Identification of Protein Networks and Biological Pathways Driving the Progression of Atherosclerosis in Human Carotid Arteries Through Mass Spectrometry-Based Proteomics
Abstract
1. Introduction
2. Results and Discussion
2.1. The DDA-Based Shotgun Proteomics Dataset
2.2. The DIA-Based Shotgun Proteomics Dataset
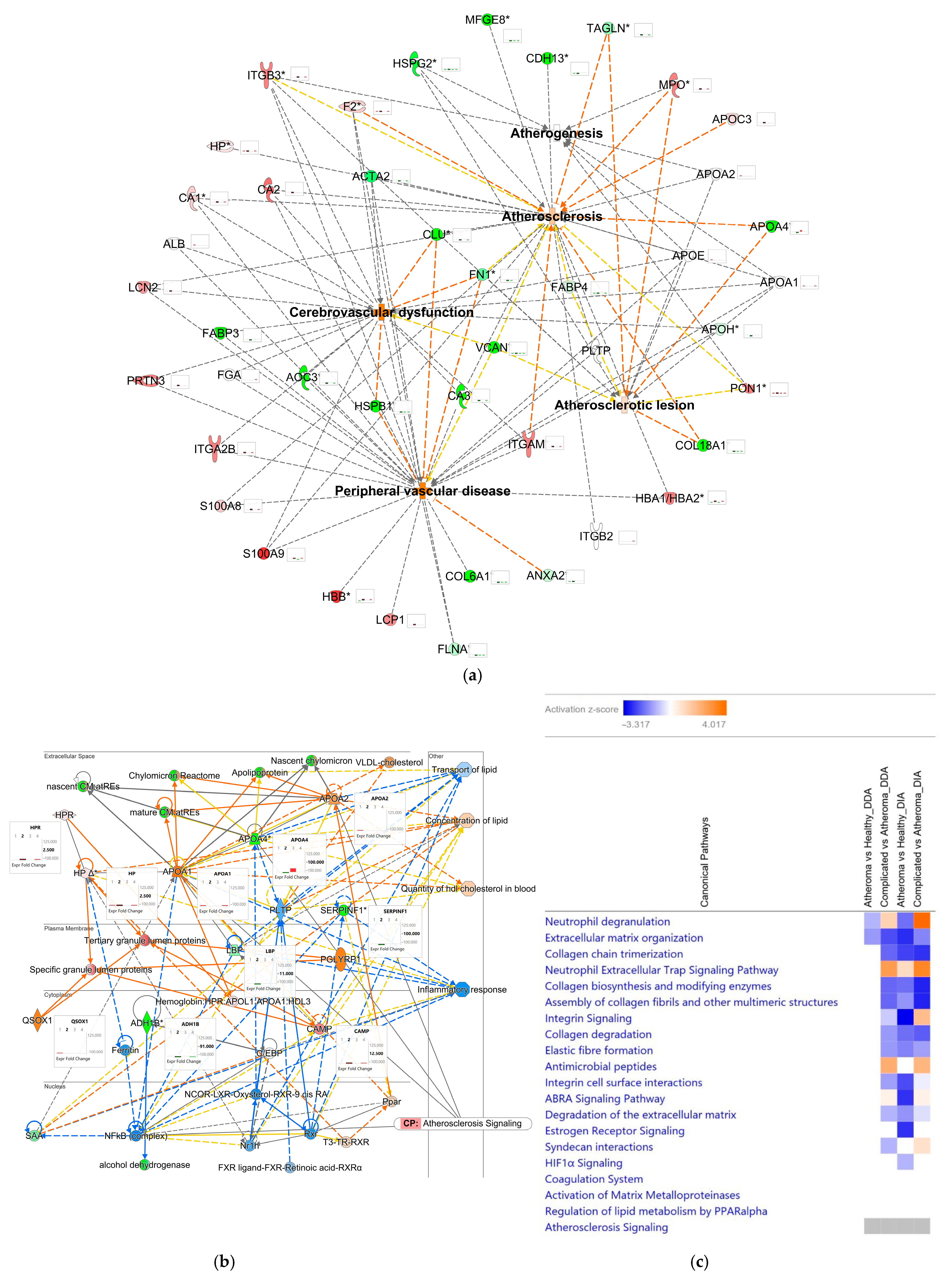
2.3. Comparative and Comprehensive Analyses of the DDA and DIA Results by Ingenuity Pathway Analysis®
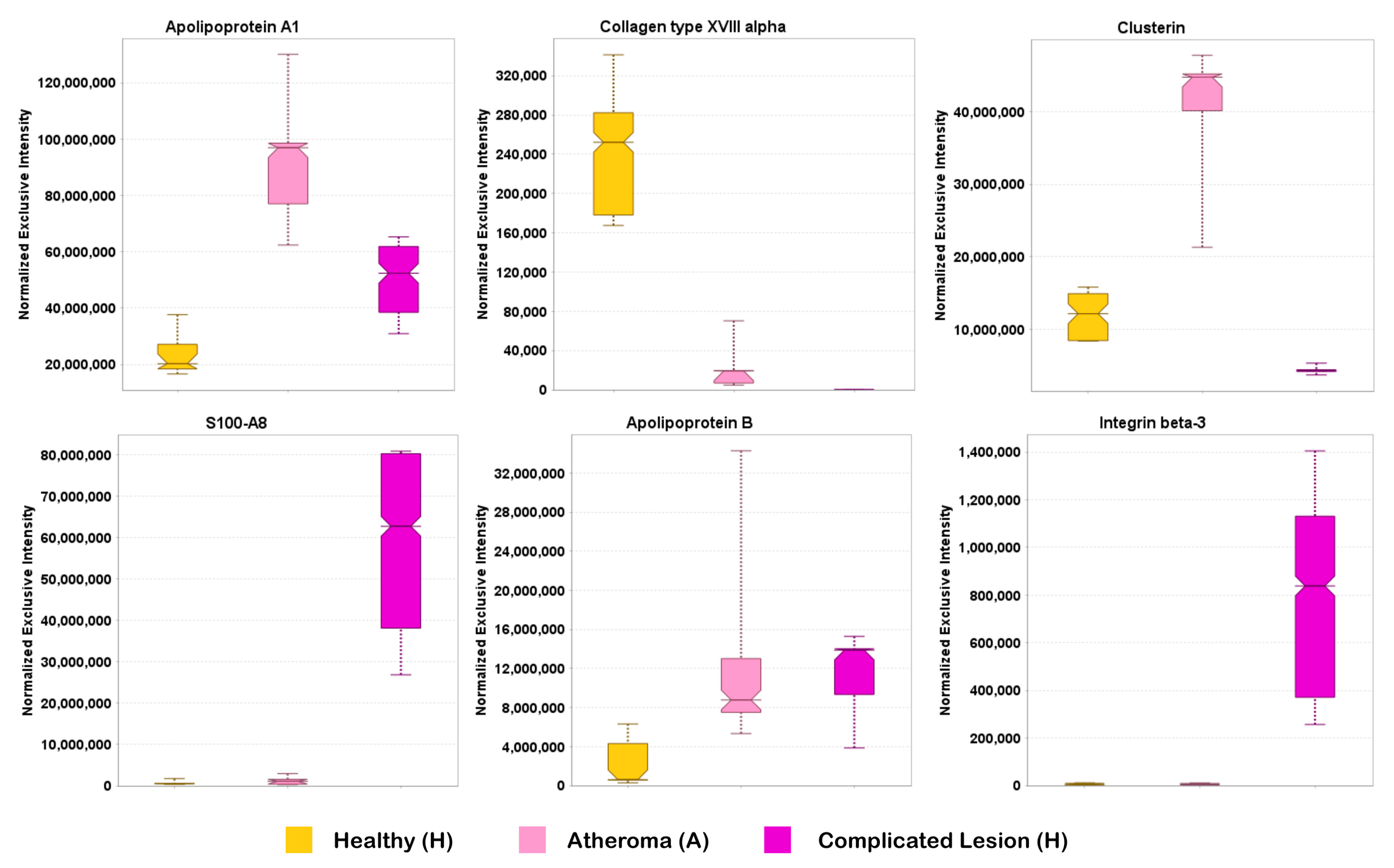
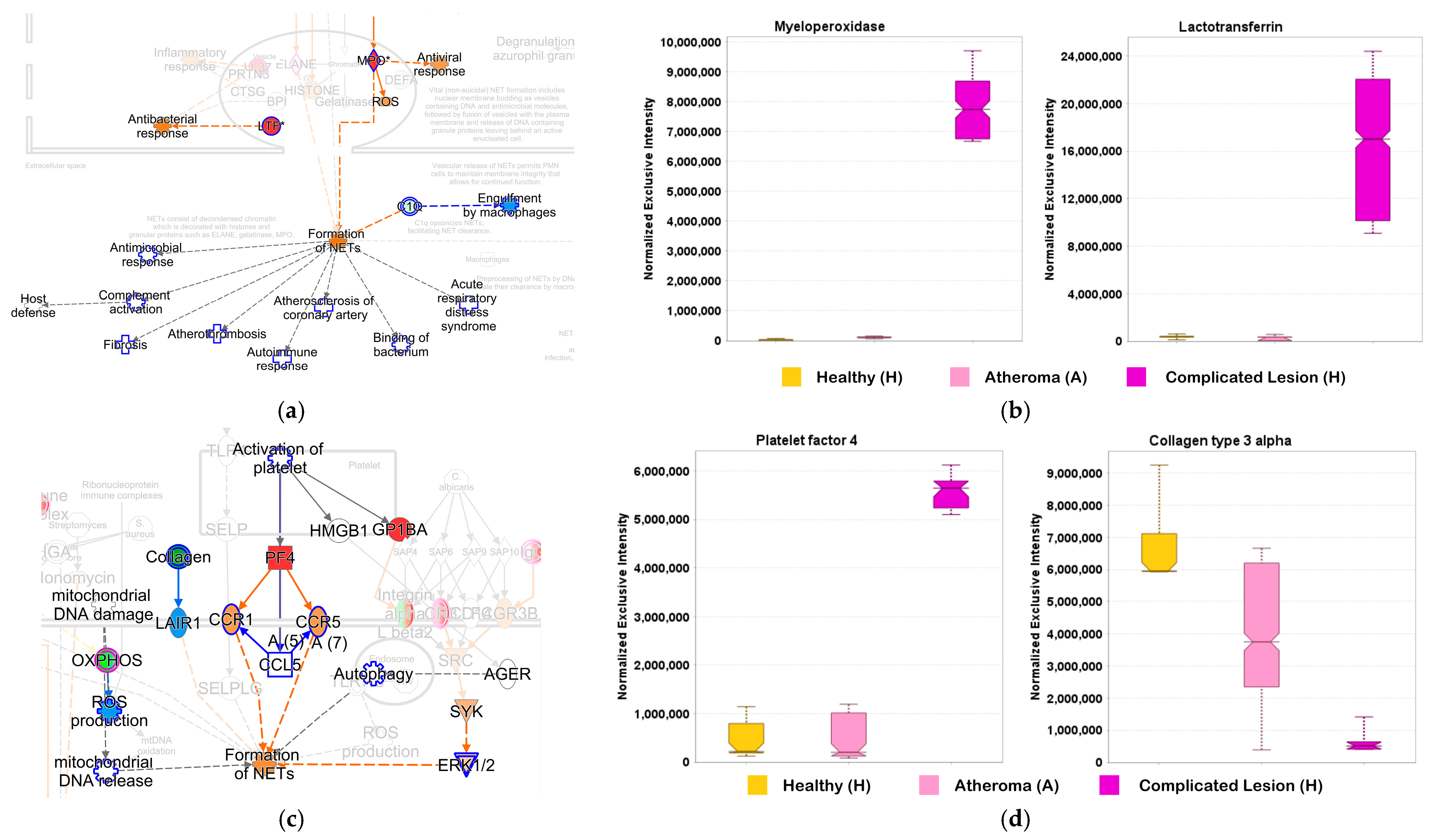
2.4. Examination of the Complex Landscape of Complicated Atherosclerotic Lesions and Identification of Potential Biomarkers from Selected Canonical Pathways
2.5. Comparative Analysis of Transcriptomic and Proteomic Datasets
3. Materials and Methods
3.1. Study Approval and Sample Collection
3.2. Sample Preparation for Shotgun Proteomics
3.3. LC–MS/MS Analyses
3.4. Data Analysis
3.4.1. DDA-Based Shotgun Proteomics
3.4.2. DIA-Based Shotgun Proteomics
3.4.3. Processing DIA Raw Data with Focus on Selected Proteins
3.5. Bioinformatics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stefanadis, C.; Antoniou, C.-K.; Tsiachris, D.; Pietri, P. Coronary atherosclerotic vulnerable plaque: Current perspectives. J. Am. Heart Assoc. 2017, 6, e005543. [Google Scholar] [CrossRef]
- Hafiane, A. Vulnerable plaque, characteristics, detection, and potential therapies. J. Cardiovasc. Dev. Dis. 2019, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Gaba, P.; Gersh, B.J.; Muller, J.; Narula, J.; Stone, G.W. Evolving concepts of the vulnerable atherosclerotic plaque and the vulnerable patient: Implications for patient care and future research. Nat. Rev. Cardiol. 2023, 20, 181–196. [Google Scholar] [CrossRef]
- Kurihara, O.; Takano, M.; Miyauchi, Y.; Mizuno, K.; Shimizu, W. Vulnerable atherosclerotic plaque features: Findings from coronary imaging. J. Geriatr. Cardiol. 2021, 18, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Chiorescu, R.M.; Mocan, M.; Inceu, A.I.; Buda, A.P.; Blendea, D.; Vlaicu, S.I. Vulnerable atherosclerotic plaque: Is there a molecular signature? Int. J. Mol. Sci. 2022, 23, 13638. [Google Scholar] [CrossRef]
- Hu, W.; Wei, R.; Wang, L.; Lu, J.; Liu, H.; Zhang, W. Correlations of MMP-1, MMP-3, and MMP-12 with the degree of atherosclerosis, plaque stability and cardiovascular and cerebrovascular events. Exp. Ther. Med. 2018, 15, 1994–1998. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tang, B.; Liu, X.; Wu, X.; Wang, H.; Xu, D.; Guo, Y. Increased monomeric CRP levels in acute myocardial infarction: A possible new and specific biomarker for diagnosis and severity assessment of disease. Atherosclerosis 2015, 239, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Siasos, G.; Tousoulis, D.; Antoniades, C.; Stefanadi, E.; Stefanadis, C. l-Arginine, the substrate for NO synthesis: An alternative treatment for premature atherosclerosis? Int. J. Cardiol. 2007, 116, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; de Ronde, M.W.J.; Kok, M.G.M.; Beijk, M.A.; De Winter, R.J.; van der Wal, A.C.; Sondermeijer, B.M.; Meijers, J.C.M.; Creemers, E.E.; Pinto-Sietsma, S.-J. MiR-223-3p and miR-122-5p as circulating biomarkers for plaque instability. Open Heart 2020, 7, e001223. [Google Scholar] [CrossRef] [PubMed]
- Shalhoub, J.; Sikkel, M.B.; Davies, K.J.; Vorkas, P.A.; Want, E.J.; Davies, A.H. Systems biology of human atherosclerosis. Vasc. Endovasc. Surg. 2014, 48, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Potor, L.; Hendrik, Z.; Patsalos, A.; Katona, É.; Méhes, G.; Póliska, S.; Csősz, É.; Kalló, G.; Komáromi, I.; Combi, Z.; et al. oxidation of hemoglobin drives a proatherogenic polarization of macrophages in human atherosclerosis. Antioxid. Redox Signal. 2021, 35, 917–950. [Google Scholar] [CrossRef]
