Biological Function of Prophage-Related Gene Cluster ΔVpaChn25_RS25055~ΔVpaChn25_0714 of Vibrio parahaemolyticus CHN25
Abstract
1. Introduction
2. Results
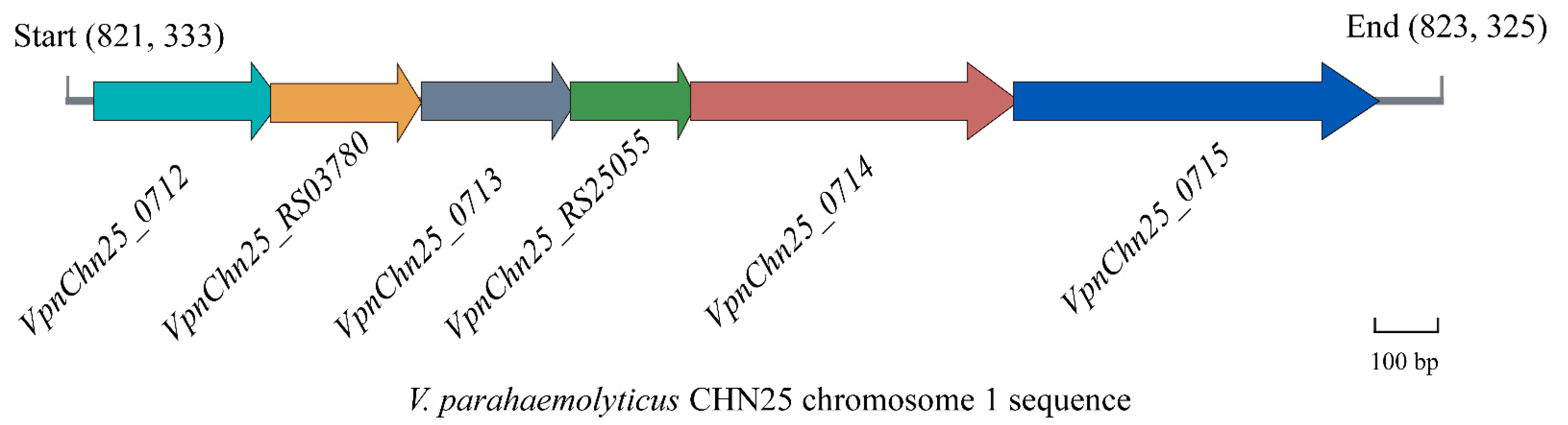
2.1. Prophage-Related Genes VpaChn25_RS25055, VpaChn25_0713, and VpaChn25_0714 in V. parahaemolyticus CHN25
2.2. Deletion and Reverse Complementation of the VpaChn25_RS25055, VpaChn25_0713, and VpaChn25_0714 Genes in V. parahaemolyticus CHN25 Genome
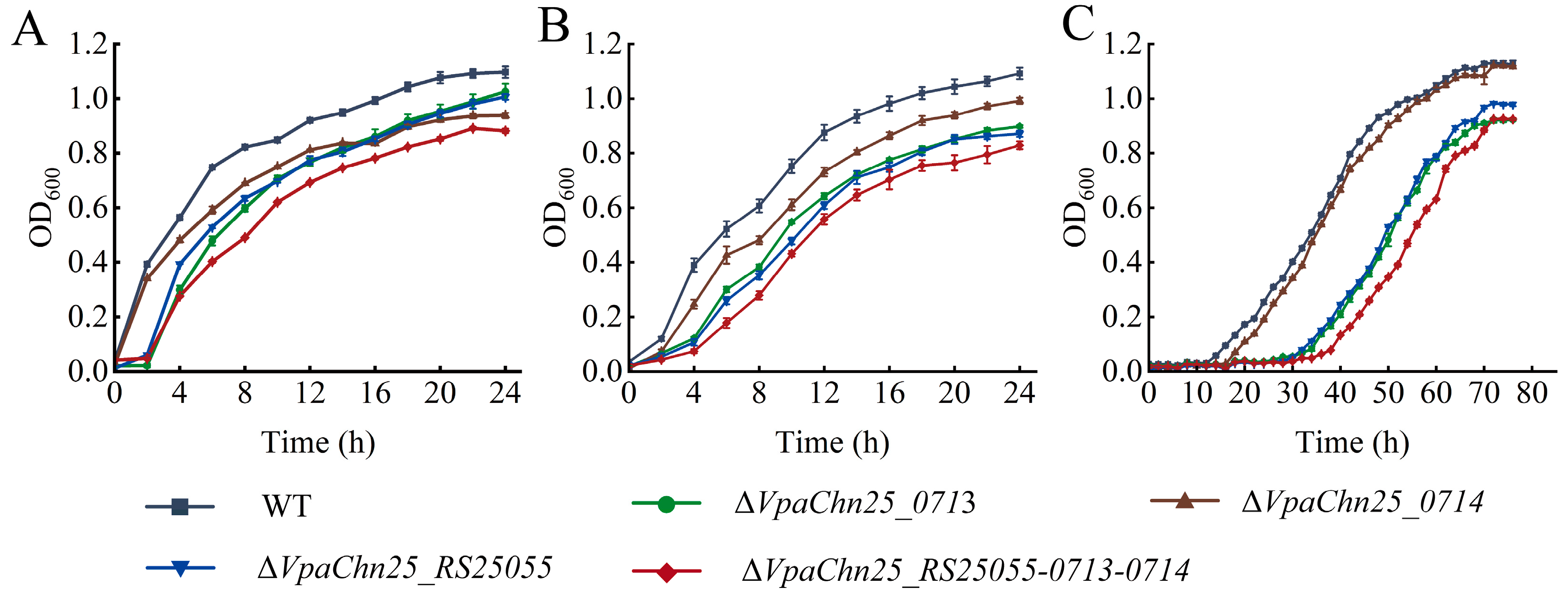
2.3. Survival of ΔVpaChn25_0713, ΔVpaChn25_0714, ΔVpaChn25_RS25055, and ΔVpaChn25_RS25055-0713-0714 Mutants at Different Temperatures and pH Conditions
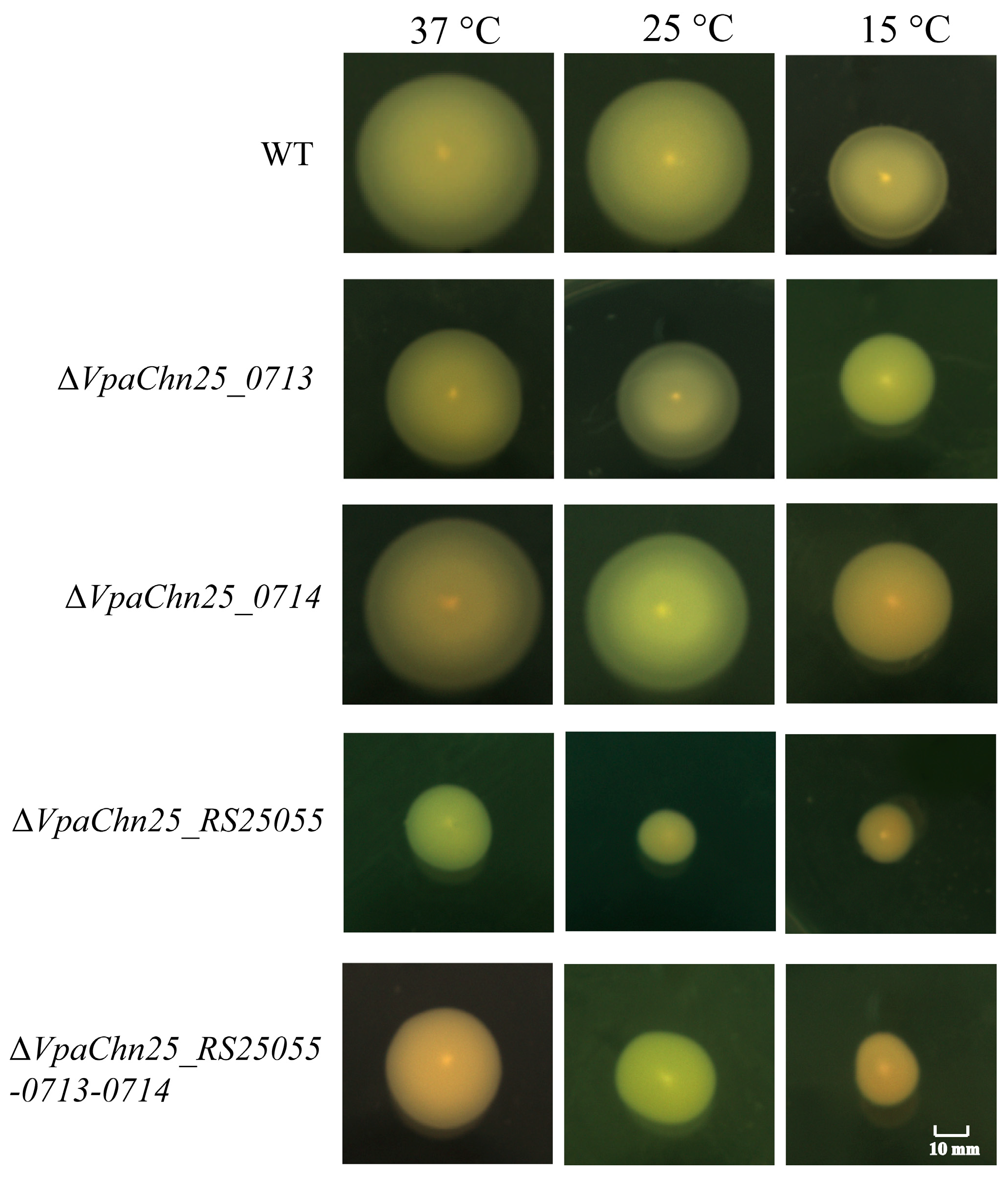
2.4. Swimming Motility of the ΔVpaChn25_RS25055, ΔVpaChn25_0713, ΔVpaChn25_0714, and ΔVpaChn25_RS25055-0713-0714 Mutants
2.5. Biofilm Formation of the ΔVpaChn25_RS25055, ΔVpaChn25_0713, ΔVpaChn25_0714, and ΔVpaChn25_RS25055-0713-0714 Mutants
2.6. Cell Surface Hydrophobicity, Cell Membrane Permeability, and Fluidity of the ΔVpaChn25_RS25055, ΔVpaChn25_0713, ΔVpaChn25_0714, and ΔVpaChn25_RS25055-0713-0714 Mutants
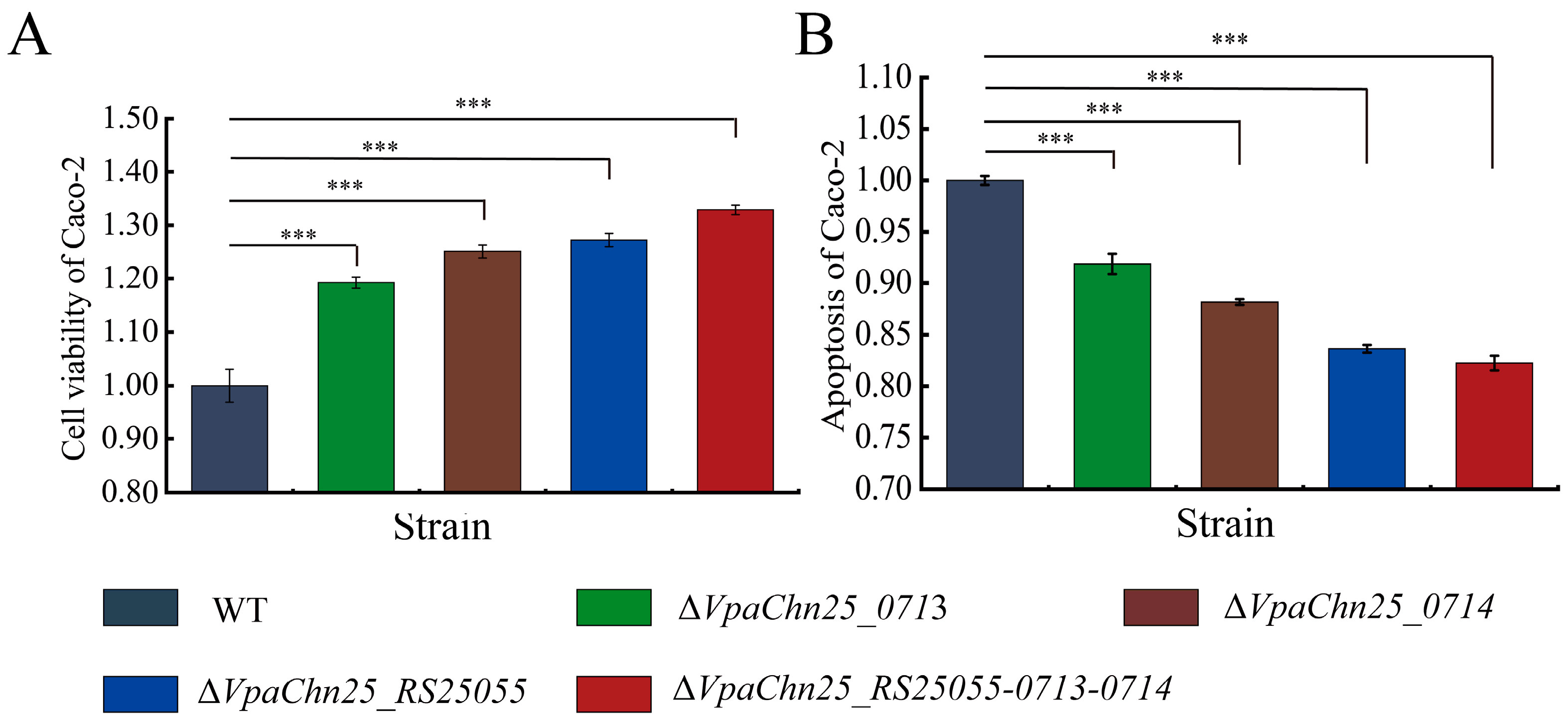
2.7. Interaction between the ΔVpaChn25_RS25055, ΔVpaChn25_0713, ΔVpaChn25_0714, and ΔVpaChn25_RS25055-0713-0714 Mutants and Host Intestinal Epithelial Cells
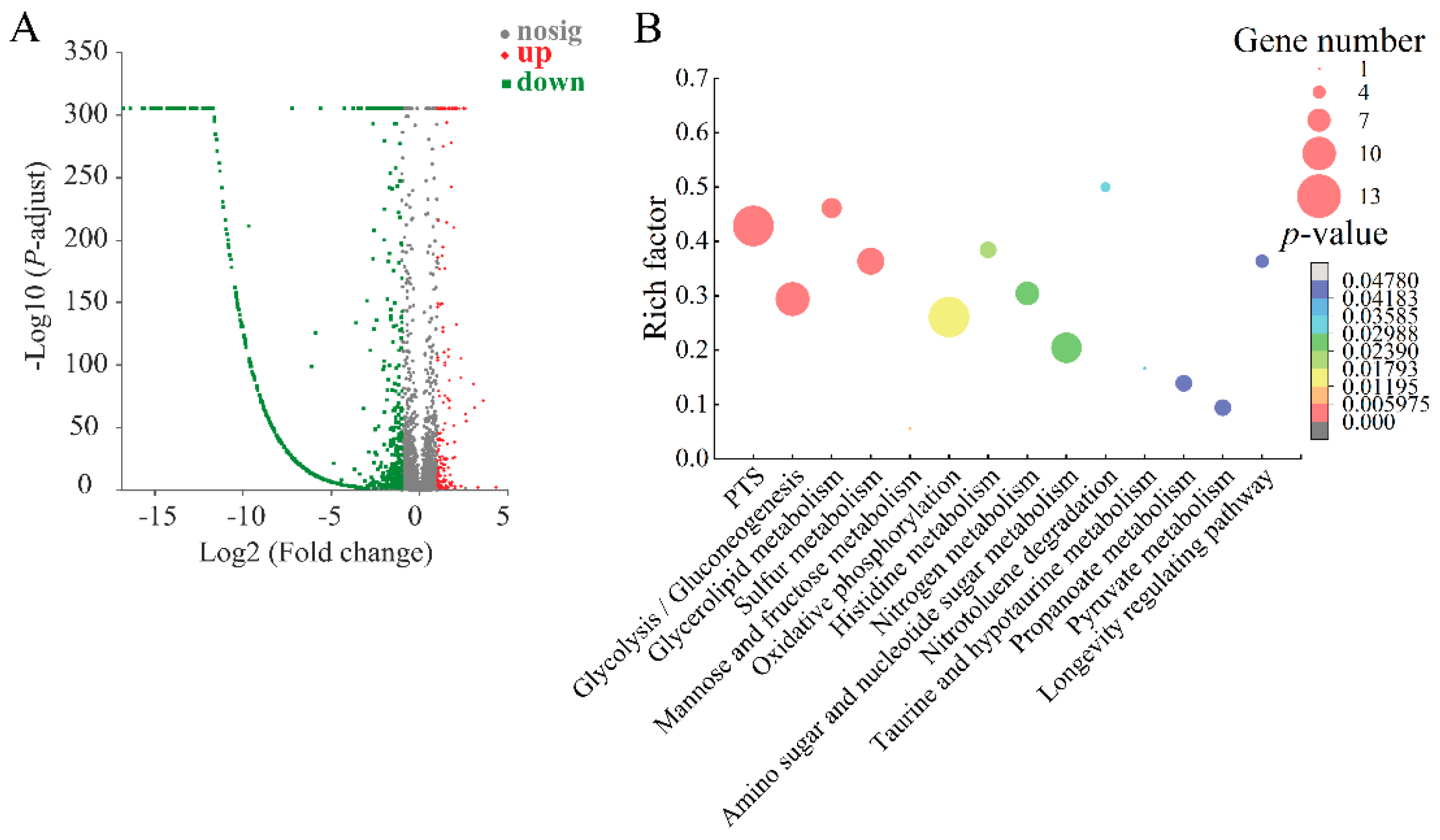
2.8. The Major Changed Metabolic Pathways in the ΔVpaChn25_RS25055, ΔVpaChn25_0713, ΔVpaChn25_0714, and ΔVpaChn25_RS25055-0713-0714 Mutants
2.8.1. The Major Changed Metabolic Pathways in the ΔVpaChn25_0713 Mutant
2.8.2. The Major Changed Metabolic Pathways in the ΔVpaChn25_0714 Mutant
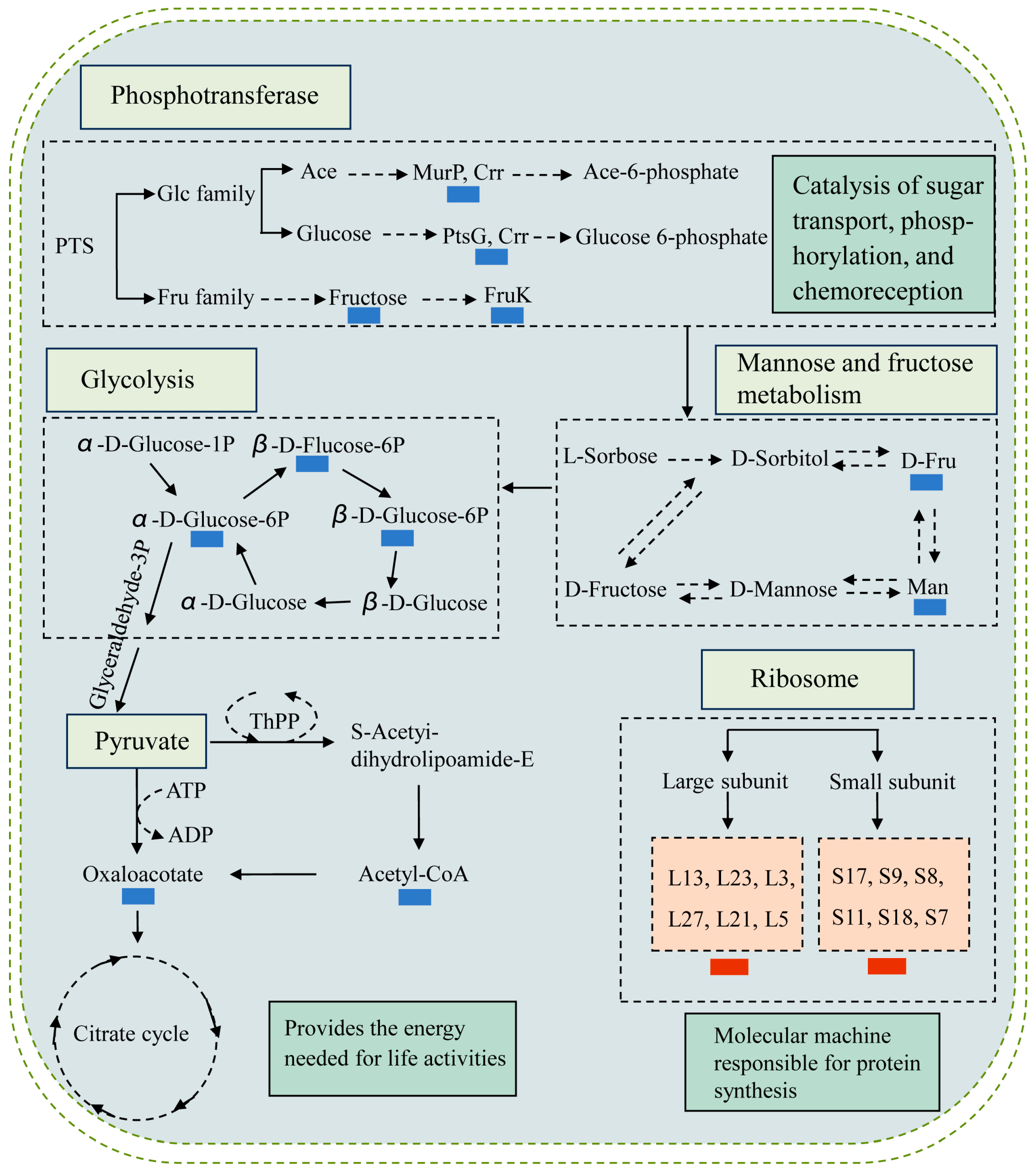
2.8.3. The Major Changed Metabolic Pathways in the ΔVpaChn25_RS25055 Mutant
2.8.4. The Major Changed Metabolic Pathways in the ΔVpaChn25_RS25055-0713-0714 Mutant
2.8.5. Possible Molecular Mechanisms of the ΔVpaChn25_RS25055, ΔVpaChn25_0713, ΔVpaChn25_0714, and ΔVpaChn25_RS25055-0713-0714 Mutants
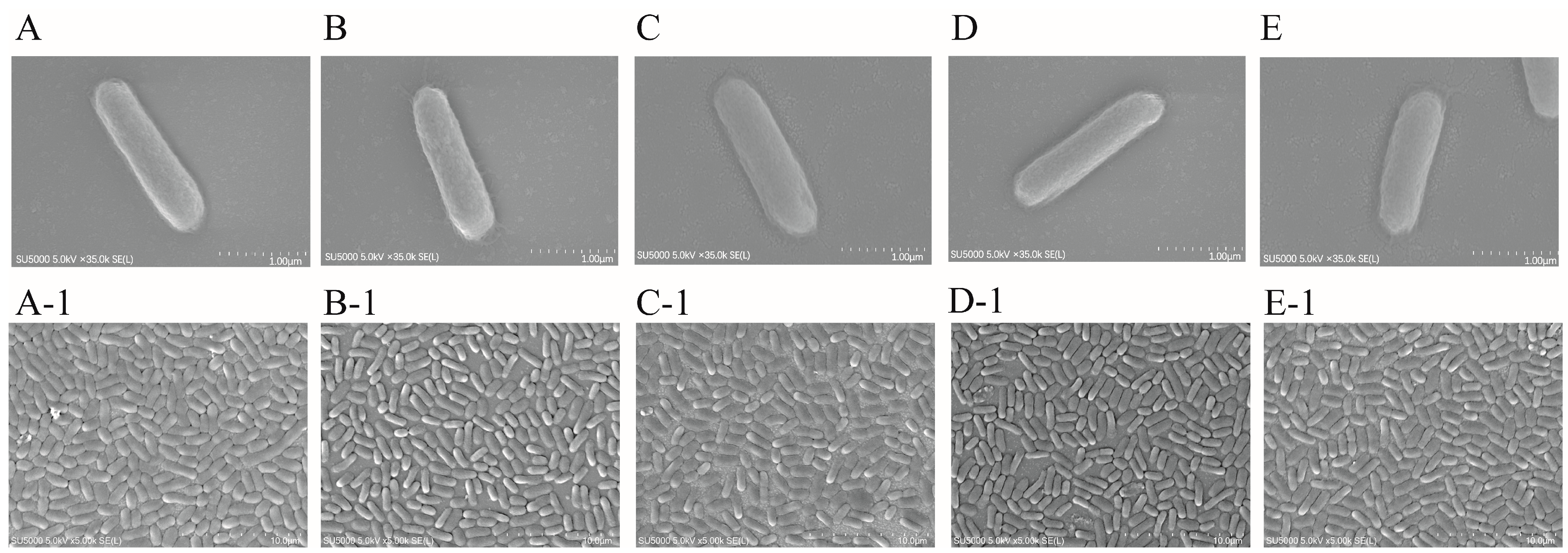
2.9. SEM Observation of Cell Structure of the ΔVpaChn25_RS25055, ΔVpaChn25_0713, ΔVpaChn25_0714, and ΔVpaChn25_RS25055-0713-0714 Mutants
2.10. Distribution of the VpaChn25_RS25055, VpaChn25_0713, and VpaChn25_0714 Genes in Bacteria
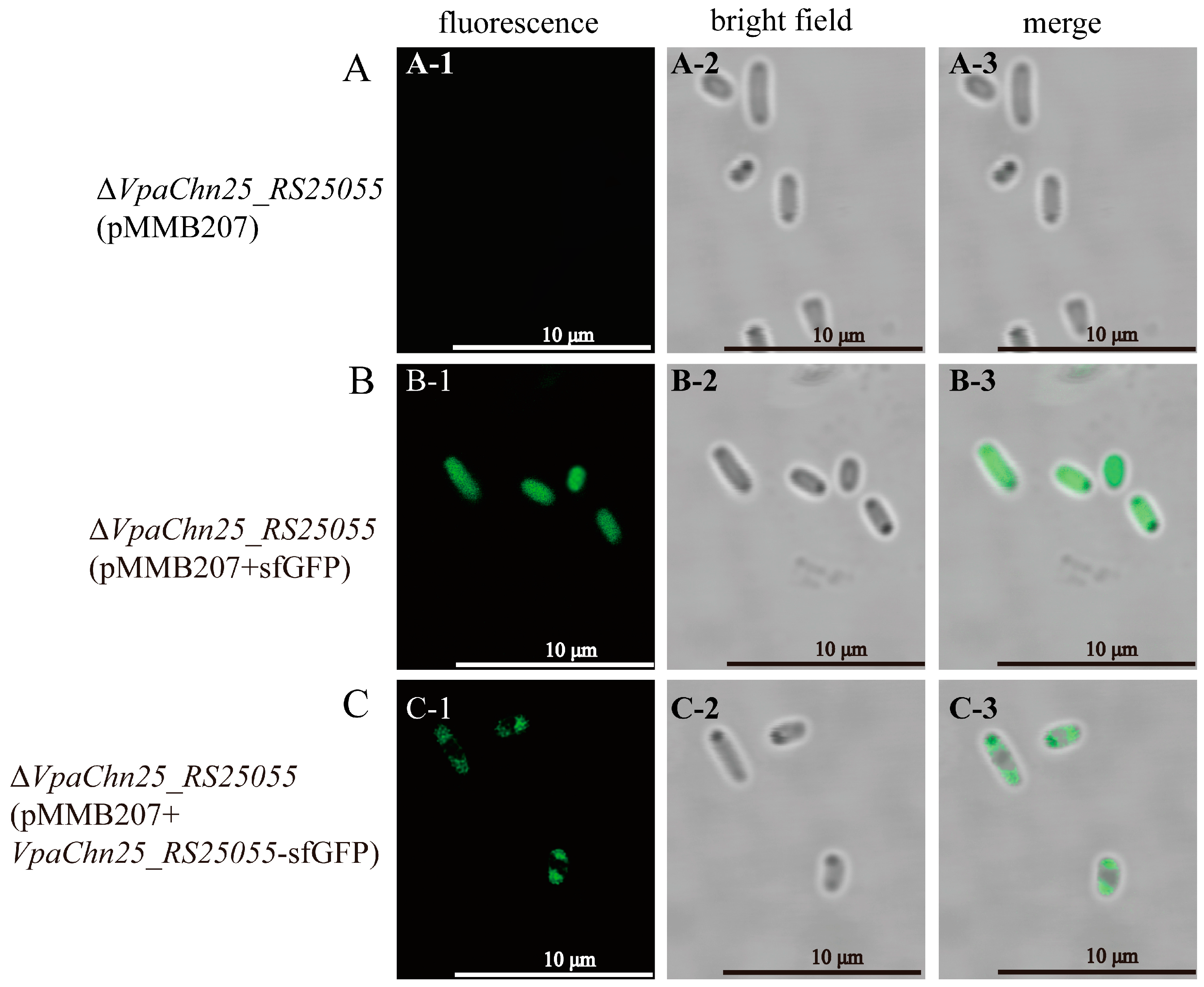
2.11. Cellular Localization of VpaChn25_RS25055 in V. parahaemolyticus CHN25
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Plasmids, and Culture Conditions
4.2. Construction of the Gene Deletion Mutants and Reverse Complementation
4.3. Growth Curve Assay
4.4. Swimming Motility Assays
4.5. Biofilm Formation Assay
4.6. Bacterial Cell Membrane Damage, Hydrophobicity, and Fluidity Assays
4.7. Human Intestinal Epithelial Cell Viability and Apoptosis Assay
4.8. Illumina RNA Sequencing
4.9. Scanning Electron Microscopy (SEM) Analysis
4.10. Real-Time Reverse Transcription-PCR Assay
4.11. Construction of Recombinant Vectors for Cell Localization Experiments
4.12. Preparation of Cells for Microscopy
4.13. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Liu, J.F.; Fu, K.F.; Qin, K.W.; Wu, C.L.; Yu, X.J.; Zhou, S.; Zhou, L.J. Rapid identification and detection of Vibrio parahaemolyticus via different types of modus operandi with LAMP method in vivo. Ann Microbiol. 2020, 70, 40. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, L.; Pei, J. Application of bayesian statistics to model incidence of Vibrio parahaemolyticus associated with fishery products and their geographical distribution in China. LWT 2020, 130, 109662. [Google Scholar] [CrossRef]
