Time-Restricted Feeding Ameliorates Methionine–Choline Deficient Diet-Induced Steatohepatitis in Mice
Abstract
1. Introduction
2. Results
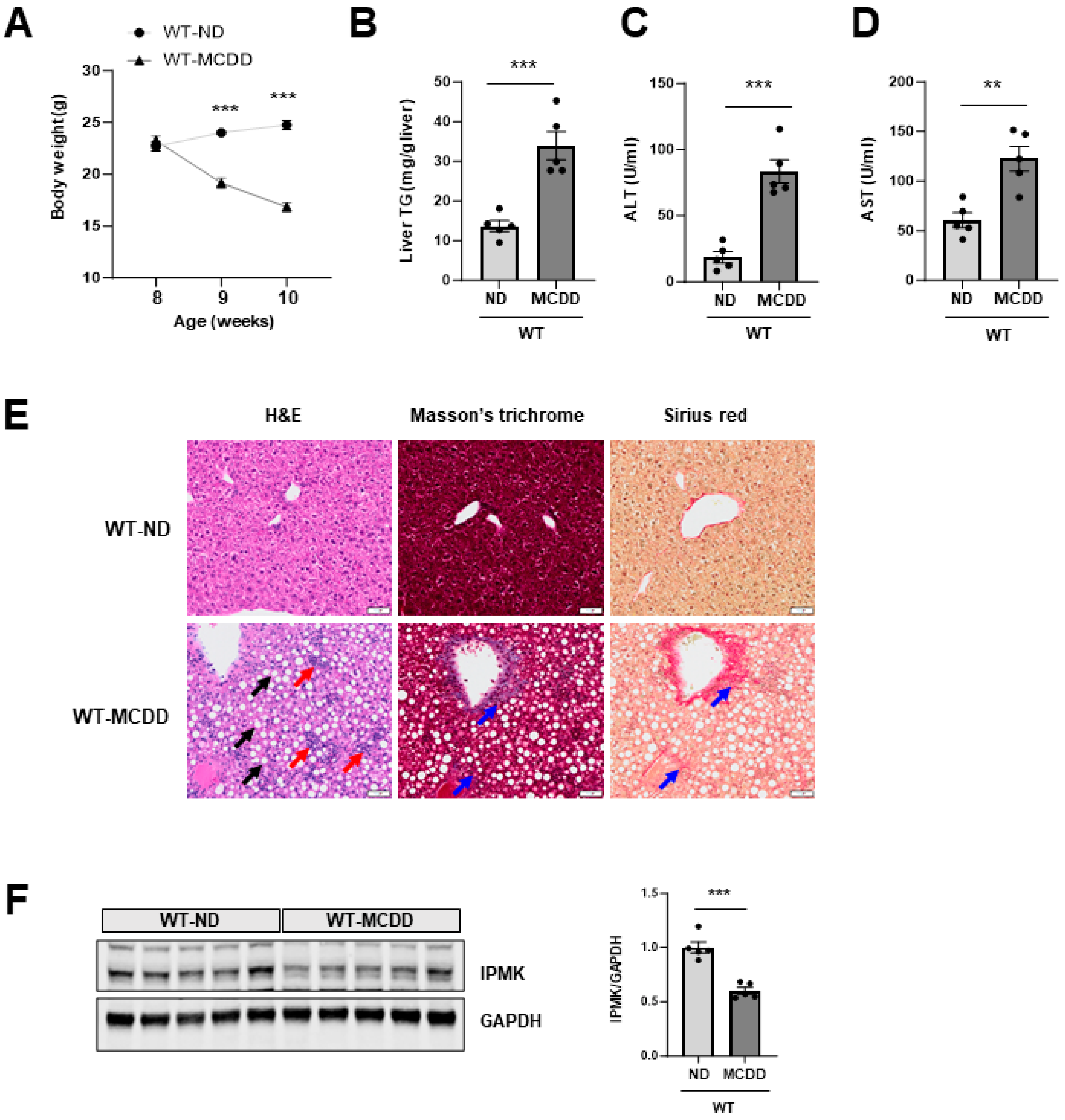
2.1. IPMK Is Decreased in the Liver of NASH Mice
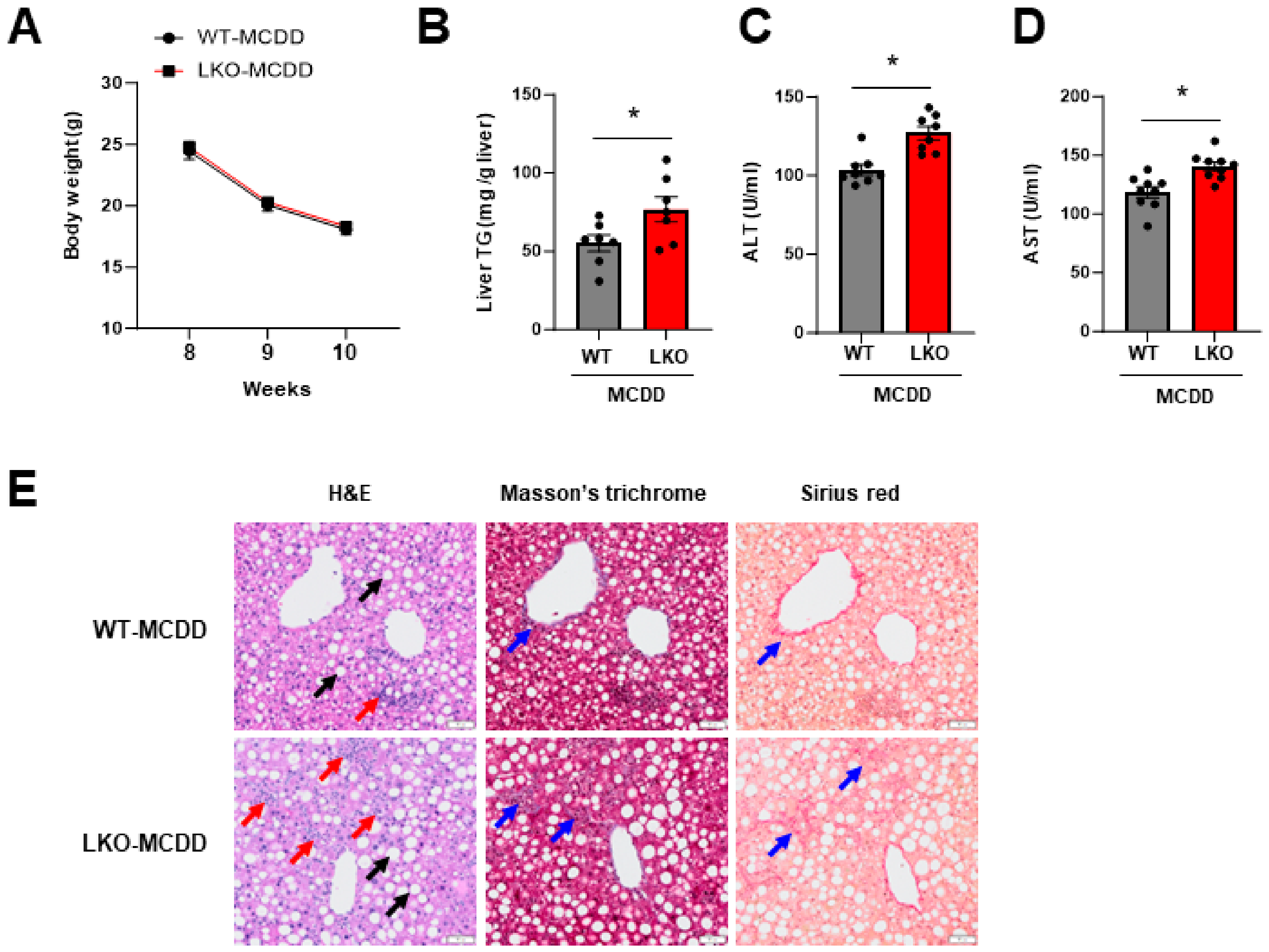
2.2. Loss of Hepatic IPMK Exacerbates NASH Progression
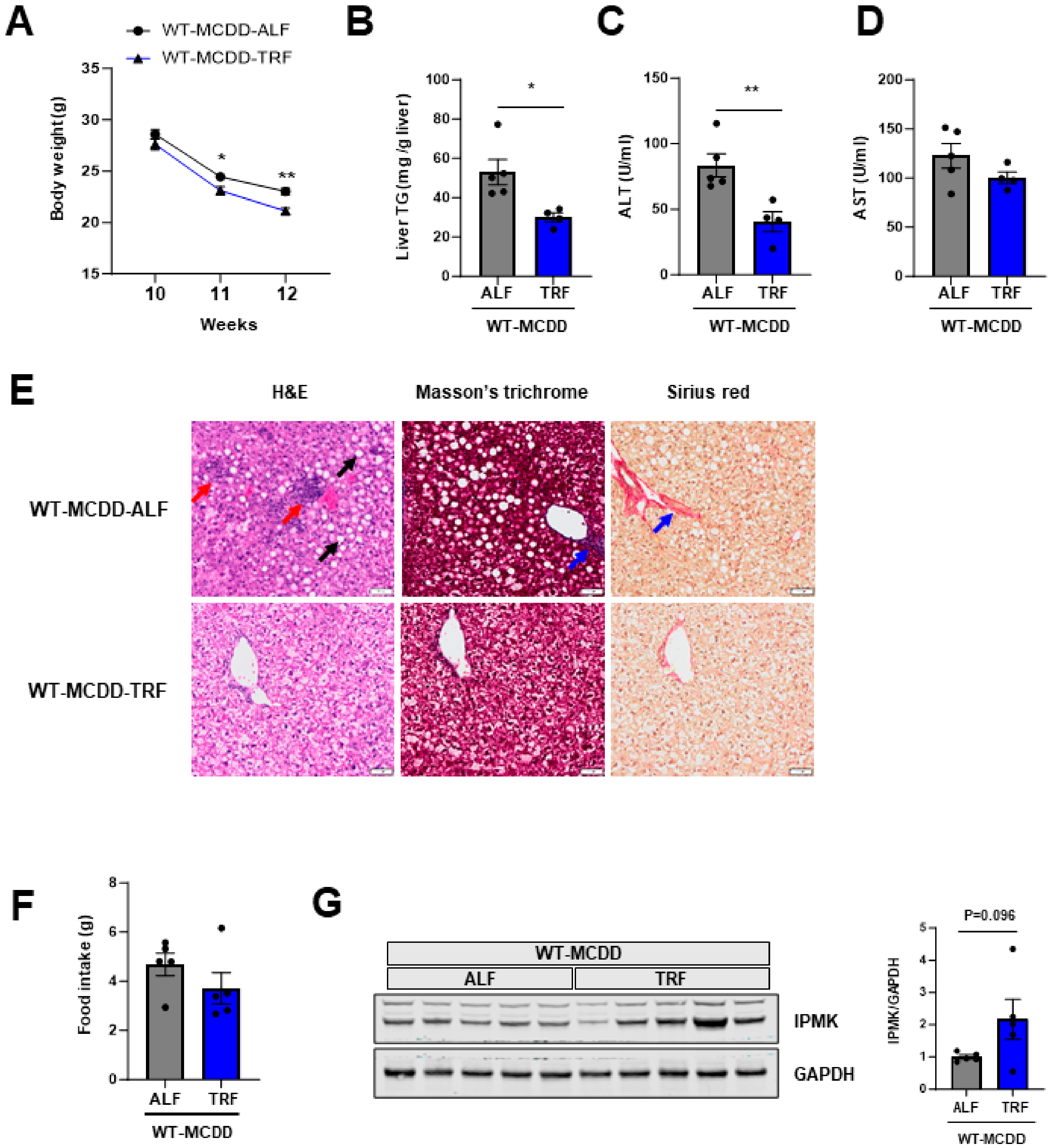
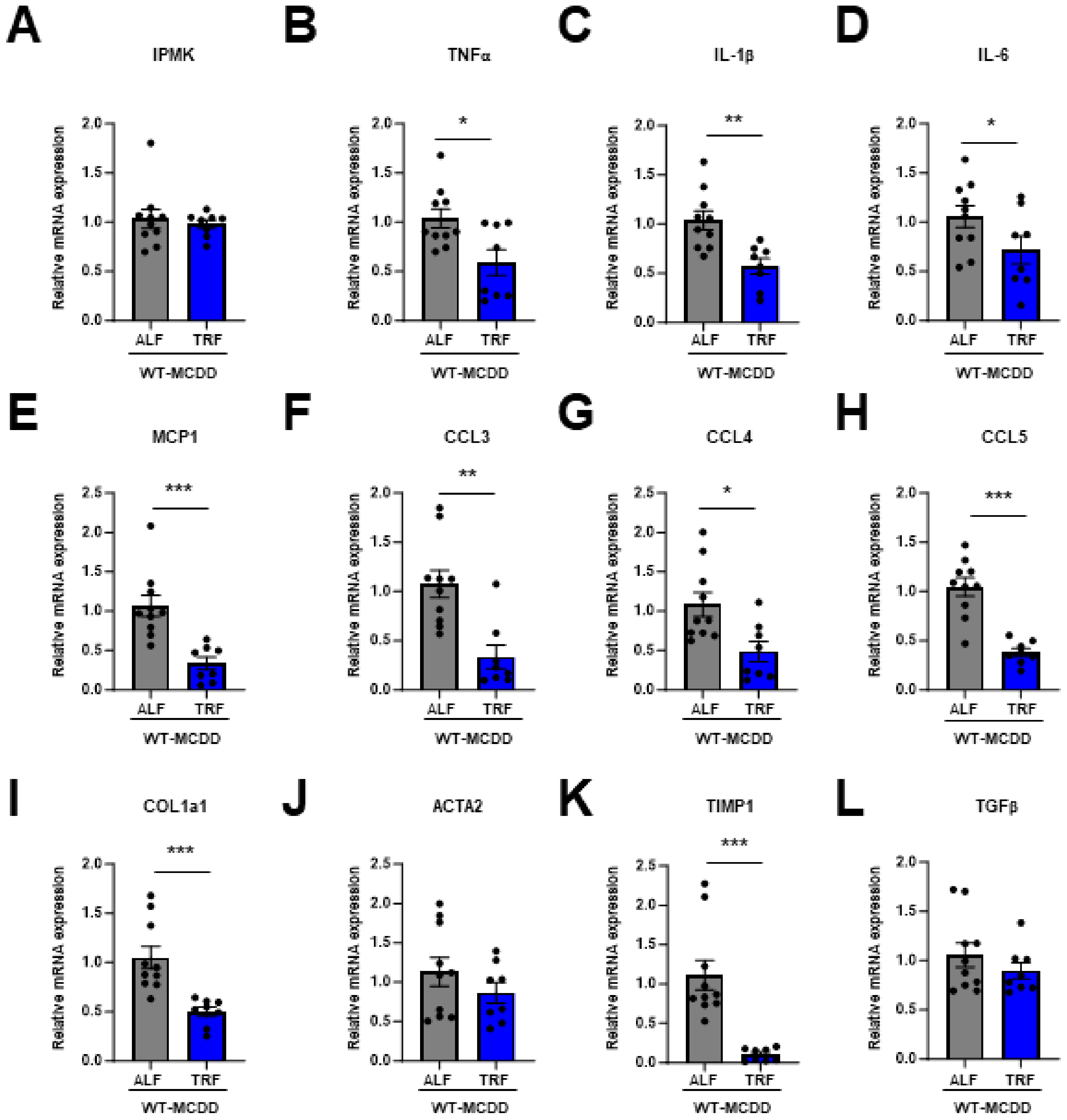
2.3. TRF Prevents NASH Progression and Restores IPMK Expression Level
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Liver Triglyceride Measurement
4.3. ALT and AST Measurement
4.4. Liver Histology
4.5. Immunoblotting
4.6. Quantitative Real-Time PCR
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loomba, R.; Friedman, S.L.; Shulman, G.I. Mechanisms and Disease Consequences of Nonalcoholic Fatty Liver Disease. Cell 2021, 184, 2537–2564. [Google Scholar] [CrossRef]
- Chitturi, S.; Abeygunasekera, S.; Farrell, G.C.; Holmes-Walker, J.; Hui, J.M.; Fung, C.; Karim, R.; Lin, R.; Samarasinghe, D.; Liddle, C.; et al. NASH and Insulin Resistance: Insulin Hypersecretion and Specific Association with the Insulin Resistance Syndrome. Hepatology 2002, 35, 373–379. [Google Scholar] [CrossRef]
- Masarone, M.; Rosato, V.; Dallio, M.; Gravina, A.G.; Aglitti, A.; Loguercio, C.; Federico, A.; Persico, M. Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxid. Med. Cell. Longev. 2018, 2018, 9547613. [Google Scholar] [CrossRef]
- Begriche, K.; Igoudjil, A.; Pessayre, D.; Fromenty, B. Mitochondrial Dysfunction in NASH: Causes, Consequences and Possible Means to Prevent It. Mitochondrion 2006, 6, 1–28. [Google Scholar] [CrossRef]
- Kanda, T.; Matsuoka, S.; Yamazaki, M.; Shibata, T.; Nirei, K.; Takahashi, H.; Kaneko, T.; Fujisawa, M.; Higuchi, T.; Nakamura, H.; et al. Apoptosis and Non-Alcoholic Fatty Liver Diseases. World J. Gastroenterol. 2018, 24, 2661–2672. [Google Scholar] [CrossRef]
- Huby, T.; Gautier, E.L. Immune Cell-Mediated Features of Non-Alcoholic Steatohepatitis. Nat. Rev. Immunol. 2022, 22, 429–443. [Google Scholar] [CrossRef]
- Jonas, W.; Schürmann, A. Genetic and Epigenetic Factors Determining NAFLD Risk. Mol. Metab. 2021, 50, 101111. [Google Scholar] [CrossRef]
- Yin, J.; Freedman, N.D.; Liu, Y.; Dawsey, S.M.; Yang, H.; Taylor, P.R.; Yin, L.; Liu, B.; Cui, J.; Fan, J.; et al. Associations between Serum Glucose, Insulin, Insulin Resistance and the Risk of Incident Primary Liver Cancer or Chronic Liver Disease Mortality: A Nested Case-Control Study. Br. J. Cancer 2023, 128, 275–284. [Google Scholar] [CrossRef]