- Yan, J.; Risacher, S.L.; Shen, L.; Saykin, A.J. Network approaches to systems biology analysis of complex disease: Integrative methods for multi-omics data. Brief. Bioinform. 2018, 19, 1370–1381. [Google Scholar] [CrossRef]
- Stahl, D.C.; Swiderek, K.M.; Davis, M.T.; Lee, T.D. Data-controlled automation of liquid chromatography/tandem mass spectrometry analysis of peptide mixtures. J. Am. Soc. Mass Spectrom. 1996, 7, 532–540. [Google Scholar] [CrossRef]
- Michalski, A.; Cox, J.; Mann, M. More than 100,000 detectable peptide species elute in single shotgun proteomics runs but the majority is inaccessible to data-dependent LC−MS/MS. J. Proteome Res. 2011, 10, 1785–1793. [Google Scholar] [CrossRef]
- Liu, H.; Sadygov, R.G.; Yates, J.R. A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal. Chem. 2004, 76, 4193–4201. [Google Scholar] [CrossRef]
- Michalski, A.; Damoc, E.; Hauschild, J.-P.; Lange, O.; Wieghaus, A.; Makarov, A.; Nagaraj, N.; Cox, J.; Mann, M.; Horning, S. Mass spectrometry-based proteomics using Q Exactive, a high-performance benchtop quadrupole Orbitrap mass spectrometer. Mol. Cell. Proteom. 2011, 10, M111.011015. [Google Scholar] [CrossRef]
- Huang, P.; Liu, C.; Gao, W.; Chu, B.; Cai, Z.; Tian, R. Synergistic optimization of liquid chromatography and mass spectrometry parameters on Orbitrap tribrid mass spectrometer for high efficient data-dependent proteomics. J. Mass Spectrom. 2021, 56, e4653. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.G. Fast Proteome Identification and Quantification from data-dependent acquisition–tandem mass spectrometry (DDA MS/MS) using free software tools. Methods Protoc. 2019, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Kalli, A.; Smith, G.T.; Sweredoski, M.J.; Hess, S. Evaluation and optimization of mass spectrometric settings during data-dependent acquisition mode: Focus on LTQ-Orbitrap mass analyzers. J. Proteome Res. 2013, 12, 3071–3086. [Google Scholar] [CrossRef]
- Jiang, Y.; Rex, D.A.B.; Schuster, D.; Neely, B.A.; Rosano, G.L.; Volkmar, N.; Momenzadeh, A.; Peters-Clarke, T.M.; Egbert, S.B.; Kreimer, S.; et al. Comprehensive overview of bottom-up proteomics using mass spectrometry. ACS Meas. Sci. Au 2024, 4, 338–417. [Google Scholar] [CrossRef]
- Lou, R.; Shui, W. Acquisition and analysis of DIA-based proteomic data: A comprehensive survey in 2023. Mol. Cell. Proteom. 2024, 23, 100712. [Google Scholar] [CrossRef] [PubMed]
- Egertson, J.D.; Kuehn, A.; Merrihew, G.E.; Bateman, N.W.; MacLean, B.X.; Ting, Y.S.; Canterbury, J.D.; Marsh, D.M.; Kellmann, M.; Zabrouskov, V.; et al. Multiplexed MS/MS for improved data-independent acquisition. Nat. Methods 2013, 10, 744–746. [Google Scholar] [CrossRef]
- Gillet, L.C.; Navarro, P.; Tate, S.; Röst, H.; Selevsek, N.; Reiter, L.; Bonner, R.; Aebersold, R. Targeted data extraction of the MS/MS Spectra generated by data-independent acquisition: A new concept for consistent and accurate proteome analysis. Mol. Cell. Proteom. 2012, 11, O111.016717. [Google Scholar] [CrossRef] [PubMed]
- Venable, J.D.; Dong, M.-Q.; Wohlschlegel, J.; Dillin, A.; Yates, J.R. Automated approach for quantitative analysis of complex peptide mixtures from tandem mass spectra. Nat. Methods 2004, 1, 39–45. [Google Scholar] [CrossRef]
- Purvine, S.; Eppel, J.-T.; Yi, E.C.; Goodlett, D.R. Shotgun collision-induced dissociation of peptides using a time of flight mass analyzer. Proteomics 2003, 3, 847–850. [Google Scholar] [CrossRef]
- Suomi, T.; Elo, L.L. Enhanced differential expression statistics for data-independent acquisition proteomics. Sci. Rep. 2017, 7, 5869. [Google Scholar] [CrossRef]
- Blattmann, P.; Heusel, M.; Aebersold, R. SWATH2stats: An R/Bioconductor package to process and convert quantitative SWATH-MS proteomics data for downstream analysis tools. PLoS ONE 2016, 11, e0153160. [Google Scholar] [CrossRef] [PubMed]
