D.; et al. Prodrugs of pyrazolo[3,4-d]pyrimidines: From library synthesis to evaluation as potential anticancer agents in an orthotopic glioblastoma model. J. Med. Chem. 2017, 60, 6305–6320. [Google Scholar] [CrossRef]
- Nešović, M.; Divac Rankov, A.; Podolski-Renić, A.; Nikolić, I.; Tasić, G.; Mancini, A.; Schenone, S.; Pešić, M.; Dinić, J. Src Inhibitors Pyrazolo[3,4-d]pyrimidines, Si306 and Pro-Si306, inhibit focal adhesion kinase and suppress human glioblastoma invasion in vitro and in vivo. Cancers 2020, 12, 1570. [Google Scholar] [CrossRef]
- Kostić, A.; Jovanović Stojanov, S.; Podolski-Renić, A.; Nešović, M.; Dragoj, M.; Nikolić, I.; Tasić, G.; Schenone, S.; Pešić, M.; Dinić, J. Pyrazolo[3,4-d]pyrimidine tyrosine kinase inhibitors induce oxidative stress in patient-derived glioblastoma cells. Brain Sci. 2021, 11, 884. [Google Scholar] [CrossRef]
- Jovanović Stojanov, S.; Kostić, A.; Ljujić, M.; Lupšić, E.; Schenone, S.; Pešić, M.; Dinić, J. Autophagy inhibition enhances anti-glioblastoma effects of pyrazolo[3,4-d]pyrimidine tyrosine kinase inhibitors. Life 2022, 12, 1503. [Google Scholar] [CrossRef] [PubMed]
- Contadini, C.; Cirotti, C.; Carbone, A.; Norouzi, M.; Cianciusi, A.; Crespan, E.; Perini, C.; Maga, G.; Barilà, D.; Musumeci, F.; et al. Identification and biological characterization of the pyrazolo[3,4-d]pyrimidine derivative SI388 active as src inhibitor. Pharmaceuticals 2023, 16, 958. [Google Scholar] [CrossRef] [PubMed]
- Poggialini, F.; Vagaggini, C.; Brai, A.; Pasqualini, C.; Crespan, E.; Maga, G.; Perini, C.; Cabella, N.; Botta, L.; Musumeci, F.; et al. Biological evaluation and in vitro characterization of adme profile of in-house pyrazolo[3,4-d]pyrimidines as dual tyrosine kinase inhibitors active against glioblastoma multiforme. Pharmaceutics 2023, 15, 453. [Google Scholar] [CrossRef] [PubMed]
- Jaraíz-Rodríguez, M.; Rocío Talaverón, R.; García-Vicente, L.; Pelaz, S.G.; Domínguez-Prieto, M.; Álvarez-Vázquez, A.; Flores-Hernández, R.; Sin, W.C.; Bechberger, J.; Medina, J.M.; et al. Connexin43 peptide, TAT-Cx43266–283, selectively targets glioma cells, impairs malignant growth, and enhances survival in mouse models in vivo. Neuro-Oncology 2020, 15, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Pelaz, S.G.; Jaraíz-Rodríguez, M.; Álvarez-Vázquez, A.; Talaverón, R.; García-Vicente, L.; Flores-Hernández, R.; Gómez de Cedrón, M.; Tabernero, M.; Ramírez de Molina, A.; Lillo, C.; et al. Targeting metabolic plasticity in glioma stem cells in vitro and in vivo through specific inhibition of c-Src by TAT-Cx43266-283. EBioMedicine 2020, 62, 103134. [Google Scholar] [CrossRef] [PubMed]
- Abbassi, R.; Johns, T.G.; Kassiou, M.; Munoz, L. DYRK1A in neurodegeneration and cancer: Molecular basis and clinical implications. Pharmacol. Ther. 2015, 151, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Litovchick, L.; Florens, L.A.; Swanson, S.K.; Washburn, M.P.; DeCaprio, J.A. DYRK1A protein kinase promotes quiescence and senescence through DREAM complex assembly. Genes Dev. 2011, 25, 801–813. [Google Scholar] [CrossRef]
- Recasens, A.; Humphrey, S.J.; Ellis, M.; Hoque, M.; Abbassi, R.H.; Chen, B.; Longworth, M.; Needham, E.J.; James, D.E.; Johns, T.G.; et al. Global phosphoproteomics reveals DYRK1A regulates CDK1 activity in glioblastoma cells. Cell Death Discov. 2021, 7, 81. [Google Scholar] [CrossRef]