- Li, H.; Tang, R.; Lou, Y.; Cui, Z.L.; Chen, W.J.; Hong, Q.; Zhang, Z.H.; Malakar, P.K.; Pan, Y.J.; Zhao, Y. A comprehensive epidemiological research for clinical Vibrio parahaemolyticus in Shanghai. Front. Microbiol. 2017, 8, 1043. [Google Scholar] [CrossRef] [PubMed]
- Fujino, T.; Okuno, Y.; Nakada, D.; Aoyama, A.; Ueho, T. On the bacteriological examination of shirasu food poisoning. Med. J. Osaka Univ. 1953, 4, 299–304. [Google Scholar]
- Narayanan, S.V.; Joseph, T.C.; Peeralil, S.; Mothadaka, M.P.; Lalitha, K.V. Prevalence, virulence characterization, AMR pattern and genetic relatedness of Vibrio parahaemolyticus isolates from retail seafood of Kerala, India. Front. Microbiol. 2020, 11, 592. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.W.; Gu, X.K.; Zhong, Q.P.; Duan, L.J.; Zhou, A.M. Absolute quantification of Vibrio parahaemolyticus by multiplex droplet digital PCR for simultaneous detection of tlh, tdh and ureR based on single intact cell. Food Control. 2020, 114, 107207. [Google Scholar] [CrossRef]
- Nair, G.B.; Hormazábal, J.C. The Vibrio parahaemolyticus pandemic. Rev. Chilena Infectol. 2005, 22, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Ling, N.; Shen, J.L.; Guo, J.; Zeng, D.X.; Ren, J.L.; Sun, L.X.; Jiang, Y.; Xue, F.; Dai, J.J.; Li, B.G. Rapid and accurate detection of viable Vibrio parahaemolyticus by sodium deoxycholate-propidium monoazide-qPCR in shrimp. Food Control. 2020, 109, 106883. [Google Scholar] [CrossRef]
- Chen, L.; Wang, J.; Chen, J.; Zhang, R.; Zhang, H.; Qi, X.; He, Y. Epidemiological characteristics of Vibrio parahaemolyticus outbreaks, Zhejiang, China, 2010–2022. Front. Microbiol. 2023, 14, 1171350. [Google Scholar] [CrossRef]
- Pérez-Reytor, D.; García, K. Galleria mellonella: A model of infection to discern novel mechanisms of pathogenesis of non-toxigenic Vibrio parahaemolyticus strains. Virulence 2018, 9, 22–24. [Google Scholar] [CrossRef]
- Hu, J.; Ye, H.; Wang, S.L.; Wang, J.J.; Han, D.D. Prophage activation in the intestine: Insights into functions and possible applications. Front. Microbiol. 2021, 12, 785634. [Google Scholar] [CrossRef]
- Fortier, L.C.; Sekulovic, O. Importance of prophages to evolution and virulence of bacterial pathogens. Virulence 2013, 4, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Esteves, N.C.; Scharf, B.E. Flagellotropic bacteriophages: Opportunities and challenges for antimicrobial applications. Int. J. Mol. Sci. 2022, 23, 7084. [Google Scholar] [CrossRef] [PubMed]
- Owen, S.V.; Wenner, N.; Dulberger, C.L.; Rodwell, E.V.; Bowers-Barnard, A.; Quinones-Olvera, N.; Rigden, D.J.; Rubin, E.J.; Garner, E.C.; Baym, M.; et al. Prophages encode phage-defense systems with cognate self-immunity. Cell Host Microbe 2021, 29, 1620–1633.e8. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yang, L.; Wang, Y.; Zhu, Z.; Yan, J.; Qin, S.; Chen, L. Prophage-encoded gene VpaChn25_0734 amplifies ecological persistence of Vibrio parahaemolyticus CHN25. Curr. Genet. 2022, 68, 267–287. [Google Scholar] [CrossRef] [PubMed]