- Kitata, R.B.; Yang, J.-C.; Chen, Y.-J. Advances in data-independent acquisition mass spectrometry towards comprehensive digital proteome landscape. Mass Spectrom. Rev. 2023, 42, 2324–2348. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, Y.; Watanabe, E.; Umeyama, T.; Nakajima, D.; Hattori, M.; Honda, K.; Ohara, O. Optimization of data-independent acquisition mass spectrometry for deep and highly sensitive proteomic analysis. Int. J. Mol. Sci. 2019, 20, 5932. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.P.; Bruderer, R.; Abbott, J.; Arthur, J.S.C.; Brenes, A.J. Optimizing Spectronaut search parameters to improve data quality with minimal proteome coverage reductions in DIA analyses of heterogeneous samples. J. Proteome Res. 2024, 23, 1926–1936. [Google Scholar] [CrossRef]
- Mehta, D.; Scandola, S.; Uhrig, R.G. BoxCar and library-free data-independent acquisition substantially improve the depth, range, and completeness of label-free quantitative proteomics. Anal. Chem. 2022, 94, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Megger, D.A.; Bracht, T.; Meyer, H.E.; Sitek, B. Label-free quantification in clinical proteomics. Biochim. Biophys. Acta 2013, 1834, 1581–1590. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Cox, J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 2016, 11, 2301–2319. [Google Scholar] [CrossRef]
- Cox, J.; Hein, M.Y.; Luber, C.A.; Paron, I.; Nagaraj, N.; Mann, M. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol. Cell. Proteom. 2014, 13, 2513–2526. [Google Scholar] [CrossRef]
- Shah, A.D.; Goode, R.J.A.; Huang, C.; Powell, D.R.; Schittenhelm, R.B. LFQ-Analyst: An easy-to-use interactive web platform to analyze and visualize label-free proteomics data preprocessed with MaxQuant. J. Proteome Res. 2020, 19, 204–211. [Google Scholar] [CrossRef]
- Nieddu, G.; Formato, M.; Lepedda, A.J. Searching for atherosclerosis biomarkers by proteomics: A focus on lesion pathogenesis and vulnerability. Int. J. Mol. Sci. 2023, 24, 15175. [Google Scholar] [CrossRef] [PubMed]
- Nahnsen, S.; Bielow, C.; Reinert, K.; Kohlbacher, O. Tools for label-free peptide quantification. Mol. Cell. Proteom. 2013, 12, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Smith, M.; Ying, Y.-L.; Makridakis, M.; Noonan, J.; Kanellakis, P.; Rai, A.; Salim, A.; Murphy, A.; Bobik, A.; et al. Quantitative proteomic landscape of unstable atherosclerosis identifies molecular signatures and therapeutic targets for plaque stabilization. Commun. Biol. 2023, 6, 265. [Google Scholar] [CrossRef] [PubMed]
- Herrington, D.M.; Mao, C.; Parker, S.J.; Fu, Z.; Yu, G.; Chen, L.; Venkatraman, V.; Fu, Y.; Wang, Y.; Howard, T.D.; et al. Proteomic architecture of human coronary and aortic atherosclerosis. Circulation 2018, 137, 2741–2756. [Google Scholar] [CrossRef]
- Hansmeier, N.; Buttigieg, J.; Kumar, P.; Pelle, S.; Choi, K.Y.; Kopriva, D.; Chao, T.-C. Identification of mature atherosclerotic plaque proteome signatures using data-independent acquisition mass spectrometry. J. Proteome Res. 2018, 17, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Feng, Y.; Lai, Z.; Chen, J.; Sun, W.; Liu, B. Proteomic insights into carotid atherosclerotic plaque progression: Unraveling molecular mechanisms from American Heart Association Classifications IV to VI. J. Vasc. Surg. 2024, 79, e313–e314. [Google Scholar] [CrossRef]
- Kohler, D.; Kaza, M.; Pasi, C.; Huang, T.; Staniak, M.; Mohandas, D.; Sabido, E.; Choi, M.; Vitek, O. MSstatsShiny: A GUI for versatile, scalable, and reproducible statistical analyses of quantitative proteomic experiments. J. Proteome Res. 2023, 22, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Camacho, J.; Picó, J.; Ferrer, A. Data understanding with PCA: Structural and variance information plots. Chemom. Intell. Lab. Syst. 2010, 100, 48–56. [Google Scholar] [CrossRef]
