The RAGE Pathway in Skin Pathology Development: A Comprehensive Review of Its Role and Therapeutic Potential
Abstract
1. Introduction
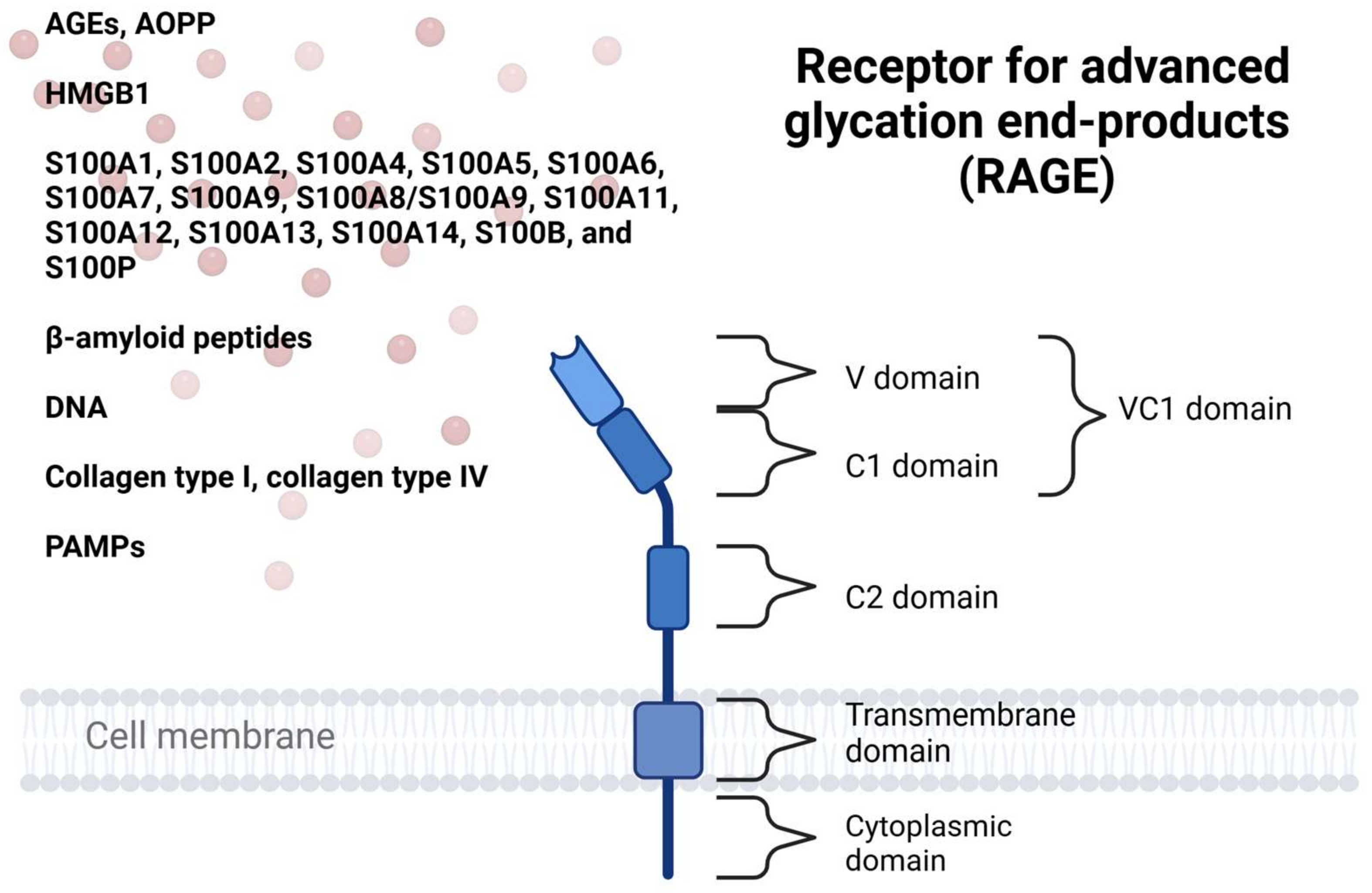
2. Receptor and Its Ligands
3. Molecular Pathway and Isoforms of RAGE
4. RAGE Involvement in Skin Pathologies
4.1. Psoriasis
| Material | Method | Study Size | Markers | Year | Authors [Ref.] |
|---|---|---|---|---|---|
| Serum | ELISA | 60 | ↑AGEs | 2023 | Kang et al. [45] |
| Serum | ELISA | 75 | ↑CML, ↑CEL, ↑sRAGE | 2022 | Damasiewicz-Bodzek et al. [47] |
| Serum | ELISA | 160 | ↑AGEs, ↑sRAGE | 2022 | Karas et al. [48] |
| Serum | ELISA | 158 | ↑HMGB1, ↑S100A7, ↑S100A12 | 2020 | Borsky et al. [54] |
| Serum | ELISA | 80 | ↑AGEs, ↓sRAGE, ↓esRAGE | 2017 | Papagrigoraki et al. [46] |
| Serum | ELISA | 50 | ↑S100B | 2017 | Salem et al. [51] |
| Serum | ELISA | 55 | ↑S100A7, ↑S100A8/A9, ↑S100A12 | 2016 | Wilsmann-Theis et al. [55] |
| Serum | ELISA | 14 | ↑S100A8/S100A9, ↓S10A7 | 2009 | Anderson et al. [59] |
| Peripheral leukocytes | Flow cytometry | 32 | ↑RAGE | 2019 | Strohbuecker et al. [49] |
| Peripheral leukocytes | PCR | 272 | ↑2184G RAGE allele | 2002 | Vasku et al. [50] |
| Skin biopsy | IHC | 10 | ↑AGEs | 2023 | Kang et al. [45] |
| Skin biopsy | IHC | 14 | ↑HMGB1 | 2019 | Strohbuecker et al. [49] |
| Skin biopsy | IHC | 80 | ↑AGEs | 2017 | Papagrigoraki et al. [46] |
| Skin biopsy | IHC | 50 | ↑S100B | 2017 | Salem et al. [51] |
| Skin biopsy | PCR | 341 | ↑S100A7, ↑S100A8, ↑S100A9, ↑S100A12 | 2016 | Wilsmann-Theis et al. [55] |
| Skin biopsy | Western Blot, PCR | 21 | ↑S100A4 | 2010 | Zibert et al. [53] |
| Skin biopsy | IHC | 14 | ↑S100A7 | 2009 | Anderson et al. [59] |
4.2. Atopic Dermatitis
| Material | Method | Study Size | Markers | Year | Authors [Ref.] |
|---|---|---|---|---|---|
| Serum | ELISA | 65 | ↓sRAGE | 2023 | Eke-Gungor et al. [61] |
| Serum | ELISA | 41 | ↓sRAGE, ↓esRAGE | 2020 | Hong et al. [60] |
| Serum | ELISA | 15 | ↑S100A9, ↑S100A8/A9 | 2014 | Jin et al. [63] |
| Lesion washing fluids | ELISA | 12 | ↑S100A7 | 2009 | Gläser et al. [65] |
| Skin biopsy | ELISA | 41 | ↑AGEs | 2020 | Hong et al. [60] |
| Skin biopsy | IHC | 15 | ↑S100A9, ↑S100A8/A9 | 2014 | Jin et al. [63] |
| Skin biopsy | IHC, PCR | 51 | ↑S100A12 | 2013 | Suárez-Fariñas et al. [64] |
| Skin biopsy | IHC | 4 | ↑S100A7 | 2009 | Gläser et al. [65] |
4.3. Lichen Planus
4.4. Cholesteatoma
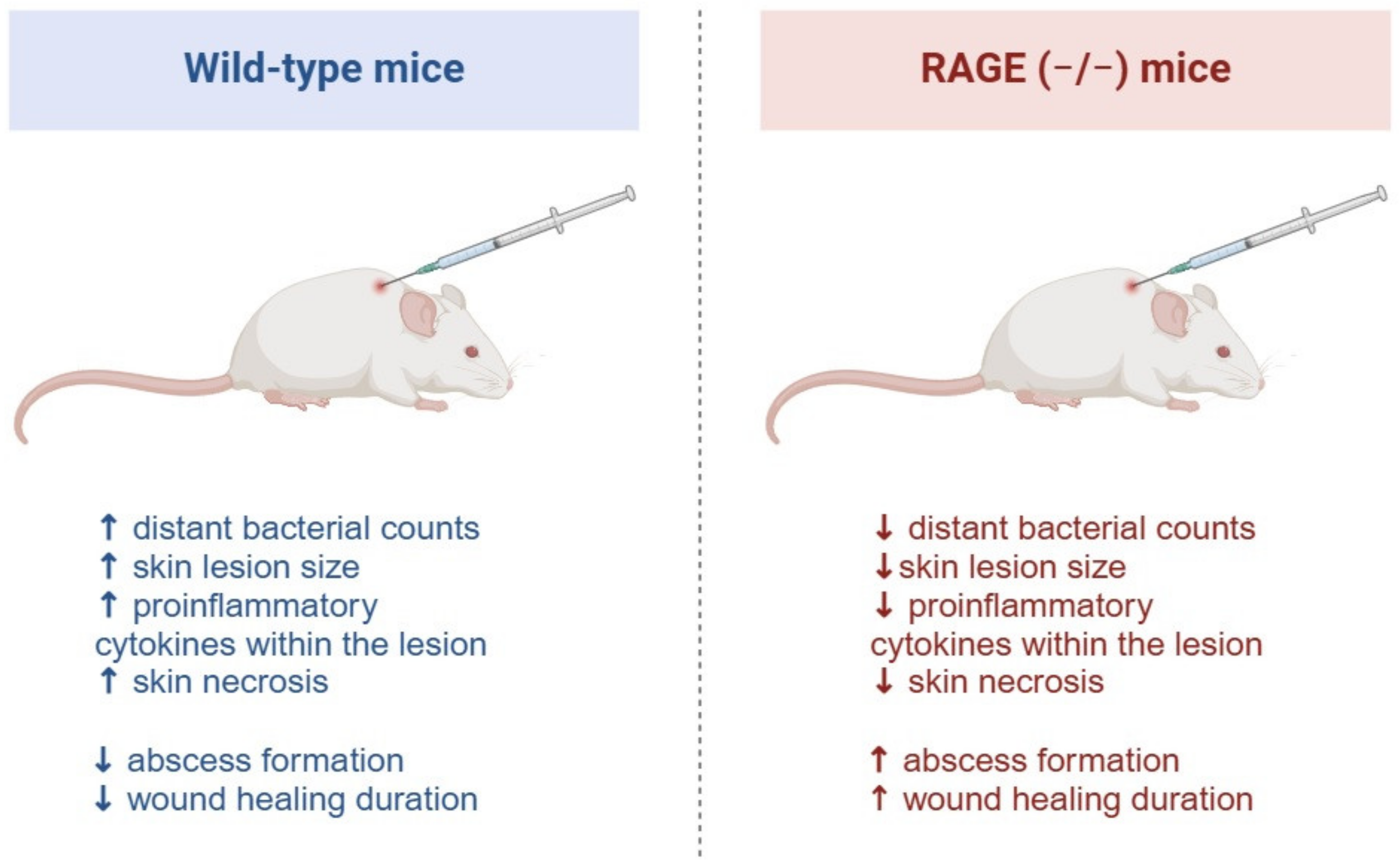
4.5. Skin Infection
4.6. Skin Fibrosis
| Model | Material | Condition | Method | Markers | Year | Authors [Ref.] |
|---|---|---|---|---|---|---|
| Human | Serum | Systemic sclerosis | ELISA, PCR | ↑S100A8, ↑S100A9 | 2013 | Xu et al. [90] |
| Human | Serum | Systemic sclerosis | ELISA | ↑HMGB1, ↑sRAGE, ↑IgG, ↑CRP | 2009 | Yoshizaki et al. [91] |
| Human | Serum | Systemic sclerosis | ELISA | ↑CML | 2007 | Kaloudi et al. [92] |
| Human | Skin biopsy | Systemic sclerosis | IHC, PCR, Western Blot | ↑S100A4 | 2015 | Tomcik et al. [84] |
| Human | Skin biopsy | Systemic sclerosis | IHC | ↑S100A8, ↑S100A9, ↑RAGE | 2013 | Xu et al. [90] |
| Human | Skin biopsy | Systemic sclerosis | IHC | ↑HMGB1, ↑RAGE | 2009 | Yoshizaki et al. [91] |
| Human | Skin biopsy | Hypertrophic scar and keloid | IHC | ↑S100A12 | 2017 | Zhao et al. [88] |
| Murine | Serum | Bleomycin-induced fibrosis | ELISA | ↑HMGB1, ↑sRAGE | 2009 | Yoshizaki et al. [91] |
| Murine | Skin biopsy | Bleomycin-induced fibrosis | IHC, Western Blot | ↑HMGB1, ↑iNOS, ↑IL-4, ↑Arg-1, ↑α2AP, ↑α-SMA, ↑type I collagen | 2020 | Kanno et al. [89] |
| Murine | Skin biopsy | Bleomycin-induced fibrosis | PCR, Western Blot | ↑S100A9, ↑IL-6, ↑IL-1β, ↑IL-8, ↑TNF-α | 2018 | Xu et al. [87] |
| Murine | Skin biopsy | Bleomycin-induced fibrosis | IHC, PCR, Western Blot | ↑S100A4 | 2015 | Tomcik et al. [84] |
4.7. Diabetic Wound Formation and Healing
4.8. Skin Cancer Progression
4.9. Skin Aging
4.10. Other Cutaneous Conditions
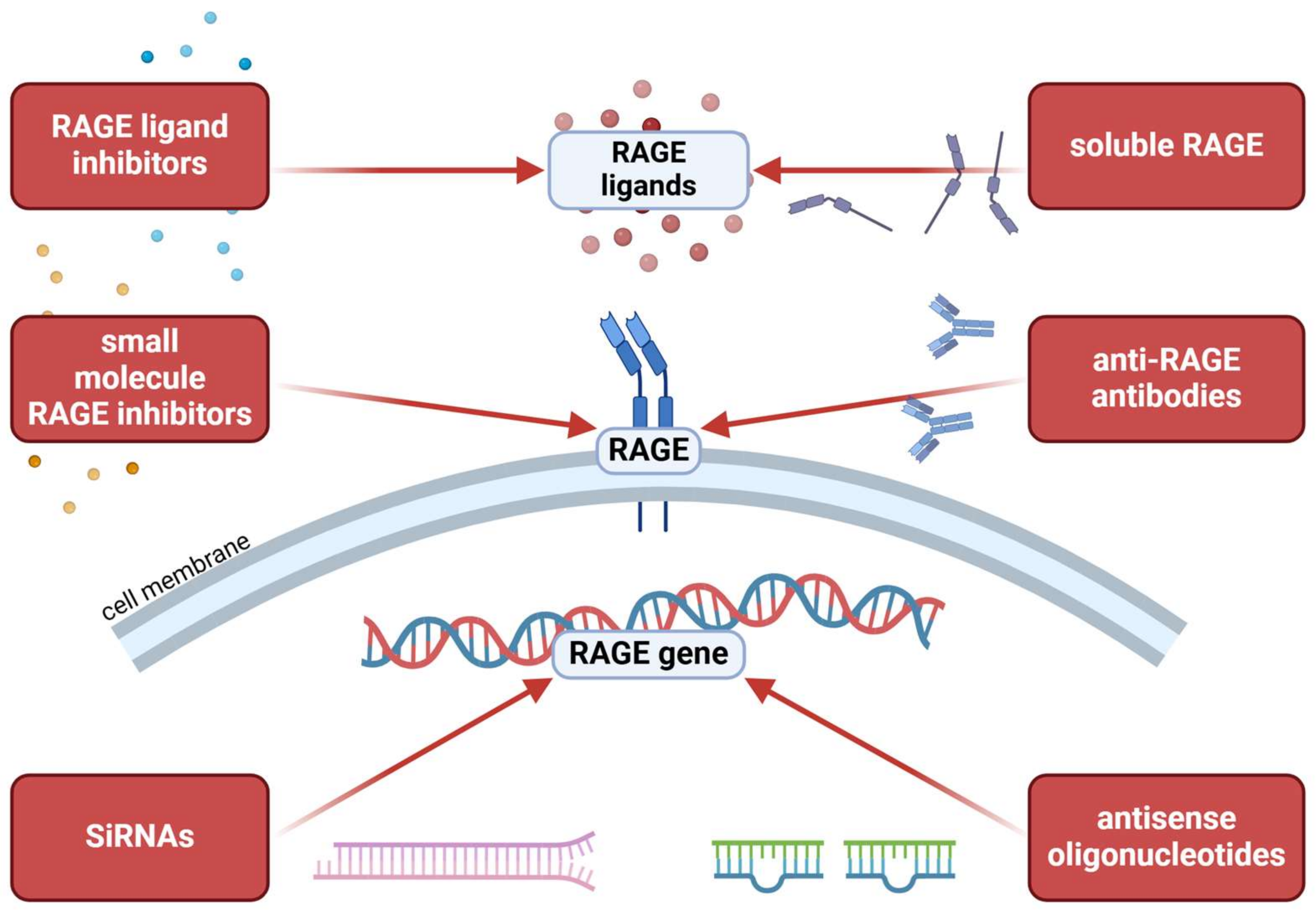
5. RAGE as a Treatment Target
5.1. RAGE-Modulating Therapies in Dermatology
| Condition | Model | Medication | Type of Medication | Outcome | Year | Authors [Ref.] |
|---|---|---|---|---|---|---|
| Psoriasis | HSFs, HaCaTs | Rutin | Non-specific RAGE modulator | ↓proliferation, ↓TNFα, ↓IL-6 | 2023 | Wang et al. [158] |
| Psoriasis | NHEKs | Lipoxin A4 | Non-specific RAGE modulator | ↓HMGB1, ↓RAGE, ↓TLR4, ↓ERK1/2 | 2017 | Liu et al. [161] |
| Psoriasis | Murine | Shenling Baizhu powder | Non-specific RAGE modulator | ↓PASI, ↓skin thickness, ↓proliferation, ↓IL-17 | 2024 | Tang et al. [160] |
| Psoriasis | Murine | Xijiao Dihuang Decoction | Non-specific RAGE modulator | ↓PASI, ↓skin thickness, ↓angiogenesis, ↓MMP9, ↓STAS3, ↓VEGFA, ↓TNFα, ↓IL-6 | 2023 | Guo et al. [159] |
| Psoriasis | Murine | Rutin | Non-specific RAGE modulator | ↓PASI, ↓skin thickness, ↓inflammatory cells infiltration, ↓NFκB, ↓RAGE | 2023 | Wang et al. [158] |
| Psoriasis | Murine | BML-111 | Non-specific RAGE modulator | ↓PASI, ↓erythema, ↓skin thickness, ↓HMGB1, ↓RAGE, ↓TLR4, ↓ERK1/2, ↓NF-κB, ↓IL-1β, ↓TNFα, ↓IL-6, ↓IL-17a, ↓IL-17c, ↓IL-23, ↓IL-22 | 2017 | Liu et al. [161] |
| Atopic dermatitis | HaCaTs | Coffea arabica | Non-specific RAGE modulator | ↓ROS, ↓ERK1/2, ↓p38, ↓NFκB, ↓NLRP3, ↓TNFα, ↓IL-6, ↓HMGB1, ↓RAGE, ↑filaggrin, ↑claudin-1 | 2023 | Chang et al. [166] |
| Atopic dermatitis | Mouse mastocytoma cell line | Glycyrrhizin | Non-specific RAGE modulator | ↓ERK1/2, ↓PI3K, ↓RAGE, ↓NFκB, ↓TNFα, ↓IL-6, ↓pAKT, ↓MC tryptase | 2018 | Wang et al. [164] |
| Atopic dermatitis | Murine | Coffea arabica | Non-specific RAGE modulator | ↓skin thickness, ↓erythema, ↓TNFα, ↓TSLP | 2023 | Chang et al. [166] |
| Atopic dermatitis | Murine | Glycyrrhizin | Non-specific RAGE modulator | ↓IgE, ↓dermatitis, ↓mast cells, ↓HMGB1, ↓RAGE, ↓NFκB, ↓TNFα, ↓IL-6 | 2018 | Wang et al. [164] |
| Atopic dermatitis | Murine | Tannic acid | Non-specific RAGE modulator | ↓cell proliferation, ↓skin thickness, ↓neutrophil and mast cells infiltration, ↑PPARγ, ↓TNFα, ↓HMGB1, ↓RAGE, ↓ERK1/2, ↓NFκB, ↓COX2, ↓IL-1β, ↓IFNγ, ↓IL-4 | 2015 | Karuppagounder et al. [165] |
| Atopic dermatitis | Murine | Quercetin | Non-specific RAGE modulator | ↓skin thickness, ↓inflammatory cells infiltration, ↑Nrf2, ↓HMGB1, ↓RAGE, ↓NFκB, ↓ERK1/2, ↓COX2, ↓TNFα, ↓IL-1β, ↓IL-2Rα, ↓IFNγ, ↓IL-4 | 2015 | Karuppagounder et al. [163] |
| Atopic dermatitis | Murine | Resveratrol | Non-specific RAGE modulator | ↓skin thickness, ↓inflammatory and mast cells infiltration, ↓GRP78, ↓CHOP, ↓cleaved caspase-7, ↓HMGB1, ↓RAGE, ↓NFκB, ↓PI3K, ↓ERK1/2, ↓COX2, ↓TNFα, ↓IL-1β, ↓IL-2Rα, ↓IFNγ, ↓IL-4 | 2014 | Karuppagounder et al. [162] |
| Lichen planus | CD3+ T lymphocytes | Quercetin | Non-specific RAGE modulator | ↓proliferation, ↓migration, ↑apoptosis, ↑IFN-γ, ↓IL-6 | 2022 | Zhao et al. [169] |
5.2. Targeting RAGE to Improve Wound Healing
| Condition | Model | Medication | Type of Medication | Outcome | Year | Authors [Ref.] |
|---|---|---|---|---|---|---|
| Non-diabetic wound | HSFs | THC/HGF | Non-specific RAGE modulator | ↓HMGB1, ↓RAGE, ↓pNF-κB, ↑BCL-2, ↓BAX, ↓cleaved caspase-3, ↓TNFα, ↓IL-6, ↓TGF-β, ↓α-SMA, ↑COL3A1, ↓FN1, ↓COL1A1 | 2024 | Xing et al. [176] |
| Diabetic wound | HSFs | Dang-Gui-Si-Ni decoction | Non-specific RAGE modulator | ↓α-SMA, ↓collagen I, ↓Smad2, ↓AGEs, ↓RAGE, ↑TGF-β1, ↑Smad3 | 2024 | Zhang et al. [181] |
| Diabetic wound | HSFs | dMSC-sEVs | Non-specific RAGE modulator | ↑proliferation, ↑migration, ↑α-SMA, ↑collagen I, ↑Smad, ↓RAGE | 2020 | Bian et al. [188] |
| Diabetic wound | HaCaTs | N-acetyl-L-cysteine | Non-specific RAGE modulator | ↓AGEs, ↑cell viability, ↑cell migration ↓IL-6, ↓IL-8, ↓MMP9, ↓NF-κB, | 2017 | Yang et al. [187] |
| Diabetic wound | Porcine | Anti-RAGE antibody | RAGE-specific inhibitor | ↓wound size, ↑collagen, ↓RAGE, ↓Mac, ↓IL-6 | 2022 | Johnson et al. [170] |
| Non-diabetic wound | Murine | THC/HGF | Non-specific RAGE modulator | ↓fibrosis, ↑regular collagen fibers, ↑epidermis thickness, ↑angiogenesis, ↑CD31, ↑CD206, ↓INOS, ↓HMGB1, ↓RAGE, ↓TGF-β | 2024 | Xing et al. [176] |
| Diabetic wound | Murine | Dang-Gui-Si-Ni decoction | Non-specific RAGE modulator | ↓wound size, ↓IL-1β, ↓IL-6, ↓TNFα, ↓AGEs, ↓RAGE, ↑INS, ↑Ang-1, ↑VEGF, ↑Tie-2, ↑TGF-β1, ↑Smad3, ↓Smad2 | 2024 | Zhang et al. [181] |
| Non-diabetic wound | Murine | Cucurbitaceae seed oils | Non-specific RAGE modulator | ↓wound size, ↑epidermis thickness, ↓AGEs, ↓RAGE, ↑Nrf2, ↑HO-1, ↓TNF-α, ↓NF-κB, ↓NLRP3, ↓CX-43, ↓EGF | 2024 | Emad et al. [182] |
