Fingolimod Inhibits Exopolysaccharide Production and Regulates Relevant Genes to Eliminate the Biofilm of K. pneumoniae
Abstract
1. Introduction
2. Results
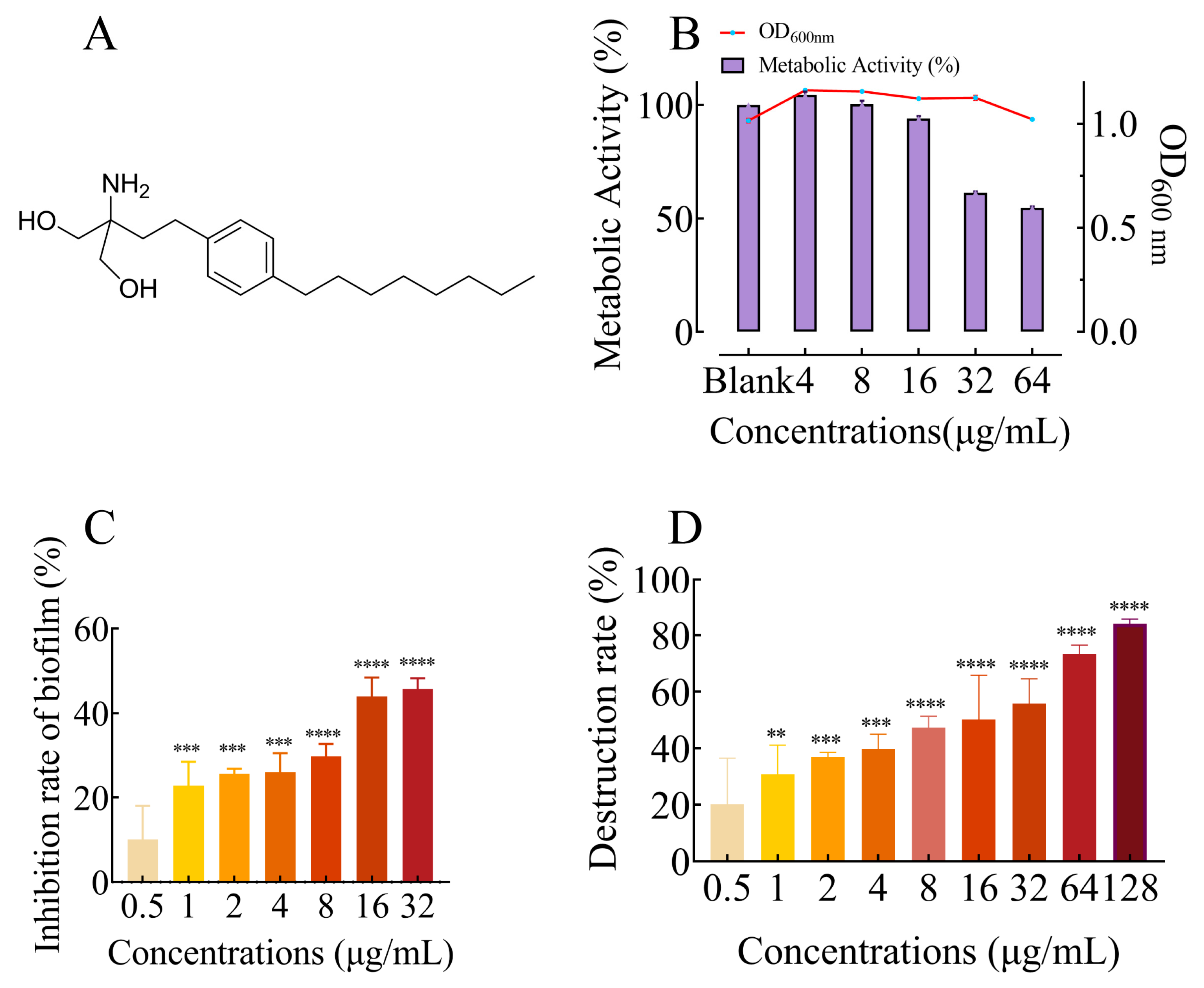
2.1. FLD Had no Effect on Growth and Metabolic Activity
2.2. FLD Can Inhibit Biofilm Formation and Eradicate Mature Biofilm
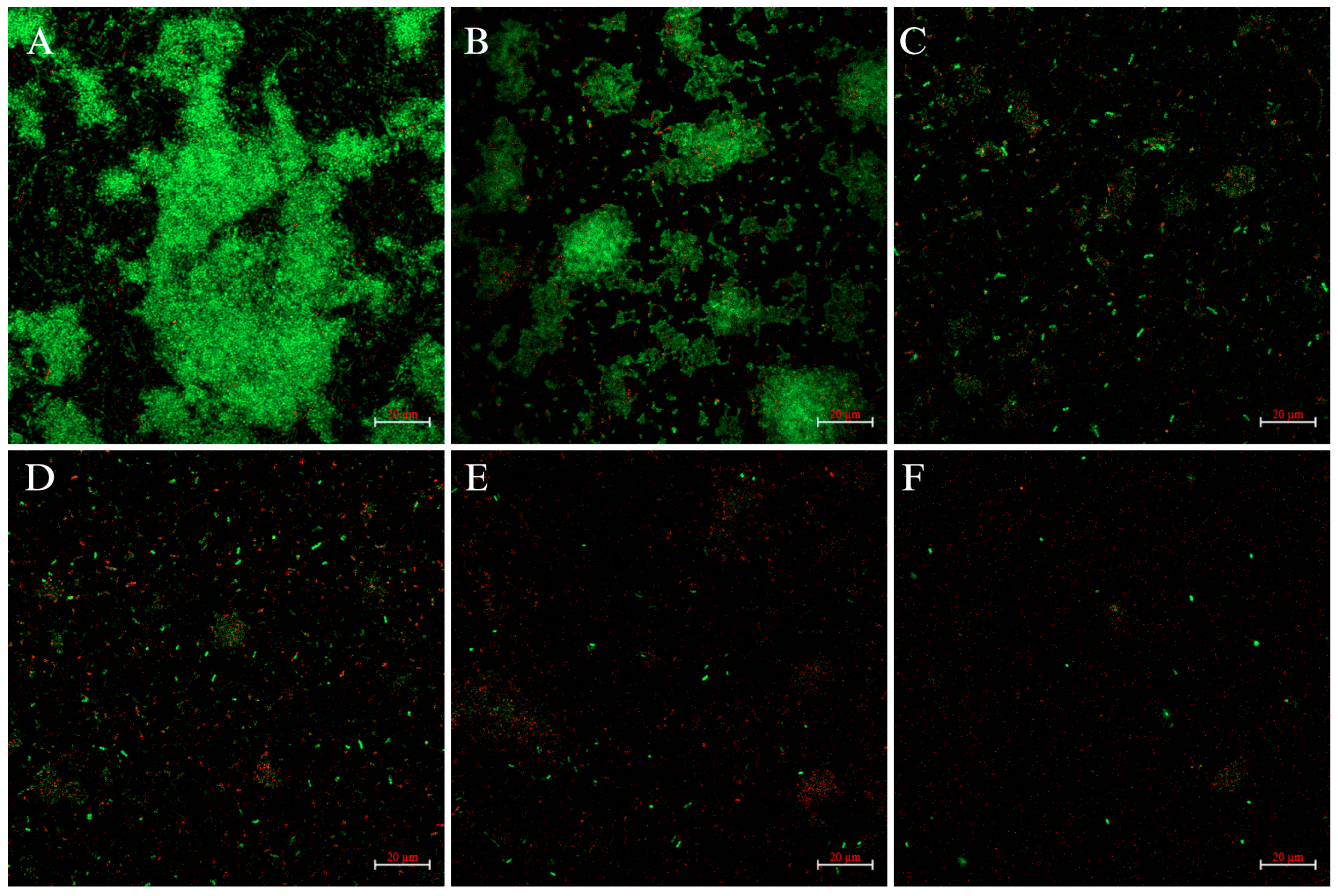
2.3. CLSM Analysis
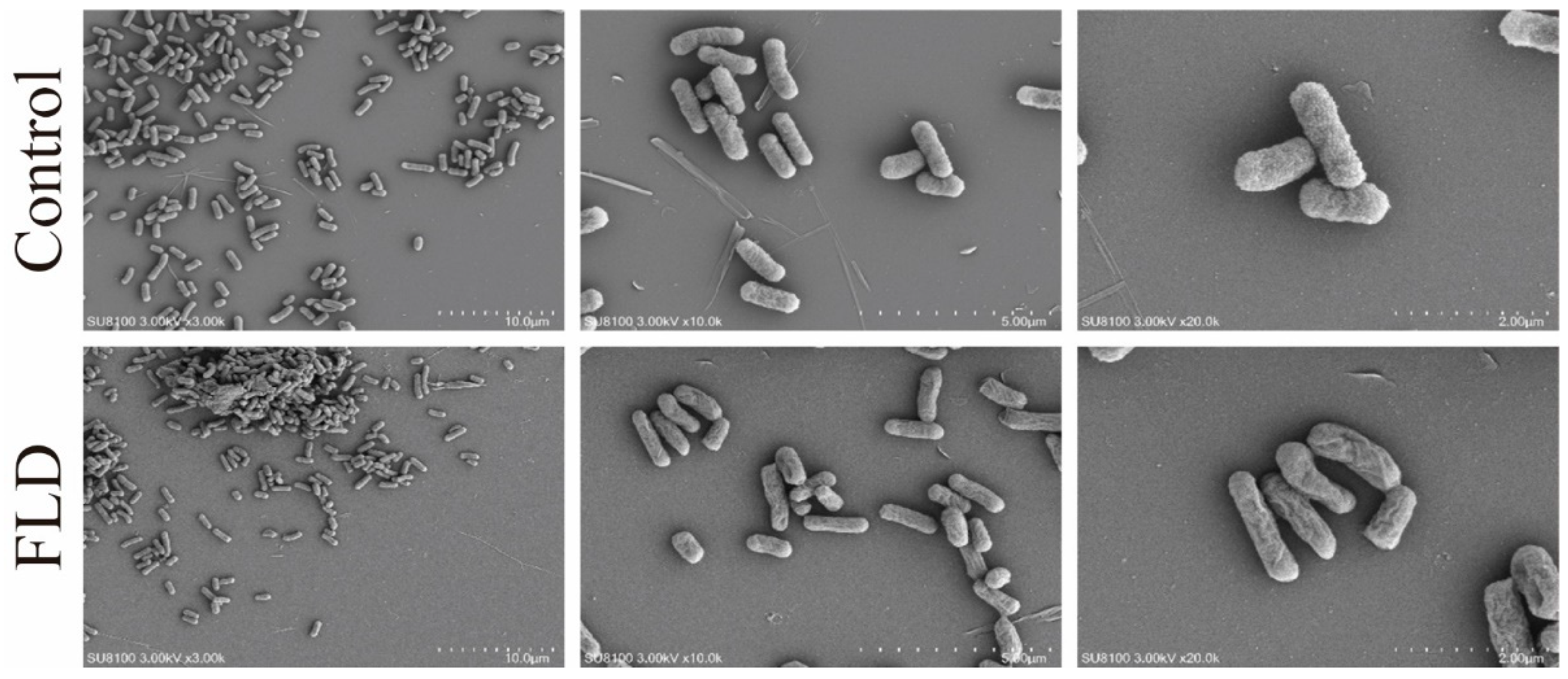
2.4. SEM Analysis
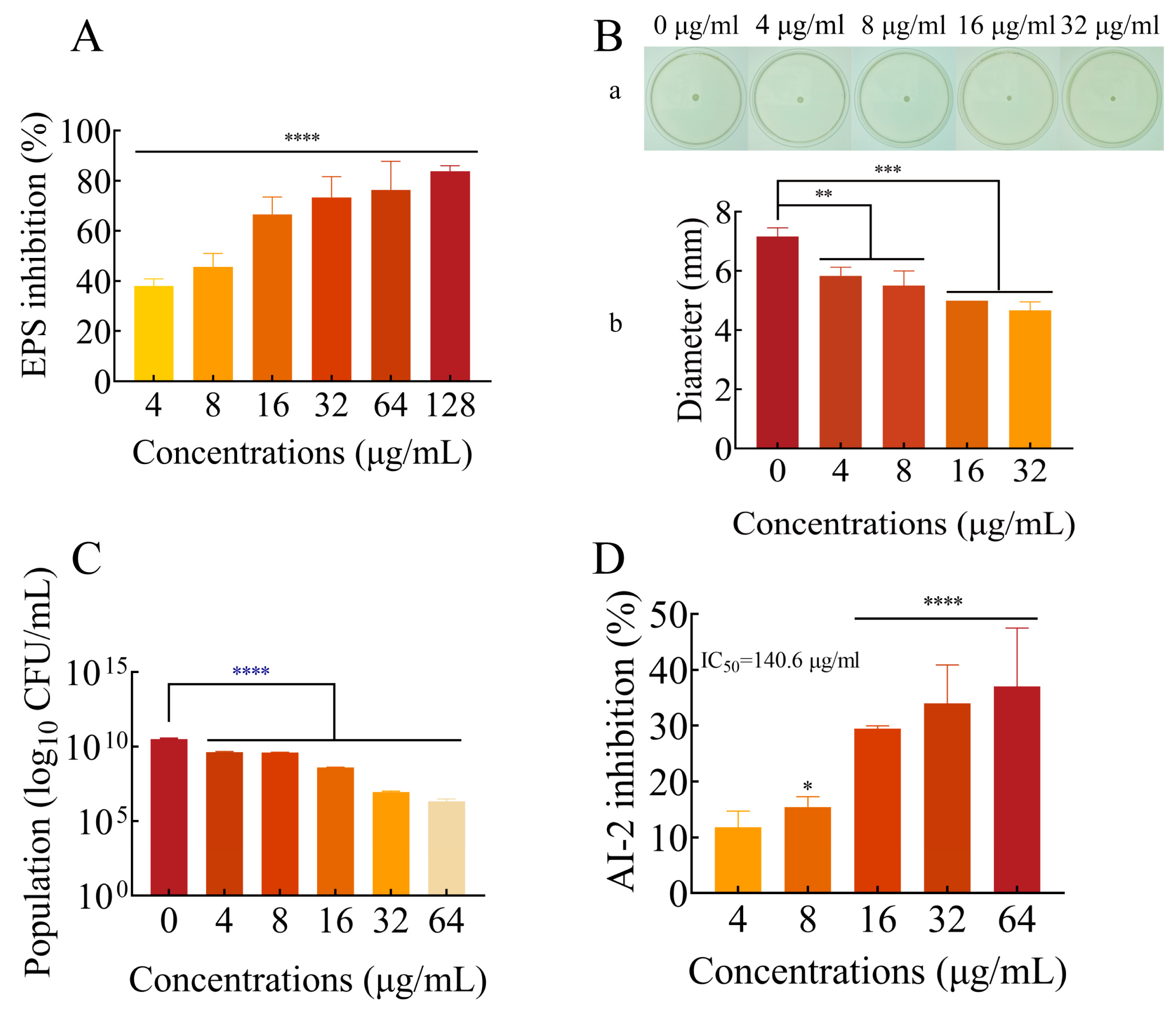
2.5. FLD Can Decrease the Production of EPS
2.6. Effects of FLD Can Inhibit the Motility of K. pneumonia
2.7. FLD Can Reduce the Count of Viable Cells in Biofilms
2.8. Effect of FLD on AI-2 Production
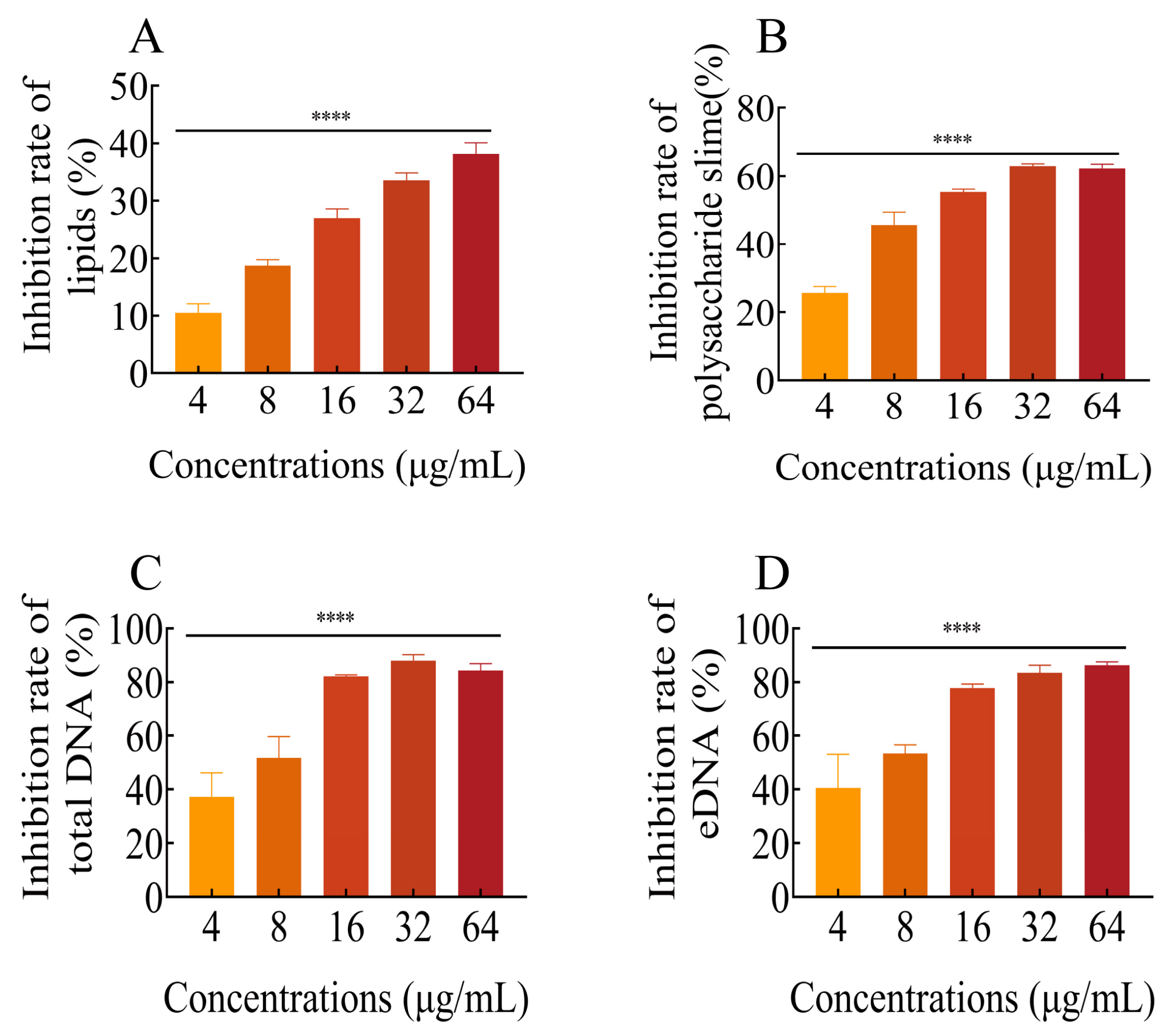
2.9. FLD Diminishes the Content of Biofilm Components
2.9.1. Total Lipids
2.9.2. Polysaccharide Slime
2.9.3. Total DNA and eDNA
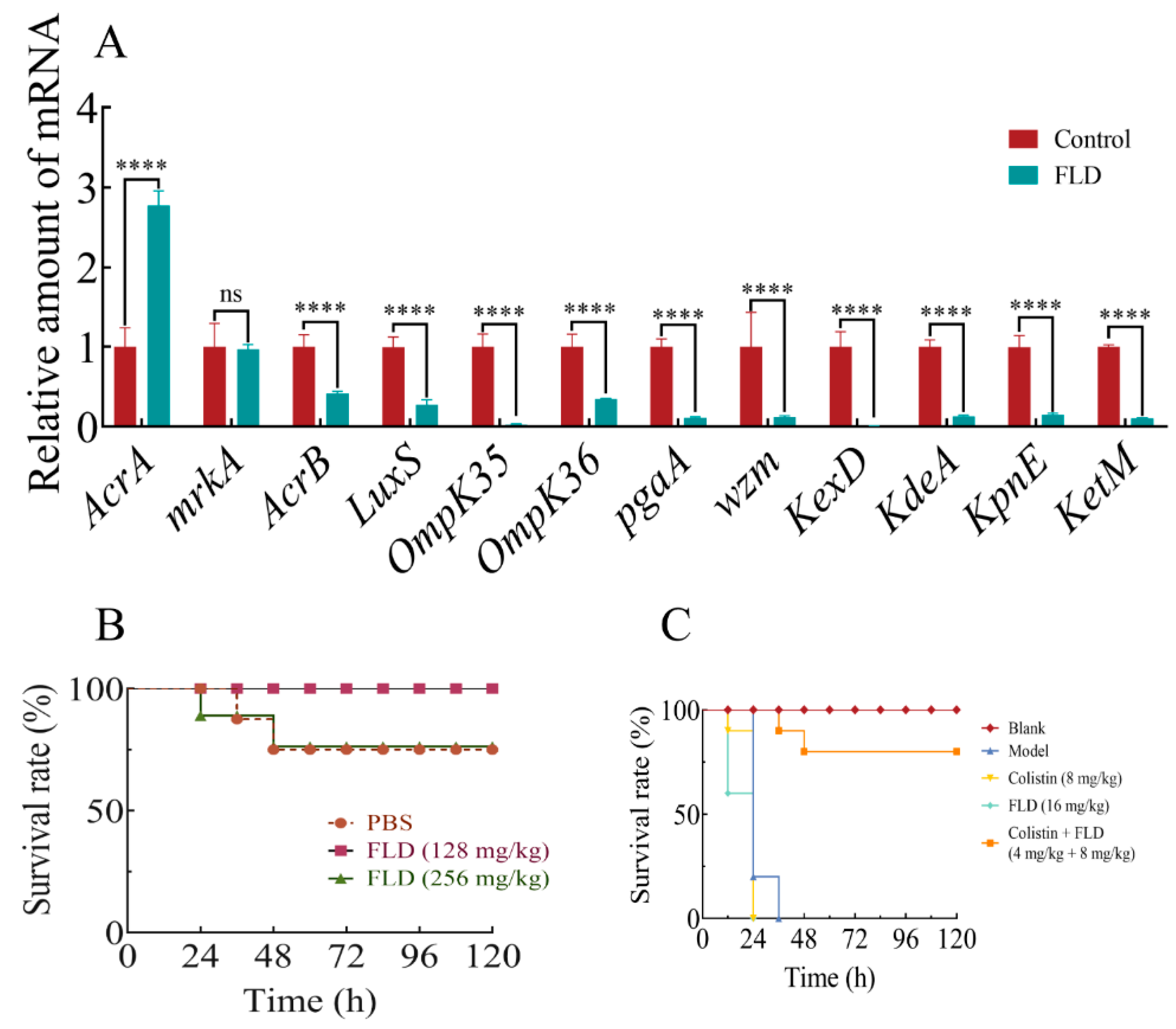
2.10. Effects of Drug Treatments on Biofilm-Associated Gene Expression
2.11. Safety Assay
2.12. Survival Rate of G. mellonella
3. Discussion
4. Materials and Methods
4.1. Reagents and Bacterial Strains
4.2. Growth and Metabolic Activity
4.3. Biofilm Formation and Quantitative Crystal Violet Assay
4.4. Eradication of Mature Biofilms
4.5. Confocal Laser Scanning Microscopy Analysis
4.6. Scanning Electron Microscopy (SEM) Analysis
4.7. EPS Production
4.8. Motility Assay
4.9. Viable Bacteria in Biofilms
4.10. AI-2 Bioluminescence Assay
4.11. Determination of Lipids, Polysaccharide Slime, and DNA in Biofilm
4.11.1. Lipids
4.11.2. Polysaccharide Slimes
4.11.3. Total DNA and eDNA
4.12. Relative Expression of Genes
4.13. Safety Assessment
4.14. G. Mellonella Infection Model
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FLD | Fingolimod |
| MDR | Multidrug-resistant |
| CV | Crystal violet |
| EPS | Exopolysaccharide |
| QS | Quorum sensing |
| OM | Outer membrane |
| LPS | Lipopolysaccharide |
| CLSI | Clinical & Laboratory Standards Institute |
References
- Bachman, M.A.; Breen, P.; Deornellas, V.; Mu, Q.; Zhao, L.; Wu, W.; Cavalcoli, J.D.; Mobley, H.L.T. Genome-wide identification of Klebsiella pneumoniae fitness genes during lung infection. mBio 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Allen, I.C. Using Klebsiella pneumoniae to Model Acute Lung Inflammation in Mice; Springer: New York, NY, USA, 2019; Volume 1960, pp. 169–180. [Google Scholar]