- Magaziner, S.J.; Zeng, Z.; Chen, B.; Salmond, G.P.C. The prophages of citrobacter rodentium represent a conserved Family of horizontally acquired mobile genetic elements associated with enteric evolution towards pathogenicity. J. Bacteriol. 2019, 201, e00638-18. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.V.; Depasquale, S.M.; Harbolick, E.A.; Hong, T.; Kernell, A.L.; Kruchko, D.H.; Modise, T.; Smith, C.E.; McCarter, L.L.; Stevens, A.M. Complete genome sequence of prepandemic Vibrio parahaemolyticus BB22OP. Genome Announc. 2013, 1, e00002-12. [Google Scholar] [CrossRef] [PubMed]
- Zabala, B.; García, K.; Espejo, R.T. Enhancement of UV light sensitivity of a Vibrio parahaemolyticus O3:K6 pandemic strain due to natural lysogenization by a telomeric phage. Appl. Environ. Microbiol. 2009, 75, 1697–1702. [Google Scholar] [CrossRef]
- Pazhani, G.P.; Chowdhury, G.; Ramamurthy, T. Adaptations of Vibrio parahaemolyticus to stress during environmental survival, host colonization, and infection. Front. Microbiol. 2021, 12, 737299. [Google Scholar] [CrossRef]
- Yang, Q.; Dong, X.; Xie, G.; Fu, S.; Zou, P.; Sun, J.; Wang, Y.; Huang, J. Comparative genomic analysis unravels the transmission pattern and intra-species divergence of acute hepatopancreatic necrosis disease (AHPND)-causing Vibrio parahaemolyticus strains. Mol. Genet. Genom. 2019, 294, 1007–1022. [Google Scholar] [CrossRef]
- Casjens, S. Prophages and bacterial genomics: What have we learned so far? Mol. Microbiol. 2003, 49, 277–300. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.J.; Liu, T.G.; Peng, X.; Chen, L.M. Insights into Vibrio parahaemolyticus CHN25 response to artificial gastric fluid stress by transcriptomic analysis. Int. J. Mol. Sci. 2014, 15, 22539–22562. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, H.; Chen, L. Comparative secretomics reveals novel virulence-associated factors of Vibrio parahaemolyticus. Front. Microbiol. 2015, 6, 707. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Sun, B.; Liu, T.; Zheng, H.; Gu, W.; He, W.; Sun, F.; Wang, Y.; Yang, M.; Bei, W.; et al. Genomic and transcriptomic analyses reveal distinct biological functions for cold shock proteins (VpaCspA and VpaCspD) in Vibrio parahaemolyticus CHN25 during low-temperature survival. BMC Genom. 2017, 18, 436. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.Z.; Wang, Y.P.; Yu, P.; Ren, S.L.; Zhu, Z.Y.; Jin, Y.Z.; Yan, J.Z.; Peng, X.; Chen, L.M. Prophage-related gene VpaChn25_0724 contributes to cell membrane integrity and growth of Vibrio parahaemolyticus CHN25. Front. Cell. Infect Microbiol. 2020, 10, 595709. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.Y.; Yang, L.Z.; Yu, P.; Wang, Y.P.; Peng, X.; Chen, L.M. Comparative proteomics and secretomics revealed virulence and antibiotic resistance-associated factors in Vibrio parahaemolyticus recovered from commonly consumed aquatic products. Front. Microbiol. 2020, 11, 1453. [Google Scholar] [CrossRef]
- Gao, C.; Yang, X.; Zhao, C.; Li, C.; Wang, S.; Zhang, X.; Xue, B.; Cao, Z.; Zhou, H.; Yang, Y.; et al. Characterization of a novel Vibrio parahaemolyticus host-phage pair and antibacterial effect against the host. Arch. Virol. 2022, 167, 531–544. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kimura, B.; Takahashi, H.; Watanabe, T.; Obata, H.; Kai, A.; Morozumi, S.; Fujii, T. Lysine decarboxylase of Vibrio parahaemolyticus: Kinetics of transcription and role in acid resistance. J. Appl. Microbiol. 2008, 104, 1283–1293. [Google Scholar] [CrossRef]
- Li, M.Z.; Meng, H.M.; Li, Y.; Gu, D. A novel transcription factor VPA0041 was identified to regulate the swarming motility in Vibrio parahaemolyticus. Pathogens 2022, 11, 453. [Google Scholar] [CrossRef]