- Radwan, H.A.; Hamed, E.H.; Saleh, O.M. Significance of Serum Adiponectin and Insulin Resistance Levels in Diagnosis of Egyptian Patients with Chronic Liver Disease and HCC. Asian Pac. J. Cancer Prev. 2019, 20, 1833–1839. [Google Scholar] [CrossRef][Green Version]
- Hung, C.-H.; Wang, J.-H.; Hu, T.-H.; Chen, C.-H.; Chang, K.-C.; Yen, Y.-H.; Kuo, Y.-H.; Tsai, M.-C.; Lu, S.-N.; Lee, C.-M. Insulin Resistance Is Associated with Hepatocellular Carcinoma in Chronic Hepatitis C Infection. World J. Gastroenterol. 2010, 16, 2265–2271. [Google Scholar] [CrossRef]
- Tarantino, G.; Crocetto, F.; Di Vito, C.; Creta, M.; Martino, R.; Pandolfo, S.D.; Pesce, S.; Napolitano, L.; Capone, D.; Imbimbo, C. Association of NAFLD and Insulin Resistance with Non Metastatic Bladder Cancer Patients: A Cross-Sectional Retrospective Study. J. Clin. Med. 2021, 10, 346. [Google Scholar] [CrossRef] [PubMed]
- Baumeier, C.; Kaiser, D.; Heeren, J.; Scheja, L.; John, C.; Weise, C.; Eravci, M.; Lagerpusch, M.; Schulze, G.; Joost, H.-G.; et al. Caloric Restriction and Intermittent Fasting Alter Hepatic Lipid Droplet Proteome and Diacylglycerol Species and Prevent Diabetes in NZO Mice. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2015, 1851, 566–576. [Google Scholar] [CrossRef] [PubMed]
- De Castro-de-Paiva, P.; Marinho, T.d.S.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Intermittent Fasting, High-Intensity Interval Training, or a Combination of Both Have Beneficial Effects in Obese Mice with Nonalcoholic Fatty Liver Disease. J. Nutr. Biochem. 2022, 104, 108997. [Google Scholar] [CrossRef] [PubMed]
- Van der Windt, D.J.; Sud, V.; Zhang, H.; Tsung, A.; Huang, H. The Effects of Physical Exercise on Fatty Liver Disease. Gene Expr. 2018, 18, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Golbidi, S.; Mesdaghinia, A.; Laher, I. Exercise in the Metabolic Syndrome. Oxid. Med. Cell. Longev. 2012, 2012, 349710. [Google Scholar] [CrossRef] [PubMed]
- Oseini, A.M.; Sanyal, A.J. Therapies in Non-Alcoholic Steatohepatitis (NASH). Liver Int. 2017, 37 (Suppl. 1), 97–103. [Google Scholar] [CrossRef] [PubMed]