- Kumar, A.; Doan, V.M.; Kunkli, B.; Csősz, É. Construction of unified human antimicrobial and immunomodulatory peptide database and examination of antimicrobial and immunomodulatory peptides in Alzheimer’s disease using network analysis of proteomics datasets. Front. Genet. 2021, 12, 633050. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Xie, Q.; Hu, T.; Yao, Q.; Zhao, J.; Wu, Q.; Tang, Q. S100A8/A9 in myocardial infarction: A promising biomarker and therapeutic target. Front. Cell Dev. Biol. 2020, 8, 603902. [Google Scholar] [CrossRef]
- Shi, F.; Sun, L.; Kaptoge, S. Association of beta-2-microglobulin and cardiovascular events and mortality: A systematic review and meta-analysis. Atherosclerosis 2021, 320, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Tuttolomondo, A.; Puleo, M.G.; Velardo, M.C.; Corpora, F.; Daidone, M.; Pinto, A. Molecular biology of atherosclerotic ischemic strokes. Int. J. Mol. Sci. 2020, 21, 9372. [Google Scholar] [CrossRef] [PubMed]
- Poredos, P.; Gregoric, I.D.; Jezovnik, M.K. Inflammation of carotid plaques and risk of cerebrovascular events. Ann. Transl. Med. 2020, 8, 1281. [Google Scholar] [CrossRef]
- Montanaro, M.; Scimeca, M.; Anemona, L.; Servadei, F.; Giacobbi, E.; Bonfiglio, R.; Bonanno, E.; Urbano, N.; Ippoliti, A.; Santeusanio, G.; et al. The Paradox Effect of calcification in carotid atherosclerosis: Microcalcification is correlated with plaque instability. Int. J. Mol. Sci. 2021, 22, 395. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Gao, J.; Lv, Q.; Cai, H.; Wang, F.; Ye, R.; Liu, X. Calcification in atherosclerotic plaque vulnerability: Friend or foe? Front. Physiol. 2020, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Simone, S.; Chieti, A.; Pontrelli, P.; Rascio, F.; Castellano, G.; Stallone, G.; Infante, B.; Gesualdo, L.; Grandaliano, G.; Pertosa, G. On-line hemodiafiltration modulates atherosclerosis signaling in peripheral lymphomonocytes of hemodialysis patients. J. Nephrol. 2021, 34, 1989–1997. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Val, A.; Fort, K.; Koenig, C.; Van der Hoeven, L.; Franciosa, G.; Moehring, T.; Ishihama, Y.; Chen, Y.; Makarov, A.; Xuan, Y.; et al. Hybrid-DIA: Intelligent data acquisition integrates targeted and discovery proteomics to analyze phospho-signaling in single spheroids. Nat. Commun. 2023, 14, 3599. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.; Wang, C.; Liu, X.; Sun, H.; Guo, Z.; Shao, J.; Li, K.; Chen, J.; Wang, J.; Lei, X.; et al. Characterization of the proteome of stable and unstable carotid atherosclerotic plaques using data-independent acquisition mass spectrometry. J. Transl. Med. 2024, 22, 247. [Google Scholar] [CrossRef]
- Lorentzen, L.G.; Yeung, K.; Eldrup, N.; Eiberg, J.P.; Sillesen, H.H.; Davies, M.J. Proteomic analysis of the extracellular matrix of human atherosclerotic plaques shows marked changes between plaque types. Matrix Biol. Plus 2024, 21, 100141. [Google Scholar] [CrossRef] [PubMed]
- Kunjathoor, V.V.; Febbraio, M.; Podrez, E.A.; Moore, K.J.; Andersson, L.; Koehn, S.; Rhee, J.S.; Silverstein, R.; Hoff, H.F.; Freeman, M.W. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J. Biol. Chem. 2002, 277, 49982–49988. [Google Scholar] [CrossRef] [PubMed]
- Potor, L.; Bányai, E.; Becs, G.; Soares, M.P.; Balla, G.; Balla, J.; Jeney, V. Atherogenesis may involve the prooxidant and proinflammatory effects of ferryl hemoglobin. Oxidative Med. Cell. Longev. 2013, 2013, 676425. [Google Scholar] [CrossRef]
- Posta, N.; Csősz, É.; Oros, M.; Pethő, D.; Potor, L.; Kalló, G.; Hendrik, Z.; Sikura, K.É.; Méhes, G.; Tóth, C.; et al. Hemoglobin oxidation generates globin-derived peptides in atherosclerotic lesions and intraventricular hemorrhage of the brain, provoking endothelial dysfunction. Lab. Investig. 2020, 100, 986–1002. [Google Scholar] [CrossRef]
