Abstract
Peripheral inflammation and gait speed alterations are common in several neurological disorders and in the aging process, but the association between the two is not well established. The aim of this systematic literary review is to determine whether proinflammatory markers are a positive predictor for gait impairments and their complications, such as falls in older adults, and may represent a risk factor for slow gait speed and its complications. The systematic review was performed in line with the Preferred Report Items for Systematic Review and Meta-Analyses (PRISMA). A protocol for literature searches was structured a priori and designed according to the International Perspective Register of Systemic Review (PROSPERO: CRD42023451108). Peer-reviewed original articles were identified by searching seven electronic databases: Excerpta Medica Database (EMBASE), SciVerse (ScienceDirect), Scopus, PubMed, Medline, Web of Science, and the Cochrane Library. The search strategy was formulated based on a combination of controlled descriptors and/or keywords related to the topic and a manual search was conducted of the reference lists from the initially selected studies to identify other eligible studies. The studies were thoroughly screened using the following inclusion criteria: older adults, spatiotemporal gait characteristics, and proinflammatory markers. A meta-analysis was not performed due to the heterogeneity of the studies, and the results were narratively synthesized. Due to the clinical and methodological heterogeneity, the studies were combined in a narrative synthesis, grouped by the type of biomarkers evaluated. A standardized data extraction form was used to collect the following methodological outcome variables from each of the included studies: author, year, population, age, sample size, spatiotemporal gait parameters such as gait velocity, and proinflammatory markers such as TNF-α, high sensitivity C-reactive (CRP) proteins, and IL-6. We included 21 out of 51 studies in our review, which examined the association between inflammatory biomarkers and gait impairment. This review highlights the role of TNF-α, CRP, and IL-6 in gait impairment. Biomarkers play an important role in the decision-making process, and IL-6 can be an effective biomarker in establishing the diagnosis of slow gait speed. Further longitudinal research is needed to establish the use of molecular biomarkers in monitoring gait impairment.
1. Introduction
Gait velocity is a simple screen of functional status in older adults, and predicts major adverse outcomes in older individuals such as falls, dementia, and death [1,2,3,4,5,6]. A gait speed lower than 0.8 m/s is a reliable cut-off for identifying subjects at increased risk of disability, and evidence on the relationship between circulating IL-6 levels and gait speed suggests that higher IL-6 levels may be associated with poorer performance, hospitalization, institutionalization, and death in older adults [7,8,9,10,11]. Falls are common in older adults, but little is known about accidental falls and their relationship to movement disorders and chronic inflammation. It has been estimated that adults older than 60 years of age suffer the most fatal falls, therefore as the prevalence of older fallers is predicted to increase with changes in demography, prevention strategies should prioritize fall-related research and establish effective strategies to reduce risk [12]. According to the World Health Organization, approximately 684,000 fatal falls occur each year, making it the second leading cause of unintentional injury death after road traffic injuries [13]. The increasing number of people who suffer falls is also an economic problem for health services worldwide, with an estimated cost for the EU of 25 billion Euros for treating fall-related injuries [14]. In addition, people who have experienced a fall develop fear of falling, which is a serious issue that negatively impacts their physical and mental health [15]. Movement disorders and chronic inflammation are frequently comorbid and underdiagnosed [7]. Systemic inflammation is closely associated with central neuroinflammation [8]. The accessibility and practicality of using blood samples have led to numerous studies measuring the profile of serum or plasma immune markers in several neurological disorders, and many of these studies have found a significant association between those markers and disease severity. Other spatiotemporal parameters, such as a stride length of 0.64 m, accurately predict major adverse events such as physical disability, falls, institutionalization, and mortality [16]. In addition, C-reactive protein (CRP) has been significantly and negatively associated with the total number of daily strides [17].
Previous studies of older adults have linked gait and mobility problems to high CRP levels, and chronic inflammation may be a factor affecting denervation of muscles and changes in the neuromuscular junction [18,19]. Inflammation also plays a role in the initiation and progression of knee osteoarthritis, and studies have shown that osteoarthritis is associated with high serum levels of inflammatory markers that alter gait mechanism [20,21,22,23,24,25].
Recent developments in drug therapies with biological agents such as anti-tumor necrosis factor alpha (TNF-α), have provided great benefits in terms of reductions in joint inflammation, pain, and improved gait function [26]. Levels of pro-inflammatory cytokines, and in particular, elevated IL-6 and CRP have been identified as independent predictors of impaired mobility, disability, and slow walking speed in older adults [7,19,27,28,29,30,31,32,33,34,35].
There is growing evidence of associations between elevated levels of inflammatory cytokines such as IL-6, TNF-α, or the acute phase CRP and several chronic health conditions or adverse aging outcomes including muscle loss and cognitive impairment (Figure 1) [34,35].
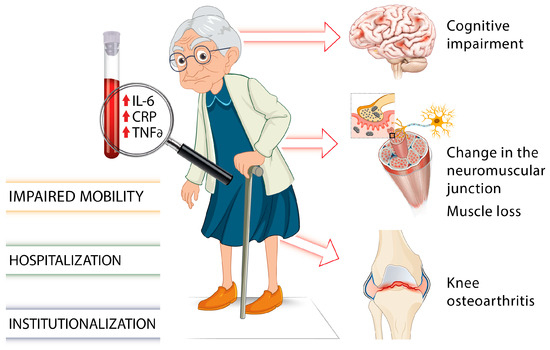
Figure 1.
Involvement of proinflammatory markers on gait impairments and frailty in older individuals [7,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35].
However, there are only a few studies focusing on peripheral inflammatory processes and gait impairment. The aim of this systematic review is therefore to analyze current scientific knowledge and to investigate the reason that proinflammatory markers represent a positive predictor for gait impairment and its complications. According to the results of this study, there will be further opportunities to develop insights into the pathophysiological mechanisms that make proinflammatory markers such a powerful tool for identifying high-risk people with gait impairments. The results of this study could also highlight the importance of gait impairments and enhance our knowledge of gait assessment protocols as a simple screen to predict major adverse outcomes.