| Diabetic wound | Murine | Resveratrol | Non-specific RAGE modulator | ↓wound size, ↑epidermis thickness, ↓IL-1β, ↓IL-6, ↓IL-18, ↓TNF-α, ↓RAGE, ↓NF-κB | 2023 | Youjun et al. [183] |
| Diabetic wound | Murine | vRAGE-ELP/SDF1-ELP | Decoy receptor/angiogenic chemokine | ↓wound size, ↑skin thickness, ↑CD31 | 2023 | Kang et al. [177] |
| Diabetic wound | Murine | Polygonatum kingianum (polygonati rhizome) | Non-specific RAGE modulator | ↓wound size, ↓inflammatory infiltration, ↑angiogenesis, ↑skin thickness, ↓AGEs, ↓RAGE, ↑Nrf2, ↑HO-1, ↑CD34, ↑bFGF, ↑VEGF, ↓SOD, ↓GSH, ↓MMP-9, ↓MMP-2, ↑TIMP-2, ↓GSP, ↓GHb, ↓ICAM-1, ↑T-AOC, ↑SOD, ↑FINS, ↓MDA, ↓TNFα, ↓IL-6, ↓IL-2, ↓IFN-γ | 2022 | Pan-Yue et al. [180] |
| Diabetic wound | Murine | vRAGE-ELP | Decoy receptor | ↓wound size, ↓epithelial gap | 2021 | Kang et al. [173] |
| Diabetic wound | Murine | dMSC-sEVs | Non-specific RAGE modulator | ↓wound size, ↑collagen, ↑PCNA, ↑CXCR4, ↑α-SMA, ↓p21 | 2020 | Bian et al. [188] |
| Diabetic and non-diabetic wound | Murine | miRNA-221-3p | Non-specific RAGE modulator | ↓wound size, ↑VEGF, ↑CD31, ↑Ki67 | 2020 | Xu et al. [189] |
| Diabetic wound | Murine | Ibrutinib | Bruton tyrosine kinase inhibitor | ↓wound size, ↓IL-1β, ↓TNF-α, ↓IL-6, ↓TLR2, ↓TLR4, ↓RAGE, ↓NF-κB | 2019 | Yang et al. [186] |
| Diabetic wound | Murine | sRAGE/SDF-1 | Decoy receptor/angiogenic chemokine | ↓wound size | 2016 | Olekson et al. [172] |
| Burn wound | Murine | Thymosin beta 4 | Non-specific RAGE modulator | ↓wound size, ↑granulation, ↑Ki67, ↑angiogenesis, ↓TNFα, ↓IL-1β, ↑VEGF, ↓RAGE | 2014 | Kim et al. [190] |
| Diabetic wound | Murine | Aminoguanidine | Non-specific RAGE modulator | ↓inflammatory infiltrate, ↓AGEs, ↓RAGE, ↓NF-κB | 2013 | Tian et al. [184] |
| Diabetic wound | Murine | AuNP/EGCG/ALA | Non-specific RAGE modulator | ↓wound size, ↓RAGE, ↑VEGF, ↓monocyte infiltration | 2012 | Chen et al. [175] |
| Diabetic wound | Murine | sRAGE | Decoy receptor | ↑neovascularization, ↑granulation, ↓epithelial gap | 2004 | Wear-Maggitti et al. [174] |
| Diabetic wound | Murine | sRAGE | Decoy receptor | ↓wound size, ↓TNFα, ↓IL-6, ↓MMP2, ↓MMP3, ↓MMP9, ↑PDGF-B, ↑VEGF | 2001 | Goova et al. [171] |
5.3. Anti-RAGE Therapy in Skin Cancer
| Condition | Model | Medication | Type of Medication | Outcome | Year | Authors [Ref.] |
|---|---|---|---|---|---|---|
| Melanoma | Human melanoma cell line A375 | sRAGE | Decoy receptor | ↓cell migration | 2016 | Herwig et al. [120] |
| Melanoma | Human melanoma cell line SK-MEL28 | MK615 | Non-specific RAGE modulator | ↓proliferation, ↑apoptosis, ↓RAGE, ↓HMGB1 | 2010 | Matsushita et al. [196] |
| Melanoma | Human melanoma cell line G361 and A375 | Anti-RAGE antibody | RAGE-specific inhibitor | ↓proliferation | 2004 | Abe et al. [113] |
| Melanoma | Murine | Anti-RAGE antibody | RAGE-specific inhibitor | ↓tumor size, ↓lung metastasis, ↑mice surivial | 2004 | Abe et al. [113] |
| Tumor angiogenesis | HUVECs | Centella asiatica | Non-specific RAGE modulator | ↓proliferation, ↓cell migration, ↓vascular tube formation | 2013 | Zhu et al. [122] |
5.4. Targeting RAGE to Decelerate Skin Aging
| Condition | Model | Medication | Type of Medication | Outcome | Year | Authors [Ref.] |
|---|---|---|---|---|---|---|
| AGEs exposure, UVB irradiation | HaCaTs | Plantamajoside | Non-specific RAGE modulator | ↑cell viability, ↓ROS, ↓RAGE, ↓MMP1, ↓TNFα, ↓IL-1β, ↓NF-ĸB/p65 | 2016 | Han et al. [197] |
| AGEs exposure, UVB irradiation | HSFs | Plantamajoside | Non-specific RAGE modulator | ↓RAGE, ↓MMP1 | 2016 | Han et al. [197] |
| AGEs exposure, UVB irradiation | HSFs | Chenopodium formosanum | Non-specific RAGE modulator | ↓ROS, ↑Nrf2, ↑HO-1, ↓MMP1, ↓MMP3, ↓MMP9, ↑TIMP-1, ↓MAPK, ↓AP-1, ↓RAGE, ↑collagen | 2022 | Lyu et al. [199] |
| UVB irradiation | HaCaTs | Ba Zhen Tang | Non-specific RAGE modulator | ↓SA-β-gal, ↓p16INK4a | 2022 | Han et al. [201] |
| UVB irradiation | Murine | Schizonepeta tenuifolia | Non-specific RAGE modulator | ↓skin wrinkles, ↓skin thickness, ↑collagen, ↓MMPs, ↑TIMP-1, ↓skin dehydration, ↑hyaluronic acid, ↓MAPK, ↓NF-κB, ↓AGEs ↓RAGE | 2023 | Gu et al. [203] |
| MGO exposure | CCC-ESF-1 | Gentiopicroside | Non-specific RAGE modulator | ↓CML, ↑cell viability, ↑FN-1, ↑LM-5, ↑COL-1, ↓MMP2, ↓MMP9, ↓ROS, ↓IL-6, ↓IL-8, ↓IL-1β, ↓NF-κB, ↓RAGE | 2024 | Chen et al. [198] |
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hudson, B.I.; Lippman, M.E. Targeting RAGE Signaling in Inflammatory Disease. Annu. Rev. Med. 2018, 69, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Guarneri, F.; Custurone, P.; Papaianni, V.; Gangemi, S. Involvement of RAGE and Oxidative Stress in Inflammatory and Infectious Skin Diseases. Antioxidants 2021, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Tampa, M.; Neagu, M.; Caruntu, C.; Constantin, C.; Georgescu, S.R. Skin Inflammation-A Cornerstone in Dermatological Conditions. J. Pers. Med. 2022, 12, 1370. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Jiang, H.; Ren, H.; Hu, X.; Wang, X.; Han, C. AGEs and chronic subclinical inflammation in diabetes: Disorders of immune system. Diabetes Metab. Res. Rev. 2015, 31, 127–137. [Google Scholar] [CrossRef]
- Senatus, L.M.; Schmidt, A.M. The AGE-RAGE Axis: Implications for Age-Associated Arterial Diseases. Front. Genet. 2017, 8, 187. [Google Scholar] [CrossRef]
- Neeper, M.; Schmidt, A.M.; Brett, J.; Yan, S.D.; Wang, F.; Pan, Y.C.; Elliston, K.; Stern, D.; Shaw, A. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J. Biol. Chem. 1992, 267, 14998–15004. [Google Scholar] [CrossRef]
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced Glycation End Products (AGEs): Biochemistry, Signaling, Analytical Methods, and Epigenetic Effects. Oxid. Med. Cell Longev. 2020, 2020, 3818196. [Google Scholar] [CrossRef]
- Kierdorf, K.; Fritz, G. RAGE regulation and signaling in inflammation and beyond. J. Leukoc. Biol. 2013, 94, 55–68. [Google Scholar] [CrossRef]
- Suresh, R.; Mosser, D.M. Pattern recognition receptors in innate immunity, host defense, and immunopathology. Adv. Physiol. Educ. 2013, 37, 284–291. [Google Scholar] [CrossRef]
- Teissier, T.; Boulanger, É. The receptor for advanced glycation end-products (RAGE) is an important pattern recognition receptor (PRR) for inflammaging. Biogerontology 2019, 20, 279–301. [Google Scholar] [CrossRef]
- Chavakis, T.; Bierhaus, A.; Nawroth, P.P. RAGE (receptor for advanced glycation end products): A central player in the inflammatory response. Microbes Infect. 2004, 6, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Yue, Q.; Song, Y.; Liu, Z.; Zhang, L.; Yang, L.; Li, J. Receptor for Advanced Glycation End Products (RAGE): A Pivotal Hub in Immune Diseases. Molecules 2022, 27, 4922. [Google Scholar] [CrossRef] [PubMed]
- Scaffidi, P.; Misteli, T.; Bianchi, M.E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002, 418, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Gąsiorowski, K.; Brokos, B.; Echeverria, V.; Barreto, G.E.; Leszek, J. RAGE-TLR Crosstalk Sustains Chronic Inflammation in Neurodegeneration. Mol. Neurobiol. 2018, 55, 1463–1476. [Google Scholar] [CrossRef]
- Andersson, U.; Yang, H.; Harris, H. Extracellular HMGB1 as a therapeutic target in inflammatory diseases. Expert. Opin. Ther. Targets. 2018, 22, 263–277. [Google Scholar] [CrossRef]
- Kang, R.; Zhang, Q.; Zeh, H.J., 3rd; Lotze, M.T.; Tang, D. HMGB1 in cancer: Good, bad, or both? Clin. Cancer Res. 2013, 19, 4046–4057. [Google Scholar] [CrossRef]
- Naglova, H.; Bucova, M. HMGB1 and its physiological and pathological roles. Bratisl. Lek. Listy 2012, 113, 163–171. [Google Scholar] [CrossRef]
- Cerón, J.J.; Ortín-Bustillo, A.; López-Martínez, M.J.; Martínez-Subiela, S.; Eckersall, P.D.; Tecles, F.; Tvarijonaviciute, A.; Muñoz-Prieto, A. S-100 Proteins: Basics and Applications as Biomarkers in Animals with Special Focus on Calgranulins (S100A8, A9, and A12). Biology 2023, 12, 881. [Google Scholar] [CrossRef]
- Gonzalez, L.L.; Garrie, K.; Turner, M.D. Role of S100 proteins in health and disease. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118677. [Google Scholar] [CrossRef]
- Hattori, F.; Kiatsurayanon, C.; Okumura, K.; Ogawa, H.; Ikeda, S.; Okamoto, K.; Niyonsaba, F. The antimicrobial protein S100A7/psoriasin enhances the expression of keratinocyte differentiation markers and strengthens the skin’s tight junction barrier. Br. J. Dermatol. 2014, 171, 742–753. [Google Scholar] [CrossRef]