- Liu, S.; Zhang, P.; Liu, Y.; Gao, X.; Hua, J.; Li, W. Metabolic regulation protects mice against Klebsiella pneumoniae lung infection. Exp. Lung Res. 2018, 44, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Castaneda, J.A.; Ruano-Ravina, A.; Barbosa-Lorenzo, R.; Paillier-Gonzalez, J.E.; Saldana-Campos, J.C.; Salinas, D.F.; Lemos-Luengas, E.V. Mortality due to KPC carbapenemase-producing Klebsiella pneumoniae infections: Systematic review and meta-analysis: Mortality due to KPC Klebsiella pneumoniae infections. J. Infect. 2018, 76, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Veselova, V.O.; Plyuta, V.A.; Kostrov, A.N.; Vtyurina, D.N.; Abramov, V.O.; Abramova, A.V.; Voitov, Y.I.; Padiy, D.A.; Thu, V.T.H.; Hue, L.T.; et al. Long-term antimicrobial performance of textiles coated with ZnO and TiO2 nanoparticles in a tropical climate. J. Funct. Biomater. 2022, 13, 233. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Liu, J.; Teng, S.; Xie, Z. Identification of a novel cathelicidin from the deinagkistrodon acutus genome with antibacterial activity by multiple mechanisms. Toxins 2020, 12, 771. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.; Soopramanien, M.; Alharbi, A.M.; Alfahemi, H.; Khan, N.A. Novel sources of bioactive molecules: Gut microbiome of species routinely exposed to microorganisms. Vet. Sci. 2022, 9, 380. [Google Scholar] [CrossRef]
- Carlson, R.P.; Taffs, R.; Davison, W.M.; Stewart, P.S. Anti-biofilm properties of chitosan-coated surfaces. J. Biomater. Sci. Polym. Ed. 2008, 19, 1035–1046. [Google Scholar] [CrossRef]
- Lebeaux, D.; Ghigo, J.M.; Beloin, C. Biofilm-related infections: Bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef]
- Tan, S.; Gao, J.; Li, Q.; Guo, T.; Dong, X.; Bai, X.; Yang, J.; Hao, S.; He, F. Synergistic effect of chlorogenic acid and levofloxacin against Klebsiella pneumonia infection in vitro and in vivo. Sci. Rep. 2020, 10, 20013. [Google Scholar] [CrossRef]
- Freitas, A.I.; Lopes, N.; Oliveira, F.; Brás, S.; França, Â.; Vasconcelos, C.; Vilanova, M.; Cerca, N. Comparative analysis between biofilm formation and gene expression in Staphylococcus epidermidis isolates. Future Microbiol. 2018, 13, 415–427. [Google Scholar] [CrossRef]
- Czuban, M.; Srinivasan, S.; Yee, N.A.; Agustin, E.; Koliszak, A.; Miller, E.; Khan, I.; Quinones, I.; Noory, H.; Motola, C.; et al. Bio-orthogonal chemistry and reloadable biomaterial enable local activation of antibiotic prodrugs and enhance treatments against Staphylococcus aureus infections. ACS Cent. Sci. 2018, 4, 1624–1632. [Google Scholar] [CrossRef] [PubMed]