- Ashrafudoulla, M.; Mizan, M.F.R.; Park, H.; Byun, K.H.; Lee, N.; Park, S.H.; Ha, S.D. Genetic relationship, virulence factors, drug resistance profile and biofilm formation ability of Vibrio parahaemolyticus isolated from mussel. Front. Microbiol. 2019, 10, 513. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, C.; Cai, N.; Yang, R.; Chen, J.; Kahramanoǧlu, İ.; Okatan, V.; Rengasamy, K.R.R.; Wan, C. The antifungal activity of loquat (Eriobotrya japonica Lindl.) leaves extract against Penicillium digitatum. Front. Nutr. 2021, 8, 663584. [Google Scholar] [CrossRef] [PubMed]
- Deutscher, J.; Aké, F.M.; Derkaoui, M.; Zébré, A.C.; Cao, T.N.; Bouraoui, H.; Kentache, T.; Mokhtari, A.; Milohanic, E.; Joyet, P. The bacterial phosphoenolpyruvate: Carbohydrate phosphotransferase system: Regulation by protein phosphorylation and phosphorylation-dependent protein-protein interactions. Microbiol. Mol. Biol. Rev. 2014, 78, 231–256. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Han, H.; Lv, Z.; Lin, Z.; Shang, Y.; Xu, T.; Wu, Y.; Zhang, Y.; Qu, D. PhoU2 but not PhoU1 as an important regulator of biofilm formation and tolerance to multiple stresses by participating in various fundamental metabolic processes in Staphylococcus epidermidis. J. Bacteriol. 2017, 199, e00219-17. [Google Scholar] [CrossRef] [PubMed]
- Moody, N.R.; Phansopal, C.; Reid, J.D. An in vitro coupled assay for PEPC with control of bicarbonate concentration. Bio. Protoc. 2021, 11, e4264. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.W.; Nishimoto, M.; Arakawa, T.; Kitaoka, M.; Fushinobu, S. Structural basis for broad substrate specificity of UDP-glucose 4-epimerase in the human milk oligosaccharide catabolic pathway of Bifidobacterium longum. Sci. Rep. 2019, 9, 11081. [Google Scholar] [CrossRef] [PubMed]
- Mattila, J.; Hietakangas, V. Regulation of carbohydrate energy metabolism in Drosophila melanogaster. Genetics 2017, 207, 1231–1253. [Google Scholar] [CrossRef]
- Hagen, W.R.; Vanoni, M.A.; Rosenbaum, K.; Schnackerz, K.D. On the iron sulfur clusters in the complex redox enzyme dihydropyrimidine. Eur. J. Biochem. 2000, 267, 3640–3646. [Google Scholar] [CrossRef]
- Theodoulou, F.L.; Kerr, I.D. ABC transporter research: Going strong 40 years on. Biochem. Soc. Trans. 2015, 43, 1033–1040. [Google Scholar] [CrossRef]
- Singh, P.; Brooks, J.F.; Ray, V.A., 2nd; Mandel, M.J.; Visick, K.L. CysK plays a role in biofilm formation and colonization by Vibrio fischeri. Appl. Environ. Microbiol. 2015, 81, 5223–5234. [Google Scholar] [CrossRef]
- Yu, Y.M.; Liang, Y.H.; Brostromer, E.; Quan, J.M.; Panjikar, S.; Dong, Y.H.; Su, X.D. A catalytic mechanism revealed by the crystal structures of the imidazolonepropionase from Bacillus subtilis. J. Biol. Chem. 2006, 281, 36929–36936. [Google Scholar] [CrossRef]
- Voelker, D.R. Glycerolipid Structure, Function, and Synthesis in Eukaryotes. In Encyclopedia of Biological Chemistry II; Elsevier: Amsterdam, The Netherlands, 2013; pp. 412–418. [Google Scholar] [CrossRef]
- Bryant, C.; DeLuca, M. Purification and characterization of an oxygen-insensitive NAD(P)H nitroreductase from Enterobacter cloacae. J. Biol. Chem. 1991, 266, 4119–4125. [Google Scholar] [CrossRef] [PubMed]
- Barrière, C.; Veiga-da-Cunha, M.; Pons, N.; Guédon, E.; van Hijum, S.A.; Kok, J.; Kuipers, O.P.; Ehrlich, D.S.; Renault, P. Fructose utilization in Lactococcus lactis as a model for low-GC gram-positive bacteria: Its regulator, signal, and DNA-binding site. J. Bacteriol. 2005, 187, 3752–3761. [Google Scholar] [CrossRef] [PubMed]