- Zhang, G.; Li, M.; Jin, J.; Bai, Y.; Yang, C. Knockdown of S100A4 decreases tumorigenesis and metastasis in osteosarcoma cells by repression of matrix metalloproteinase-9. Asian Pac. J. Cancer Prev. 2011, 12, 2075–2080. [Google Scholar] [PubMed]
- Ligthart, S.; Sedaghat, S.; Ikram, M.A.; Hofman, A.; Franco, O.H.; Dehghan, A. EN-RAGE: A novel inflammatory marker for incident coronary heart disease. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2695–2699. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.D.; Chen, X.; Fu, J.; Chen, M.; Zhu, H.; Roher, A.; Slattery, T.; Zhao, L.; Nagashima, M.; Morser, J.; et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature 1996, 382, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Lue, L.F.; Walker, D.G.; Brachova, L.; Beach, T.G.; Rogers, J.; Schmidt, A.M.; Stern, D.M.; Yan, S.D. Involvement of microglial receptor for advanced glycation endproducts (RAGE) in Alzheimer’s disease: Identification of a cellular activation mechanism. Exp. Neurol. 2001, 171, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Sparvero, L.J.; Asafu-Adjei, D.; Kang, R.; Tang, D.; Amin, N.; Im, J.; Rutledge, R.; Lin, B.; Amoscato, A.A.; Zeh, H.J.; et al. RAGE (Receptor for Advanced Glycation Endproducts), RAGE ligands, and their role in cancer and inflammation. J. Transl. Med. 2009, 7, 17. [Google Scholar] [CrossRef]
- Liu, L.; Yang, M.; Kang, R.; Dai, Y.; Yu, Y.; Gao, F.; Wang, H.; Sun, X.; Li, X.; Li, J.; et al. HMGB1-DNA complex-induced autophagy limits AIM2 inflammasome activation through RAGE. Biochem. Biophys. Res. Commun. 2014, 450, 851–856. [Google Scholar] [CrossRef]
- Fritz, G. RAGE: A single receptor fits multiple ligands. Trends Biochem. Sci. 2011, 36, 625–632. [Google Scholar] [CrossRef]
- Rouhiainen, A.; Kuja-Panula, J.; Tumova, S.; Rauvala, H. RAGE-mediated cell signaling. Methods Mol. Biol. 2013, 963, 239–263. [Google Scholar]
- Gutowska, K.; Czajkowski, K.; Kuryłowicz, A. Receptor for the Advanced Glycation End Products (RAGE) Pathway in Adipose Tissue Metabolism. Int. J. Mol. Sci. 2023, 24, 10982. [Google Scholar] [CrossRef]
- Schmidt, A.M. Soluble RAGEs—Prospects for treating & tracking metabolic and inflammatory disease. Vascul Pharmacol. 2015, 72, 1–8. [Google Scholar]
- Santilli, F.; Vazzana, N.; Bucciarelli, L.G.; Davì, G. Soluble forms of RAGE in human diseases: Clinical and therapeutical implications. Curr. Med. Chem. 2009, 16, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Sabbatinelli, J.; Castiglione, S.; Macrì, F.; Giuliani, A.; Ramini, D.; Vinci, M.C.; Tortato, E.; Bonfigli, A.R.; Olivieri, F.; Raucci, A. Circulating levels of AGEs and soluble RAGE isoforms are associated with all-cause mortality and development of cardiovascular complications in type 2 diabetes: A retrospective cohort study. Cardiovasc. Diabetol. 2022, 21, 95. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Wang, R.; Li, T.; Zhou, J.P.; Chang, G.Q.; Zhao, N.; Yang, L.N.; Zhai, H.; Yang, L. Reduced soluble RAGE is associated with disease severity of axonal Guillain-Barré syndrome. Sci. Rep. 2016, 6, 21890. [Google Scholar] [CrossRef]
- Tesarová, P.; Kalousová, M.; Jáchymová, M.; Mestek, O.; Petruzelka, L.; Zima, T. Receptor for advanced glycation end products (RAGE)—Soluble form (sRAGE) and gene polymorphisms in patients with breast cancer. Cancer Investig. 2007, 25, 720–725. [Google Scholar] [CrossRef]
- Downs, C.A. Analysis of RAGE Proteome and Interactome in Lung Adenocarcinoma Using PANTHER and STRING Databases. Biol. Res. Nurs. 2021, 23, 698–707. [Google Scholar] [CrossRef]
- Yonekura, H.; Yamamoto, Y.; Sakurai, S.; Petrova, R.G.; Abedin, M.J.; Li, H.; Yasui, K.; Takeuchi, M.; Makita, Z.; Takasawa, S.; et al. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem. J. 2003, 370 Pt 3, 1097–1109. [Google Scholar] [CrossRef]
- Qin, J.; Goswami, R.; Dawson, S.; Dawson, G. Expression of the receptor for advanced glycation end products in oligodendrocytes in response to oxidative stress. J. Neurosci. Res. 2008, 86, 2414–2422. [Google Scholar] [CrossRef]
- Grossin, N.; Wautier, M.P.; Picot, J.; Stern, D.M.; Wautier, J.L. Differential effect of plasma or erythrocyte AGE-ligands of RAGE on expression of transcripts for receptor isoforms. Diabetes Metab. 2009, 35, 410–417. [Google Scholar] [CrossRef]
- Iwamura, M.; Yamamoto, Y.; Kitayama, Y.; Higuchi, K.; Fujimura, T.; Hase, T.; Yamamoto, H. Epidermal expression of receptor for advanced glycation end products (RAGE) is related to inflammation and apoptosis in human skin. Exp. Dermatol. 2016, 25, 235–237. [Google Scholar] [CrossRef]
- Leibold, J.S.; Riehl, A.; Hettinger, J.; Durben, M.; Hess, J.; Angel, P. Keratinocyte-specific deletion of the receptor RAGE modulates the kinetics of skin inflammation in vivo. J. Investig. Dermatol. 2013, 133, 2400–2406. [Google Scholar] [CrossRef]
- Sultana, R.; Parveen, A.; Kang, M.C.; Hong, S.M.; Kim, S.Y. Glyoxal-derived advanced glycation end products (GO-AGEs) with UVB critically induce skin inflammaging: In vitro and in silico approaches. Sci. Rep. 2024, 14, 1843. [Google Scholar] [CrossRef] [PubMed]
- Wolf, R.; Howard, O.M.; Dong, H.F.; Voscopoulos, C.; Boeshans, K.; Winston, J.; Divi, R.; Gunsior, M.; Goldsmith, P.; Ahvazi, B.; et al. Chemotactic activity of S100A7 (Psoriasin) is mediated by the receptor for advanced glycation end products and potentiates inflammation with highly homologous but functionally distinct S100A15. J. Immunol. 2008, 181, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Sun, Y.; Wu, T.; Hou, X.; Zheng, S.; Zhang, H.; Lin, T.; Liu, H.; Sun, T. Echinacoside-Zinc Nanomaterial Inhibits Skin Glycation by Suppressing the Transcriptional Activation of the Receptor for Advanced Glycation End-Products. ACS Nano 2023, 17, 14123–14135. [Google Scholar] [CrossRef]
- Lamore, S.D.; Cabello, C.M.; Wondrak, G.T. HMGB1-directed drug discovery targeting cutaneous inflammatory dysregulation. Curr. Drug Metab. 2010, 11, 250–265. [Google Scholar] [CrossRef]
- Kang, P.; Chen, J.; Wang, S.; Zhang, S.; Li, S.; Guo, S.; Song, P.; Liu, L.; Wang, G.; Gao, T.; et al. Advanced Glycation End Products-Induced Activation of Keratinocytes: A Mechanism Underlying Cutaneous Immune Response in Psoriasis. J. Innate Immun. 2023, 15, 876–892. [Google Scholar] [CrossRef]
- Papagrigoraki, A.; Del Giglio, M.; Cosma, C.; Maurelli, M.; Girolomoni, G.; Lapolla, A. Advanced Glycation End Products are Increased in the Skin and Blood of Patients with Severe Psoriasis. Acta Derm. Venereol. 2017, 97, 782–787. [Google Scholar] [CrossRef]
- Damasiewicz-Bodzek, A.; Nowak, A. Concentrations of N6-Carboxymethyllysine (CML), N6-Carboxyethyllysine (CEL), and Soluble Receptor for Advanced Glycation End-Products (sRAGE) Are Increased in Psoriatic Patients. Biomolecules 2022, 12, 1870. [Google Scholar] [CrossRef]
- Karas, A.; Holmannova, D.; Borsky, P.; Fiala, Z.; Andrys, C.; Hamakova, K.; Svadlakova, T.; Palicka, V.; Krejsek, J.; Rehacek, V.; et al. Significantly Altered Serum Levels of NAD, AGE, RAGE, CRP, and Elastin as Potential Biomarkers of Psoriasis and Aging-A Case-Control Study. Biomedicines 2022, 10, 1133. [Google Scholar] [CrossRef]
- Strohbuecker, L.; Koenen, H.; van Rijssen, E.; van Cranenbroek, B.; Fasse, E.; Joosten, I.; Körber, A.; Bergmann, C. Increased dermal expression of chromatin-associated protein HMGB1 and concomitant T-cell expression of the DNA RAGE in patients with psoriasis vulgaris. Psoriasis 2019, 9, 7–17. [Google Scholar] [CrossRef]
- Vasků, V.; Kanková, K.; Vasků, A.; Muzík, J.; Izakovicová Hollá, L.; Semrádová, V.; Vácha, J. Gene polymorphisms (G82S, 1704G/T, 2184A/G and 2245G/A) of the receptor of advanced glycation end products (RAGE) in plaque psoriasis. Arch. Dermatol Res. 2002, 294, 127–130. [Google Scholar] [CrossRef]
- Salem, S.A.M.; El-Khateeb, E.A.; Harvy, M.; Emam, H.M.E.; Abdelaal, W.; Nemr, R.E.; El-Hagry, O.O. Study of serum levels and skin expression of S100B protein in psoriasis. An. Bras. Dermatol. 2017, 92, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, M.; Sawada, Y.; Saito-Sasaki, N.; Yoshioka, H.; Hama, K.; Omoto, D.; Ohmori, S.; Okada, E.; Nakamura, M. High S100A2 expression in keratinocytes in patients with drug eruption. Sci. Rep. 2021, 11, 5493. [Google Scholar] [CrossRef] [PubMed]