- Cargill, J.S.; Upton, M. Low concentrations of vancomycin stimulate biofilm formation in some clinical isolates of Staphylococcus epidermidis. J. Clin. Pathol. 2009, 62, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.; Cataneli, P.V.; Pinheiro, L.; Moraes, R.D.; Benini, M.K.; Ribeiro, D.S.D.C. Antimicrobial resistance profile of planktonic and biofilm cells of Staphylococcus aureus and coagulase-negative Staphylococci. Int. J. Mol. Sci. 2016, 17, 1423. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.M.; Lanigan, N.; O’Neill, I.J.; Bottacini, F.; Lugli, G.A.; Viappiani, A.; Turroni, F.; Ventura, M.; van Sinderen, D. Bifidobacterial biofilm formation is a multifactorial adaptive phenomenon in response to bile exposure. Sci. Rep. 2020, 10, 11598. [Google Scholar] [CrossRef] [PubMed]
- Jekely, G. Origin of phagotrophic eukaryotes as social cheaters in microbial biofilms. Biol. Direct 2007, 2, 3. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yu, S.; Su, T.; Wu, H.; Liu, S.; Wang, D.; Zhao, T.; Jin, Z.; Du, W.; Zhu, M.J.; Chua, S.L.; et al. PslG, a self-produced glycosyl hydrolase, triggers biofilm disassembly by disrupting exopolysaccharide matrix. Cell Res. 2015, 25, 1352–1367. [Google Scholar] [CrossRef]
- Tian, S.; Su, L.; Liu, Y.; Cao, J.; Yang, G.; Ren, Y.; Huang, F.; Liu, J.; An, Y.; van der Mei, H.C.; et al. Self-targeting, zwitterionic micellar dispersants enhance antibiotic killing of infectious biofilms—An intravital imaging study in mice. Sci. Adv. 2020, 6, b1112. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Y.; Shah, S.; Kim, D.; Simon-Soro, A.; Ito, T.; Hajfathalian, M.; Li, Y.; Hsu, J.C.; Nieves, L.M.; et al. Precision targeting of bacterial pathogen via bi-functional nanozyme activated by biofilm microenvironment. Biomaterials 2021, 268, 120581. [Google Scholar] [CrossRef]
- Marvasi, M.; Visscher, P.T.; Casillas, M.L. Exopolymeric substances (EPS) from Bacillus subtilis: Polymers and genes encoding their synthesis. FEMS Microbiol. Lett. 2010, 313, 1–9. [Google Scholar] [CrossRef]
- Fanaei, P.R.; Emaneini, M.; Beigverdi, R.; Banar, M.; van Leeuwen, W.B.; Jabalameli, F. Combinatorial effects of antibiotics and enzymes against dual-species Staphylococcus aureus and Pseudomonas aeruginosa biofilms in the wound-like medium. PLoS ONE 2020, 15, e235093. [Google Scholar] [CrossRef]
- Bellich, B.; Lagatolla, C.; Tossi, A.; Benincasa, M.; Cescutti, P.; Rizzo, R. Influence of bacterial biofilm polysaccharide structure on interactions with antimicrobial peptides: A study on Klebsiella pneumoniae. Int. J. Mol. Sci. 2018, 19, 1685. [Google Scholar] [CrossRef] [PubMed]
- Whitchurch, C.B.; Tolker-Nielsen, T.; Ragas, P.C.; Mattick, J.S. Extracellular DNA required for bacterial biofilm formation. Science 2002, 295, 1487. [Google Scholar] [CrossRef] [PubMed]