- Massey, A.J.; Benwell, K.; Burbridge, M.; Kotschy, A.; Walmsley, D.L. Targeting DYRK1A/B kinases to modulate p21-cyclin D1-p27 signalling and induce anti-tumour activity in a model of human glioblastoma. J. Cell Mol. Med. 2021, 25, 10650–10662. [Google Scholar] [CrossRef]
- Akinleye, A.; Furqan, M.; Mukhi, N.; Ravella, P.; Liu, D. MEK and the inhibitors: From bench to bedside. J. Hematol. Oncol. 2013, 6, 27. [Google Scholar] [CrossRef]
- Selvasaravanan, K.D.; Wiederspohn, N.; Hadzalic, A.; Strobel, H.; Payer, C.; Schuster, A.; Karpel-Massler, G.; Siegelin, M.D.; Halatsch, M.E.; Debatin, K.M.; et al. The limitations of targeting MEK signalling in Glioblastoma therapy. Sci. Rep. 2020, 10, 7401. [Google Scholar] [CrossRef] [PubMed]
- Monk, B.J.; Grisham, R.N.; Banerjee, S.; Kalbacher, E.; Mirza, M.R.; Romero, I.; Vuylsteke, P.; Coleman, R.L.; Hilpert, F.; Oza, A.M.; et al. MILO/ENGOT-ov11: Binimetinib versus physician’s choice chemotherapy in recurrent or persistent low-grade serous carcinomas of the ovary, fallopian tube, or primary peritoneum. J. Clin. Oncol. 2020, 38, 3753–3762. [Google Scholar] [CrossRef] [PubMed]
- Woodfield, S.E.; Zhang, L.; Scorsone, K.A.; Liu, Y.; Zage, P.E. Binimetinib inhibits MEK and is effective against neuroblastoma tumor cells with low NF1 expression. BMC Cancer 2016, 16, 172. [Google Scholar] [CrossRef] [PubMed]
- Bikhezar, F.; de Kruijff, R.M.; van der Meer, A.J.G.M.; Torrelo Villa, G.; van der Pol, S.M.A.; Becerril Aragon, G.; Gasol Garcia, A.; Narayan, R.S.; de Vries, H.E.; Slotman, B.J.; et al. Preclinical evaluation of binimetinib (MEK162) delivered via polymeric nanocarriers in combination with radiation and temozolomide in glioma. J. Neurooncol. 2020, 146, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R.S.; Gasol, A.; Slangen, P.L.G.; Cornelissen, F.M.G.; Lagerweij, T.; Veldman, H.Y.Y.E.; Dik, R.; van den Berg, J.; Slotman, B.J.; Würdinger, T.; et al. Identification of MEK162 as a radiosensitizer for the treatment of glioblastoma. Mol. Cancer Ther. 2018, 17, 347–354. [Google Scholar] [CrossRef]
- Vinitsky, A.; Chiang, J.; Bag, A.K.; Campagne, O.; Stewart, C.F.; Dunphy, P.; Shulkin, B.; Li, Q.; Lin, T.; Hoehn, M.E.; et al. LGG-22. SJ901: Phase I/II evaluation of single agent mirdametinib (PD-0325901), a brain-penetrant MEK1/2 inhibitor, for the treatment of children, adolescents, and young adults with low-grade glioma (LGG). Neuro-Oncology 2022, 24, i92. [Google Scholar] [CrossRef]
- Houweling, M.; Abdul, U.K.; Brahm, C.; Lagerweij, T.; Heukelom, S.; Koken, P.W.; Honeywell, R.; Wedekind, L.E.; Peters, G.J.; Verheul, H.; et al. Radio-sensitizing effect of MEK inhibition in glioblastoma in vitro and in vivo. J. Cancer Res. Clin. Oncol. 2023, 149, 297–305. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, A.; Lin, J.; Wu, L.; Zhang, H.; Yang, X.; Wan, X.; Miao, R.; Sang, X.; Zhao, H. Mps1/TTK: A novel target and biomarker for cancer. J. Drug Target 2017, 25, 112–118. [Google Scholar] [CrossRef]
- Wang, J.; Xie, Y.; Bai, X.; Wang, N.; Yu, H.; Deng, Z.; Lian, M.; Yu, S.; Liu, H.; Xie, W.; et al. Targeting dual specificity protein kinase TTK attenuates tumorigenesis of glioblastoma. Oncotarget 2018, 9, 3081–3088. [Google Scholar] [CrossRef]