- Zibert, J.R.; Skov, L.; Thyssen, J.P.; Jacobsen, G.K.; Grigorian, M. Significance of the S100A4 protein in psoriasis. J. Investig. Dermatol. 2010, 130, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Borsky, P.; Fiala, Z.; Andrys, C.; Beranek, M.; Hamakova, K.; Malkova, A.; Svadlakova, T.; Krejsek, J.; Palicka, V.; Borska, L.; et al. Alarmins HMGB1, IL-33, S100A7, and S100A12 in Psoriasis Vulgaris. Mediators Inflamm. 2020, 2020, 8465083. [Google Scholar] [CrossRef]
- Wilsmann-Theis, D.; Wagenpfeil, J.; Holzinger, D.; Roth, J.; Koch, S.; Schnautz, S.; Bieber, T.; Wenzel, J. Among the S100 proteins, S100A12 is the most significant marker for psoriasis disease activity. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1165–1170. [Google Scholar] [CrossRef]
- Wolf, R.; Mirmohammadsadegh, A.; Walz, M.; Lysa, B.; Tartler, U.; Remus, R.; Hengge, U.; Michel, G.; Ruzicka, T. Molecular cloning and characterization of alternatively spliced mRNA isoforms from psoriatic skin encoding a novel member of the S100 family. FASEB J. 2003, 17, 1969–1971. [Google Scholar] [CrossRef]
- Lei, H.; Li, X.; Jing, B.; Xu, H.; Wu, Y. Human S100A7 Induces Mature Interleukin1α Expression by RAGE-p38 MAPK-Calpain1 Pathway in Psoriasis. PLoS ONE 2017, 12, e0169788. [Google Scholar] [CrossRef]
- Son, E.D.; Kim, H.J.; Kim, K.H.; Bin, B.H.; Bae, I.H.; Lim, K.M.; Yu, S.J.; Cho, E.G.; Lee, T.R. S100A7 (psoriasin) inhibits human epidermal differentiation by enhanced IL-6 secretion through IκB/NF-κB signalling. Exp. Dermatol. 2016, 25, 636–641. [Google Scholar] [CrossRef]
- Anderson, K.S.; Wong, J.; Polyak, K.; Aronzon, D.; Enerbäck, C. Detection of psoriasin/S100A7 in the sera of patients with psoriasis. Br. J. Dermatol. 2009, 160, 325–332. [Google Scholar] [CrossRef]
- Hong, J.Y.; Kim, M.J.; Hong, J.K.; Noh, H.H.; Park, K.Y.; Lee, M.K.; Seo, S.J. In vivo quantitative analysis of advanced glycation end products in atopic dermatitis-Possible culprit for the comorbidities? Exp. Dermatol. 2020, 29, 1012–1016. [Google Scholar] [CrossRef]
- Eke-Gungor, H.; Sagiroglu, B.; Can-Coskun, Z.N.; Kocer, D.; Karakukcu, Ç. Serum sRAGE levels in children with atopic dermatitis: A prospective study. Postepy Dermatol. Alergol. 2023, 40, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Kasperska-Zajac, A.; Damasiewicz-Bodzek, A.; Tyrpień-Golder, K.; Zamlyński, J.; Grzanka, A. Circulating soluble receptor for advanced glycation end products is decreased and inversely associated with acute phase response in chronic spontaneous urticaria. Inflamm. Res. 2016, 65, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Park, C.O.; Shin, J.U.; Noh, J.Y.; Lee, Y.S.; Lee, N.R.; Kim, H.R.; Noh, S.; Lee, Y.; Lee, J.H.; et al. DAMP molecules S100A9 and S100A8 activated by IL-17A and house-dust mites are increased in atopic dermatitis. Exp. Dermatol. 2014, 23, 938–941. [Google Scholar] [CrossRef]
- Suárez-Fariñas, M.; Dhingra, N.; Gittler, J.; Shemer, A.; Cardinale, I.; de Guzman Strong, C.; Krueger, J.G.; Guttman-Yassky, E. Intrinsic atopic dermatitis shows similar TH2 and higher TH17 immune activation compared with extrinsic atopic dermatitis. J. Allergy Clin. Immunol. 2013, 132, 361–370. [Google Scholar] [CrossRef]
- Gläser, R.; Meyer-Hoffert, U.; Harder, J.; Cordes, J.; Wittersheim, M.; Kobliakova, J.; Fölster-Holst, R.; Proksch, E.; Schröder, J.M.; Schwarz, T. The antimicrobial protein psoriasin (S100A7) is upregulated in atopic dermatitis and after experimental skin barrier disruption. J. Investig. Dermatol. 2009, 129, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Usatine, R.P.; Tinitigan, M. Diagnosis and treatment of lichen planus. Am. Fam. Physician 2011, 84, 53–60. [Google Scholar]
- Lodi, G.; Giuliani, M.; Majorana, A.; Sardella, A.; Bez, C.; Demarosi, F.; Carrassi, A. Lichen planus and hepatitis C virus: A multicentre study of patients with oral lesions and a systematic review. Br. J. Dermatol. 2004, 151, 1172–1181. [Google Scholar] [CrossRef]
- De Vries, H.J.; van Marle, J.; Teunissen, M.B.; Picavet, D.; Zorgdrager, F.; Bos, J.D.; Weel, J.; Cornelissen, M. Lichen planus is associated with human herpesvirus type 7 replication and infiltration of plasmacytoid dendritic cells. Br. J. Dermatol. 2006, 154, 361–364. [Google Scholar] [CrossRef]
- De Carvalho, G.C.; Domingues, R.; de Sousa Nogueira, M.A.; Calvielli Castelo Branco, A.C.; Gomes Manfrere, K.C.; Pereira, N.V.; Aoki, V.; Sotto, M.N.; Da Silva Duarte, A.J.; Sato, M.N. Up-regulation of Proinflammatory Genes and Cytokines Induced by S100A8 in CD8+ T Cells in Lichen Planus. Acta Derm. Venereol. 2016, 96, 485–489. [Google Scholar] [CrossRef]
- De Carvalho, G.C.; Hirata, F.Y.A.; Domingues, R.; Figueiredo, C.A.; Zaniboni, M.C.; Pereira, N.V.; Sotto, M.N.; Aoki, V.; da Silva Duarte, A.J.; Sato, M.N. Up-regulation of HMGB1 and TLR4 in skin lesions of lichen planus. Arch. Dermatol. Res. 2018, 310, 523–528. [Google Scholar] [CrossRef]
- Salem, A.; Almahmoudi, R.; Vehviläinen, M.; Salo, T. Role of the high mobility group box 1 signalling axes via the receptor for advanced glycation end-products and toll-like receptor-4 in the immunopathology of oral lichen planus: A potential drug target? Eur J Oral Sci. 2018, 126, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, J.; Özgirgin, N.; Olszewska, E. Cholesteatoma Definition and Classification: A Literature Review. J. Int. Adv. Otol. 2017, 13, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Szczepanski, M.J.; Luczak, M.; Olszewska, E.; Molinska-Glura, M.; Zagor, M.; Krzeski, A.; Skarzynski, H.; Misiak, J.; Dzaman, K.; Bilusiak, M.; et al. Molecular signaling of the HMGB1/RAGE axis contributes to cholesteatoma pathogenesis. J. Mol. Med. 2015, 93, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Łuczak, M.W.; Dżaman, K.; Zaręba, Ł.; Czerwaty, K.; Siewiera, J.; Głuszko, A.; Olszewska, E.; Brzost, J.; Kantor, I.; Szczepański, M.J.; et al. HMGB1 Carried by Small Extracellular Vesicles Potentially Plays a Role in Promoting Acquired Middle Ear Cholesteatoma. Diagnostics 2023, 13, 3469. [Google Scholar] [CrossRef]
- Achouiti, A.; Van’t Veer, C.; de Vos, A.F.; van der Poll, T. The receptor for advanced glycation end products promotes bacterial growth at distant body sites in Staphylococcus aureus skin infection. Microbes Infect. 2015, 17, 622–627. [Google Scholar] [CrossRef]
- Na, M.; Mohammad, M.; Fei, Y.; Wang, W.; Holdfeldt, A.; Forsman, H.; Ali, A.; Pullerits, R.; Jin, T. Lack of Receptor for Advanced Glycation End Products Leads to Less Severe Staphylococcal Skin Infection but More Skin Abscesses and Prolonged Wound Healing. J. Infect. Dis. 2018, 218, 791–800. [Google Scholar] [CrossRef]
- Kim, M.H.; Choi, Y.W.; Choi, H.Y.; Myung, K.B.; Cho, S.N. The expression of RAGE and EN-RAGE in leprosy. Br. J. Dermatol. 2006, 154, 594–601. [Google Scholar] [CrossRef]
- Taslimi, Y.; Agbajogu, C.; Brynjolfsson, S.F.; Masoudzadeh, N.; Mashayekhi, V.; Gharibzadeh, S.; Östensson, M.; Nakka, S.S.; Mizbani, A.; Rafati, S.; et al. Profiling inflammatory response in lesions of cutaneous leishmaniasis patients using a non-invasive sampling method combined with a high-throughput protein detection assay. Cytokine 2020, 130, 155056. [Google Scholar] [CrossRef]
- Van Zoelen, M.A.; Schouten, M.; de Vos, A.F.; Florquin, S.; Meijers, J.C.; Nawroth, P.P.; Bierhaus, A.; van der Poll, T. The receptor for advanced glycation end products impairs host defense in pneumococcal pneumonia. J. Immunol. 2009, 182, 4349–4356. [Google Scholar] [CrossRef]
- Achouiti, A.; de Vos, A.F.; de Beer, R.; Florquin, S.; van ‘t Veer, C.; van der Poll, T. Limited role of the receptor for advanced glycation end products during Streptococcus pneumoniae bacteremia. J. Innate Immun. 2013, 5, 603–612. [Google Scholar] [CrossRef]
- O’Reilly, S. S100A4 a classical DAMP as a therapeutic target in fibrosis. Matrix Biol. 2024, 127, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, J.C.; Lee, M.H.; Yang, C.E.; Lee, J.H.; Lee, W.J. High-Mobility Group Box 1 Mediates Fibroblast Activity via RAGE-MAPK and NF-κB Signaling in Keloid Scar Formation. Int. J. Mol. Sci. 2017, 19, 76. [Google Scholar] [CrossRef] [PubMed]
- Yammani, R.R.; Carlson, C.S.; Bresnick, A.R.; Loeser, R.F. Increase in production of matrix metalloproteinase 13 by human articular chondrocytes due to stimulation with S100A4: Role of the receptor for advanced glycation end products. Arthritis Rheum. 2006, 54, 2901–2911. [Google Scholar] [CrossRef]
