Characterizing the D-Amino Acid Position in Peptide Epimers by Using Higher-Energy Collisional Dissociation Tandem Mass Spectrometry: A Case Study of Liraglutide
Abstract
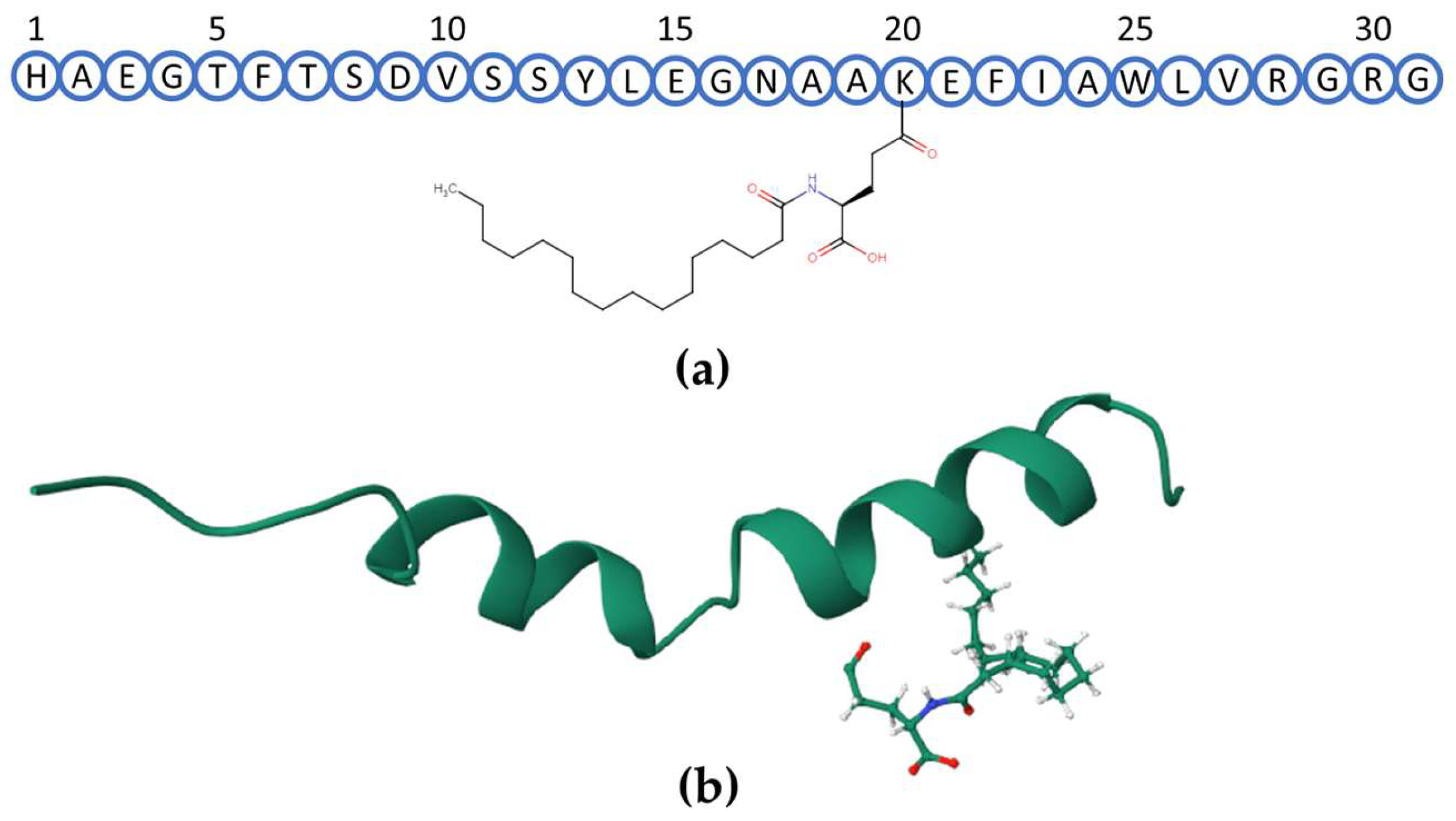
1. Introduction
2. Results
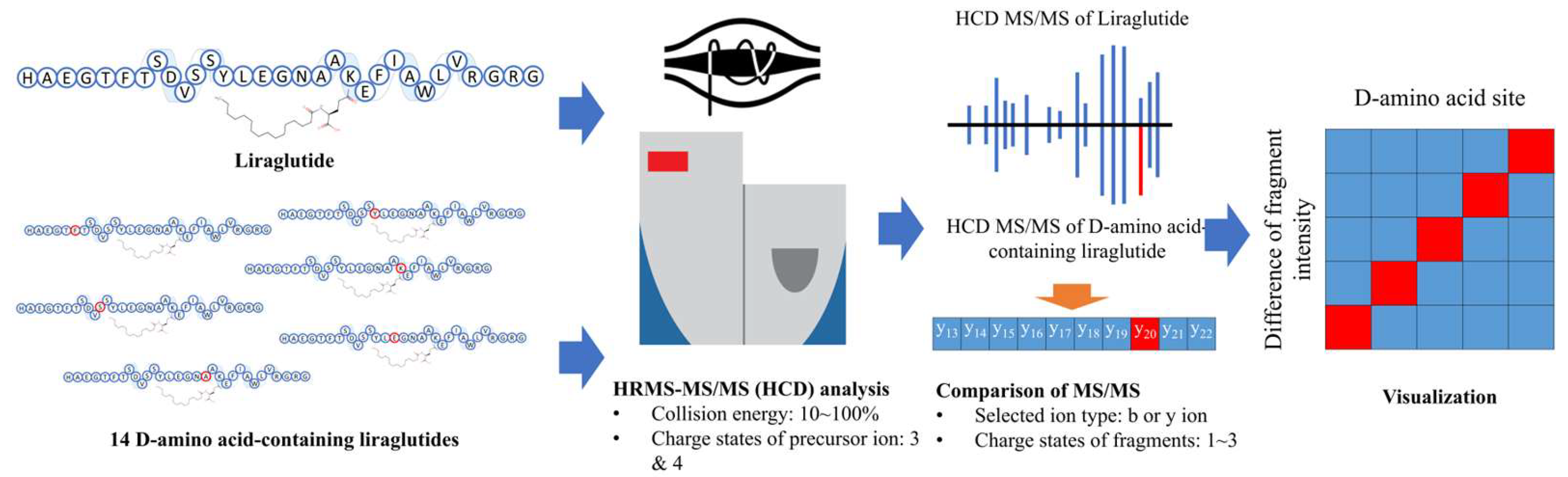
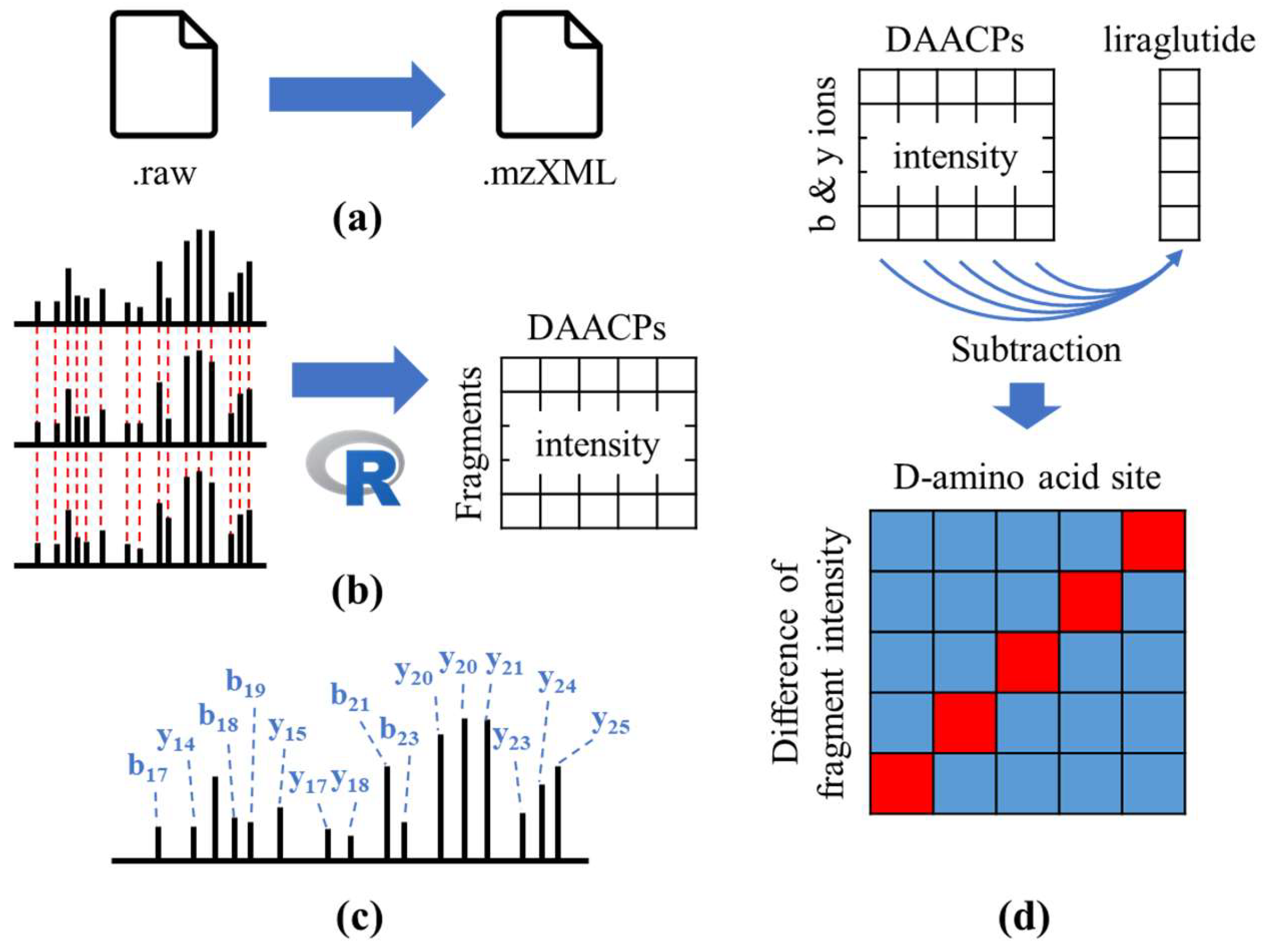
2.1. Analytical Procedure
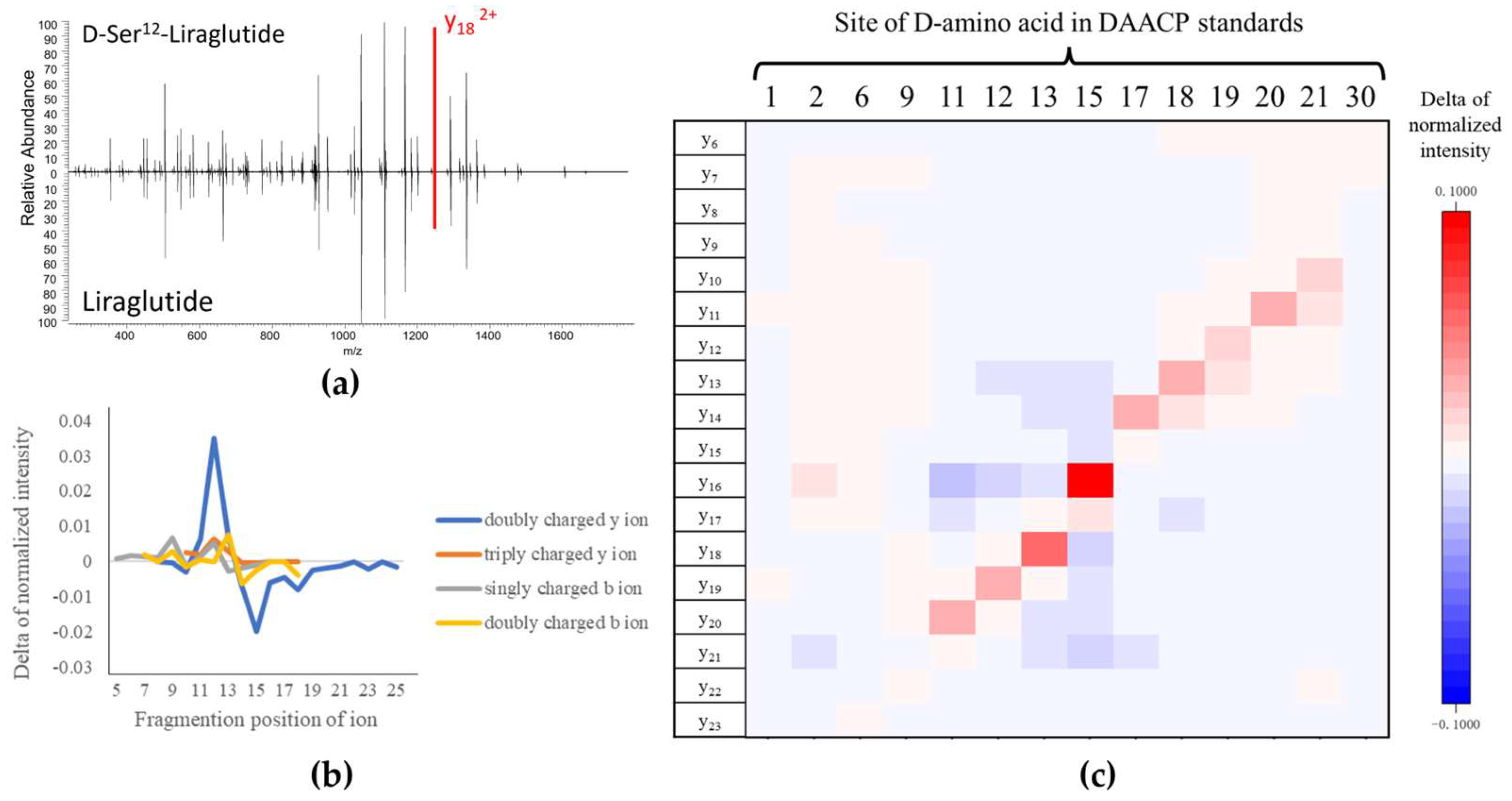
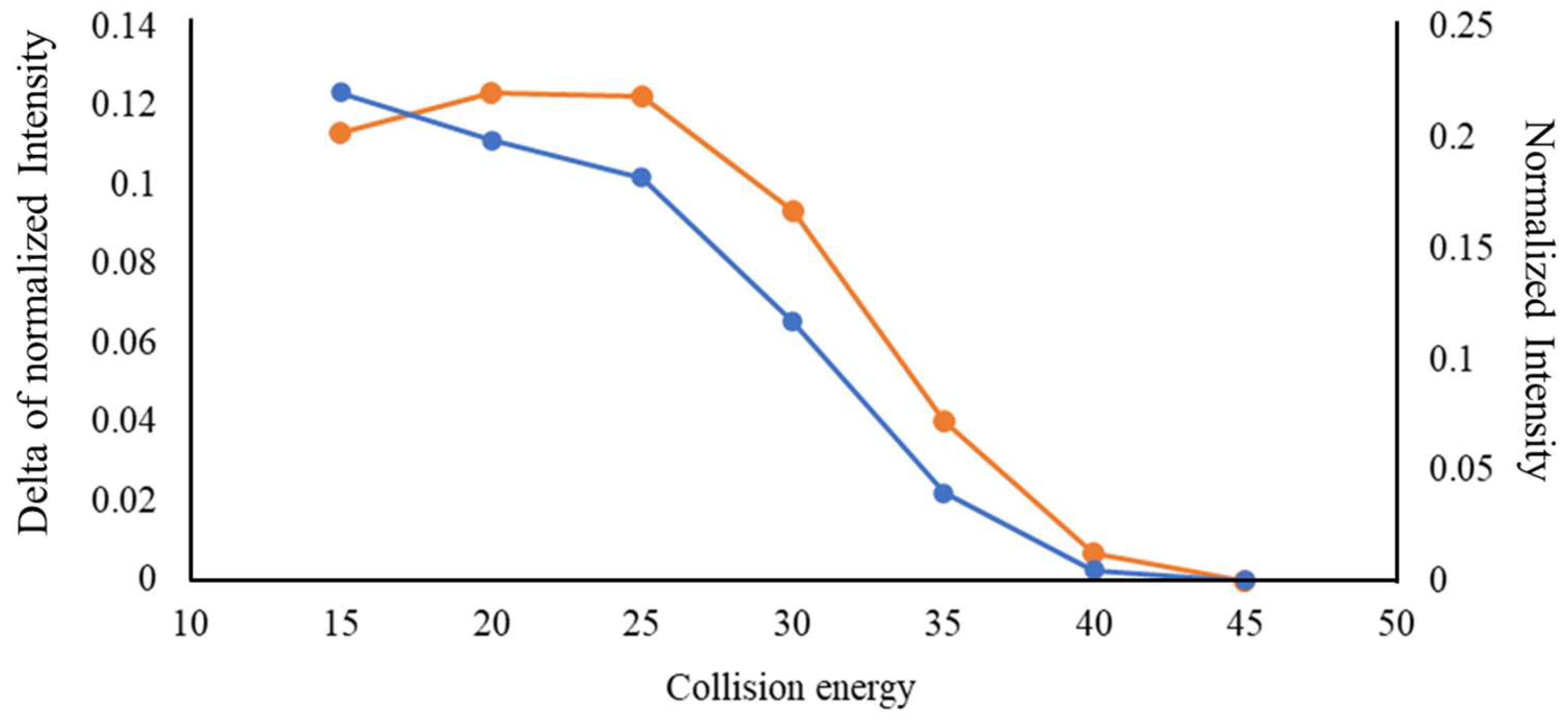
2.2. Comparison between the MS/MS Spectra of Liraglutide and DAACPs
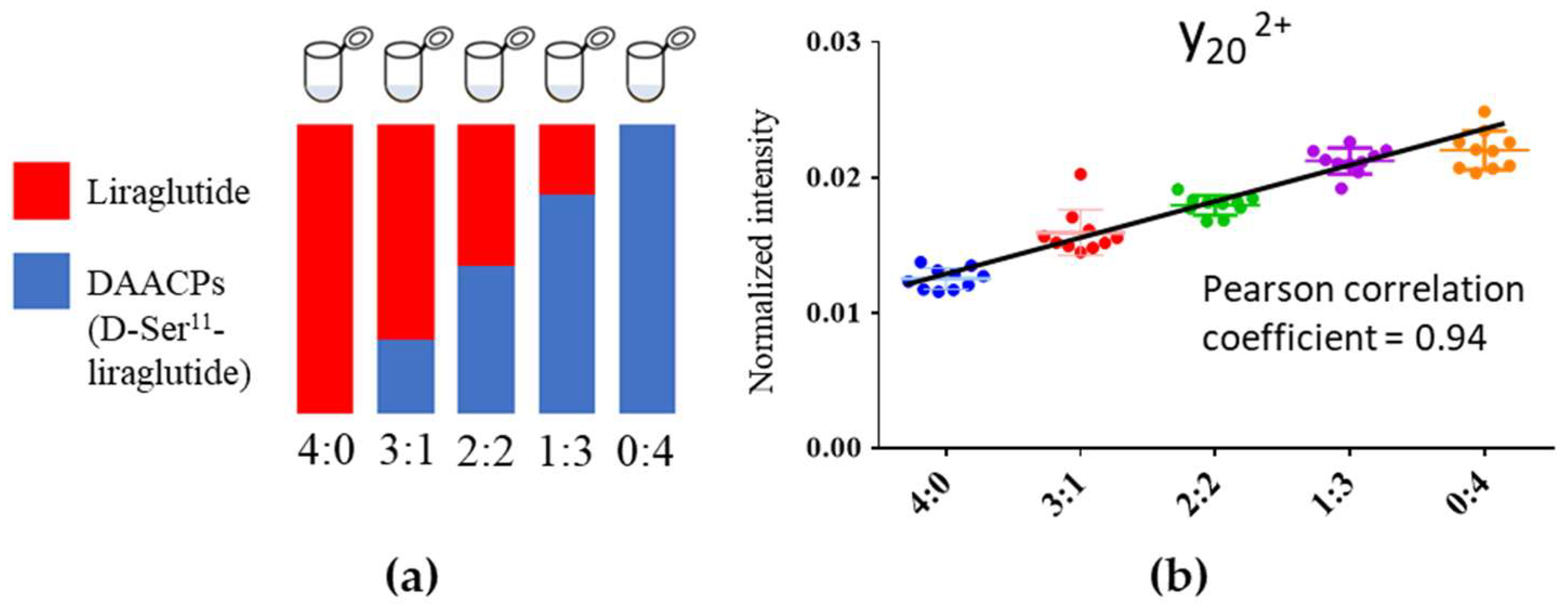
2.3. Linearity between Fragment Ion Intensity and Proportion of DAACPs
3. Discussion
3.1. Capability of HCD to Characterize DAACPs
3.2. Possible Mechanism Leading to the Higher Ion Intensity at the Site-Related Fragment