- Atrafi, F.; Boix, O.; Subbiah, V.; Diamond, J.R.; Chawla, S.P.; Tolcher, A.W.; LoRusso, P.M.; Eder, J.P.; Gutierrez, M.; Sankhala, K.; et al. A phase I study of an MPS1 inhibitor (BAY 1217389) in combination with paclitaxel using a novel randomized continual reassessment method for dose escalation. Clin. Cancer Res. 2021, 27, 6366–6375. [Google Scholar] [CrossRef]
- Yu, J.; Gao, G.; Wei, X.; Wang, Y. TTK Protein Kinase promotes temozolomide resistance through inducing autophagy in glioblastoma. BMC Cancer 2022, 22, 786. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Laufer, R.; Patel, N.K.; Ng, G.; Sampson, P.B.; Li, S.W.; Lang, Y.; Feher, M.; Brokx, R.; Beletskaya, I.; et al. Discovery of pyrazolo[1,5-a]pyrimidine TTK Inhibitors: CFI-402257 is a potent, selective, bioavailable anticancer agent. ACS Med. Chem. Lett. 2016, 7, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.M.; Wei, X.; Fletcher, G.C.; Kiarash, R.; Brokx, R.; Hodgson, R.; Beletskaya, I.; Bray, M.R.; Mak, T.W. Functional characterization of CFI-402257, a potent and selective Mps1/TTK kinase inhibitor, for the treatment of cancer. Proc. Natl. Acad. Sci. USA 2017, 114, 3127–3132. [Google Scholar] [CrossRef] [PubMed]
- Garuti, L.; Roberti, M.; Bottegoni, G. Multi-kinase inhibitors. Curr. Med. Chem. 2015, 22, 695–712. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Zheng, F.; Ren, D.; Du, F.; Dong, Q.; Wang, Z.; Zhao, F.; Ahmad, R.; Zhao, J. Anlotinib: A novel multi-targeting tyrosine kinase inhibitor in clinical development. J. Hematol. Oncol. 2018, 11, 120. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhou, A.; Zhang, W.; Jiang, Z.; Chen, B.; Zhao, J.; Li, Z.; Wang, L.; Bi, X.; Zhao, H.; et al. Anlotinib in the treatment of advanced hepatocellular carcinoma: An open-label phase II study (ALTER-0802 study). Hepatol. Int. 2021, 15, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wang, H.; Pan, H.; Chen, J.; Deng, C. Anlotinib combined with temozolomide suppresses glioblastoma growth via mediation of JAK2/STAT3 signaling pathway. Cancer Chemother. Pharmacol. 2022, 89, 183–196. [Google Scholar] [CrossRef]
- Sun, Y.; Niu, W.; Du, F.; Du, C.; Li, S.; Wang, J.; Li, L.; Wang, F.; Hao, Y.; Li, C.; et al. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J. Hematol. Oncol. 2016, 9, 105. [Google Scholar] [CrossRef]
- Galimberti, C.; Piepoli, T.; Letari, O.; Artusi, R.; Persiani, S.; Caselli, G.; Rovati, L.C. CR13626: A novel oral brain penetrant tyrosine kinase inhibitor that reduces tumor growth and prolongs survival in a mouse model of glioblastoma. Am. J. Cancer Res. 2021, 11, 3558–3574. [Google Scholar]
- Chen, H.M.; Tsai, C.H.; Hung, W.C. Foretinib inhibits angiogenesis, lymphangiogenesis and tumor growth of pancreatic cancer in vivo by decreasing VEGFR-2/3 and TIE-2 signaling. Oncotarget 2015, 6, 14940–14952. [Google Scholar] [CrossRef]
- Gortany, N.K.; Panahi, G.; Ghafari, H.; Shekari, M.; Ghazi-Khansari, M. Foretinib induces G2/M cell cycle arrest, apoptosis, and invasion in human glioblastoma cells through c-MET inhibition. Cancer Chemother. Pharmacol. 2021, 87, 827–842. [Google Scholar] [CrossRef] [PubMed]