2. Methods
2.1. Identifying the Research Question
This review primarily aimed to synthesize all of the published evidence on associations between gait impairment and inflammatory markers in older adults. The review question was formulated using the PCC strategy (population, concept, and context): Population: older adults; Concept: Peripheral inflammation and gait impairment; Context: Emerging proinflammatory markers as a powerful tool for identifying high-risk people with gait impairments. The review questions were therefore:
- Are proinflammatory markers a positive predictor and risk factor for gait impairment?
- Which proinflammatory markers represent a risk factor for slow gait speed and its complications?
2.2. Literature Search Methodology
The following systematic review was performed in line with the Preferred Report Items for Systematic Review and Meta-Analyses (PRISMA). A protocol was structured a priori and designed according to the International Perspective Register of Systemic Review (PROSPERO: CRD42023451108). The research question was developed using the PICO framework. The study analyzed every original article published up to July 2023 that met the following inclusion criteria: (1) full text in English; (2) primary articles only; and (3) presentation of identifiable data measuring gait and inflammatory markers. Studies were searched in the following databases: Excerpta Medica Database (EMBASE), SciVerse (ScienceDirect), Scopus, PubMed, Medline, Web of Science, and the Cochrane Library. The search strategy was formulated based on a combination of controlled descriptors and/or keywords related to the topic and, in addition, a manual search was conducted of the reference lists from the initially selected studies to identify other eligible studies.
An electronic search in PubMed was performed on 25 July 2023 using the following search terms: (interleukin 6[MeSH Terms]) OR (IL-6[MeSH Terms]) AND (gait[MeSH Terms]); (CRP[MeSH Terms]) OR (C reactive protein[MeSH Terms]) OR (protein, C reactive[MeSH Terms]) AND (gait[MeSH Terms]); (tumor necrosis factor[MeSH Terms]) OR (TNFalpha[MeSH Terms]) AND (gait[MeSH Terms]).
When determining which articles to include, we analyzed their title and abstract, then retreived the full text for articles that met the inclusion criteria. Duplicate studies, editorials, conference abstracts, and non-English references were removed using the Rayyan software (https://www.rayyan.ai/) package, and other studies without duplicates were selected based on eligibility criteria by two (L.B. and O.C.-L.) independent and blinded reviewers by reading the abstracts and followed by reading the full text. Another two expert reviewers assessed the internal quality and resolved disagreements in this study selection process (O.C. and F.T.). Finally, the reference lists of all the relevant articles were manually cross-referenced in order to identify any additional articles. These guidelines ensure an adequate evaluation of the research from a methodological point of view in order to exclude possible replication of the methods or results.
Finally, the reference lists of all the relevant articles were manually cross-referenced in order to identify any additional articles. In this study, the following data were extracted: (1) characteristics of the studies (name of the study, authors, year of publication); (2) demographic information of the samples (sample size, participant characteristics of mean age, gender distribution); (3) gait assessment; (4) serum inflammatory parameters (expression level, standard deviation or closest equivalent, correspondent technologies). We excluded conference proceedings, articles reporting results from less than ten elderly patients, that did not assess gait or that assessed gait over a walking distance shorter than five meters
3. Results
A number of articles (51) were initially identified while searching the scientific literature regarding this topic, and 21 articles were selected and included in this review. Figure 2 presents a flowchart of the review process (PRISMA diagram).
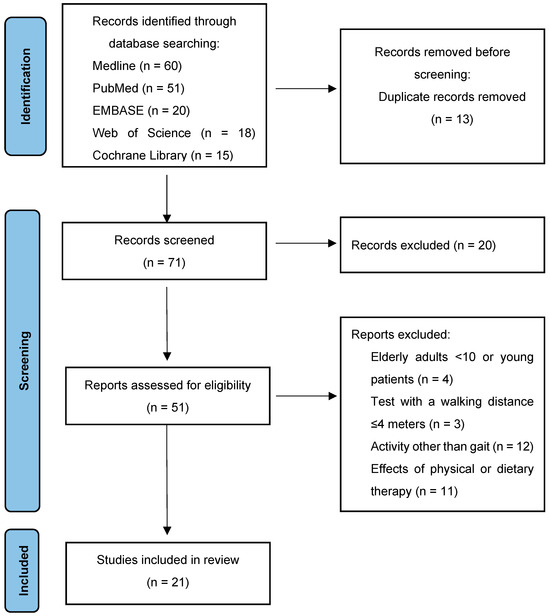
Figure 2.
PRISMA diagram: the figure represents the study selection flow through identification, screening, eligibility and inclusion.
A qualitative synthesis of the data from the selected studies is provided, describing the inflammatory biomarkers, gait dysfunction, and gait assessment protocol used in each study. Our search selected 21 articles. They are summarized in Table 1. We reported the main findings concerning the relationship between IL-6, CRP, TNFα, and gait parameters. We listed the instruments used and evaluation protocol for gait assessment.

Table 1.
Characteristics of inflammatory biomarkers and gait parameters: an overview of the selected studies.
3.1. CRP
CRP as an inflammatory marker predictive of decreased gait was the most frequently used study parameter, and was included in 13 of the 21 articles analyzed. High sensitivity CRP (hs-CRP) was used for the relationship with gait parameters in five of them [43,44,45,48,50].
In one study [41], several groups were categorized according to their initial CRP, taking 1.33 mg/dL as a low value, and 2.7 mg/dL as a high value. Values higher than 2.7 mg/dL were associated with a greater probability of subsequent disability. They were also associated with the following: activities of daily living (ADL), instrumental activities of daily living (IADL), impaired balance, impaired walking speed, and arthritis.
Elevated CRP levels were related to low vitamin D levels [42], with a higher probability of slow walking speed, which was attenuated when vitamin D deficiency was absent, although the relevant factor inducing slow gait was CRP. Another study [36] investigated major mobility impairment (MMD) and reported consistent relationships with elevated CRP (>3.0 mg/dL) and a higher risk of MMD, which increased the risk by 38% with a sensitivity of 63% and specificity of 54% as a predictive value over 5 years.