- Tomcik, M.; Palumbo-Zerr, K.; Zerr, P.; Avouac, J.; Dees, C.; Sumova, B.; Distler, A.; Beyer, C.; Cerezo, L.A.; Becvar, R.; et al. S100A4 amplifies TGF-β-induced fibroblast activation in systemic sclerosis. Ann. Rheum. Dis. 2015, 74, 1748–1755. [Google Scholar] [CrossRef]
- Švec, X.; Štorkánová, H.; Trinh-Minh, T.; Tran, M.C.; Štorkánová, L.; Hulejová, H.; Oreská, S.; Heřmánková, B.; Bečvář, R.; Pavelka, K.; et al. S100A4-neutralizing monoclonal antibody 6B12 counteracts the established experimental skin fibrosis induced by bleomycin. Rheumatology 2024, 63, 817–825. [Google Scholar] [CrossRef]
- Trinh-Minh, T.; Györfi, A.H.; Tomcik, M.; Tran-Manh, C.; Zhou, X.; Dickel, N.; Tümerdem, B.S.; Kreuter, A.; Burmann, S.N.; Borchert, S.V.; et al. Effect of Anti-S100A4 Monoclonal Antibody Treatment on Experimental Skin Fibrosis and Systemic Sclerosis-Specific Transcriptional Signatures in Human Skin. Arthritis Rheumatol. 2024, 76, 783–795. [Google Scholar] [CrossRef]
- Xu, X.; Chen, Z.; Zhu, X.; Wang, D.; Liang, J.; Zhao, C.; Feng, X.; Wang, J.; Zou, H.; Sun, L. S100A9 aggravates bleomycin-induced dermal fibrosis in mice via activation of ERK1/2 MAPK and NF-κB pathways. Iran. J. Basic. Med. Sci. 2018, 21, 194–201. [Google Scholar]
- Zhao, J.; Zhong, A.; Friedrich, E.E.; Jia, S.; Xie, P.; Galiano, R.D.; Mustoe, T.A.; Hong, S.J. S100A12 Induced in the Epidermis by Reduced Hydration Activates Dermal Fibroblasts and Causes Dermal Fibrosis. J. Investig. Dermatol. 2017, 137, 650–659. [Google Scholar] [CrossRef]
- Kanno, Y.; Shu, E.; Niwa, H.; Kanoh, H.; Seishima, M. Alternatively activated macrophages are associated with the α2AP production that occurs with the development of dermal fibrosis: The role of alternatively activated macrophages on the development of fibrosis. Arthritis Res. Ther. 2020, 22, 76. [Google Scholar] [CrossRef]
- Xu, X.; Wu, W.Y.; Tu, W.Z.; Chu, H.Y.; Zhu, X.X.; Liang, M.R.; Xue, Y.; Wang, J.C.; Zou, H.J. Increased expression of S100A8 and S100A9 in patients with diffuse cutaneous systemic sclerosis. A correlation with organ involvement and immunological abnormalities. Clin. Rheumatol. 2013, 32, 1501–1510. [Google Scholar] [CrossRef]
- Yoshizaki, A.; Komura, K.; Iwata, Y.; Ogawa, F.; Hara, T.; Muroi, E.; Takenaka, M.; Shimizu, K.; Hasegawa, M.; Fujimoto, M.; et al. Clinical significance of serum HMGB-1 and sRAGE levels in systemic sclerosis: Association with disease severity. J. Clin. Immunol. 2009, 29, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Kaloudi, O.; Basta, G.; Perfetto, F.; Bartoli, F.; Del Rosso, A.; Miniati, I.; Conforti, M.L.; Generini, S.; Guiducci, S.; Abbate, R.; et al. Circulating levels of Nepsilon-(carboxymethyl)lysine are increased in systemic sclerosis. Rheumatology 2007, 46, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Davies, C.A.; Herrick, A.L.; Cordingley, L.; Freemont, A.J.; Jeziorska, M. Expression of advanced glycation end products and their receptor in skin from patients with systemic sclerosis with and without calcinosis. Rheumatology 2009, 48, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Van Putte, L.; De Schrijver, S.; Moortgat, P. The effects of advanced glycation end products (AGEs) on dermal wound healing and scar formation: A systematic review. Scars Burn. Health 2016, 2, 2059513116676828. [Google Scholar] [CrossRef]
- Zhu, P.; Ren, M.; Yang, C.; Hu, Y.X.; Ran, J.M.; Yan, L. Involvement of RAGE, MAPK and NF-κB pathways in AGEs-induced MMP-9 activation in HaCaT keratinocytes. Exp. Dermatol. 2012, 21, 123–129. [Google Scholar] [CrossRef]
- Zhu, P.; Chen, C.; Wu, D.; Chen, G.; Tan, R.; Ran, J. AGEs-induced MMP-9 activation mediated by Notch1 signaling is involved in impaired wound healing in diabetic rats. Diabetes Res. Clin. Pract. 2022, 186, 109831. [Google Scholar] [CrossRef]
- Niu, Y.; Xie, T.; Ge, K.; Lin, Y.; Lu, S. Effects of extracellular matrix glycosylation on proliferation and apoptosis of human dermal fibroblasts via the receptor for advanced glycosylated end products. Am. J. Dermatopathol. 2008, 30, 344–351. [Google Scholar] [CrossRef]
- Loughlin, D.T.; Artlett, C.M. Precursor of advanced glycation end products mediates ER-stress-induced caspase-3 activation of human dermal fibroblasts through NAD(P)H oxidase 4. PLoS ONE 2010, 5, e11093. [Google Scholar] [CrossRef]
- Serban, A.I.; Stanca, L.; Geicu, O.I.; Munteanu, M.C.; Dinischiotu, A. RAGE and TGF-β1 Cross-Talk Regulate Extracellular Matrix Turnover and Cytokine Synthesis in AGEs Exposed Fibroblast Cells. PLoS ONE 2016, 11, e0152376. [Google Scholar] [CrossRef]
- Ren, X.; Ge, M.; Qin, X.; Xu, P.; Zhu, P.; Dang, Y.; Gu, J.; Ye, X. S100a8/NF-κB signal pathway is involved in the 800-nm diode laser-induced skin collagen remodeling. Lasers Med. Sci. 2016, 31, 673–678. [Google Scholar] [CrossRef]
- Pandolfi, F.; Altamura, S.; Frosali, S.; Conti, P. Key Role of DAMP in Inflammation, Cancer, and Tissue Repair. Clin. Ther. 2016, 38, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.F.; Tang, W.; Lin, W.D.; Liu, Z.Y.; Lu, X.X.; Zhang, B.; Ye, F.; Liu, Z.M.; Zou, J.J.; Liao, W.Q. Receptor for advanced glycation end as drug targets in diabetes-induced skin lesion. Am. J. Transl. Res. 2017, 9, 330–342. [Google Scholar] [PubMed]
- Chen, M.C.; Chen, K.C.; Chang, G.C.; Lin, H.; Wu, C.C.; Kao, W.H.; Teng, C.J.; Hsu, S.L.; Yang, T.Y. RAGE acts as an oncogenic role and promotes the metastasis of human lung cancer. Cell Death Dis. 2020, 11, 265. [Google Scholar] [CrossRef] [PubMed]
- Kwak, T.; Drews-Elger, K.; Ergonul, A.; Miller, P.C.; Braley, A.; Hwang, G.H.; Zhao, D.; Besser, A.; Yamamoto, Y.; Yamamoto, H.; et al. Targeting of RAGE-ligand signaling impairs breast cancer cell invasion and metastasis. Oncogene 2017, 36, 1559–1572. [Google Scholar] [CrossRef]
- Applegate, C.C.; Nelappana, M.B.; Nielsen, E.A.; Kalinowski, L.; Dobrucki, I.T.; Dobrucki, L.W. RAGE as a Novel Biomarker for Prostate Cancer: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 4889. [Google Scholar] [CrossRef]
- Wen, Y.; Zhu, Y.; Zhang, C.; Yang, X.; Gao, Y.; Li, M.; Yang, H.; Liu, T.; Tang, H. Chronic inflammation, cancer development and immunotherapy. Front. Pharmacol. 2022, 13, 1040163. [Google Scholar] [CrossRef]
- Gebhardt, C.; Riehl, A.; Durchdewald, M.; Németh, J.; Fürstenberger, G.; Müller-Decker, K.; Enk, A.; Arnold, B.; Bierhaus, A.; Nawroth, P.P.; et al. RAGE signaling sustains inflammation and promotes tumor development. J. Exp. Med. 2008, 205, 275–285. [Google Scholar] [CrossRef]
- Mou, K.; Liu, W.; Han, D.; Li, P. HMGB1/RAGE axis promotes autophagy and protects keratinocytes from ultraviolet radiation-induced cell death. J. Dermatol. Sci. 2017, 85, 162–169. [Google Scholar] [CrossRef]
- Iotzova-Weiss, G.; Dziunycz, P.J.; Freiberger, S.N.; Läuchli, S.; Hafner, J.; Vogl, T.; French, L.E.; Hofbauer, G.F. S100A8/A9 stimulates keratinocyte proliferation in the development of squamous cell carcinoma of the skin via the receptor for advanced glycation-end products. PLoS ONE 2015, 10, e0120971. [Google Scholar]
- Nguyen, A.H.; Detty, S.Q.; Agrawal, D.K. Clinical Implications of High-mobility Group Box-1 (HMGB1) and the Receptor for Advanced Glycation End-products (RAGE) in Cutaneous Malignancy: A Systematic Review. Anticancer. Res. 2017, 37, 1–7. [Google Scholar] [CrossRef]
- Olaoba, O.T.; Kadasah, S.; Vetter, S.W.; Leclerc, E. RAGE Signaling in Melanoma Tumors. Int. J. Mol. Sci. 2020, 21, 8989. [Google Scholar] [CrossRef] [PubMed]
- Li Pomi, F.; Borgia, F.; Custurone, P.; Vaccaro, M.; Pioggia, G.; Gangemi, S. Role of HMGB1 in Cutaneous Melanoma: State of the Art. Int. J. Mol. Sci. 2022, 23, 9327. [Google Scholar] [CrossRef] [PubMed]
- Abe, R.; Shimizu, T.; Sugawara, H.; Watanabe, H.; Nakamura, H.; Choei, H.; Sasaki, N.; Yamagishi, S.; Takeuchi, M.; Shimizu, H. Regulation of human melanoma growth and metastasis by AGE-AGE receptor interactions. J. Investig. Dermatol. 2004, 122, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Hibino, T.; Sakaguchi, M.; Miyamoto, S.; Yamamoto, M.; Motoyama, A.; Hosoi, J.; Shimokata, T.; Ito, T.; Tsuboi, R.; Huh, N.H. S100A9 is a novel ligand of EMMPRIN that promotes melanoma metastasis. Cancer Res. 2013, 73, 172–183. [Google Scholar] [CrossRef]
- Özbay Kurt, F.G.; Cicortas, B.A.; Balzasch, B.M.; De la Torre, C.; Ast, V.; Tavukcuoglu, E.; Ak, C.; Wohlfeil, S.A.; Cerwenka, A.; Utikal, J.; et al. S100A9 and HMGB1 orchestrate MDSC-mediated immunosuppression in melanoma through TLR4 signaling. J. Immunother. Cancer. 2024, 12, e009552. [Google Scholar] [CrossRef]