- Rynders, C.A.; Thomas, E.A.; Zaman, A.; Pan, Z.; Catenacci, V.A.; Melanson, E.L. Effectiveness of Intermittent Fasting and Time-Restricted Feeding Compared to Continuous Energy Restriction for Weight Loss. Nutrients 2019, 11, 2442. [Google Scholar] [CrossRef]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.J.; et al. Time-Restricted Feeding without Reducing Caloric Intake Prevents Metabolic Diseases in Mice Fed a High-Fat Diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef]
- Chaix, A.; Zarrinpar, A.; Miu, P.; Panda, S. Time-Restricted Feeding Is a Preventative and Therapeutic Intervention against Diverse Nutritional Challenges. Cell Metab. 2014, 20, 991–1005. [Google Scholar] [CrossRef]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018, 27, 1212–1221.e3. [Google Scholar] [CrossRef]
- Shu, Y.Y.; Gao, W.K.; Chu, H.K.; Yang, L.; Pan, X.L.; Ye, J. Attenuation by Time-Restricted Feeding of High-Fat and High-Fructose Diet-Induced NASH in Mice Is Related to Per2 and Ferroptosis. Oxid. Med. Cell. Longev. 2022, 2022, 8063897. [Google Scholar] [CrossRef] [PubMed]
- Tamanna, N.; Kroeker, K.; Braun, K.; Banh, S.; Treberg, J.R. The Effect of Short-Term Methionine Restriction on Glutathione Synthetic Capacity and Antioxidant Responses at the Whole Tissue and Mitochondrial Level in the Rat Liver. Exp. Gerontol. 2019, 127, 110712. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. S-Adenosylmethionine. Int. J. Biochem. Cell Biol. 2000, 32, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Vance, D.E. Role of Phosphatidylcholine Biosynthesis in the Regulation of Lipoprotein Homeostasis. Curr. Opin. Lipidol. 2008, 19, 229–234. [Google Scholar] [CrossRef]
- Corbin, K.D.; Zeisel, S.H. Choline Metabolism Provides Novel Insights into Nonalcoholic Fatty Liver Disease and Its Progression. Curr. Opin. Gastroenterol. 2012, 28, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Hebbard, L.; George, J. Animal Models of Nonalcoholic Fatty Liver Disease. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.; Claudel, T.; Baghdasaryan, A.; Mueller, M.; Halilbasic, E.; Das, S.K.; Lass, A.; Zimmermann, R.; Zechner, R.; Hoefler, G.; et al. Role of Adipose Triglyceride Lipase (PNPLA2) in Protection from Hepatic Inflammation in Mouse Models of Steatohepatitis and Endotoxemia. Hepatology 2014, 59, 858–869. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Kim, S.H.; Kim, S.-N.; Kwon, H.-J.; Kim, J.-D.; Oh, J.Y.; Jung, Y.-S. Sex-Specific Metabolic Interactions between Liver and Adipose Tissue in MCD Diet-Induced Non-Alcoholic Fatty Liver Disease. Oncotarget 2016, 7, 46959–46971. [Google Scholar] [CrossRef]