Verghese et al. [43] assessed elevated CRP in a large sample size, with a median of 5.5 mg/dL and minimum levels of 1.1 mg/dL, and found that subjects with elevated values had a reduction in gait speed of −2.46/cm/s/year.
Another study [44] established a relationship between elevated hs-CRP values and the probability of disability in IADL, social and leisure activities (LSA), lower extremity mobility (LEM), and general physical activities (GPA), with knee extensor power, gait speed, or both, explaining the association in continuous variable models with a decrease in these with increased hs-CRP.
Ravaglia et al. [34] used an hs-CRP (ELISA kit with high sensitivity), fibrinogen and leukocyte count as inflammatory markers, and only found an inverse relationship for gait and balance with hs-CRP, and an inverse relationship with CRP and with severe limitation of basic activities of daily living (ABVD) and IADL disability. An increase in hs-CRP levels was negatively associated with gait speed, but this was only significant in older adults, and not in middle-aged men (70).
The relationship between CRP and fibrinogen [49] with gait speed was studied with 373 people over 20 years, associating the two parameters with gait speed as a single task after adjusting for cardiovascular and risk parameters. However, when executive functions were taken into account, the association with CRP disappeared and the association with fibrinogen was attenuated. Dupont et al. [48] used the combination of two parameters, white blood cell count and high-sensitivity CRP, as factors related to the onset of sarcopenia, and observed a negative association with baseline physical activity, quality of life scores, and the SF-36 physical component score in middle-aged and older men. Baseline leukocyte levels were negatively associated with gait velocity, isometric 90° quadriceps and isokinetic peak torque. In another study with 421 participants [46], where CRP was related to motor cognitive risk syndrome (MCR), characterized by cognitive complaints and slow gait, patients with higher CRP levels (2.8 mg/dL) had a higher probability of MCR if memory impairment was present.
3.2. Relationship of CRP with Other Biomarkers
Langmann et al. [50] related several inflammatory markers, i.e., hs-CRP, TNFα, and its two receptors (TNFα-R1 and TNFα-R2), interleukin-6 (IL-6), soluble IL-6 receptor (sIL-6R), and interleukin-10 (IL-10) with measures of frailty, function, mobility, and falls in elderly women, and observed that frail patients had significantly higher levels of hs-CRP, TNFα-R1, TNFα-R2, IL-6, and IL-6-sR. Higher baseline levels of hs-CRP and IL-6 were associated with worse physical performance and gait speed at 12 months.
A study of patients with Parkinson’s disease determined hs-CRP levels in patients with freezing of gait (FOG). Patients with FOG showed higher levels of hs-CRP than those without FOG, establishing a FOG-No FOG cut-off level of 0.935 mg/dL, with a sensitivity of 87.1% and specificity of 89.2%.
Penninx [30] evaluated IL-6 together with CRP and the following soluble cytokine receptors (sR), IL-2sR, TNFsR1, TNFsR2, and IL-6sR. People with incident mobility had an IL-6 value of 2.18 mg/L compared to 1.70 mg/L for those without limitations. Soluble cytokine values IL-2sR, TNFsR1, TNFsR2 were also higher. Higher levels of CRP, IL-6, and TNFα were all significantly associated with an increased risk of incident mobility limitation, but appeared to be stronger for IL-6 and CRP than for TNFα. None of the interaction terms for sex or race were statistically significant.
Dupont et al. [51] also analyzed IL-6, IL-8, IL-1β, CRP, and TNFα in sarcopenic subjects. Proinflammatory IL-1β correlated positively with manual grip strength and IL-6 with myofascial autoliberation (aLM). IL-6 correlated inversely with step count. IL-8 was inversely correlated with manual grip strength in women but not in men. In contrast, the proinflammatory cytokines CRP, IL-6, and TNFα correlated inversely with the physical component score of the SF-36 in men, but not in women.
3.3. IL-6 and TNF-Alpha
Brown [47] first reported that individuals who had a high IL-6 concentration in blood (3.2 pg/mL) together with previous slow gait and depression, were associated with slow gait. Older individuals in the highest blood IL-6 quartile (more than 4.60 pg/mL) had a 1.75 cm/s/year faster decline in gait speed compared to the participants with IL-6 levels in the lowest quartile [53]. Custodero [37] evaluated the effects of physical performance intervention on 400-m gait speed at 12-month follow-up according to annual IL-6 change categorized by a 1 pg/mL increase or decrease, and observed that subjects with an annual IL-6 change of between −1 and +2 pg/mL had a significant difference in gait speed in the PA intervention group compared with the healthy educational intervention group. In a study of 333 participants with a mean follow-up of 2.3 years, Verghese et al. [7] found that each one-unit increase in log IL-6 levels was associated with a 0.98/cm/s/year increase in the rate of gait velocity decline. Participants in the highest quartile of IL-6 (4.60 pg/mL) had significantly slower gait velocity compared to the other participants. In another study [39], IL-6 levels were positively correlated with knee extensor and knee flexor power. The final model showed that the factors of plasma IL-6 concentrations and physical activity level explained 4.1% of the mean knee flexor power variability, and plasma IL-6 concentration was the variable that best explained the isokinetic variable. Interestingly, participants in the highest tertile of IL-6 (IL-6 > 2.51 pg/mL) were 1.76 times more likely to develop at least mobility-disability and 1.62 times more likely to develop mobility plus ADL-disability, compared with the lowest percentage of IL-6 (1.75 pg/mL) [31].
Nadkarni [38] analyzed IL-6 along with gait speed and white matter (WM) hyperintensities for 10 years in 179 adults. Higher sustained 10-year exposure to IL-6 was significantly associated with slower gait speed. Although attenuated by approximately 47%, this association remained statistically significant after accounting for covariates. A study with 99 participants [52] demonstrated an inverse relationship between circulating IL-6 and thigh muscle composition, strength, and specific weight. Reductions in IL-6 were associated with improvements in gait speed in individuals with higher IL-6 levels (>1.36 pg/mL).