- Ainoda, Y.; Aoki, K.; Ishii, Y.; Okuda, K.; Furukawa, H.; Manabe, R.; Sahara, T.; Nakamura-Uchiyama, F.; Kurosu, H.; Ando, Y.; et al. Klebsiella pneumoniae carbapenemase (KPC)-producing Klebsiella pneumoniae ST258 isolated from a Japanese patient without a history of foreign travel—A new public health concern in Japan: A case report. BMC Infect. Dis. 2019, 19, 20. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Speziale, P.; Montanaro, L.; Costerton, J.W. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials 2012, 33, 5967–5982. [Google Scholar] [CrossRef] [PubMed]
- Billerbeck, S.; Brisbois, J.; Agmon, N.; Jimenez, M.; Temple, J.; Shen, M.; Boeke, J.D.; Cornish, V.W. A scalable peptide-GPCR language for engineering multicellular communication. Nat. Commun. 2018, 9, 5057. [Google Scholar] [CrossRef]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef]
- Di Somma, A.; Moretta, A.; Cane, C.; Cirillo, A.; Duilio, A. Antimicrobial and antibiofilm peptides. Biomolecules 2020, 10, 652. [Google Scholar] [CrossRef]
- Gallegos-Monterrosa, R.; Mendiola, R.O.; Nuñez, Y.; Auvynet, C.; Kumar, K.M.; Tang, B.; Ruiz-Ortega, L.I.; Bustamante, V.H. Antibacterial and antibiofilm activities of ZIF-67. J. Antibiot. 2023, 76, 603–612. [Google Scholar] [CrossRef]
- Ghezzi, D.; Sassoni, E.; Boi, M.; Montesissa, M.; Baldini, N.; Graziani, G.; Cappelletti, M. Antibacterial and antibiofilm activity of nanostructured copper films prepared by ionized jet deposition. Antibiotics 2022, 12, 55. [Google Scholar] [CrossRef]
- Miranda-Cadena, K.; Marcos-Arias, C.; Mateo, E.; Aguirre-Urizar, J.M.; Quindós, G.; Eraso, E. In vitro activities of carvacrol, cinnamaldehyde and thymol against Candida biofilms. Biomed. Pharmacother. 2021, 143, 112218. [Google Scholar] [CrossRef] [PubMed]
- Gilbert-Girard, S.; Savijoki, K.; Yli-Kauhaluoma, J.; Fallarero, A. Screening of FDA-approved drugs using a 384-well plate-based biofilm platform: The case of Fingolimod. Microorganisms 2020, 8, 1834. [Google Scholar] [CrossRef] [PubMed]
- Castro-Borrero, W.; Graves, D.; Frohman, T.C.; Flores, A.B.; Hardeman, P.; Logan, D.; Orchard, M.; Greenberg, B.; Frohman, E.M. Current and Emerging Therapies in Multiple Sclerosis: A Systematic Review; SAGE Publications: London, UK, 2012; Volume 5, pp. 205–220. [Google Scholar] [CrossRef]