- Buss, K.A.; Cooper, D.R.; Ingram-Smith, C.; Ferry, J.G.; Sanders, D.A.; Hasson, M.S. Urkinase: Structure of acetate kinase, a member of the ASKHA superfamily of phosphotransferases. J. Bacteriol. 2001, 183, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, M.R.; Yao, Y.C.; Bostrom, I.K.; Wang, Y.D.; Chen, A.Q.; Li, J.X.; Gu, S.H.; Ji, C.N. Characterization and structure of glyceraldehyde-3-phosphate dehydrogenase type 1 from Escherichia coli. Acta Crystallogr. F Struct. Biol. Commun. 2020, 76, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Nauta, K.M.; Ho, T.D.; Ellermeier, C.D. The Penicillin-Binding Protein PbpP Is a Sensor of β-Lactams and Is Required for Activation of the Extracytoplasmic Function σ Factor σ(P) in Bacillus thuringiensis. mBio 2021, 12, e00179-21. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, R.L.; Kane, J.F. In vivo synthesis of histidine by a cloned histidine Ammonia-Lyase in Escherichia coli. J. Bacteriol. 1985, 162, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhu, X.; Zeng, H. Fatty acid metabolism in adaptive immunity. FEBS J. 2023, 290, 584–599. [Google Scholar] [CrossRef]
- Huang, J.; Chen, D.; Yan, H.; Xie, F.; Yu, Y.; Zhang, L.; Sun, M.; Peng, X. Acetylglutamate kinase is required for both gametophyte function and embryo development in Arabidopsis thaliana. J. Integr. Plant Biol. 2017, 59, 642–656. [Google Scholar] [CrossRef]
- Ohshima, K.; Nojima, S.; Tahara, S.; Kurashige, M.; Hori, Y.; Hagiwara, K.; Okuzaki, D.; Oki, S.; Wada, N.; Ikeda, J.I.; et al. Argininosuccinate Synthase 1-Deficiency Enhances the Cell Sensitivity to Arginine through Decreased DEPTOR Expression in Endometrial Cancer. Sci. Rep. 2017, 7, 45504. [Google Scholar] [CrossRef]
- Charlier, D.; Bervoets, I. Regulation of arginine biosynthesis, catabolism and transport in Escherichia coli. Amino Acids. 2019, 51, 1103–1127. [Google Scholar] [CrossRef]
- Jing, M.L.; Zheng, T.; Gong, T.; Yan, J.C.; Chen, J.M.; Lin, Y.W.; Tang, B.Y.; Ma, Q.Z.; Zhou, X.D.; Li, Y.Q. AhrC negatively regulates Streptococcus mutans arginine biosynthesis. Microbiol. Spectr. 2022, 10, e0072122. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Ge, X.; Stone, V.; Kong, X.; El-Rami, F.; Liu, Y.; Kitten, T.; Xu, P. ciaR impacts biofilm formation by regulating an arginine biosynthesis pathway in Streptococcus sanguinis SK36. Sci. Rep. 2017, 7, 17183. [Google Scholar] [CrossRef] [PubMed]
- Song, W.S.; Jeon, Y.J.; Namgung, B.; Hong, M.; Yoon, S.I. A conserved TLR5 binding and activation hot spot on flagellin. Sci. Rep. 2017, 7, 40878. [Google Scholar] [CrossRef] [PubMed]
- Bouteiller, M.; Dupont, C.; Bourigault, Y.; Latour, X.; Barbey, C.; Konto-Ghiorghi, Y.; Merieau, A. Pseudomonas flagella: Generalities and specificities. Int. J. Mol. Sci. 2021, 22, 3337. [Google Scholar] [CrossRef]
- Lafita-Navarro, M.C.; Conacci-Sorrell, M. Nucleolar stress: From development to cancer. Semin. Cell Dev. Biol. 2023, 136, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Aseev, L.V.; Koledinskaya, L.S.; Boni, I.V. Regulation of ribosomal protein operons rplM-rpsI, rpmB-rpmG, and rplU-rpmA at the transcriptional and translational levels. J. Bacteriol. 2016, 198, 2494–2502. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Nam, E.W.; Kim, Y.; Lee, S.; Kim, S.I.; Yoon, H. Transcriptomic approach for understanding the adaptation of Salmonella enterica to contaminated produce. J. Microbiol. Biotechnol. 2020, 30, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Chandel, N.S. Carbohydrate Metabolism. Cold Spring Harb Perspect Biol. 2021, 13, a040568. [Google Scholar] [CrossRef]