3.4. Relationship between IL-6 and TNF-Alpha and Other Biomarkers
The association between IL-6 and major mobility disability (MMD) was investigated [36] and revealed that elevated levels above 2.5 pg/mL were associated with an increased risk of MMD compared to low values ≤2.5 pg/mL. In combination with elevated CRP (>3.0 mg/dLL) alone, patients with both elevated CRP and IL-6 had a 37% increased risk of MMD with a sensitivity of 75% and specificity of 34%.
Kositsawat [40], relating levels of several inflammatory parameters in addition to vitamin D (25 hydroxyvitamin D, 25(OH)D) and insulin-like growthfctor (IGF-1), found that participants with slow walking were more likely to have low 25(OH)D (<20 ng/mL), low IGF-1 (<112 ng/mL), high CRP (≥3 mg/L) and IL- 6 (≥2.87 pg/mL) than participants without a slow walking speed.
4. Discussion
Inflammation has adverse affective, cognitive, motor, and neurostructural consequences for older adults [47,54]. In older people, levels of IL-6 higher than 2.5 pg/mL predict a future risk of gait speed decline and a higher risk of functional decline over the subsequent 4 years [7,38]. Elevated sensitive CRP concentrations (≥3 mg/L) at baseline were associated with a faster annual decline in gait velocity of 0.91 cm/s [41] and an increased likelihood of motoric cognitive risk syndrome [46]. In terms of cognitive-motor syndrome, physical function and cognitive function share common neurological processes, and a cognitive decline in tandem with a loss of muscle strength places elderly people at increased risk of personal injury, poor mobility, fall-related injuries leading to frailty, reduced independence, and poorer quality of life [53,55,56,57,58]. In fact, older adults show a high prevalence of gait disorders, and higher IL-6 is linked to a larger volume of white matter hyperintensities (in MRI) that are correlated to slow gait [38,59,60,61,62,63]. Inflammation is common both in aging and frailty, while chronic inflammation is associated with decreased muscle mass and strength, disability, dementia, increased morbidity, and mortality. Studies on the impact of exercise on proinflammatory and anti-inflammatory cytokines have shown that physical exercise in pre-frail older adults in primary care improved depression, gait speed, muscle mass indices, physical function, frailty, and had significant improvement of TNFα levels at 3 months [64,65]. An increase in hs-CRP levels was negatively associated with gait speed only in older adults, but not in middle-aged men [48]. It seems reasonable that over time, chronic inflammation might be a factor affecting denervation of muscles and changes in the neuromuscular junction [18,19,66]. Inflammation also plays a role in the initiation and progression of knee osteoarthritis, and several studies have shown that osteoarthritis is associated with high serum levels of inflammatory markers that alter gait mechanisms [20,21,22,23,24,25].
Biomechanical investigation permits the identification of gait abnormalities that may also adversely affect the quality of life. One of the most important spatiotemporal parameters that seems to significantly decline in patients with chronic inflammation is gait speed. Gait speed is emerging as one of the major determinants of health, but in this review, we did not find one particular gait assessment protocol or tool that is used by most of the studies, but instead a wide variety of combinations are described. Physical performance and gait speed was assessed from 4 m to 400 m on an instrumented walkway [29,36,38,52] making comparison of the results difficult. We excluded gait tests with a walking distance of less than 4 m in order to only include tests affected by the start and the stop phases of gait to a lesser extent.
The large number of patients analyzed in the studies included (an average of 1300 patients, with a minimum of 40 and a maximum of 5642) suggests that the peripheral inflammatory biomarkers IL-6 and CRP are related to gait impairments. A consensus among the clinical research community in this regard has yet to be achieved in order to standardize the evidence according to which higher levels of CRP, IL-6, and TNFα are all significantly associated with a faster decline in gait speed, worse physical performance, major mobility impairment, lower extremity mobility, impairments to activities of daily living, and general physical activities.
Gait speed is the most frequently recorded parameter in the literature, and 0.8 m/s is a reliable cut-off for identifying subjects at increased risk of disability but further studies should be conducted to select the most sensitive protocol for assessing and representing this biomarker of frailty in people with inflammation. Many circulating biomarkers change with age independently of disease [67,68]. Characterizing age-adjusted distributions of these biomarkers in healthy older adults would therefore be important to increase specificity of diagnosis, inform treatment and prevention, and limit unnecessary procedures and treatments. Despite the fact that people over the age of 65 are the fastest growing age segment of our population, characterization of many circulating biomarkers for the different age groups and gender remain incomplete, and changes at extreme old ages are predicted using mathematical models [69]. In this review, we only identified analysis of age groups in the study of Dupont and co-workers (2021) who performed a stratified cross-sectional analysis according to two age groups, 40–59 years (middle-aged adults) and 60–79 years (older adults), demonstrating that hs-CRP concentration in blood was significantly inversely associated with SF-36 physical component scores in both middle-aged and older adults. This aspect needs to be clearly investigated in future studies in this field.
A limitation of any systematic review is the potential omission of relevant articles. Although we tried to use exhaustive inclusion criteria, it is possible that we did not identify all publications on the subject. Our search strategy was based on MeSH and key words assigned by the authors, and we may have missed publications that were not indexed under those terms, although we tried to identify further articles through reference lists. However, the search strategy used in this work had the advantage of using five large databases, enabling an exhaustive literature review in commonly used biomedical databases for literature search retrieval.
5. Conclusions
Substantial evidence suggests that the peripheral inflammatory biomarkers IL-6 and CRP are related to frailty status and gait impairment. Cytokines are key chemical mediators of the immune response produced by cells, and an understanding of the molecular, biomechanical, and neuropsychological networks involved is required in order to enhance prevention strategies of gait impairments and its complications, such as falls among the aging population. Our findings regarding the causal relationship between chronic inflammation and gait impairment are not conclusive due to heterogeneous approaches to gait analysis in terms of the instruments used and measurement duration/length. Walking speed is a commonly used outcome in different types of studies, but the methodologies and descriptions of walking tests vary widely from study to study. We recommend standardizing the protocol for assessing walking speed in older adults, such as using a 6 or 10-Meter Walk Test or a 6-min walk test in order to understand what could also benefit from standardization [70,71,72,73].