4. Materials and Methods
4.1. Chemicals and Sample Preparation
4.2. HRMS Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ollivaux, C.; Soyez, D.; Toullec, J.Y. Biogenesis of d-Amino acid containing peptides/proteins: Where, when and how? J. Pept. Sci. 2014, 20, 595–612. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rodríguez, S.; Martínez-Gómez, A.I.; Rodríguez-Vico, F.; Clemente-Jiménez, J.M.; Las Heras-Vázquez, F.J. Natural occurrence and industrial applications of d-Amino acids: An overview. Chem. Biodivers. 2010, 7, 1531–1548. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Wey, M.; Armstrong, D.W. d-Amino acids in biological systems. Chirality 2023, 35, 508–534. [Google Scholar] [CrossRef] [PubMed]
- Fujii, N.; Satoh, K.; Harada, K.; Ishibashi, Y. Simultaneous stereoinversion and isomerization at specific aspartic acid residues in αA-crystallin from human lens. J. Biochem. 1994, 116, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Roher, A.; Lowenson, J.; Clarke, S.; Wolkow, C.; Wang, R.; Cotter, R.; Reardon, I.; Zürcher-Neely, H.; Heinrikson, R.; Ball, M. Structural alterations in the peptide backbone of beta-amyloid core protein may account for its deposition and stability in Alzheimer’s disease. J. Biol. Chem. 1993, 268, 3072–3083. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Watanabe, A.; Ogawara, M.; Mori, H.; Shirasawa, T. Isoaspartate formation and neurodegeneration in Alzheimer’s disease. Arch. Biochem. Biophys. 