- Gáll, T.; Nagy, P.; Garai, D.; Potor, L.; Balla, G.J.; Balla, G.; Balla, J. Overview on hydrogen sulfide-mediated suppression of vascular calcification and hemoglobin/heme-mediated vascular damage in atherosclerosis. Redox Biol. 2022, 57, 102504. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Melnichenko, A.A.; Myasoedova, V.A.; Grechko, A.V.; Orekhov, A.N. Mechanisms of foam cell formation in atherosclerosis. J. Mol. Med. 2017, 95, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Allahverdian, S.; Pannu, P.S.; Francis, G.A. Contribution of monocyte-derived macrophages and smooth muscle cells to arterial foam cell formation. Cardiovasc. Res. 2012, 95, 165–172. [Google Scholar] [CrossRef]
- Mortensen, M.B.; Dzaye, O.; Bøtker, H.E.; Jensen, J.M.; Maeng, M.; Bentzon, J.F.; Kanstrup, H.; Sørensen, H.T.; Leipsic, J.; Blankstein, R.; et al. Low-Density lipoprotein cholesterol is predominantly associated with atherosclerotic cardiovascular disease events in patients with evidence of coronary atherosclerosis: The Western Denmark Heart Registry. Circulation 2023, 147, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Panayiotou, A.; Griffin, M.; Georgiou, N.; Bond, D.; Tyllis, T.; Tziakouri-Shiakalli, C.; Fessas, C.; Nicolaides, A. ApoB/ApoA1 ratio and subclinical atherosclerosis. Int. Angiol. 2008, 27, 74–80. [Google Scholar] [PubMed]
- Močnik, M.; Marčun Varda, N. Lipid biomarkers and atherosclerosis—Old and new in cardiovascular risk in childhood. Int. J. Mol. Sci. 2023, 24, 2237. [Google Scholar] [CrossRef]
- Schreiner, T.G.; Ignat, B.E.; Grosu, C.; Costache, A.D.; Leon, M.M.; Mitu, F. Lipid-derived biomarkers as therapeutic targets for chronic coronary syndrome and ischemic stroke: An updated narrative review. Medicina 2024, 60, 561. [Google Scholar] [CrossRef]
- Deng, F.; Li, D.; Lei, L.; Yang, Q.; Li, Q.; Wang, H.; Deng, J.; Zheng, Q.; Jiang, W. Association between apolipoprotein B/A1 ratio and coronary plaque vulnerability in patients with atherosclerotic cardiovascular disease: An intravascular optical coherence tomography study. Cardiovasc. Diabetol. 2021, 20, 188. [Google Scholar] [CrossRef]
- Finamore, F.; Nieddu, G.; Rocchiccioli, S.; Spirito, R.; Guarino, A.; Formato, M.; Lepedda, A.J. Apolipoprotein signature of HDL and LDL from atherosclerotic patients in relation with carotid plaque typology: A preliminary report. Biomedicines 2021, 9, 1156. [Google Scholar] [CrossRef] [PubMed]
- Davidson, W.S.; Shah, A.S.; Sexmith, H.; Gordon, S.M. The HDL proteome watch: Compilation of studies leads to new insights on HDL function. Biochim. Biophys. Acta. Mol. Cell Biol. Lipids 2022, 1867, 159072. [Google Scholar] [CrossRef]
- Nicoll, R. Plaque collagen synthesis and calcification: Working together to protect against instability and rupture. In Cardiovascular Calcification; Henein, M., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–15. ISBN 978-3-030-81515-8. [Google Scholar]
- Nakagawa, K.; Tanaka, M.; Hahm, T.-H.; Nguyen, H.-N.; Matsui, T.; Chen, Y.-X.; Nakashima, Y. Accumulation of plasma-derived lipids in the lipid core and necrotic core of human atheroma: Imaging mass spectrometry and histopathological analyses. Arterioscler. Thromb. Vasc. Biol. 2021, 41, e498–e511. [Google Scholar] [CrossRef]
- Vootukuri, S.; Li, J.; Nedelman, M.; Thomas, C.; Jiang, J.-K.; Babayeva, M.; Coller, B.S. Preclinical Studies of RUC-4, a novel platelet αIIbβ3 antagonist, in non-human primates and with human platelets. J. Clin. Transl. Sci. 2019, 3, 65–74. [Google Scholar] [CrossRef]
- Huilcaman, R.; Venturini, W.; Fuenzalida, L.; Cayo, A.; Segovia, R.; Valenzuela, C.; Brown, N.; Moore-Carrasco, R. Platelets, a key cell in inflammation and atherosclerosis progression. Cells 2022, 11, 1014. [Google Scholar] [CrossRef]