Author Contributions
L.B., O.C.L., F.T. and O.C. contributed to the data collection, validation, writing, and original draft preparation. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting reported results can be found writing at lorenzo.brognara2@unibo.it.
Acknowledgments
The authors would like to thank MariaPia Cumani (University of Bologna) for Figure 1 editing.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Montero-Odasso, M.; Schapira, M.; Soriano, E.R.; Varela, M.; Kaplan, R.; Camera, L.A.; Mayorga, L.M. Gait Velocity as a Single Predictor of Adverse Events in Healthy Seniors Aged 75 Years and Older. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Abellan Van Kan, G.; Rolland, Y.; Andrieu, S.; Bauer, J.; Beauchet, O.; Bonnefoy, M.; Cesari, M.; Donini, L.; Gillette-Guyonnet, S.; Inzitari, M.; et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J. Nutr. Health Aging 2009, 13, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Verghese, J.; Holtzer, R.; Lipton, R.B.; Wang, C. Quantitative Gait Markers and Incident Fall Risk in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64A, 896–901. [Google Scholar] [CrossRef]
- Verghese, J.; LeValley, A.; Hall, C.B.; Katz, M.J.; Ambrose, A.F.; Lipton, R.B. Epidemiology of Gait Disorders in Community-Residing Older Adults. J. Am. Geriatr. Soc. 2006, 54, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ruiz, L.; Jimenez, A.R.; Garcia-Villamil, G.; Seco, F. Detecting Fall Risk and Frailty in Elders with Inertial Motion Sensors: A Survey of Significant Gait Parameters. Sensors 2021, 21, 6918. [Google Scholar] [CrossRef]
- Studenski, S. Gait Speed and Survival in Older Adults. JAMA 2011, 305, 50. [Google Scholar] [CrossRef]
- Verghese, J.; Holtzer, R.; Oh-Park, M.; Derby, C.A.; Lipton, R.B.; Wang, C. Inflammatory Markers and Gait Speed Decline in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66A, 1083–1089. [Google Scholar] [CrossRef]
- Hardy, S.E.; Perera, S.; Roumani, Y.F.; Chandler, J.M.; Studenski, S.A. Improvement in Usual Gait Speed Predicts Better Survival in Older Adults. J. Am. Geriatr. Soc. 2007, 55, 1727–1734. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Pamoukdjian, F.; Lévy, V.; Sebbane, G.; Boubaya, M.; Landre, T.; Bloch-Queyrat, C.; Paillaud, E.; Zelek, L. Slow gait speed is an independent predictor of early death in older cancer outpatients: Results from a prospective cohort study. J. Nutr. Health Aging 2017, 21, 202–206. [Google Scholar] [CrossRef]
- Zhu, S.; Patel, K.V.; Bandinelli, S.; Ferrucci, L.; Guralnik, J.M. Predictors of Interleukin-6 Elevation in Older Adults. J. Am. Geriatr. Soc. 2009, 57, 1672–1677. [Google Scholar] [CrossRef]
- Hacıdursunoğlu Erbaş, D.; Çınar, F.; Eti Aslan, F. Elderly patients and falls: A systematic review and meta-analysis. Aging Clin. Exp. Res. 2021, 33, 2953–2966. [Google Scholar] [CrossRef]
- World Health Organization. Falls. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/falls (accessed on 20 July 2023).
- Almada, M.; Brochado, P.; Portela, D.; Midão, L.; Costa, E. Prevalence of falls and associated factors among community-dwelling older adults: A cross-sectional study. J. Frailty Aging 2021, 10, 10–16. [Google Scholar] [CrossRef]
- Gambaro, E.; Gramaglia, C.; Azzolina, D.; Campani, D.; Molin, A.D.; Zeppegno, P. The complex associations between late life depression, fear of falling and risk of falls. A systematic review and meta-analysis. Ageing Res. Rev. 2022, 73, 101532. [Google Scholar] [CrossRef] [PubMed]
- Bytyçi, I.; Henein, M.Y. Stride Length Predicts Adverse Clinical Events in Older Adults: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 2670. [Google Scholar] [CrossRef]
- Gardner, A.W.; Parker, D.E.; Montgomery, P.S.; Blevins, S.M.; Teague, A.M.; Casanegra, A.I. Monitored Daily Ambulatory Activity, Inflammation, and Oxidative Stress in Patients with Claudication. Angiology 2014, 65, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Freire, M.; de Cabo, R.; Studenski, S.A.; Ferrucci, L. The Neuromuscular Junction: Aging at the Crossroad between Nerves and Muscle. Front. Aging Neurosci. 2014, 6, 208. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.C.P.A.; Zunzunegui, M.-V.; Li, A.; Phillips, S.P.; Guralnik, J.M.; Guerra, R.O. Association between C-reactive protein and physical performance in older populations: Results from the International Mobility in Aging Study (IMIAS). Age Ageing 2016, 45, 274–280. [Google Scholar] [CrossRef]
- Edd, S.N.; Favre, J.; Blazek, K.; Omoumi, P.; Asay, J.L.; Andriacchi, T.P. Altered gait mechanics and elevated serum pro-inflammatory cytokines in asymptomatic patients with MRI evidence of knee cartilage loss. Osteoarthr. Cartil. 2017, 25, 899–906. [Google Scholar] [CrossRef]
- Mabey, T. Cytokines as biochemical markers for knee osteoarthritis. World J. Orthop. 2015, 6, 95. [Google Scholar] [CrossRef]