2000, 381, 225–234. [Google Scholar] [CrossRef]
- Fujii, N.; Ishibashi, Y.; Satoh, K.; Fujino, M.; Harada, K. Simultaneous racemization and isomerization at specific aspartic acid residues in αB-crystallin from the aged human lens. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 1994, 1204, 157–163. [Google Scholar] [CrossRef]
- Shimizu, T.; Fukuda, H.; Murayama, S.; Izumiyama, N.; Shirasawa, T. Isoaspartate formation at position 23 of amyloid beta peptide enhanced fibril formation and deposited onto senile plaques and vascular amyloids in Alzheimer’s disease. J. Neurosci. Res. 2002, 70, 451–461. [Google Scholar] [CrossRef]
- Nishimura, Y.; Francis, J.N.; Donau, O.K.; Jesteadt, E.; Sadjadpour, R.; Smith, A.R.; Seaman, M.S.; Welch, B.D.; Martin, M.A.; Kay, M.S. Prevention and treatment of SHIVAD8 infection in rhesus macaques by a potent d-peptide HIV entry inhibitor. Proc. Natl. Acad. Sci. USA 2020, 117, 22436–22442. [Google Scholar] [CrossRef]
- Feng, Z.; Xu, B. Inspiration from the mirror: d-Amino acid containing peptides in biomedical approaches. Biomol. Concepts 2016, 7, 179–187. [Google Scholar] [CrossRef]
- D’Hondt, M.; Bracke, N.; Taevernier, L.; Gevaert, B.; Verbeke, F.; Wynendaele, E.; De Spiegeleer, B. Related impurities in peptide medicines. J. Pharm. Biomed. Anal. 2014, 101, 2–30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xu, W.; Yin, C.; Tang, Y. Characterization of low-level d-Amino acid isomeric impurities of Semaglutide using liquid chromatography-high resolution tandem mass spectrometry. J. Pharm. Biomed. Anal. 2023, 224, 115164. [Google Scholar] [CrossRef] [PubMed]
- Riester, D.; Wiesmüller, K.-H.; Stoll, D.; Kuhn, R. Racemization of amino acids in solid-phase peptide synthesis investigated by capillary electrophoresis. Anal. Chem. 1996, 68, 2361–2365. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. ANDAs for Certain Highly Purified Synthetic Peptide Drug Products That Refer to Listed Drugs of rDNA Origin Guidance for Industry. 2021. Available online: https://www.fda.gov/media/107622/download (accessed on 22 January 2024).