- Canet, M.J.; Hardwick, R.N.; Lake, A.D.; Dzierlenga, A.L.; Clarke, J.D.; Cherrington, N.J. Modeling Human Nonalcoholic Steatohepatitis-Associated Changes in Drug Transporter Expression Using Experimental Rodent Models. Drug Metab. Dispos. 2014, 42, 586–595. [Google Scholar] [CrossRef]
- Rinella, M.E.; Green, R.M. The Methionine-Choline Deficient Dietary Model of Steatohepatitis Does Not Exhibit Insulin Resistance. J. Hepatol. 2004, 40, 47–51. [Google Scholar] [CrossRef]
- Kirsch, R.; Clarkson, V.; Shephard, E.G.; Marais, D.A.; Jaffer, M.A.; Woodburne, V.E.; Kirsch, R.E.; de la M Hall, P. Rodent Nutritional Model of Non-Alcoholic Steatohepatitis: Species, Strain and Sex Difference Studies. J. Gastroenterol. Hepatol. 2003, 18, 1272–1282. [Google Scholar] [CrossRef]
- Li, H.; Toth, E.; Cherrington, N.J. Asking the Right Questions with Animal Models: Methionine- and Choline-Deficient Model in Predicting Adverse Drug Reactions in Human NASH. Toxicol. Sci. 2018, 161, 23–33. [Google Scholar] [CrossRef]
- Frederick, J.P.; Mattiske, D.; Wofford, J.A.; Megosh, L.C.; Drake, L.Y.; Chiou, S.-T.; Hogan, B.L.M.; York, J.D. An Essential Role for an Inositol Polyphosphate Multikinase, Ipk2, in Mouse Embryogenesis and Second Messenger Production. Proc. Natl. Acad. Sci. USA 2005, 102, 8454–8459. [Google Scholar] [CrossRef] [PubMed]
- Maag, D.; Maxwell, M.J.; Hardesty, D.A.; Boucher, K.L.; Choudhari, N.; Hanno, A.G.; Ma, J.F.; Snowman, A.S.; Pietropaoli, J.W.; Xu, R.; et al. Inositol Polyphosphate Multikinase Is a Physiologic PI3-Kinase That Activates Akt/PKB. Proc. Natl. Acad. Sci. USA 2011, 108, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, S.F.; Maag, D.; Maxwell, M.J.; Resnick, A.C.; Juluri, K.R.; Chakraborty, A.; Koldobskiy, M.A.; Cha, S.H.; Barrow, R.; et al. Amino Acid Signaling to MTOR Mediated by Inositol Polyphosphate Multikinase. Cell Metab. 2011, 13, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Tyagi, R.; Lee, J.Y.; Park, J.; Kim, Y.R.; Beon, J.; Chen, P.Y.; Cha, J.Y.; Snyder, S.H.; Kim, S. Inositol Polyphosphate Multikinase Is a Coactivator for Serum Response Factor-Dependent Induction of Immediate Early Genes. Proc. Natl. Acad. Sci. USA 2013, 110, 19938–19943. [Google Scholar] [CrossRef]