- Petersen, B.D.; Liu, M.S.; Podicheti, R.; Yang, A.Y.; Simpson, C.A.; Hemmerich, C.; Rusch, D.B.; van Kessel, J.C. The polar flagellar transcriptional regulatory network in Vibrio campbellii deviates from canonical Vibrio species. J. Bacteriol. 2021, 203, e0027621. [Google Scholar] [CrossRef]
- Sheng, Q.; Liu, S.M.; Cheng, J.H.; Li, C.Y.; Fu, H.H.; Zhang, X.Y.; Song, X.Y.; McMinn, A.; Zhang, Y.Z.; Su, H.N.; et al. Lack of N-terminal segment of the flagellin protein results in the production of a shortened polar flagellum in the deep-sea sedimentary bacterium Pseudoalteromonas sp. strain SM9913. Appl. Environ. Microbiol. 2021, 87, e0152721. [Google Scholar] [CrossRef]
- Chebly, H.; Marvaud, J.C.; Safa, L.; Elkak, A.K.; Kobeissy, P.H.; Kansau, I.; Larrazet, C. Clostridioides difficile Flagellin Activates the Intracellular NLRC4 Inflammasome. Int. J. Mol. Sci. 2022, 23, 12366. [Google Scholar] [CrossRef] [PubMed]
- Schauder, S.; Nunn, R.S.; Lanz, R.; Erni, B.; Schirmer, T. Crystal structure of the IIB subunit of a fructose permease (IIBLev) from Bacillus subtilis. J. Mol. Biol. 1998, 276, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Su, C.L.; Chen, L.M. Virulence, resistance, and genetic diversity of Vibrio parahaemolyticus recovered from commonly consumed aquatic products in Shanghai, China. Mar. Pollut. Bull. 2020, 160, 111554. [Google Scholar] [CrossRef]
- Zheng, X.T.; Han, B.; Kumar, V.; Feyaerts, A.F.; Van Dijck, P.; Bossier, P. Essential oils improve the survival of gnotobiotic brine shrimp (Artemia franciscana) challenged with Vibrio campbellii. Front. Immunol. 2021, 12, 693932. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tang, R.X.; Zhu, D.W.; Wang, W.F.; Yi, L.; Ma, L.X. Non-peptide guided auto-secretion of recombinant proteins by super-folder green fluorescent protein in Escherichia coli. Sci. Rep. 2017, 7, 6990. [Google Scholar] [CrossRef]
- Ding, G.Y.; Zhao, L.; Xu, J.; Cheng, J.Y.; Cai, Y.Y.; Du, H.H.; Xiao, G.S.; Zhao, F. Quantitative risk assessment of Vibrio parahaemolyticus in shellfish from retail to consumption in coastal city of east China. J. Food Prot. 2022, 85, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- McCarter, L.L. Polar flagellar motility of the Vibrionaceae. Microbiol. Mol. Biol. Rev. 2001, 65, 445–462. [Google Scholar] [CrossRef]
- Matsuzaki, T.; Matsumoto, S.; Kasai, T.; Yoshizawa, E.; Okamoto, S.; Yoshikawa, H.Y.; Taniguchi, H.; Takebe, T. Defining lineage-specific membrane fluidity signatures that regulate adhesion kinetics. Stem. Cell Rep. 2018, 11, 852–860. [Google Scholar] [CrossRef]
- Salas-Tovar, J.A.; Escobedo-García, S.; Olivas, G.I.; Acosta-Muñiz, C.H.; Harte, F.; Sepulveda, D.R. Method-induced variation in the bacterial cell surface hydrophobicity MATH test. J. Microbiol. Methods 2021, 185, 106234. [Google Scholar] [CrossRef]
- Deutscher, J.; Francke, C.; Postma, P.W. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol. Mol. Biol. Rev. 2006, 70, 939–1031. [Google Scholar] [CrossRef]