- Shultz, S.P.; Buck, A.N.; Fink, P.W.; Kung, S.M.; Ward, M.J.; Antal, Z.; Backus, S.I.; Kraszewski, A.P.; Hillstrom, H.J. Body mass affects kinetic symmetry and inflammatory markers in adolescent knees during gait. Clin. Biomech. 2023, 102, 105887. [Google Scholar] [CrossRef]
- Addison, O.; Drummond, M.J.; Lastayo, P.C.; Dibble, L.E.; Wende, A.R.; McClain, D.A.; Marcus, R.L. Intramuscular fat and inflammation differ in older adults: The impact of frailty and inactivity. J. Nutr. Health Aging 2014, 18, 532–538. [Google Scholar] [CrossRef]
- Bautmans, I.; Njemini, R.; Predom, H.; Lemper, J.-C.; Mets, T. Muscle Endurance in Elderly Nursing Home Residents Is Related to Fatigue Perception, Mobility, and Circulating Tumor Necrosis Factor-Alpha, Interleukin-6, and Heat Shock Protein 70. J. Am. Geriatr. Soc. 2008, 56, 389–396. [Google Scholar] [CrossRef]
- Broström, E.; Esbjörnsson, A.-C.; von Heideken, J.; Larsson, P.; Wretenberg, P.; Iversen, M. Change in Gait Deviation Index after anti-tumour necrosis factor-α treatment in individuals with rheumatoid arthritis: A pilot study. Scand. J. Rheumatol. 2013, 42, 356–361. [Google Scholar] [CrossRef]
- Oda, R.; Fujiwara, H.; Tokunaga, D.; Nakamura, S.; Taniguchi, D.; Kawahito, Y.; Seno, T.; Matsui, T.; Kubo, T. How do anti-TNF therapies affect gait function in patients with rheumatoid arthritis? Int. J. Rheum. Dis. 2014, 17, 57–62. [Google Scholar] [CrossRef]
- McDermott, M.M.; Liu, K.; Ferrucci, L.; Tian, L.; Guralnik, J.M.; Green, D.; Tan, J.; Liao, Y.; Pearce, W.H.; Schneider, J.R.; et al. Circulating Blood Markers and Functional Impairment in Peripheral Arterial Disease. J. Am. Geriatr. Soc. 2008, 56, 1504–1510. [Google Scholar] [CrossRef]
- Brinkley, T.E.; Leng, X.; Miller, M.E.; Kitzman, D.W.; Pahor, M.; Berry, M.J.; Marsh, A.P.; Kritchevsky, S.B.; Nicklas, B.J. Chronic Inflammation Is Associated with Low Physical Function in Older Adults Across Multiple Comorbidities. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64A, 455–461. [Google Scholar] [CrossRef]
- Hsu, F.-C.; Kritchevsky, S.B.; Liu, Y.; Kanaya, A.; Newman, A.B.; Perry, S.E.; Visser, M.; Pahor, M.; Harris, T.B.; Nicklas, B.J.; et al. Association Between Inflammatory Components and Physical Function in the Health, Aging, and Body Composition Study: A Principal Component Analysis Approach. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64A, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Penninx, B.W.J.H.; Kritchevsky, S.B.; Newman, A.B.; Nicklas, B.J.; Simonsick, E.M.; Rubin, S.; Nevitt, M.; Visser, M.; Harris, T.; Pahor, M. Inflammatory Markers and Incident Mobility Limitation in the Elderly. J. Am. Geriatr. Soc. 2004, 52, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Harris, T.B.; Guralnik, J.M.; Tracy, R.P.; Corti, M.-C.; Cohen, H.J.; Penninx, B.; Pahor, M.; Wallace, R.; Havlik, R.J. Serum IL-6 Level and the Development of Disability in Older Persons. J. Am. Geriatr. Soc. 1999, 47, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Cesari, M.; Anton, S.; Marzetti, E.; Giovannini, S.; Seo, A.Y.; Carter, C.; Yu, B.P.; Leeuwenburgh, C. Molecular inflammation: Underpinnings of aging and age-related diseases. Ageing Res. Rev. 2009, 8, 18–30. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, S4–S9. [Google Scholar] [CrossRef]
- Ravaglia, G.; Forti, P.; Maioli, F.; Brunetti, N.; Martelli, M.; Talerico, T.; Bastagli, L.; Muscari, A.; Mariani, E. Peripheral blood markers of inflammation and functional impairment in elderly community-dwellers. Exp. Gerontol. 2004, 39, 1415–1422. [Google Scholar] [CrossRef]
- Michaud, M.; Balardy, L.; Moulis, G.; Gaudin, C.; Peyrot, C.; Vellas, B.; Cesari, M.; Nourhashemi, F. Proinflammatory Cytokines, Aging, and Age-Related Diseases. J. Am. Med. Dir. Assoc. 2013, 14, 877–882. [Google Scholar] [CrossRef]
- Beavers, D.P.; Kritchevsky, S.B.; Gill, T.M.; Ambrosius, W.T.; Anton, S.D.; Fielding, R.A.; King, A.C.; Rejeski, W.J.; Lovato, L.; McDermott, M.M.; et al. Elevated IL-6 and CRP Levels Are Associated with Incident Self-Reported Major Mobility Disability: A Pooled Analysis of Older Adults with Slow Gait Speed. J. Gerontol. Ser. A 2021, 76, 2293–2299. [Google Scholar] [CrossRef]
- Custodero, C.; Pahor, M.; Mazzoccoli, C.; Manini, T.M.; Anton, S.D.; Mazzocca, A.; Lozupone, M.; Panza, F.; Sabbà, C.; Solfrizzi, V. Effect of change of interleukin-6 over time on gait speed response: Results from the lifestyle interventions and independence for elders study. Mech. Ageing Dev. 2023, 210, 111763. [Google Scholar] [CrossRef]
- Nadkarni, N.K.; Boudreau, R.M.; Studenski, S.A.; Lopez, O.L.; Liu, G.; Kritchevsky, S.; Yaffe, K.; Newman, A.B.; Rosano, C. Slow gait, white matter characteristics, and prior 10-year interleukin-6 levels in older adults. Neurology 2016, 87, 1993–1999. [Google Scholar] [CrossRef]