- Tedesco-Silva, H.; Mourad, G.; Kahan, B.D.; Boira, J.G.; Weimar, W.; Mulgaonkar, S.; Nashan, B.; Madsen, S.; Charpentier, B.; Pellet, P.; et al. FTY720, a novel immunomodulator: Efficacy and safety results from the first phase 2A study in de novo renal transplantation. Transplantation 2005, 79, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Jayathilake, P.G.; Jana, S.; Rushton, S.; Swailes, D.; Bridgens, B.; Curtis, T.; Chen, J. Extracellular polymeric substance production and aggregated bacteria colonization influence the competition of microbes in biofilms. Front. Microbiol. 2017, 8, 1865. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wang, W.; Shi, M.; Wei, X.; Zhou, X.; Li, B.; Zhang, J. Novel antibiofilm inhibitor Ginkgetin as an antibacterial synergist against Escherichia coli. Int. J. Mol. Sci. 2022, 23, 8809. [Google Scholar] [CrossRef] [PubMed]
- Herigstad, B.; Hamilton, M.; Heersink, J. How to optimize the drop plate method for enumerating bacteria. J. Microbiol. Methods 2001, 44, 121–129. [Google Scholar] [CrossRef]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.M.; Wertheim, H.F.L.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance—The need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef]
- Spellberg, B.; Bartlett, J.G.; Gilbert, D.N. The future of antibiotics and resistance. N. Engl. J. Med. 2013, 368, 299–302. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Yang, N.; Wen, Z.; Sun, X.; Chai, Y.; Ma, Z. Wheat microbiome bacteria can reduce virulence of a plant pathogenic fungus by altering histone acetylation. Nat. Commun. 2018, 9, 3414–3429. [Google Scholar] [CrossRef]
- Han, J.; Luo, J.; Du, Z.; Chen, Y.; Liu, T. Synergistic effects of baicalin and levofloxacin against hypervirulent Klebsiella pneumoniae biofilm in vitro. Curr. Microbiol. 2023, 80, 126. [Google Scholar] [CrossRef] [PubMed]
- Vuotto, C.; Longo, F.; Pascolini, C.; Donelli, G.; Balice, M.P.; Libori, M.F.; Tiracchia, V.; Salvia, A.; Varaldo, P.E. Biofilm formation and antibiotic resistance in Klebsiella pneumoniae urinary strains. J. Appl. Microbiol. 2017, 123, 1003–1018. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Ag. 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Dynes, J.J.; Lawrence, J.R.; Korber, D.R.; Swerhone, G.D.W.; Leppard, G.G.; Hitchcock, A.P. Morphological and biochemical changes in Pseudomonas fluorescens biofilms induced by sub-inhibitory exposure to antimicrobial agents. Can. J. Microbiol. 2009, 55, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Prakash, B.; Veeregowda, B.M.; Krishnappa, G. Biofilms: A survival strategy of bacteria. Curr. Sci. 2003, 85, 1299–1307. [Google Scholar]
- Alonso, V.P.P.; Harada, A.M.M.; Kabuki, D.Y. Competitive and/or cooperative interactions of listeria monocytogenes with bacillus cereus in dual-species biofilm formation. Front. Microbiol. 2020, 11, 177. [Google Scholar] [CrossRef]