- Guha, P.; Tyagi, R.; Chowdhury, S.; Reilly, L.; Fu, C.; Xu, R.; Resnick, A.C.; Snyder, S.H. IPMK Mediates Activation of ULK Signaling and Transcriptional Regulation of Autophagy Linked to Liver Inflammation and Regeneration. Cell Rep. 2019, 26, 2692–2703.e7. [Google Scholar] [CrossRef]
- Bang, S.; Kim, S.; Dailey, M.J.; Chen, Y.; Moran, T.H.; Snyder, S.H.; Kim, S.F. AMP-Activated Protein Kinase Is Physiologically Regulated by Inositol Polyphosphate Multikinase. Proc. Natl. Acad. Sci. USA 2012, 109, 616–620. [Google Scholar] [CrossRef]
- Kim, E.; Beon, J.; Lee, S.; Park, S.J.; Ahn, H.; Kim, M.G.; Park, J.E.; Kim, W.; Yuk, J.-M.; Kang, S.-J.; et al. Inositol Polyphosphate Multikinase Promotes Toll-like Receptor–Induced Inflammation by Stabilizing TRAF6. Sci. Adv. 2017, 3, e1602296. [Google Scholar] [CrossRef]
- Lee, H.; Kim, E.; Kim, S. MiRNA-Induced Downregulation of IPMK in Macrophages Mediates Lipopolysaccharide-Triggered TLR4 Signaling. Biomolecules 2023, 13, 332. [Google Scholar] [CrossRef]
- Jung, I.-R.; Anokye-Danso, F.; Jin, S.; Ahima, R.S.; Kim, S.F. IPMK Modulates Hepatic Glucose Production and Insulin Signaling. J. Cell. Physiol. 2022, 237, 3421–3432. [Google Scholar] [CrossRef]
- Rizki, G.; Arnaboldi, L.; Gabrielli, B.; Yan, J.; Lee, G.S.; Ng, R.K.; Turner, S.M.; Badger, T.M.; Pitas, R.E.; Maher, J.J. Mice Fed a Lipogenic Methionine-Choline-Deficient Diet Develop Hypermetabolism Coincident with Hepatic Suppression of SCD-1. J. Lipid Res. 2006, 47, 2280–2290. [Google Scholar] [CrossRef]
- Machado, M.V.; Michelotti, G.A.; Xie, G.; Almeida Pereira, T.; Boursier, J.; Bohnic, B.; Guy, C.D.; Diehl, A.M. Mouse Models of Diet-Induced Nonalcoholic Steatohepatitis Reproduce the Heterogeneity of the Human Disease. PLoS ONE 2015, 10, e0127991. [Google Scholar] [CrossRef]
- Xie, Z.; Sun, Y.; Ye, Y.; Hu, D.; Zhang, H.; He, Z.; Zhao, H.; Yang, H.; Mao, Y. Randomized Controlled Trial for Time-Restricted Eating in Healthy Volunteers without Obesity. Nat. Commun. 2022, 13, 1003. [Google Scholar] [CrossRef]
- Marjot, T.; Tomlinson, J.W.; Hodson, L.; Ray, D.W. Timing of Energy Intake and the Therapeutic Potential of Intermittent Fasting and Time-Restricted Eating in NAFLD. Gut 2023, 72, 1607–1619. [Google Scholar] [CrossRef]