- Felicio, D.C.; Pereira, D.S.; Assumpção, A.M.; de Jesus-Moraleida, F.R.; de Queiroz, B.Z.; da Silva, J.P.; Rosa, N.M.d.B.; Dias, J.M.D.; Pereira, L.S.M. Inflammatory mediators, muscle and functional performance of community-dwelling elderly women. Arch. Gerontol. Geriatr. 2014, 59, 549–553. [Google Scholar] [CrossRef]
- Kositsawat, J.; Kuo, C.-L.; Barry, L.C.; Melzer, D.; Bandinelli, S.; Ferrucci, L.; Wu, R.; Kuchel, G.A. Interaction Between Vitamin D and Interleukin 6 on Slow Gait Speed: 6-Year Follow-up Data of Older Adults from InCHIANTI. J. Gerontol. Ser. A 2020, 75, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Lassale, C.; Batty, G.D.; Steptoe, A.; Cadar, D.; Akbaraly, T.N.; Kivimäki, M.; Zaninotto, P. Association of 10-Year C-Reactive Protein Trajectories with Markers of Healthy Aging: Findings from the English Longitudinal Study of Aging. J. Gerontol. Ser. A 2019, 74, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Kositsawat, J.; Barry, L.C.; Kuchel, G.A. C-Reactive Protein, Vitamin D Deficiency, and Slow Gait Speed. J. Am. Geriatr. Soc. 2013, 61, 1574–1579. [Google Scholar] [CrossRef] [PubMed]
- Verghese, J.; Holtzer, R.; Lipton, R.B.; Wang, C. High-sensitivity C-reactive protein and mobility disability in older adults. Age Ageing 2012, 41, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.-K.; Bean, J.F.; Yen, C.-J.; Leveille, S.G. Linking C-Reactive Protein to Late-Life Disability in the National Health and Nutrition Examination Survey (NHANES) 1999–2002. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yin, W.; Zhou, C.; Zhu, Y.; Gu, M.; Liu, B.; Ren, H.; Yang, X. Association between levels of high-sensitivity C-reactive protein in plasma and freezing of gait in Parkinson’s disease. Aging Clin. Exp. Res. 2022, 34, 1865–1872. [Google Scholar] [CrossRef]
- Bai, A.; Shi, H.; Huang, X.; Xu, W.; Deng, Y. Association of C-Reactive Protein and Motoric Cognitive Risk Syndrome in Community-Dwelling Older Adults: The China Health and Retirement Longitudinal Study. J. Nutr. Health Aging 2021, 25, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.J.; Roose, S.P.; Zhang, J.; Wall, M.; Rutherford, B.R.; Ayonayon, H.N.; Butters, M.A.; Harris, T.; Newman, A.B.; Satterfield, S.; et al. Inflammation, Depression, and Slow Gait: A High Mortality Phenotype in Later Life. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J.; Antonio, L.; Dedeyne, L.; O’Neill, T.W.; Vanderschueren, D.; Rastrelli, G.; Maggi, M.; Bártfai, G.; Casanueva, F.F.; Giwercman, A.; et al. Inflammatory markers are associated with quality of life, physical activity, and gait speed but not sarcopenia in aged men (40–79 years). J. Cachexia Sarcopenia Muscle 2021, 12, 1818–1831. [Google Scholar] [CrossRef] [PubMed]
- Heumann, Z.; Youssim, I.; Kizony, R.; Friedlander, Y.; Shochat, T.; Weiss, R.; Hochner, H.; Agmon, M. The Relationships of Fibrinogen and C-Reactive Protein with Gait Performance: A 20-Year Longitudinal Study. Front. Aging Neurosci. 2022, 14, 761948. [Google Scholar] [CrossRef]
- Langmann, G.A.; Perera, S.; Ferchak, M.A.; Nace, D.A.; Resnick, N.M.; Greenspan, S.L. Inflammatory Markers and Frailty in Long-Term Care Residents. J. Am. Geriatr. Soc. 2017, 65, 1777–1783. [Google Scholar] [CrossRef]
- Dupont, J.; Vercauteren, L.; Amini, N.; Lapauw, L.; De Schaepdryver, M.; Poesen, K.; Dedeyne, L.; Verschueren, S.; Tournoy, J.; Koppo, K.; et al. Are inflammatory markers associated with sarcopenia-related traits in older adults with sarcopenia?—A cross-sectional analysis of the ENHANce study. Exp. Gerontol. 2023, 178, 112196. [Google Scholar] [CrossRef]
- Grosicki, G.J.; Barrett, B.B.; Englund, D.A.; Liu, C.; Travison, T.G.; Cederholm, T.; Koochek, A.; von Berens, Å.; Gustafsson, T.; Benard, T.; et al. Circulating interleukin-6 is associated with skeletal muscle strength, quality, and functional adaptation with exercise training in mobility-limited older adults. J. Frailty Aging 2020, 9, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Won, C.W. Sarcopenia Is Associated with Cognitive Impairment Mainly Due to Slow Gait Speed: Results from the Korean Frailty and Aging Cohort Study (KFACS). Int. J. Environ. Res. Public Health 2019, 16, 1491. [Google Scholar] [CrossRef] [PubMed]
- Mezuk, B.; Lohman, M.; Dumenci, L.; Lapane, K.L. Are Depression and Frailty Overlapping Syndromes in Mid- and Late-life? A Latent Variable Analysis. Am. J. Geriatr. Psychiatry 2013, 21, 560–569. [Google Scholar] [CrossRef]
- Sipilä, S.; Tirkkonen, A.; Savikangas, T.; Hänninen, T.; Laukkanen, P.; Alen, M.; Fielding, R.A.; Kivipelto, M.; Kulmala, J.; Rantanen, T.; et al. Effects of physical and cognitive training on gait speed and cognition in older adults: A randomized controlled trial. Scand. J. Med. Sci. Sports 2021, 31, 1518–1533. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.J.H.; Caspi, A.; Ambler, A.; Broadbent, J.M.; Cohen, H.J.; d’Arbeloff, T.; Elliott, M.; Hancox, R.J.; Harrington, H.; Hogan, S.; et al. Association of Neurocognitive and Physical Function with Gait Speed in Midlife. JAMA Netw. Open 2019, 2, e1913123. [Google Scholar] [CrossRef]