- Ruma, I.M.; Putranto, E.W.; Kondo, E.; Murata, H.; Watanabe, M.; Huang, P.; Kinoshita, R.; Futami, J.; Inoue, Y.; Yamauchi, A.; et al. MCAM, as a novel receptor for S100A8/A9, mediates progression of malignant melanoma through prominent activation of NF-κB and ROS formation upon ligand binding. Clin. Exp. Metastasis. 2016, 33, 609–627. [Google Scholar] [CrossRef]
- Wang, W.; Chapman, N.M.; Zhang, B.; Li, M.; Fan, M.; Laribee, R.N.; Zaidi, M.R.; Pfeffer, L.M.; Chi, H.; Wu, Z.H. Upregulation of PD-L1 via HMGB1-Activated IRF3 and NF-κB Contributes to UV Radiation-Induced Immune Suppression. Cancer Res. 2019, 79, 2909–2922. [Google Scholar] [CrossRef]
- Zhang, K.; Anumanthan, G.; Scheaffer, S.; Cornelius, L.A. HMGB1/RAGE Mediates UVB-Induced Secretory Inflammatory Response and Resistance to Apoptosis in Human Melanocytes. J. Investig. Dermatol. 2019, 139, 202–212. [Google Scholar] [CrossRef]
- Haase-Kohn, C.; Wolf, S.; Lenk, J.; Pietzsch, J. Copper-mediated cross-linking of S100A4, but not of S100A2, results in proinflammatory effects in melanoma cells. Biochem. Biophys. Res. Commun. 2011, 413, 494–498. [Google Scholar] [CrossRef]
- Herwig, N.; Belter, B.; Wolf, S.; Haase-Kohn, C.; Pietzsch, J. Interaction of extracellular S100A4 with RAGE prompts prometastatic activation of A375 melanoma cells. J. Cell Mol. Med. 2016, 20, 825–835. [Google Scholar] [CrossRef]
- Herwig, N.; Belter, B.; Pietzsch, J. Extracellular S100A4 affects endothelial cell integrity and stimulates transmigration of A375 melanoma cells. Biochem. Biophys. Res. Commun. 2016, 477, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Ito, T.; Nakahara, T.; Nagae, K.; Fuyuno, Y.; Nakao, M.; Akahoshi, M.; Nakagawa, R.; Tu, Y.; Uchi, H.; et al. Upregulation of S100P, receptor for advanced glycation end products and ezrin in malignant melanoma. J. Dermatol. 2013, 40, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, E.; Heizmann, C.W.; Vetter, S.W. RAGE and S100 protein transcription levels are highly variable in human melanoma tumors and cells. Gen. Physiol. Biophys. 2009, 28, F65–F75. [Google Scholar] [PubMed]
- Meghnani, V.; Wagh, A.; Indurthi, V.S.; Koladia, M.; Vetter, S.W.; Law, B.; Leclerc, E. The receptor for advanced glycation end products influences the expression of its S100 protein ligands in melanoma tumors. Int. J. Biochem. Cell Biol. 2014, 57, 54–62. [Google Scholar] [CrossRef]
- Wagner, N.B.; Weide, B.; Reith, M.; Tarnanidis, K.; Kehrel, C.; Lichtenberger, R.; Pflugfelder, A.; Herpel, E.; Eubel, J.; Ikenberg, K.; et al. Diminished levels of the soluble form of RAGE are related to poor survival in malignant melanoma. Int. J. Cancer. 2015, 137, 2607–2617. [Google Scholar] [CrossRef]
- Gkogkolou, P.; Böhm, M. Advanced glycation end products: Key players in skin aging? Dermatoendocrinology 2012, 4, 259–270. [Google Scholar] [CrossRef]
- Lohwasser, C.; Neureiter, D.; Weigle, B.; Kirchner, T.; Schuppan, D. The receptor for advanced glycation end products is highly expressed in the skin and upregulated by advanced glycation end products and tumor necrosis factor-alpha. J. Investig. Dermatol. 2006, 126, 291–299. [Google Scholar] [CrossRef]
- Pageon, H.; Zucchi, H.; Rousset, F.; Girardeau-Hubert, S.; Tancrede, E.; Asselineau, D. Glycation stimulates cutaneous monocyte differentiation in reconstructed skin in vitro. Mech. Ageing Dev. 2017, 162, 18–26. [Google Scholar] [CrossRef]
- Hiramoto, K.; Imai, M.; Tanaka, S.; Ooi, K. Changes in the AGE/Macrophage/TNF-α Pathway Affect Skin Dryness during KK-Ay/Tajcl Mice Aging. Life 2023, 13, 1339. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, J.Y.; Oh, S.H. Advanced glycation end products (AGEs) promote melanogenesis through receptor for AGEs. Sci. Rep. 2016, 6, 27848. [Google Scholar] [CrossRef]
- Wondrak, G.T.; Roberts, M.J.; Jacobson, M.K.; Jacobson, E.L. Photosensitized growth inhibition of cultured human skin cells: Mechanism and suppression of oxidative stress from solar irradiation of glycated proteins. J. Investig. Dermatol. 2002, 119, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Yao, A.; Zhang, Y.; Ouyang, M.; Wen, L.; Lai, W. Expression profiles and functional analysis of transfer RNA-derived small RNAs (tsRNAs) in photoaged human dermal fibroblasts. Photochem. Photobiol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.M.; El-Fallah, A.A.; Shaker, R. HMGB1-RAGE-moesin axis may be indicted for acne vulgaris. J. Cosmet. Dermatol. 2022, 21, 1642–1646. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Zhang, F.; Qiao, Z.; Jin, Y.; Zheng, R.; Wu, J. Investigating the mechanism of PAD in the treatment of acne based on network pharmacology and molecular docking: A review. Medicine 2024, 103, e38785. [Google Scholar] [CrossRef] [PubMed]
- Urbonaviciute, V.; Voll, R.E. High-mobility group box 1 represents a potential marker of disease activity and novel therapeutic target in systemic lupus erythematosus. J. Intern. Med. 2011, 270, 309–318. [Google Scholar] [CrossRef]
- Abdulahad, D.A.; Westra, J.; Limburg, P.C.; Kallenberg, C.G.; Bijl, M. HMGB1 in systemic lupus Erythematosus: Its role in cutaneous lesions development. Autoimmun. Rev. 2010, 9, 661–665. [Google Scholar] [CrossRef]
- Akoglu, G.; Sungu, N.; Karaismailoglu, E.; Aktas, A. Expression of the receptor for advanced glycation end products in acquired reactive perforating collagenosis. Indian. J. Dermatol. Venereol. Leprol. 2017, 83, 432–435. [Google Scholar] [CrossRef]
- Han, E.C.; Cho, S.B.; Ahn, K.J.; Oh, S.H.; Kim, J.; Kim, D.S.; Lee, K.H.; Bang, D. Expression of Pro-inflammatory Protein S100A12 (EN-RAGE) in Behçet’s Disease and Its Association with Disease Activity: A Pilot Study. Ann. Dermatol. 2011, 23, 313–320. [Google Scholar] [CrossRef]
- Birgin, E.; Gebhardt, C.; Hetjens, S.; Fischer, S.; Rückert, F.; Reichenberger, M.A. Extracorporal Shock Wave Therapy Enhances Receptor for Advanced Glycated End-Product-Dependent Flap Survival and Angiogenesis. Ann. Plast. Surg. 2018, 80, 424–431. [Google Scholar] [CrossRef]
- Joshi, A.A.; Wu, Y.; Deng, S.; Preston-Hurlburt, P.; Forbes, J.M.; Herold, K.C. RAGE antagonism with azeliragon improves xenograft rejection by T cells in humanized mice. Clin. Immunol. 2022, 245, 109165. [Google Scholar] [CrossRef]
- Hong, Y.; Shen, C.; Yin, Q.; Sun, M.; Ma, Y.; Liu, X. Effects of RAGE-Specific Inhibitor FPS-ZM1 on Amyloid-β Metabolism and AGEs-Induced Inflammation and Oxidative Stress in Rat Hippocampus. Neurochem. Res. 2016, 41, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Jia, P.; Zhang, D.; Yao, Z. TTP488 ameliorates NLRP3-associated inflammation, viability, apoptosis, and ROS production in an Alzheimer’s disease cell model by mediating the JAK1/STAT3/NFκB/IRF3 pathway. Cell Biochem. Funct. 2021, 39, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.L.; Johnson, J.; Ober, R.; Holland, A.; Zhang, G.; Backer, M.; Backer, J.; Ali, Z.; Tekabe, Y. Novel Receptor for Advanced Glycation End Products-Blocking Antibody to Treat Diabetic Peripheral Artery Disease. J. Am. Heart Assoc. 2021, 10, e016696. [Google Scholar] [CrossRef] [PubMed]
- Pullerits, R.; Bokarewa, M.; Dahlberg, L.; Tarkowski, A. Synovial fluid expression of autoantibodies specific for RAGE relates to less erosive course of rheumatoid arthritis. Rheumatology 2007, 46, 1367–1371. [Google Scholar] [CrossRef][Green Version]
- Jeong, S.J.; Lim, B.J.; Park, S.; Choi, D.; Kim, H.W.; Ku, N.S.; Han, S.H.; Kim, C.O.; Choi, J.Y.; Song, Y.G.; et al. The effect of sRAGE-Fc fusion protein attenuates inflammation and decreases mortality in a murine cecal ligation and puncture model. Inflamm. Res. 2012, 61, 1211–1218. [Google Scholar] [CrossRef]
- Ooi, H.; Nasu, R.; Furukawa, A.; Takeuchi, M.; Koriyama, Y. Pyridoxamine and Aminoguanidine Attenuate the Abnormal Aggregation of β-Tubulin and Suppression of Neurite Outgrowth by Glyceraldehyde-Derived Toxic Advanced Glycation End-Products. Front. Pharmacol. 2022, 13, 921611. [Google Scholar] [CrossRef]
- Bresnick, A.R. S100 proteins as therapeutic targets. Biophys. Rev. 2018, 10, 1617–1629. [Google Scholar] [CrossRef]
- Cai, X.G.; Xia, J.R.; Li, W.D.; Lu, F.L.; Liu, J.; Lu, Q.; Zhi, H. Anti-fibrotic effects of specific-siRNA targeting of the receptor for advanced glycation end products in a rat model of experimental hepatic fibrosis. Mol. Med. Rep. 2014, 10, 306–314. [Google Scholar] [CrossRef]