- Saier, M.H., Jr. The bacterial phosphotransferase system: New frontiers 50 years after its discovery. J. Mol. Microbiol. Biotechnol. 2015, 25, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Boreiko, S.; Silva, M.; Melo, R.d.F.P.; Silber, A.M.; Iulek, J. Structure of urocanate hydratase from the protozoan trypanosoma cruzi. Int. J. Biol. Macromol. 2020, 146, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Matsuoka, H.; Hirooka, K. Regulation of fatty acid metabolism in bacteria. Mol. Microbiol. 2007, 66, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, K.B.; Gatto, C.; Wilkinson, B.J. Interrelationships between fatty acid composition, staphyloxanthin content, fluidity, and carbon flow in the Staphylococcus aureus membrane. Molecules 2018, 23, 1201. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; Xu, Z.Y.; Ge, X.L.; Sanyal, S.; Lu, Z.J.; Javid, B. Selective translation by alternative bacterial ribosomes. Proc. Natl. Acad. Sci. USA 2020, 117, 19487–19496. [Google Scholar] [CrossRef] [PubMed]
- Bowman, G.R.; Lyuksyutova, A.I.; Shapiro, L. Bacterial polarity. Curr. Opin. Cell Biol. 2011, 23, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.H.; Chen, W.C.; Lin, C.W.; Lin, Y.T.; Ning, H.C.; Chang, Y.C.; Yang, T.C. Relationship of the CreBC two-component regulatory system and inner membrane protein CreD with swimming motility in Stenotrophomonas maltophilia. PLoS ONE 2017, 12, e0174704. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Tang, W.S.; Si, T.Y.; Tang, J.X. Influence of physical effects on the swarming motility of Pseudomonas aeruginosa. Biophys J. 2017, 112, 1462–1471. [Google Scholar] [CrossRef]
- Tan, L.; Zhao, F.; Han, Q.; Zhao, A.J.; Malakar, P.K.; Liu, H.Q.; Pan, Y.J.; Zhao, Y. High correlation between structure development and chemical variation during biofilm formation by Vibrio parahaemolyticus. Front. Microbiol. 2018, 9, 1881. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, J.; Sun, M.; Yu, P.; Ma, Y.; Xie, L.; Chen, L. Comparative secretomic and proteomic analysis reveal multiple defensive strategies developed by Vibrio cholerae against the heavy metal (Cd2+, Ni2+, Pb2+, and Zn2+) stresses. Front. Microbiol. 2023, 14, 1294177. [Google Scholar] [CrossRef]
- Arroyo-Pérez, E.E.; Ringgaard, S. Interdependent Polar Localization of FlhF and FlhG and Their Importance for Flagellum Formation of Vibrio parahaemolyticus. Front. Microbiol. 2021, 12, 655239. [Google Scholar] [CrossRef] [PubMed]
- Renzette, N.; Gumlaw, N.; Nordman, J.T.; Krieger, M.; Yeh, S.P.; Long, E.; Centore, R.; Boonsombat, R.; Sandler, S.J. Localization of RecA in Escherichia coli K-12 using RecA-GFP. Mol. Microbiol. 2005, 57, 1074–1085. [Google Scholar] [CrossRef] [PubMed]
- Sleight, S.C.; Bartley, B.A.; Lieviant, J.A.; Sauro, H.M. In-Fusion BioBrick assembly and re-engineering. Nucleic Acids Res. 2010, 38, 2624–2636. [Google Scholar] [CrossRef] [PubMed]






| Primer | Sequence (5′-3′) | Product Size (bp) | Reference |
|---|---|---|---|
| VpaChn25_0713-up-F | GCTCTAGATCACCCTTCACGCTAT | 454 | This study |
| VpaChn25_0713-up-R | CTCGCTCATTTTCGTTACCCATTGATAGCC | ||
| VpaChn25_0713-down-F | GGGTAACGAAAATGAGCGAGACAGCGAGGA | 322 | This study |
| VpaChn25_0713-down-R | CGAGCTCATTCAGACACTCGCACT | ||
| VpaChn25_0713-up-ex-F | TTGGTGGCAAGAAAGG | 1673 | This study |
| VpaChn25_0713-down-ex-R | ACAAAATCGGGTAGGC | ||
| VpaChn25_0713-com-F | CGAGCTCATGGGTAACGAACTGCAACGT | 234 | This study |
| VpaChn25_0713-com-R | GCTCTAGATTAGGCCGCTTCCTCGCT | ||