- Kyrdalen, I.L.; Thingstad, P.; Sandvik, L.; Ormstad, H. Associations between gait speed and well-known fall risk factors among community-dwelling older adults. Physiother. Res. Int. 2019, 24, e1743. [Google Scholar] [CrossRef]
- Sui, S.X.; Holloway-Kew, K.L.; Hyde, N.K.; Williams, L.J.; Leach, S.; Pasco, J.A. Muscle strength and gait speed rather than lean mass are better indicators for poor cognitive function in older men. Sci. Rep. 2020, 10, 10367. [Google Scholar] [CrossRef]
- Fornage, M.; Chiang, Y.A.; O’Meara, E.S.; Psaty, B.M.; Reiner, A.P.; Siscovick, D.S.; Tracy, R.P.; Longstreth, W.T., Jr. Biomarkers of Inflammation and MRI-Defined Small Vessel Disease of the Brain. Stroke 2008, 39, 1952–1959. [Google Scholar] [CrossRef]
- Satizabal, C.L.; Zhu, Y.C.; Mazoyer, B.; Dufouil, C.; Tzourio, C. Circulating IL-6 and CRP are associated with MRI findings in the elderly: The 3C-Dijon Study. Neurology 2012, 78, 720–727. [Google Scholar] [CrossRef]
- Wersching, H.; Duning, T.; Lohmann, H.; Mohammadi, S.; Stehling, C.; Fobker, M.; Conty, M.; Minnerup, J.; Ringelstein, E.; Berger, K.; et al. Serum C-reactive protein is linked to cerebral microstructural integrity and cognitive function. Neurology 2010, 74, 1022–1029. [Google Scholar] [CrossRef]
- Benson, R.R.; Guttmann, C.R.G.; Wei, X.; Warfield, S.K.; Hall, C.; Schmidt, J.A.; Kikinis, R.; Wolfson, L.I. Older people with impaired mobility have specific loci of periventricular abnormality on MRI. Neurology 2002, 58, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Rosano, C.; Kuller, L.H.; Chung, H.; Arnold, A.M.; Longstreth, W.T.; Newman, A.B. Subclinical Brain Magnetic Resonance Imaging Abnormalities Predict Physical Functional Decline in High-Functioning Older Adults. J. Am. Geriatr. Soc. 2005, 53, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.F.; Chan, Y.H.; Seetharaman, S.; Denishkrshna, A.; Au, L.; Kwek, S.C.; Chen, M.Z.; Ng, S.E.; Hui, R.J.Y.; Merchant, R.A. Impact of Exercise and Cognitive Stimulation Therapy on Physical Function, Cognition and Muscle Mass in Pre-Frail Older Adults in the Primary Care Setting: A Cluster Randomized Controlled Trial. J. Nutr. Health Aging 2023, 27, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Renner, S.W.; Qiao, Y.; Gmelin, T.; Santanasto, A.J.; Boudreau, R.M.; Walston, J.D.; Perls, T.T.; Christensen, K.; Newman, A.B.; Glynn, N.W.; et al. Association of fatigue, inflammation, and physical activity on gait speed: The Long Life Family Study. Aging Clin. Exp. Res. 2022, 34, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Scalzo, P.; Kümmer, A.; Cardoso, F.; Teixeira, A.L. Serum levels of interleukin-6 are elevated in patients with Parkinson’s disease and correlate with physical performance. Neurosci. Lett. 2010, 468, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Kadomura, T.; Ohta, K.; Koyama, K.; Okuda, H.; Kobayashi, M.; Ishii, C.; Fujiwara, Y.; Nishioka, T.; Ohmae, Y.; et al. Analyses of laboratory data and establishment of reference values and intervals for healthy elderly people. J. Nutr. Health Aging 2012, 16, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, P.; Thyagarajan, B.; Sun, F.; Honig, L.S.; Schupf, N.; Cosentino, S.; Feitosa, M.F.; Wojczynski, M.; Newman, A.B.; Montano, M.; et al. Age and Sex Distributions of Age-Related Biomarker Values in Healthy Older Adults from the Long Life Family Study. J. Am. Geriatr. Soc. 2016, 64, e189–e194. [Google Scholar] [CrossRef]
- Arbeev, K.G.; Ukraintseva, S.V.; Akushevich, I.; Kulminski, A.M.; Arbeeva, L.S.; Akushevich, L.; Culminskaya, I.V.; Yashin, A.I. Age trajectories of physiological indices in relation to healthy life course. Mech. Ageing Dev. 2011, 132, 93–102. [Google Scholar] [CrossRef]
- Rikli, R.E.; Jones, C.J. The Reliability and Validity of a 6-Minute Walk Test as a Measure of Physical Endurance in Older Adults. J. Aging Phys. Act. 1998, 6, 363–375. [Google Scholar] [CrossRef]
- Peters, D.M.; Fritz, S.L.; Krotish, D.E. Assessing the Reliability and Validity of a Shorter Walk Test Compared with the 10-Meter Walk Test for Measurements of Gait Speed in Healthy, Older Adults. J. Geriatr. Phys. Ther. 2013, 36, 24–30. [Google Scholar] [CrossRef]
- Giannouli, V.; Markopoulou, A.; Kiosseoglou, G.; Kosmidis, M.H. Neuropsychological functioning in patients with interstitial lung disease. Appl. Neuropsychol. Adult 2022, 29, 1290–1295. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.L.S.; Pin, T.W. Reliability, validity and minimal detectable change of 2-minute walk test, 6-minute walk test and 10-meter walk test in frail older adults with dementia. Exp. Gerontol. 2019, 115, 9–18. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).