- Aragonès, G.; Auguet, T.; Guiu-Jurado, E.; Berlanga, A.; Curriu, M.; Martinez, S.; Alibalic, A.; Aguilar, C.; Hernández, E.; Camara, M.-L.; et al. Proteomic profile of unstable atheroma plaque: Increased neutrophil defensin 1, clusterin, and apolipoprotein E levels in carotid secretome. J. Proteome Res. 2016, 15, 933–944. [Google Scholar] [CrossRef]
- Theofilatos, K.; Stojkovic, S.; Hasman, M.; van der Laan, S.W.; Baig, F.; Barallobre-Barreiro, J.; Schmidt, L.E.; Yin, S.; Yin, X.; Burnap, S.; et al. Proteomic atlas of atherosclerosis: The contribution of proteoglycans to sex differences, plaque phenotypes, and outcomes. Circ. Res. 2023, 133, 542–558. [Google Scholar] [CrossRef] [PubMed]
- Schiopu, A.; Cotoi, O.S. S100A8 and S100A9: DAMPs at the crossroads between innate immunity, traditional risk factors, and cardiovascular disease. Mediat. Inflamm. 2013, 2013, 828354. [Google Scholar] [CrossRef]
- Drechsler, M.; Megens, R.T.A.; van Zandvoort, M.; Weber, C.; Soehnlein, O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation 2010, 122, 1837–1845. [Google Scholar] [CrossRef] [PubMed]
- McCormick, M.M.; Rahimi, F.; Bobryshev, Y.V.; Gaus, K.; Zreiqat, H.; Cai, H.; Lord, R.S.A.; Geczy, C.L. S100A8 and S100A9 in human arterial wall: Implications for atherogenesis. J. Biol. Chem. 2005, 280, 41521–41529. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Pang, B.; Sun, S.; An, C.; Wu, M.; Wang, N.; Yuan, Y.; Liu, G. Neutrophil extracellular traps contributing to atherosclerosis: From pathophysiology to clinical implications. Exp. Biol. Med. 2023, 248, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, C.; Li, J. Neutrophil extracellular traps: A catalyst for atherosclerosis. Mol. Cell. Biochem. 2024, 479, 3213–3227. [Google Scholar] [CrossRef]
- Frangie, C.; Daher, J. Role of myeloperoxidase in inflammation and atherosclerosis (Review). Biomed. Rep. 2022, 16, 53. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, S.J.; Kettle, A.J.; Rosen, H.; Winterbourn, C.C.; Nauseef, W.M. Myeloperoxidase: A front-line defender against phagocytosed microorganisms. J. Leukoc. Biol. 2013, 93, 185–198. [Google Scholar] [CrossRef]
- Delporte, C.; Van Antwerpen, P.; Vanhamme, L.; Roumeguère, T.; Zouaoui Boudjeltia, K. Low-density lipoprotein modified by myeloperoxidase in inflammatory pathways and clinical studies. Mediat. Inflamm. 2013, 2013, 971579. [Google Scholar] [CrossRef]
- Nadel, J.; Tumanov, S.; Kong, S.M.Y.; Chen, W.; Giannotti, N.; Sivasubramaniam, V.; Rashid, I.; Ugander, M.; Jabbour, A.; Stocker, R. Intraplaque myeloperoxidase activity as biomarker of unstable atheroma and adverse clinical outcomes in human atherosclerosis. JACC. Adv. 2023, 2, 100310. [Google Scholar] [CrossRef]
- Chen, C.; Lu, M.; Zhang, Z.; Qin, L. The role of lactoferrin in atherosclerosis. Biometals 2023, 36, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Vengen, I.T.; Dale, A.C.; Wiseth, R.; Midthjell, K.; Videm, V. Lactoferrin is a novel predictor of fatal ischemic heart disease in diabetes mellitus type 2: Long-term follow-up of the HUNT 1 study. Atherosclerosis 2010, 212, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Vasilyev, V.B.; Sokolov, A.V.; Kostevich, V.A.; Elizarova, A.Y.; Gorbunov, N.P.; Panasenko, O.M. Binding of lactoferrin to the surface of low-density lipoproteins modified by myeloperoxidase prevents intracellular cholesterol accumulation by human blood monocytes. Biochem. Cell Biol. 2021, 99, 109–116. [Google Scholar] [CrossRef]
- Badimon, L.; Vilahur, G. Platelets, arterial thrombosis and cerebral ischemia. Cerebrovasc. Dis. 2007, 24 (Suppl. S1), 30–39. [Google Scholar] [CrossRef]
- Estevez, B.; Du, X. New concepts and mechanisms of platelet activation signaling. Physiology 2017, 32, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Huseynov, A.; Reinhardt, J.; Chandra, L.; Dürschmied, D.; Langer, H.F. Novel aspects targeting platelets in atherosclerotic cardiovascular disease—A translational perspective. Int. J. Mol. Sci. 2023, 24, 6280. [Google Scholar] [CrossRef] [PubMed]