- Chung, H.; Chou, W.; Sears, D.D.; Patterson, R.E.; Webster, N.J.G.; Ellies, L.G. Time-Restricted Feeding Improves Insulin Resistance and Hepatic Steatosis in a Mouse Model of Postmenopausal Obesity. Metabolism 2016, 65, 1743–1754. [Google Scholar] [CrossRef]
- Wei, X.; Lin, B.; Huang, Y.; Yang, S.; Huang, C.; Shi, L.; Liu, D.; Zhang, P.; Lin, J.; Xu, B.; et al. Effects of Time-Restricted Eating on Nonalcoholic Fatty Liver Disease: The TREATY-FLD Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e233513. [Google Scholar] [CrossRef]
- Cholankeril, G.; Patel, R.; Khurana, S.; Satapathy, S.K. Hepatocellular Carcinoma in Non-Alcoholic Steatohepatitis: Current Knowledge and Implications for Management. World J. Hepatol. 2017, 9, 533–543. [Google Scholar] [CrossRef]
- Lowe, D.A.; Wu, N.; Rohdin-Bibby, L.; Moore, A.H.; Kelly, N.; Liu, Y.E.; Philip, E.; Vittinghoff, E.; Heymsfield, S.B.; Olgin, J.E.; et al. Effects of Time-Restricted Eating on Weight Loss and Other Metabolic Parameters in Women and Men with Overweight and Obesity: The TREAT Randomized Clinical Trial. JAMA Intern. Med. 2020, 180, 1491–1499. [Google Scholar] [CrossRef]
- Rivera, C.A.; Adegboyega, P.; van Rooijen, N.; Tagalicud, A.; Allman, M.; Wallace, M. Toll-like Receptor-4 Signaling and Kupffer Cells Play Pivotal Roles in the Pathogenesis of Non-Alcoholic Steatohepatitis. J. Hepatol. 2007, 47, 571–579. [Google Scholar] [CrossRef]
- Parlati, L.; Régnier, M.; Guillou, H.; Postic, C. New Targets for NAFLD. JHEP Rep. 2021, 3, 100346. [Google Scholar] [CrossRef] [PubMed]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Animal Models of Fibrosis in Nonalcoholic Steatohepatitis: Do They Reflect. Human. Disease? Adv. Nutr. 2020, 11, 1696–1711. [Google Scholar] [CrossRef]
- Kim, J.W.; Hahn, K.R.; Yoo, D.Y.; Jung, H.Y.; Hwang, I.K.; Seong, J.K.; Yoon, Y.S. Methionine-Choline Deprivation Impairs Adult Hippocampal Neurogenesis in C57BL/6 Mice. J. Med. Food 2019, 22, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Takahashi, S.; Fang, Z.-Z.; Matsubara, T.; Krausz, K.W.; Qu, A.; Gonzalez, F.J. Role of White Adipose Lipolysis in the Development of NASH Induced by Methionine- and Choline-Deficient Diet. Biochim. Biophys. Acta 2014, 1841, 1596–1607. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Li, H.; Zhou, J.; Li, Q.; Glaser, S.; Francis, H.; Alpini, G.; Wu, C. Methionine- and Choline-Deficient Diet-Induced Nonalcoholic Steatohepatitis Is Associated with Increased Intestinal Inflammation. Am. J. Pathol. 