| VpaChn25_RS25055-up-F | GCTCTAGAACATCGTGACGGTTTAT | 491 | This study |
| VpaChn25_RS25055-up-R | TCACTCATTTCTTGTTAGGCCGCTTCCTCG | ||
| VpaChn25_RS25055-down-F | GCCTAACAAGAAATGAGTGAAGTTAAAGGT | 482 | This study |
| VpaChn25_RS25055-down-R | CGAGCTCTCATAGCGTTTCCTCTT | ||
| VpaChn25_RS25055- up-ex-F | GGCGTTTCTTTCACCT | 1983 | This study |
| VpaChn25_RS25055-down-ex-R | TCAACAACTTTCGGATT | ||
| VpaChn25_RS25055-com-F | CGAGCTCATGAGCGAGACAGCGAGG | 186 | This study |
| VpaChn25_RS25055-com-R | GCTCTAGATCATTTTTCCCATTCCTT | ||
| VpaChn25_0714-up-F | GCTCTAGAACAGCCTTTCCAGATT | 308 | This study |
| VpaChn25_0714-up-R | AGTTTCATAGTTACCTTTAACTTCACTCAT | ||
| VpaChn25_0714-down-F | TTAAAGGTAACTATGAAACTAACCCGTTGC | 473 | This study |
| VpaChn25_0714-down-R | CGAGCTCACCTACAGCCAGCATT | ||
| VpaChn25_0714- up-ex-F | GCAACGAGTGGGATTT | 1757 | This study |
| VpaChn25_0714-down-ex- R | TTGGTGCTCTGCGGTA | ||
| VpaChn25_0714-com-F | CGAGCTCATGAGTGAAGTTAAAGGTAAG | 480 | This study |
| VpaChn25_0714-com-R | GCTCTAGATCATAGCGTTTCCTCTTTAAG | ||
| VpaChn25_RS25055-0713-0714-up-F | GCTCTAGATATCAGAGTCACCCTTCA | 468 | This study |
| VpaChn25_RS25055-0713-0714-up-R | TTTCCTCTTTTTGCAGTTCGTTACCCATGT | ||
| VpaChn25_RS25055-0713-0714-down-F | CGAACTGCAAAAAGAGGAAACGCTATGAAA | 485 | This study |
| VpaChn25_RS25055-0713-0714-down-R | CGAGCTCACCTACAGCCAGCATT | ||
| VpaChn25_RS25055-0713-0714 up-ex-F | GGCGTTTCTTTCACCT | 1780 | This study |
| VpaChn25_RS25055-0713-0714-down-ex-R | CAGCGTATCTTGAGGC | ||
| VpaChn25_RS25055-0713-0714-com-F | CGAGCTCATGGGTAACGAACTGCAACGTT | 867 | This study |
| VpaChn25_RS25055-0713-0714-com-R | GCTCTAGATCATA GCGTTTCCTCTTTAAGGTCTAGG | ||
| RS-sfGFP-F | ACACAGGAAACAGAATTCGTGAAGAGTACGAGGACATGATCAATG | This study | |
| RS-R | TTTTTCCCATTCCTTCTCATTGCTCG | ||
| sfGFP-F | CGAGCAATGAGAAGGAATGGGAAAAACGTGGTTCTGGTGGTGAAGC | This study | |
| RS-sfGFP-R | CTGCAGGTCGACTCTAGATTATTTATATAATTCATCCATACCATGAGTAATACCTGC | ||
| sfGFP-F2 | ACACAGGAAACAGAATTCTATGAGCAAAGGAGAAGAACTTTTCACTG | This study | |
| sfGFP-R2 | CTGCAGGTCGACTCTAGATTATTTATATAATTCATCCATACCATGAG | ||
| pMMB207-F | GAGCTGTTGACAATTAATCATCGGC | This study | |
| pMMB207-R | CTACGGCGTTTCACTTCTGAGTTC | ||
| tlh-F | AAAGCGGATTATGCAGAAGCACTG | 596 | [15] |
| tlh-R | ACTTTCTAGCATTTTCTCTGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Xu, Y.; Yang, L.; Wang, Y.; Li, M.; Chen, L. Biological Function of Prophage-Related Gene Cluster ΔVpaChn25_RS25055~ΔVpaChn25_0714 of Vibrio parahaemolyticus CHN25. Int. J. Mol. Sci. 2024, 25, 1393. https://doi.org/10.3390/ijms25031393
Zhao H, Xu Y, Yang L, Wang Y, Li M, Chen L. Biological Function of Prophage-Related Gene Cluster ΔVpaChn25_RS25055~ΔVpaChn25_0714 of Vibrio parahaemolyticus CHN25. International Journal of Molecular Sciences. 2024; 25(3):1393. https://doi.org/10.3390/ijms25031393
Chicago/Turabian StyleZhao, Hui, Yingwei Xu, Lianzhi Yang, Yaping Wang, Mingyou Li, and Lanming Chen. 2024. "Biological Function of Prophage-Related Gene Cluster ΔVpaChn25_RS25055~ΔVpaChn25_0714 of Vibrio parahaemolyticus CHN25" International Journal of Molecular Sciences 25, no. 3: 1393. https://doi.org/10.3390/ijms25031393
APA StyleZhao, H., Xu, Y., Yang, L., Wang, Y., Li, M., & Chen, L. (2024). Biological Function of Prophage-Related Gene Cluster ΔVpaChn25_RS25055~ΔVpaChn25_0714 of Vibrio parahaemolyticus CHN25. International Journal of Molecular Sciences, 25(3), 1393. https://doi.org/10.3390/ijms25031393