- Han, K.M.; Kang, R.J.; Jeon, H.; Lee, H.J.; Lee, J.S.; Park, H.; Gak Jeon, S.; Suk, K.; Seo, J.; Hoe, H.S. Regorafenib regulates ad pathology, neuroinflammation, and dendritic spinogenesis in cells and a mouse model of AD. Cells 2020, 9, 1655. [Google Scholar] [CrossRef] [PubMed]
- Chiang, I.T.; Liu, Y.C.; Liu, H.S.; Ali, A.A.A.; Chou, S.Y.; Hsu, T.I.; Hsu, F.T. Regorafenib Reverses Temozolomide-Induced CXCL12/CXCR4 signaling and triggers apoptosis mechanism in glioblastoma. Neurotherapeutics 2022, 19, 616–634. [Google Scholar] [CrossRef] [PubMed]
- Zeiner, P.S.; Kinzig, M.; Divé, I.; Maurer, G.D.; Filipski, K.; Harter, P.N.; Senft, C.; Bähr, O.; Hattingen, E.; Steinbach, J.P.; et al. Regorafenib CSF penetration, efficacy, and mri patterns in recurrent malignant glioma patients. J. Clin. Med. 2019, 8, 2031. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhan, Y.; Wu, Z.; Lin, M.; Jin, X.; Jiang, L.; Qiu, Y. A novel multitarget kinase inhibitor BZG with potent anticancer activity in vitro and vivo enhances efficacy of sorafenib through PI3K pathways in hepatocellular carcinoma cells. Biomed. Pharmacother. 2020, 125, 110033. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Jo, Y.; Oh, H.K.; Kim, E.H. Sorafenib increases tumor treating fields-induced cell death in glioblastoma by inhibiting STAT3. Am. J. Cancer Res. 2020, 10, 3475–3486. [Google Scholar] [PubMed]
- Zajak, A.; Sumorek-Wiadro, J.; Maciejczik, A.; Langner, E.; Wertel, I.; Rzeski, W.; Jakubowicz-Gil, J. LY294002 and sorafenib as inhibitors of intracellular survival pathways in the elimination of human glioma cells by programmed cell death. Cell Tissue Res. 2021, 386, 17–28. [Google Scholar] [CrossRef]
- Sweeney, W.E.; Frost, P.; Avner, E.D. Tesevatinib ameliorates progression of polycystic kidney disease in rodent models of autosomal recessive polycystic kidney disease. World J. Nephrol. 2017, 6, 188–200. [Google Scholar] [CrossRef]
- Kizilbash, S.H.; Gupta, S.K.; Parrish, K.E.; Laramy, J.K.; Kim, M.; Gampa, G.; Carlson, B.L.; Bakken, K.K.; Mladek, A.C.; Schroeder, M.A.; et al. In vivo efficacy of Tesevatinib in EGFR-amplified patient-derived xenograft glioblastoma models may be limited by tissue binding and compensatory signaling. Mol. Cancer Ther. 2021, 20, 1009–1018. [Google Scholar] [CrossRef]
- Brar, H.K.; Jose, J.; Wu, Z.; Sharma, M. Tyrosine kinase inhibitors for glioblastoma multiforme: Challenges and opportunities for drug delivery. Pharmaceutics 2022, 15, 59. [Google Scholar] [CrossRef]
- Cooper, E.; Choi, P.J.; Denny, W.A.; Jose, J.; Dragunow, M.; Park, T.I. The use of heptamethine cyanine dyes as drug-conjugate systems in the treatment of primary and metastatic brain tumors. Front. Oncol. 2021, 11, 654921. [Google Scholar] [CrossRef] [PubMed]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed]