- Patel, C.N.; Bauer, S.P.; Davies, J.; Durbin, J.D.; Shiyanova, T.L.; Zhang, K.; Tang, J.X. N+ 1 engineering of an aspartate isomerization hotspot in the complementarity-determining region of a monoclonal antibody. J. Pharm. Sci. 2016, 105, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Nowak, C.; Ponniah, G.; Neill, A.; Liu, H. Characterization of succinimide stability during trypsin digestion for LC-MS analysis. Anal. Biochem. 2017, 526, 1–8. [Google Scholar] [CrossRef] [PubMed]
- MONTECUCCHI, P.C.; DE CASTIGLIONE, R.; PIANI, S.; GOZZINI, L.; ERSPAMER, V. Amino acid composition and sequence of dermorphin, a novel opiate-like peptide from the skin of Phyllomedusa sauvagei. Int. J. Pept. Protein Res. 1981, 17, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Livnat, I.; Tai, H.-C.; Jansson, E.T.; Bai, L.; Romanova, E.V.; Chen, T.-T.; Yu, K.; Chen, S.-A.; Zhang, Y.; Wang, Z.-y. A d-Amino acid-containing neuropeptide discovery funnel. Anal. Chem. 2016, 88, 11868–11876. [Google Scholar] [CrossRef]
- Ewing, M.A.; Wang, J.; Sheeley, S.A.; Sweedler, J.V. Detecting d-Amino acid-containing neuropeptides using selective enzymatic digestion. Anal. Chem. 2008, 80, 2874–2880. [Google Scholar] [CrossRef]
- Bai, L.; Livnat, I.; Romanova, E.V.; Alexeeva, V.; Yau, P.M.; Vilim, F.S.; Weiss, K.R.; Jing, J.; Sweedler, J.V. Characterization of GdFFD, a d-Amino acid-containing neuropeptide that functions as an extrinsic modulator of the Aplysia feeding circuit. J. Biol. Chem. 2013, 288, 32837–32851. [Google Scholar] [CrossRef]
- Bai, L.; Romanova, E.V.; Sweedler, J.V. Distinguishing endogenous d-Amino acid-containing neuropeptides in individual neurons using tandem mass spectrometry. Anal. Chem. 2011, 83, 2794–2800. [Google Scholar] [CrossRef]
- Aki, K.; Okamura, E. Kinetics of the competitive reactions of isomerization and peptide bond cleavage at l-α-and d-β-aspartyl residues in an αA-crystallin fragment. J. Pept. Sci. 2017, 23, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Ludewig, R.; Dong, J.; Zou, H.; Scriba, G.K. Separation of peptide diastereomers using CEC and a hydrophobic monolithic column. J. Sep. Sci. 2010, 33, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Jeanne Dit Fouque, K.; Garabedian, A.; Porter, J.; Baird, M.; Pang, X.; Williams, T.D.; Li, L.; Shvartsburg, A.; Fernandez-Lima, F. Fast and effective ion mobility–mass spectrometry separation of d-amino-acid-containing peptides. Anal. Chem. 2017, 89, 11787–11794. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Deng, L.; Baker, E.S.; Ibrahim, Y.M.; Petyuk, V.A.; Smith, R.D. Distinguishing d-and l-aspartic and isoaspartic acids in amyloid β peptides with ultrahigh resolution ion mobility spectrometry. Chem. Commun. 2017, 53, 7913–7916. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Siems, W.F.; Klasmeier, J.; Hill, H.H. Separation of isomeric peptides using electrospray ionization/high-resolution ion mobility spectrometry. Anal. Chem. 2000, 72, 391–395. [Google Scholar] [CrossRef]
- Li, G.; Delafield, D.G.; Li, L. Improved structural elucidation of peptide isomers and their receptors using advanced ion mobility-mass spectrometry. TrAC Trends Anal. Chem. 2020, 124, 115546. [Google Scholar] [CrossRef]
- Jia, C.; Lietz, C.B.; Yu, Q.; Li, L. Site-specific characterization of d-Amino acid containing peptide epimers by ion mobility spectrometry. Anal. Chem. 2014, 86, 2972–2981. [Google Scholar] [CrossRef]
- Koehbach, J.; Gruber, C.W.; Becker, C.; Kreil, D.P.; Jilek, A. MALDI TOF/TOF-based approach for the identification of d-Amino acids in biologically active peptides and proteins. J. Proteome Res. 2016, 15, 1487–1496. [Google Scholar] [CrossRef]
- Adams, C.M.; Zubarev, R.A. Distinguishing and quantifying peptides and proteins containing d-Amino acids by tandem mass spectrometry. Anal. Chem. 2005, 77, 4571–4580. [Google Scholar] [CrossRef]