- Husain, F.M.; Ahmad, I.; Khan, M.S.; Ahmad, E.; Tahseen, Q.; Khan, M.S.; Alshabib, N.A. Sub-MICs of mentha piperita essential oil and menthol inhibits AHL mediated quorum sensing and biofilm of Gram-negative bacteria. Front. Microbiol. 2015, 6, 420. [Google Scholar] [CrossRef]
- Alotaibi, G.F. Occurrence of potentially pathogenic bacteria in epilithic biofilm forming bacteria isolated from Porter Brook River-stones, Sheffield, UK. Saudi J. Biol. Sci. 2020, 27, 3405–3414. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, H.; Li, L.; Wang, E.; Zhou, X.; Gu, Y.; Wu, X.; Shen, L.; Zeng, W. Whole genome sequencing and comparative genomic analyses of Lysinibacillus pakistanensis LZH-9, a halotolerant strain with excellent COD removal capability. Microorganisms 2020, 8, 716. [Google Scholar] [CrossRef]
- Coughlan, L.M.; Cotter, P.D.; Hill, C.; Alvarez-Ordonez, A. New weapons to fight old enemies: Novel strategies for the (Bio)control of bacterial biofilms in the food industry. Front. Microbiol. 2016, 7, 1641. [Google Scholar] [CrossRef]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J.; Ghannoum, M.; Parsek, M.; Whiteley, M.; Mukherjee, P. Bacterial extracellular polysaccharides in biofilm formation and function. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.A.; Marr, C.M. Hypervirulent Klebsiella pneumoniae. Clin. Microbiol. Rev. 2019, 32. [Google Scholar] [CrossRef] [PubMed]
- Kobylka, J.; Kuth, M.S.; Muller, R.T.; Geertsma, E.R.; Pos, K.M. AcrB: A mean, keen, drug efflux machine. Ann. N. Y. Acad. Sci. 2020, 1459, 38–68. [Google Scholar] [CrossRef] [PubMed]
- Hasdemir, U.O.; Chevalier, J.; Nordmann, P.; Pages, J. Detection and prevalence of active drug efflux mechanism in various multidrug-resistant Klebsiella pneumoniae strains from Turkey. J. Clin. Microbiol. 2004, 42, 2701–2706. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiari, R.; Javadi, A.; Aminzadeh, M.; Molaee-Aghaee, E.; Shaffaghat, Z. Association between presence of RmpA, MrkA and MrkD genes and antibiotic resistance in clinical Klebsiella pneumoniae isolates from hospitals in Tehran, Iran. Iran. J. Public Health 2021, 50, 1009–1016. [Google Scholar] [CrossRef]
- Camejo, P.Y.; Santo, D.J.; McMahon, K.D.; Noguera, D.R. Genome-enabled insights into the ecophysiology of the comammox bacterium “Candidatus nitrospira nitrosa”. mSystems 2017, 2, e00059-17. [Google Scholar] [CrossRef]
- Maldonado, R.F.; Sá-Correia, I.; Valvano, M.A.; Whitfield, C. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol. Rev. 2016, 40, 480–493. [Google Scholar] [CrossRef]