- Lakkadwala, S.; Singh, J. Co-delivery of doxorubicin and erlotinib through liposomal nanoparticles for glioblastoma tumor regression using an in vitro brain tumor model. Colloids Surf. B Biointerfaces 2019, 173, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Rehman, U.; Parveen, N.; Sheikh, A.; Abourehab, M.A.S.; Sahebkar, A.; Kesharwani, P. Polymeric nanoparticles-siRNA as an emerging nano-polyplexes against ovarian cancer. Colloids Surf. B Biointerfaces 2022, 218, 112766. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.M.; Ahmad, F.J.; Panda, A.K.; Talegaonkar, S. Investigation of imatinib loaded surface decorated biodegradable nanocarriers against glioblastoma cell lines: Intracellular uptake and cytotoxicity studies. Int. J. Pharm. 2016, 507, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.K.; Labhasetwar, V. Nanotech approaches to drug delivery and imaging. Drug Discov. Today 2003, 8, 1112–1120. [Google Scholar] [CrossRef]
- Xu, W.; Ye, C.; Qing, X.; Liu, S.; Lv, X.; Wang, W.; Dong, X.; Zhang, Y. Multi-target tyrosine kinase inhibitor nanoparticle delivery systems for cancer therapy. Mater. Today Bio 2022, 16, 100358. [Google Scholar] [CrossRef]
- Greish, K.; Jasim, A.; Parayath, N.; Abdelghany, S.; Alkhateeb, A.; Taurin, S.; Nehoff, H. Micellar formulations of Crizotinib and Dasatinib in the management of glioblastoma multiforme. J. Drug Target 2018, 26, 692–708. [Google Scholar] [CrossRef]
- Kratz, F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Control Release 2008, 132, 171–183. [Google Scholar] [CrossRef]
- Yang, Z.; Du, Y.; Lei, L.; Xia, X.; Wang, X.; Tong, F.; Li, Y.; Gao, H. Co-delivery of ibrutinib and hydroxychloroquine by albumin nanoparticles for enhanced chemotherapy of glioma. Int. J. Pharm. 2023, 630, 122436. [Google Scholar] [CrossRef]
- Zhou, X.; Shi, K.; Hao, Y.; Yang, C.; Zha, R.; Yi, C.; Qian, Z. Advances in nanotechnology-based delivery systems for EGFR tyrosine kinases inhibitors in cancer therapy. Asian J. Pharm. Sci. 2020, 15, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.L.; Grimes, S.W.; Lewis, R.L.; Alexis, F. Multilayered polymer-coated carbon nanotubes to deliver dasatinib. Mol. Pharm. 2014, 11, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Saliou, B.; Thomas, O.; Lautram, N.; Clavreul, A.; Hureaux, J.; Urban, T.; Benoit, J.P.; Lagarce, F. Development and in vitro evaluation of a novel lipid nanocapsule formulation of etoposide. Eur. J. Pharm. Sci. 2013, 50, 172–180. [Google Scholar] [CrossRef]
- Clavreul, A.; Roger, E.; Pourbaghi-Masouleh, M.; Lemaire, L.; Tétaud, C.; Menei, P. Development and characterization of sorafenib-loaded lipid nanocapsules for the treatment of glioblastoma. Drug Deliv. 2018, 25, 1756–1765. [Google Scholar] [CrossRef]
- Sanai, N.; Li, J.; Boerner, J.; Stark, K.; Wu, J.; Kim, S.; Derogatis, A.; Mehta, S.; Dhruv, H.D.; Heilbrun, L.K.; et al. Phase 0 Trial of AZD1775 in first-recurrence glioblastoma patients. Clin. Cancer Res. 2018, 24, 3820–3828. [Google Scholar] [CrossRef] [PubMed]
- Duerinck, J.; Du Four, S.; Bouttens, F.; Andre, C.; Verschaeve, V.; Van Fraeyenhove, F.; Chaskis, C.; D’Haene, N.; Le Mercier, M.; Rogiers, A.; et al. Randomized phase II trial comparing axitinib with the combination of axitinib and lomustine in patients with recurrent glioblastoma. J. Neurooncol. 2018, 136, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Hainsworth, J.D.; Becker, K.P.; Mekhail, T.; Chowdhary, S.A.; Eakle, J.F.; Wright, D.; Langdon, R.M.; Yost, K.J.; Padula, G.D.A.; West-Osterfield, K.; et al. Phase I/II study of bevacizumab with BKM120, an oral PI3K inhibitor, in patients with refractory solid tumors (phase I) and relapsed/refractory glioblastoma (phase II). J. Neurooncol. 2019, 144, 303–311. [Google Scholar] [CrossRef]