2021, 191, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Schroder, J.D.; Falqueto, H.; Mânica, A.; Zanini, D.; de Oliveira, T.; de Sá, C.A.; Cardoso, A.M.; Manfredi, L.H. Effects of Time-Restricted Feeding in Weight Loss, Metabolic Syndrome and Cardiovascular Risk in Obese Women. J. Transl. Med. 2021, 19, 3. [Google Scholar] [CrossRef] [PubMed]
- Braunersreuther, V.; Viviani, G.L.; Mach, F.; Montecucco, F. Role of Cytokines and Chemokines in Non-Alcoholic Fatty Liver Disease. World J. Gastroenterol. 2012, 18, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Sahin, H.; Trautwein, C.; Wasmuth, H.E. Functional Role of Chemokines in Liver Disease Models. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 682–690. [Google Scholar] [CrossRef]
- Cao, S.; Liu, M.; Sehrawat, T.S.; Shah, V.H. Regulation and Functional Roles of Chemokines in Liver Diseases. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 630–647. [Google Scholar] [CrossRef]
- Berres, M.-L.; Koenen, R.R.; Rueland, A.; Zaldivar, M.M.; Heinrichs, D.; Sahin, H.; Schmitz, P.; Streetz, K.L.; Berg, T.; Gassler, N.; et al. Antagonism of the Chemokine Ccl5 Ameliorates Experimental Liver Fibrosis in Mice. J. Clin. Investig. 2010, 120, 4129–4140. [Google Scholar] [CrossRef]
- Sadeghi, M.; Lahdou, I.; Oweira, H.; Daniel, V.; Terness, P.; Schmidt, J.; Weiss, K.-H.; Longerich, T.; Schemmer, P.; Opelz, G.; et al. Serum Levels of Chemokines CCL4 and CCL5 in Cirrhotic Patients Indicate the Presence of Hepatocellular Carcinoma. Br. J. Cancer 2015, 113, 756–762. [Google Scholar] [CrossRef]
- Xu, L.; Chen, Y.; Nagashimada, M.; Ni, Y.; Zhuge, F.; Chen, G.; Li, H.; Pan, T.; Yamashita, T.; Mukaida, N.; et al. CC Chemokine Ligand 3 Deficiency Ameliorates Diet-Induced Steatohepatitis by Regulating Liver Macrophage Recruitment and M1/M2 Status in Mice. Metabolism 2021, 125, 154914. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, Y.; Lee, M.N.; Nah, J.; Yun, N.; Wu, D.; Pae, M. Time-Restricted Feeding Reduces Monocyte Production by Controlling Hematopoietic Stem and Progenitor Cells in the Bone Marrow during Obesity. Front. Immunol. 2022, 13, 1054875. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, Y.; Lee, M.; Wu, D.; Pae, M. Time-Restricted Feeding Restores Obesity-Induced Alteration in Adipose Tissue Immune Cell Phenotype. Nutrients 2021, 13, 3780. [Google Scholar] [CrossRef]