- Wasfi, R.; Hamed, S.M.; Amer, M.A.; Fahmy, L.I. Proteus mirabilis biofilm: Development and therapeutic strategies. Front. Cell Infect. Microbiol. 2020, 10, 414. [Google Scholar] [CrossRef]
- De Araujo, C.; Balestrino, D.; Roth, L.; Charbonnel, N.; Forestier, C. Quorum sensing affects biofilm formation through lipopolysaccharide synthesis in Klebsiella pneumoniae. Res. Microbiol. 2010, 161, 595–603. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Clinical and Laboratory Standards Institute: Wanyne, PA, USA, 2018. [Google Scholar]
- Swetha, T.K.; Pooranachithra, M.; Subramenium, G.A.; Divya, V.; Balamurugan, K.; Pandian, S.K. Umbelliferone impedes biofilm formation and virulence of methicillin-resistant Staphylococcus epidermidis via impairment of initial attachment and intercellular adhesion. Front. Cell Infect. Microbiol. 2019, 9, 357. [Google Scholar] [CrossRef]
- Zuo, J.; Yin, H.; Hu, J.; Miao, J.; Chen, Z.; Qi, K.; Wang, Z.; Gong, J.; Phouthapane, V.; Jiang, W.; et al. Lsr operon is associated with AI-2 transfer and pathogenicity in avian pathogenic Escherichia coli. Vet. Res. 2019, 50, 109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Xu, C.; Zhou, X.; Qi, R.; Liu, L.; Lv, F.; Li, Z.; Wang, S. Cationic conjugated polymers for enhancing beneficial bacteria adhesion and biofilm formation in gut microbiota. Colloids Surf. B Biointerfaces 2020, 188, 110815. [Google Scholar] [CrossRef]
- Adnan, M.; Patel, M.; Deshpande, S.; Alreshidi, M.; Siddiqui, A.J.; Reddy, M.N.; Emira, N.; De Feo, V. Effect of adiantum philippense extract on biofilm formation, adhesion with its antibacterial activities against foodborne pathogens, and characterization of bioactive metabolites: An in vitro-in silico approach. Front. Microbiol. 2020, 11, 823. [Google Scholar] [CrossRef] [PubMed]
- Vikram, A.; Jayaprakasha, G.K.; Uckoo, R.M.; Patil, B.S. Inhibition of Escherichia coli O157:H7 motility and biofilm by β-Sitosterol glucoside. Biochimica et Biophysica Acta (BBA) Gen. Subj. 2013, 1830, 5219–5228. [Google Scholar] [CrossRef] [PubMed]
- Byreddy, A.R.; Gupta, A.; Barrow, C.J.; Puri, M. A quick colorimetric method for total lipid quantification in microalgae. J. Microbiol. Methods 2016, 125, 28–32. [Google Scholar] [CrossRef]
- Kang, J.; Li, Q.; Liu, L.; Jin, W.; Wang, J.; Sun, Y. The specific effect of gallic acid on Escherichia coli biofilm formation by regulating pgaABCD genes expression. Appl. Microbiol. Biotechnol. 2018, 102, 1837–1846. [Google Scholar] [CrossRef]