- Yeh, W.J.; Hsia, S.M.; Lee, W.H.; Wu, C.H. Polyphenols with antiglycation activity and mechanisms of action: A review of recent findings. J. Food Drug Anal. 2017, 25, 84–92. [Google Scholar] [CrossRef]
- Zhou, Z.; Tang, Y.; Jin, X.; Chen, C.; Lu, Y.; Liu, L.; Shen, C. Metformin Inhibits Advanced Glycation End Products-Induced Inflammatory Response in Murine Macrophages Partly through AMPK Activation and RAGE/NFκB Pathway Suppression. J. Diabetes Res. 2016, 2016, 4847812. [Google Scholar] [CrossRef]
- Huang, J.S.; Guh, J.Y.; Chen, H.C.; Hung, W.C.; Lai, Y.H.; Chuang, L.Y. Role of receptor for advanced glycation end-product (RAGE) and the JAK/STAT-signaling pathway in AGE-induced collagen production in NRK-49F cells. J. Cell Biochem. 2001, 81, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, N.; Bahl, A.; Dhawan, V. C-reactive protein (CRP) up-regulates expression of receptor for advanced glycation end products (RAGE) and its inflammatory ligand EN-RAGE in THP-1 cells: Inhibitory effects of atorvastatin. Int. J. Cardiol. 2010, 142, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Matsui, T.; Nakamura, K.; Takeuchi, M.; Inoue, H. Telmisartan inhibits advanced glycation end products (AGEs)-elicited endothelial cell injury by suppressing AGE receptor (RAGE) expression via peroxisome proliferator-activated receptor-gammaactivation. Protein Pept. Lett. 2008, 15, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Ramadass, V.; Vaiyapuri, T.; Tergaonkar, V. Small Molecule NF-κB Pathway Inhibitors in Clinic. Int. J. Mol. Sci. 2020, 21, 5164. [Google Scholar] [CrossRef]
- Sabbagh, M.N.; Agro, A.; Bell, J.; Aisen, P.S.; Schweizer, E.; Galasko, D. PF-04494700, an oral inhibitor of receptor for advanced glycation end products (RAGE), in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2011, 25, 206–212. [Google Scholar] [CrossRef]
- Galasko, D.; Bell, J.; Mancuso, J.Y.; Kupiec, J.W.; Sabbagh, M.N.; van Dyck, C.; Thomas, R.G.; Aisen, P.S.; Alzheimer’s Disease Cooperative Study. Clinical trial of an inhibitor of RAGE-Aβ interactions in Alzheimer disease. Neurology 2014, 82, 1536–1542. [Google Scholar] [CrossRef]
- Mezentsev, A.V.; Bruskin, S.A.; Soboleva, A.G.; Sobolev, V.V.; Piruzian, E.S. Pharmacological control of receptor of advanced glycation end-products and its biological effects in psoriasis. Int. J. Biomed. Sci. 2013, 9, 112–122. [Google Scholar]
- Wang, M.; Ma, X.; Gao, C.; Luo, Y.; Fei, X.; Zheng, Q.; Ma, X.; Kuai, L.; Li, B.; Wang, R.; et al. Rutin attenuates inflammation by downregulating AGE-RAGE signaling pathway in psoriasis: Network pharmacology analysis and experimental evidence. Int. Immunopharmacol. 2023, 125 Pt. A, 111033. [Google Scholar] [CrossRef]
- Guo, Y.; Gan, H.; Xu, S.; Zeng, G.; Xiao, L.; Ding, Z.; Zhu, J.; Xiong, X.; Fu, Z. Deciphering the Mechanism of Xijiao Dihuang Decoction in Treating Psoriasis by Network Pharmacology and Experimental Validation. Drug Des. Dev. Ther. 2023, 17, 2805–2819. [Google Scholar] [CrossRef]
- Tang, B.; Zheng, X.; Luo, Q.; Li, X.; Yang, Y.; Bi, Y.; Chen, Y.; Han, L.; Chen, H.; Lu, C. Network pharmacology and gut microbiota insights: Unraveling Shenling Baizhu powder’s role in psoriasis treatment. Front. Pharmacol. 2024, 15, 1362161. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Duan, X.; Poorun, D.; Xu, J.; Zhang, S.; Gan, L.; He, M.; Zhu, K.; Ming, Z.; et al. Lipoxin A4 and its analog suppress inflammation by modulating HMGB1 translocation and expression in psoriasis. Sci. Rep. 2017, 7, 7100. [Google Scholar] [CrossRef] [PubMed]
- Karuppagounder, V.; Arumugam, S.; Thandavarayan, R.A.; Pitchaimani, V.; Sreedhar, R.; Afrin, R.; Harima, M.; Suzuki, H.; Nomoto, M.; Miyashita, S.; et al. Resveratrol attenuates HMGB1 signaling and inflammation in house dust mite-induced atopic dermatitis in mice. Int. Immunopharmacol. 2014, 23, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Karuppagounder, V.; Arumugam, S.; Thandavarayan, R.A.; Pitchaimani, V.; Sreedhar, R.; Afrin, R.; Harima, M.; Suzuki, H.; Nomoto, M.; Miyashita, S.; et al. Modulation of HMGB1 translocation and RAGE/NFκB cascade by quercetin treatment mitigates atopic dermatitis in NC/Nga transgenic mice. Exp. Dermatol. 2015, 24, 418–423. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Peng, G.; Han, X. Glycyrrhizin ameliorates atopic dermatitis-like symptoms through inhibition of HMGB1. Int Immunopharmacol. 2018, 60, 9–17. [Google Scholar] [CrossRef]
- Karuppagounder, V.; Arumugam, S.; Thandavarayan, R.A.; Pitchaimani, V.; Sreedhar, R.; Afrin, R.; Harima, M.; Suzuki, H.; Nomoto, M.; Miyashita, S.; et al. Tannic acid modulates NFκB signaling pathway and skin inflammation in NC/Nga mice through PPARγ expression. Cytokine 2015, 76, 206–213. [Google Scholar] [CrossRef]
- Chang, Q.X.; Lyu, J.L.; Wu, P.Y.; Wen, K.C.; Chang, C.C.; Chiang, H.M. Coffea arabica Extract Attenuates Atopic Dermatitis-like Skin Lesions by Regulating NLRP3 Inflammasome Expression and Skin Barrier Functions. Int. J. Mol. Sci. 2023, 24, 12367. [Google Scholar] [CrossRef]
- Huang, W.; Huang, X.; Yang, L.; Han, W.; Zhu, Z.; Wang, Y.; Chen, R. Network Pharmacology and Molecular Docking Analysis Exploring the Mechanism of Tripterygium wilfordii in the Treatment of Oral Lichen Planus. Medicina 2023, 59, 1448. [Google Scholar] [CrossRef]
- Chang, W.; Shi, J.; Li, L.; Zhang, P.; Ren, Y.; Yan, Y.; Ge, Y. Network pharmacology and molecular docking analysis predict the mechanisms of Huangbai liniment in treating oral lichen planus. Medicine 2024, 103, e39352. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, L.; Zhang, M.; Zhou, C.; Wang, Y.; Ma, J.; Fan, Y. Reveals of quercetin’s therapeutic effects on oral lichen planus based on network pharmacology approach and experimental validation. Sci. Rep. 2022, 12, 1162. [Google Scholar] [CrossRef]
- Johnson, J.M.; Takebe, Y.; Zhang, G.; Ober, R.; McLuckie, A.; Niedt, G.W.; Johnson, L.L. Blocking RAGE improves wound healing in diabetic pigs. Int. Wound J. 2023, 20, 678–686. [Google Scholar] [CrossRef]
- Goova, M.T.; Li, J.; Kislinger, T.; Qu, W.; Lu, Y.; Bucciarelli, L.G.; Nowygrod, S.; Wolf, B.M.; Caliste, X.; Yan, S.F.; et al. Blockade of receptor for advanced glycation end-products restores effective wound healing in diabetic mice. Am. J. Pathol. 2001, 159, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Olekson, M.P.; Faulknor, R.A.; Hsia, H.C.; Schmidt, A.M.; Berthiaume, F. Soluble Receptor for Advanced Glycation End Products Improves Stromal Cell-Derived Factor-1 Activity in Model Diabetic Environments. Adv. Wound Care 2016, 5, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Kumar, S.; D’Elia, A.; Dash, B.; Nanda, V.; Hsia, H.C.; Yarmush, M.L.; Berthiaume, F. Self-assembled elastin-like polypeptide fusion protein coacervates as competitive inhibitors of advanced glycation end-products enhance diabetic wound healing. J. Control. Release 2021, 333, 176–187. [Google Scholar] [CrossRef]
- Wear-Maggitti, K.; Lee, J.; Conejero, A.; Schmidt, A.M.; Grant, R.; Breitbart, A. Use of topical sRAGE in diabetic wounds increases neovascularization and granulation tissue formation. Ann. Plast. Surg. 2004, 52, 519–521, discussion 522. [Google Scholar] [CrossRef]
- Chen, S.A.; Chen, H.M.; Yao, Y.D.; Hung, C.F.; Tu, C.S.; Liang, Y.J. Topical treatment with anti-oxidants and Au nanoparticles promote healing of diabetic wound through receptor for advance glycation end-products. Eur J Pharm Sci. 2012, 47, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.; Xia, G.; Tang, X.; Zhuang, Z.; Shan, J.; Fang, X.; Qiu, L.; Zha, X.; Chen, X.L. A Multifunctional Nanocomposite Hydrogel Delivery System Based on Dual-Loaded Liposomes for Scarless Wound Healing. Adv. Healthc. Mater. 2024, 13, e2401619. [Google Scholar] [CrossRef]
- Kang, H.J.; Kumar, S.; Dash, B.C.; Hsia, H.C.; Yarmush, M.L.; Berthiaume, F. Multifunctional Elastin-Like Polypeptide Fusion Protein Coacervates Inhibit Receptor-Mediated Proinflammatory Signals and Promote Angiogenesis in Mouse Diabetic Wounds. Adv Wound Care 2023, 12, 241–255. [Google Scholar] [CrossRef]
- Geng, J.; Zhou, G.; Guo, S.; Ma, C.; Ma, J. Underlying Mechanism of Traditional Herbal Formula Chuang-Ling-Ye in the Treatment of Diabetic Foot Ulcer through Network Pharmacology and Molecular Docking. Curr. Pharm. Des. 2024, 30, 448–467. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, T.; Jiang, X. Network Pharmacology Study on Herb Pair Bletilla striata-Galla chinensis in the Treatment of Chronic Skin Ulcers. Curr. Pharm. Des. 2024, 30, 1354–1376. [Google Scholar] [CrossRef]