- Koronowski, K.B.; Sassone-Corsi, P. Communicating Clocks Shape Circadian Homeostasis. Science 2021, 371, eabd0951. [Google Scholar] [CrossRef]
- He, B.; Nohara, K.; Park, N.; Park, Y.-S.; Guillory, B.; Zhao, Z.; Garcia, J.M.; Koike, N.; Lee, C.C.; Takahashi, J.S.; et al. The Small Molecule Nobiletin Targets the Molecular Oscillator to Enhance Circadian Rhythms and Protect against Metabolic Syndrome. Cell Metab. 2016, 23, 610–621. [Google Scholar] [CrossRef]
- Turek, F.W.; Joshu, C.; Kohsaka, A.; Lin, E.; Ivanova, G.; McDearmon, E.; Laposky, A.; Losee-Olson, S.; Easton, A.; Jensen, D.R.; et al. Obesity and Metabolic Syndrome in Circadian Clock Mutant Mice. Science 2005, 308, 1043–1045. [Google Scholar] [CrossRef]
- Takahashi, J.S. Transcriptional Architecture of the Mammalian Circadian Clock. Nat. Rev. Genet. 2017, 18, 164–179. [Google Scholar] [CrossRef]
- Chaix, A.; Lin, T.; Le, H.D.; Chang, M.W.; Panda, S. Time-Restricted Feeding Prevents Obesity and Metabolic Syndrome in Mice Lacking a Circadian Clock. Cell Metab. 2019, 29, 303–319.e4. [Google Scholar] [CrossRef]
- Tyagi, R.; Chakraborty, S.; Tripathi, S.J.; Jung, I.-R.; Kim, S.F.; Snyder, S.H.; Paul, B.D. Inositol Polyphosphate Multikinase Modulates Redox Signaling through Nuclear Factor Erythroid 2-Related Factor 2 and Glutathione Metabolism. iScience 2023, 26, 107199. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, I.-R.; Ahima, R.S.; Kim, S.F. Time-Restricted Feeding Ameliorates Methionine–Choline Deficient Diet-Induced Steatohepatitis in Mice. Int. J. Mol. Sci. 2024, 25, 1390. https://doi.org/10.3390/ijms25031390
Jung I-R, Ahima RS, Kim SF. Time-Restricted Feeding Ameliorates Methionine–Choline Deficient Diet-Induced Steatohepatitis in Mice. International Journal of Molecular Sciences. 2024; 25(3):1390. https://doi.org/10.3390/ijms25031390
Chicago/Turabian StyleJung, Ik-Rak, Rexford S. Ahima, and Sangwon F. Kim. 2024. "Time-Restricted Feeding Ameliorates Methionine–Choline Deficient Diet-Induced Steatohepatitis in Mice" International Journal of Molecular Sciences 25, no. 3: 1390. https://doi.org/10.3390/ijms25031390
APA StyleJung, I.-R., Ahima, R. S., & Kim, S. F. (2024). Time-Restricted Feeding Ameliorates Methionine–Choline Deficient Diet-Induced Steatohepatitis in Mice. International Journal of Molecular Sciences, 25(3), 1390. https://doi.org/10.3390/ijms25031390