- Türkel, İ.; Yıldırım, T.; Yazgan, B.; Bilgin, M.; Başbulut, E. Relationship between antibiotic resistance, efflux pumps, and biofilm formation in extended-spectrum β-lactamase producing Klebsiella pneumoniae. J. Chemother. 2018, 30, 354–363. [Google Scholar] [CrossRef]
| Gene | Primer Sequence | Base Sequence | Reference |
|---|---|---|---|
| 23srRNA | 23srRNA-F | ATCGTACCCCAAACCGACAC | [43] |
| 23srRNA-R | TTCTCCCGAAGTTACGGCAC | ||
| AcrA | AcrA-F | CTCTGGCGGTCGTTCTGATGC | |
| AcrA-R | CATGTGCTGGGCTCCCTGTTG | ||
| mrkA | mrkA-F | ACGTCTCTAACTGCCAGGC | |
| mrkA-R | TAGCCCTGTTGTTTGCTGGT | ||
| AcrB | AcrB-F | CAATACGGAAGAGTTTGGCA | [44] |
| AcrB-R | CAGACGAACCTGGGAACC | ||
| LuxS | LuxS-F | AGTGATGCCGGAACGCGG | |
| LuxS-R | CGGTGTACCAATCAGGCTC | ||
| OmpK35 | OmpK35-F | GCAATATTCTGGCAGTGGTGATC | |
| OmpK35-R | ACCATTTTTCCATAGAAGTCCAGT | ||
| OmpK36 | OmpK36-F | TTAAAGTACTGTCCCTCCTGG | |
| OmpK36-R | TCAGAGAAGTAGTGCAGACCGTCA | ||
| pgaA | pgaA-F | GCAGACGCTCTCCTATGTC- | |
| pgaA-R | GCCGAGAGCAGGGGAATC | ||
| wzm | wzm-F | TGCCAGTTCGGCCACTAAC | |
| wzm-R | GACAACAATAACCGGGATGG | ||
| KexD | KexD-F | ACCGGTTGCGCAATACCCTGA | [70] |
| KexD-R | CGTAATTGACGCCATCCCTG | ||
| KdeA | KdeA-F | GTTGTTCCCGTTATGTCTGGTGC | |
| KdeA-R | CCAGCAGCCACTGTAAAAACATGC | ||
| KpnE | KpnE-F | ATTGCTGAAATTACCGGCAC | |
| KpnE-R | AAATACCGATCCCTTCCCAC | ||
| KetM | KetM-F | TTGGCAGAGAAGGCGGTTGG | |
| KetM-R | CATGACCATCCCGGGCTTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, X.; Yang, Y.-J.; Li, Z.; Ge, W.-B.; Xu, X.; Liu, X.-W.; Li, J.-Y. Fingolimod Inhibits Exopolysaccharide Production and Regulates Relevant Genes to Eliminate the Biofilm of K. pneumoniae. Int. J. Mol. Sci. 2024, 25, 1397. https://doi.org/10.3390/ijms25031397
Geng X, Yang Y-J, Li Z, Ge W-B, Xu X, Liu X-W, Li J-Y. Fingolimod Inhibits Exopolysaccharide Production and Regulates Relevant Genes to Eliminate the Biofilm of K. pneumoniae. International Journal of Molecular Sciences. 2024; 25(3):1397. https://doi.org/10.3390/ijms25031397
Chicago/Turabian StyleGeng, Xiang, Ya-Jun Yang, Zhun Li, Wen-Bo Ge, Xiao Xu, Xi-Wang Liu, and Jian-Yong Li. 2024. "Fingolimod Inhibits Exopolysaccharide Production and Regulates Relevant Genes to Eliminate the Biofilm of K. pneumoniae" International Journal of Molecular Sciences 25, no. 3: 1397. https://doi.org/10.3390/ijms25031397
APA StyleGeng, X., Yang, Y.-J., Li, Z., Ge, W.-B., Xu, X., Liu, X.-W., & Li, J.-Y. (2024). Fingolimod Inhibits Exopolysaccharide Production and Regulates Relevant Genes to Eliminate the Biofilm of K. pneumoniae. International Journal of Molecular Sciences, 25(3), 1397. https://doi.org/10.3390/ijms25031397