- Tao, Y.; Quebbemann, N.R.; Julian, R.R. Discriminating d-Amino acid-containing peptide epimers by radical-directed dissociation mass spectrometry. Anal. Chem. 2012, 84, 6814–6820. [Google Scholar] [CrossRef]
- Lyon, Y.A.; Beran, G.; Julian, R.R. Leveraging electron transfer dissociation for site selective radical generation: Applications for peptide epimer analysis. J. Am. Soc. Mass Spectrom. 2017, 28, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-T.; Julian, R.R. Two-dimensional identification and localization of isomers in crystallin peptides using TWIM-MS. Analyst 2020, 145, 5232–5241. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-T.; Riggs, D.L.; Lyon, Y.A.; Julian, R.R. Statistical Framework for Identifying Differences in Similar Mass Spectra: Expanding Possibilities for Isomer Identification. Anal. Chem. 2023, 95, 6996–7005. [Google Scholar] [CrossRef] [PubMed]
- Muttenthaler, M.; King, G.F.; Adams, D.J.; Alewood, P.F. Trends in peptide drug discovery. Nat. Rev. Drug Discov. 2021, 20, 309–325. [Google Scholar] [CrossRef]
- Adusumilli, R.; Mallick, P. Data conversion with ProteoWizard msConvert. Proteom. Methods Protoc. 2017, 1550, 339–368. [Google Scholar]
- Malik, A.; Angel, L.A.; Spezia, R.; Hase, W.L. Collisional dynamics simulations revealing fragmentation properties of Zn (II)-bound poly-peptide. Phys. Chem. Chem. Phys. 2020, 22, 14551–14559. [Google Scholar] [CrossRef] [PubMed]
- Homayoon, Z.; Macaluso, V.; Martin-Somer, A.; Muniz, M.C.N.B.; Borges, I.; Hase, W.L.; Spezia, R. Chemical dynamics simulations of CID of peptide ions: Comparisons between TIK (H+) 2 and TLK (H+) 2 fragmentation dynamics, and with thermal simulations. Phys. Chem. Chem. Phys. 2018, 20, 3614–3629. [Google Scholar] [CrossRef]
- Pérez-Mellor, A.; Le Barbu-Debus, K.; Lepere, V.; Alata, I.; Spezia, R.; Zehnacker, A. Structure and collision-induced dissociation of the protonated cyclo His-Phe dipeptide: Mechanistic studies and stereochemical effects. Eur. Phys. J. D 2021, 75, 165. [Google Scholar] [CrossRef]
- Pérez-Mellor, A.; Alata, I.; Lepere, V.; Spezia, R.; Zehnacker-Rentien, A. Stereospecific collision-induced dissociation and vibrational spectroscopy of protonated cyclo (Tyr-Pro). Int. J. Mass Spectrom. 2021, 465, 116590. [Google Scholar] [CrossRef]
- Pérez-Mellor, A.F.; Spezia, R.; Zehnacker, A. How Symmetry Influences the Dissociation of Protonated Cyclic Peptides. Symmetry 2022, 14, 679. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Maupetit, J.; Derreumaux, P.; Tufféry, P. Improved PEP-FOLD approach for peptide and miniprotein structure prediction. J. Chem. Theory Comput. 2014, 10, 4745–4758. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-C.; Wu, H.-Y.; Lin, L.-C.; Chang, C.-W.; Liao, P.-C. Characterizing the D-Amino Acid Position in Peptide Epimers by Using Higher-Energy Collisional Dissociation Tandem Mass Spectrometry: A Case Study of Liraglutide. Int. J. Mol. Sci. 2024, 25, 1379. https://doi.org/10.3390/ijms25031379
Chen Y-C, Wu H-Y, Lin L-C, Chang C-W, Liao P-C. Characterizing the D-Amino Acid Position in Peptide Epimers by Using Higher-Energy Collisional Dissociation Tandem Mass Spectrometry: A Case Study of Liraglutide. International Journal of Molecular Sciences. 2024; 25(3):1379. https://doi.org/10.3390/ijms25031379
Chicago/Turabian StyleChen, Yuan-Chih, Hsin-Yi Wu, Lung-Cheng Lin, Chih-Wei Chang, and Pao-Chi Liao. 2024. "Characterizing the D-Amino Acid Position in Peptide Epimers by Using Higher-Energy Collisional Dissociation Tandem Mass Spectrometry: A Case Study of Liraglutide" International Journal of Molecular Sciences 25, no. 3: 1379. https://doi.org/10.3390/ijms25031379
APA StyleChen, Y.-C., Wu, H.-Y., Lin, L.-C., Chang, C.-W., & Liao, P.-C. (2024). Characterizing the D-Amino Acid Position in Peptide Epimers by Using Higher-Energy Collisional Dissociation Tandem Mass Spectrometry: A Case Study of Liraglutide. International Journal of Molecular Sciences, 25(3), 1379. https://doi.org/10.3390/ijms25031379