- Qin, P.-Y.; Xu, Y.-J.; Zuo, X.-D.; Duan, J.-H.; Qiu, B.; Li, X.-F.; Li, J.-P.; Yu, J. Effect and mechanisms of Polygonatum kingianum (polygonati rhizome) on wound healing in diabetic rats. J. Ethnopharmacol. 2022, 298, 115612. [Google Scholar]
- Zhang, S.; Xu, Y.; Zhang Junior, C.; Chen, X.; Zhu, J. Dang-Gui-Si-Ni decoction facilitates wound healing in diabetic foot ulcers by regulating expression of AGEs/RAGE/TGF-β/Smad2/3. Arch. Dermatol. Res. 2024, 316, 338. [Google Scholar] [CrossRef] [PubMed]
- Emad, A.M.; Mahrous, E.A.; Rasheed, D.M.; Gomaa, F.A.M.; Hamdan, A.M.E.; Selim, H.M.R.M.; Yousef, E.M.; Abo-Zalam, H.B.; El-Gazar, A.A.; Ragab, G.M. Wound Healing Efficacy of Cucurbitaceae Seed Oils in Rats: Comprehensive Phytochemical, Pharmacological, and Histological Studies Tackling AGE/RAGE and Nrf2/Ho-1 Cue. Pharmaceuticals 2024, 17, 733. [Google Scholar] [CrossRef] [PubMed]
- Youjun, D.; Huang, Y.; Lai, Y.; Ma, Z.; Wang, X.; Chen, B.; Ding, X.; Tan, Q. Mechanisms of resveratrol against diabetic wound by network pharmacology and experimental validation. Ann. Med. 2023, 55, 2280811. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Qing, C.; Niu, Y.; Dong, J.; Cao, X.; Song, F.; Ji, X.; Lu, S. Effect of aminoguanidine intervention on neutrophils in diabetes inflammatory cells wound healing. Exp. Clin. Endocrinol. Diabetes 2013, 121, 635–642. [Google Scholar] [CrossRef]
- Alqahtani, A.S.; Li, K.M.; Razmovski-Naumovski, V.; Kam, A.; Alam, P.; Li, G.Q. Attenuation of methylglyoxal-induced glycation and cellular dysfunction in wound healing by Centella cordifolia. Saudi J. Biol. Sci. 2021, 28, 813–824. [Google Scholar] [CrossRef]
- Yang, X.; Cao, Z.; Wu, P.; Li, Z. Effect and Mechanism of the Bruton Tyrosine Kinase (Btk) Inhibitor Ibrutinib on Rat Model of Diabetic Foot Ulcers. Med. Sci. Monit. 2019, 25, 7951–7957. [Google Scholar] [CrossRef]
- Yang, C.T.; Meng, F.H.; Chen, L.; Li, X.; Cen, L.J.; Wen, Y.H.; Li, C.C.; Zhang, H. Inhibition of Methylglyoxal-Induced AGEs/RAGE Expression Contributes to Dermal Protection by N-Acetyl-L-Cysteine. Cell Physiol. Biochem. 2017, 41, 742–754. [Google Scholar] [CrossRef]
- Bian, X.; Li, B.; Yang, J.; Ma, K.; Sun, M.; Zhang, C.; Fu, X. Regenerative and protective effects of dMSC-sEVs on high-glucose-induced senescent fibroblasts by suppressing RAGE pathway and activating Smad pathway. Stem Cell Res. Ther. 2020, 11, 166. [Google Scholar] [CrossRef]
- Xu, J.; Bai, S.; Cao, Y.; Liu, L.; Fang, Y.; Du, J.; Luo, L.; Chen, M.; Shen, B.; Zhang, Q. miRNA-221-3p in Endothelial Progenitor Cell-Derived Exosomes Accelerates Skin Wound Healing in Diabetic Mice. Diabetes Metab. Syndr. Obes. 2020, 13, 1259–1270. [Google Scholar] [CrossRef]
- Kim, S.; Kwon, J. Thymosin beta 4 improves dermal burn wound healing via downregulation of receptor of advanced glycation end products in db/db mice. Biochim. Biophys. Acta 2014, 1840, 3452–3459. [Google Scholar] [CrossRef]
- Ranzato, E.; Patrone, M.; Pedrazzi, M.; Burlando, B. Hmgb1 promotes wound healing of 3T3 mouse fibroblasts via RAGE-dependent ERK1/2 activation. Cell Biochem. Biophys. 2010, 57, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Ranzato, E.; Patrone, M.; Pedrazzi, M.; Burlando, B. HMGb1 promotes scratch wound closure of HaCaT keratinocytes via ERK1/2 activation. Mol. Cell Biochem. 2009, 332, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Tancharoen, S.; Gando, S.; Binita, S.; Nagasato, T.; Kikuchi, K.; Nawa, Y.; Dararat, P.; Yamamoto, M.; Narkpinit, S.; Maruyama, I. HMGB1 Promotes Intraoral Palatal Wound Healing through RAGE-Dependent Mechanisms. Int. J. Mol. Sci. 2016, 17, 1961. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, A.; Blood, D.C.; del Toro, G.; Canet, A.; Lee, D.C.; Qu, W.; Tanji, N.; Lu, Y.; Lalla, E.; Fu, C.; et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature 2000, 405, 354–360. [Google Scholar] [CrossRef]
- Zhao, B.; Li, Y.; Wang, B.; Liu, J.; Yang, Y.; Quan, Q.; An, Q.; Liang, R.; Liu, C.; Yang, C. Uncovering the Anti-Angiogenic Mechanisms of Centella asiatica via Network Pharmacology and Experimental Validation. Molecules 2024, 29, 362. [Google Scholar] [CrossRef]
- Matsushita, S.; Tada, K.I.; Kawahara, K.I.; Kawai, K.; Hashiguchi, T.; Maruyama, I.; Kanekura, T. Advanced malignant melanoma responds to Prunus mume Sieb. Et. Zucc (Ume) extract: Case report and in vitro study. Exp. Ther. Med. 2010, 1, 569–574. [Google Scholar] [CrossRef]
- Han, A.R.; Nam, M.H.; Lee, K.W. Plantamajoside Inhibits UVB and Advanced Glycation End Products-Induced MMP-1 Expression by Suppressing the MAPK and NF-κB Pathways in HaCaT Cells. Photochem. Photobiol. 2016, 92, 708–719. [Google Scholar] [CrossRef]
- Chen, C.; Liu, X.; Li, L.; Guo, M.; He, Y.; Dong, Y.; Meng, H.; Yi, F. Study of the mechanism by gentiopicroside protects against skin fibroblast glycation damage via the RAGE pathway. Sci. Rep. 2024, 14, 4685. [Google Scholar] [CrossRef]
- Lyu, J.L.; Liu, Y.J.; Wen, K.C.; Chiu, C.Y.; Lin, Y.H.; Chiang, H.M. Protective Effect of Djulis (Chenopodium formosanum) Extract against UV- and AGEs-Induced Skin Aging via Alleviating Oxidative Stress and Collagen Degradation. Molecules 2022, 27, 2332. [Google Scholar] [CrossRef]
- Wu, J.; Pan, L. Study on the effect of Pogostemon cablin Benth on skin aging based on network pharmacology. Curr. Comput. Aided Drug Des. 2022. [Google Scholar] [CrossRef]
- Han, M.; Li, H.; Ke, D.; Tian, L.M.; Hong, Y.; Zhang, C.; Tian, D.Z.; Chen, L.; Zhan, L.R.; Zong, S.Q. Mechanism of Ba Zhen Tang Delaying Skin Photoaging Based on Network Pharmacology and Molecular Docking. Clin. Cosmet. Investig. Dermatol. 2022, 15, 763–781. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, K.; Gao, S.; Zhao, J.; Liu, G.; Chen, Y.; Lin, H.; Zhao, W.; Hu, Z.; Xu, N. Carnosine Stimulates Macrophage-Mediated Clearance of Senescent Skin Cells Through Activation of the AKT2 Signaling Pathway by CD36 and RAGE. Front. Pharmacol. 2020, 11, 593832. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.J.; Lee, H.W.; Yoo, G.; Kim, D.; Choi, I.W.; Kim, Y.; Ha, S.K. Protective effect of Schizonepeta tenuifolia Briq. ethanolic extract against UVB-induced skin aging and photodamage in hairless mice. Front. Pharmacol. 2023, 14, 1176073. [Google Scholar] [CrossRef] [PubMed]


| Material | Method | Study Size | Markers | Year | Authors [Ref.] |
|---|---|---|---|---|---|
| Serum | ELISA | 50 | ↑S100A8/A9 | 2016 | de Carvalho et al. [69] |
| Skin biopsy (dermis) | IHC | 49 | ↑HMGB1, ↑RAGE | 2018 | de Carvalho et al. [70] |
| Mucosa biopsy | IHC | 45 | ↑HMGB1, ↑RAGE | 2018 | Salem et al. [71] |
| Skin biopsy | IHC | 50 | ↑S100A8 | 2016 | de Carvalho et al. [69] |
| Skin biopsy | PCR | 50 | ↑S100A8, ↑S100A9, ↑S100A8/A9 | 2016 | de Carvalho et al. [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radziszewski, M.; Galus, R.; Łuszczyński, K.; Winiarski, S.; Wąsowski, D.; Malejczyk, J.; Włodarski, P.; Ścieżyńska, A. The RAGE Pathway in Skin Pathology Development: A Comprehensive Review of Its Role and Therapeutic Potential. Int. J. Mol. Sci. 2024, 25, 13570. https://doi.org/10.3390/ijms252413570
Radziszewski M, Galus R, Łuszczyński K, Winiarski S, Wąsowski D, Malejczyk J, Włodarski P, Ścieżyńska A. The RAGE Pathway in Skin Pathology Development: A Comprehensive Review of Its Role and Therapeutic Potential. International Journal of Molecular Sciences. 2024; 25(24):13570. https://doi.org/10.3390/ijms252413570
Chicago/Turabian StyleRadziszewski, Marcin, Ryszard Galus, Krzysztof Łuszczyński, Sebastian Winiarski, Dariusz Wąsowski, Jacek Malejczyk, Paweł Włodarski, and Aneta Ścieżyńska. 2024. "The RAGE Pathway in Skin Pathology Development: A Comprehensive Review of Its Role and Therapeutic Potential" International Journal of Molecular Sciences 25, no. 24: 13570. https://doi.org/10.3390/ijms252413570
APA StyleRadziszewski, M., Galus, R., Łuszczyński, K., Winiarski, S., Wąsowski, D., Malejczyk, J., Włodarski, P., & Ścieżyńska, A. (2024). The RAGE Pathway in Skin Pathology Development: A Comprehensive Review of Its Role and Therapeutic Potential. International Journal of Molecular Sciences, 25(24), 13570. https://doi.org/10.3390/ijms252413570