- Mehta, S.; Fiorelli, R.; Bao, X.; Pennington-Krygier, C.; Derogatis, A.; Kim, S.; Yoo, W.; Li, J.; Sanai, N. A Phase 0 trial of ceritinib in patients with brain metastases and recurrent glioblastoma. Clin. Cancer Res. 2022, 28, 289–297. [Google Scholar] [CrossRef]
- Desai, A.V.; Robinson, G.W.; Gauvain, K.; Basu, E.M.; Macy, M.E.; Maese, L.; Whipple, N.S.; Sabnis, A.J.; Foster, J.H.; Shusterman, S.; et al. Entrectinib in children and young adults with solid or primary CNS tumors harboring NTRK, ROS1, or ALK aberrations (STARTRK-NG). Neuro-Oncology 2022, 24, 1776–1789. [Google Scholar] [CrossRef]
- Lee, E.Q.; Muzikansky, A.; Duda, D.G.; Gaffey, S.; Dietrich, J.; Nayak, L.; Chukwueke, U.N.; Beroukhim, R.; Doherty, L.; Laub, C.K.; et al. Phase II trial of ponatinib in patients with bevacizumab-refractory glioblastoma. Cancer Med. 2019, 8, 5988–5994. [Google Scholar] [CrossRef]
- Lombardi, G.; De Salvo, G.L.; Brandes, A.A.; Eoli, M.; Rudà, R.; Faedi, M.; Lolli, I.; Pace, A.; Daniele, B.; Pasqualetti, F.; et al. Regorafenib compared with lomustine in patients with relapsed glioblastoma (REGOMA): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2019, 20, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Galanis, E.; Anderson, S.K.; Twohy, E.L.; Carrero, X.W.; Dixon, J.G.; Tran, D.D.; Jeyapalan, S.A.; Anderson, D.M.; Kaufmann, T.J.; Feathers, R.W.; et al. A phase 1 and randomized, placebo-controlled phase 2 trial of bevacizumab plus dasatinib in patients with recurrent glioblastoma: Alliance/North Central Cancer Treatment Group N0872. Cancer 2019, 125, 3790–3800. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Jardim, D.L.; Johnson, F.M.; Subbiah, V.; Piha-Paul, S.; Tsimberidou, A.M.; Falchook, G.S.; Karp, D.; Zinner, R.; Wheler, J.; et al. Phase I study of the combination of crizotinib (as a MET inhibitor) and dasatinib (as a c-SRC inhibitor) in patients with advanced cancer. Invest. New Drugs 2018, 36, 416–423. [Google Scholar] [CrossRef] [PubMed]
- HMPL-813 in Treating Patients with Glioblastoma. Available online: https://clinicaltrials.gov/study/NCT03231501?cond=Glioblastoma&term=Tyrosine%20Kinase%20Inhibitor&limit=50&page=1&rank=2 (accessed on 17 January 2024).
- Han, B.; Li, K.; Zhao, Y.; Li, B.; Cheng, Y.; Zhou, J.; Lu, Y.; Shi, Y.; Wang, Z.; Jiang, L.; et al. Anlotinib as a third-line therapy in patients with refractory advanced non-small-cell lung cancer: A multicentre, randomised phase II trial (ALTER0302). Br. J. Cancer 2018, 118, 654–661. [Google Scholar] [CrossRef]
- Anlotinib Combined with Dose-Dense Temozolomide for the First Recurrent or Progressive Glioblastoma after STUPP Regimen. Available online: https://clinicaltrials.gov/study/NCT04547855?cond=Glioblastoma&term=Tyrosine%20Kinase%20Inhibitor&rank=10#publications (accessed on 17 January 2024).
- Johnson, T.S.; MacDonald, T.J.; Pacholczyk, R.; Aguilera, D.; Al-Basheer, A.; Bajaj, M.; Bandopadhayay, P.; Berrong, Z.; Bouffet, E.; Castellino, R.C.; et al. Indoximod-based chemo-immunotherapy for pediatric brain tumors: A first-in-children phase 1 trial. Neuro-Oncology 2023. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).