- Lebbink, R.J.; de Ruiter, T.; Adelmeijer, J.; Brenkman, A.B.; van Helvoort, J.M.; Koch, M.; Farndale, R.W.; Lisman, T.; Sonnenberg, A.; Lenting, P.J.; et al. Collagens are functional, high affinity ligands for the inhibitory immune receptor LAIR-1. J. Exp. Med. 2006, 203, 1419–1425. [Google Scholar] [CrossRef]
- Geerdink, R.J.; Hennus, M.P.; Westerlaken, G.H.A.; Abrahams, A.C.; Albers, K.I.; Walk, J.; Wesselink, E.; Janssen, R.; Bont, L.; Meyaard, L. LAIR-1 limits neutrophil extracellular trap formation in viral bronchiolitis. J. Allergy Clin. Immunol. 2018, 141, 811–814. [Google Scholar] [CrossRef]
- Kumawat, K.; Geerdink, R.J.; Hennus, M.P.; Roda, M.A.; van Ark, I.; Leusink-Muis, T.; Folkerts, G.; van Oort-Jansen, A.; Mazharian, A.; Watson, S.P.; et al. LAIR-1 limits neutrophilic airway inflammation. Front. Immunol. 2019, 10, 842. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Z.; Carr, S.A.; Mertins, P.; Zhang, H.; Zhang, Z.; Chan, D.W.; Ellis, M.J.C.; Townsend, R.R.; Smith, R.D.; et al. Proteome profiling outperforms transcriptome profiling for coexpression based gene function prediction. Mol. Cell. Proteom. 2017, 16, 121–134. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef]
- Moore, K.J.; Sheedy, F.J.; Fisher, E.A. Macrophages in atherosclerosis: A dynamic balance. Nat. Rev. Immunol. 2013, 13, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Barin, J.G.; Baldeviano, G.C.; Talor, M.V.; Wu, L.; Ong, S.; Quader, F.; Chen, P.; Zheng, D.; Caturegli, P.; Rose, N.R.; et al. Macrophages participate in IL-17-mediated inflammation. Eur. J. Immunol. 2012, 42, 726–736. [Google Scholar] [CrossRef]
- Makuch, M.; Stepanechko, M.; Bzowska, M. The dance of macrophage death: The interplay between the inevitable and the microenvironment. Front. Immunol. 2024, 15, 1330461. [Google Scholar] [CrossRef] [PubMed]
- Miceli, G.; Basso, M.G.; Pintus, C.; Pennacchio, A.R.; Cocciola, E.; Cuffaro, M.; Profita, M.; Rizzo, G.; Tuttolomondo, A. Molecular pathways of vulnerable carotid plaques at risk of ischemic stroke: A narrative review. Int. J. Mol. Sci. 2024, 25, 4351. [Google Scholar] [CrossRef]
- Jin, H.; Goossens, P.; Juhasz, P.; Eijgelaar, W.; Manca, M.; Karel, J.M.H.; Smirnov, E.; Sikkink, C.J.J.M.; Mees, B.M.E.; Waring, O.; et al. Integrative multiomics analysis of human atherosclerosis reveals a serum response factor-driven network associated with intraplaque hemorrhage. Clin. Transl. Med. 2021, 11, e458. [Google Scholar] [CrossRef] [PubMed]
- Meier, F.; Park, M.A.; Mann, M. Trapped ion mobility spectrometry and parallel accumulation–serial fragmentation in proteomics. Mol. Cell. Proteom. 2021, 20, 100138. [Google Scholar] [CrossRef]
- Carnielli, C.M.; Macedo, C.C.S.; De Rossi, T.; Granato, D.C.; Rivera, C.; Domingues, R.R.; Pauletti, B.A.; Yokoo, S.; Heberle, H.; Busso-Lopes, A.F.; et al. Combining discovery and targeted proteomics reveals a prognostic signature in oral cancer. Nat. Commun. 2018, 9, 3598. [Google Scholar] [CrossRef]
- Stary, H.C.; Chandler, A.B.; Dinsmore, R.E.; Fuster, V.; Glagov, S.; Insull, W.J.; Rosenfeld, M.E.; Schwartz, C.J.; Wagner, W.D.; Wissler, R.W. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1995, 92, 1355–1374. [Google Scholar] [CrossRef]
- Kong, A.T.; Leprevost, F.V.; Avtonomov, D.M.; Mellacheruvu, D.; Nesvizhskii, A.I. MSFragger: Ultrafast and comprehensive peptide identification in mass spectrometry–based proteomics. Nat. Methods 2017, 14, 513–520. [Google Scholar] [CrossRef]
- Keller, A.; Nesvizhskii, A.I.; Kolker, E.; Aebersold, R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 2002, 74, 5383–5392. [Google Scholar] [CrossRef] [PubMed]
- Nesvizhskii, A.I.; Keller, A.; Kolker, E.; Aebersold, R. A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 2003, 75, 4646–4658. [Google Scholar] [CrossRef] [PubMed]
- Old, W.M.; Meyer-Arendt, K.; Aveline-Wolf, L.; Pierce, K.G.; Mendoza, A.; Sevinsky, J.R.; Resing, K.A.; Ahn, N.G. Comparison of label-free methods for quantifying human proteins by shotgun proteomics. Mol. Cell. Proteom. 2005, 4, 1487–1502. [Google Scholar] [CrossRef]
- Mishra, P.; Singh, U.; Pandey, C.M.; Mishra, P.; Pandey, G. Application of student’s t-test, analysis of variance, and covariance. Ann. Card. Anaesth. 2019, 22, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C. A tutorial on kernel density estimation and recent advances. Biostat. Epidemiol. 2017, 1, 161–187. [Google Scholar] [CrossRef]
- Searle, B.C.; Pino, L.K.; Egertson, J.D.; Ting, Y.S.; Lawrence, R.T.; MacLean, B.X.; Villén, J.; MacCoss, M.J. Chromatogram libraries improve peptide detection and quantification by data independent acquisition mass spectrometry. Nat. Commun. 2018, 9, 5128. [Google Scholar] [CrossRef]
- Schessner, J.P.; Voytik, E.; Bludau, I. A practical guide to interpreting and generating bottom-up proteomics data visualizations. Proteomics 2022, 22, 2100103. [Google Scholar] [CrossRef]
- Vizcaíno, J.A.; Deutsch, E.W.; Wang, R.; Csordas, A.; Reisinger, F.; Ríos, D.; Dianes, J.A.; Sun, Z.; Farrah, T.; Bandeira, N.; et al. ProteomeXchange provides globally coordinated proteomics data submission and dissemination. Nat. Biotechnol. 2014, 32, 223–226. [Google Scholar] [CrossRef]


| Comparison | Statistical Analysis | Number of Differentially Expressed Proteins | Upregulated | Downregulated |
|---|---|---|---|---|
| A versus H | t-test | 28 | 7 | 31 |
| C versus H | t-test | 79 | 25 | 54 |
| C versus A | t-test | 59 | 23 | 36 |
| Comparison | Statistical Analysis | Number of Differentially Expressed Proteins | Upregulated | Downregulated |
|---|---|---|---|---|
| A versus H | t-test | 118 | 57 | 61 |
| C versus H | t-test | 317 | 95 | 222 |
| C versus A | t-test | 261 | 81 | 180 |
| One-way ANOVA | 331 |
| Comparison | Statistical Analysis | Number of Differentially Expressed Proteins | Upregulated | Downregulated |
|---|---|---|---|---|
| A versus H | t-test | 184 | 42 | 142 |
| C versus H | t-test | 280 | 140 | 140 |
| C versus A | t-test | 213 | 124 | 89 |
| One-way ANOVA | 709 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalló, G.; Zaman, K.; Potor, L.; Hendrik, Z.; Méhes, G.; Tóth, C.; Gergely, P.; Tőzsér, J.; Balla, G.; Balla, J.; et al. Identification of Protein Networks and Biological Pathways Driving the Progression of Atherosclerosis in Human Carotid Arteries Through Mass Spectrometry-Based Proteomics. Int. J. Mol. Sci. 2024, 25, 13665. https://doi.org/10.3390/ijms252413665
Kalló G, Zaman K, Potor L, Hendrik Z, Méhes G, Tóth C, Gergely P, Tőzsér J, Balla G, Balla J, et al. Identification of Protein Networks and Biological Pathways Driving the Progression of Atherosclerosis in Human Carotid Arteries Through Mass Spectrometry-Based Proteomics. International Journal of Molecular Sciences. 2024; 25(24):13665. https://doi.org/10.3390/ijms252413665
Chicago/Turabian StyleKalló, Gergő, Khadiza Zaman, László Potor, Zoltán Hendrik, Gábor Méhes, Csaba Tóth, Péter Gergely, József Tőzsér, György Balla, József Balla, and et al. 2024. "Identification of Protein Networks and Biological Pathways Driving the Progression of Atherosclerosis in Human Carotid Arteries Through Mass Spectrometry-Based Proteomics" International Journal of Molecular Sciences 25, no. 24: 13665. https://doi.org/10.3390/ijms252413665
APA StyleKalló, G., Zaman, K., Potor, L., Hendrik, Z., Méhes, G., Tóth, C., Gergely, P., Tőzsér, J., Balla, G., Balla, J., Prokai, L., & Csősz, É. (2024). Identification of Protein Networks and Biological Pathways Driving the Progression of Atherosclerosis in Human Carotid Arteries Through Mass Spectrometry-Based Proteomics. International Journal of Molecular Sciences, 25(24), 13665. https://doi.org/10.3390/ijms252413665










