Molecular Profiles of Sensitization to Non-Specific Lipid Transfer Proteins in Lithuania: Single Center Experience
Abstract
1. Introduction
2. Results
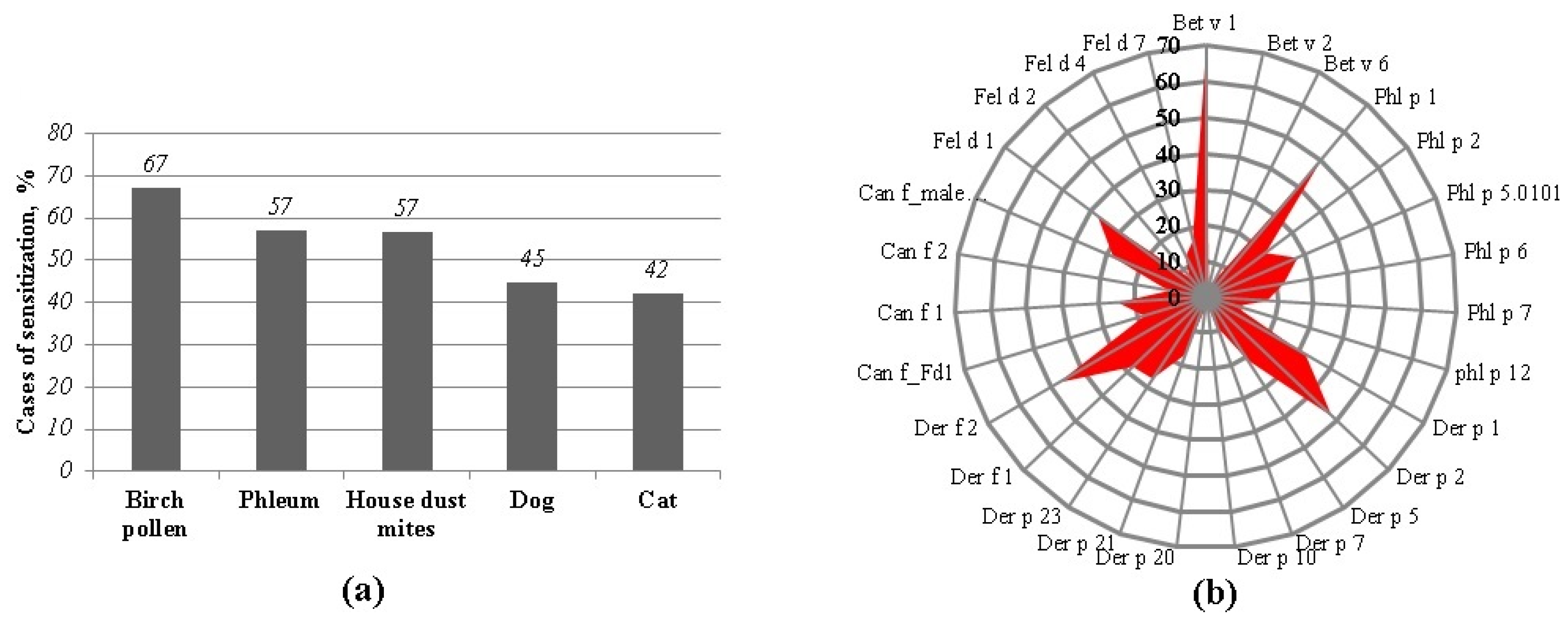
2.1. The Main Allergen Sources of the Study Group
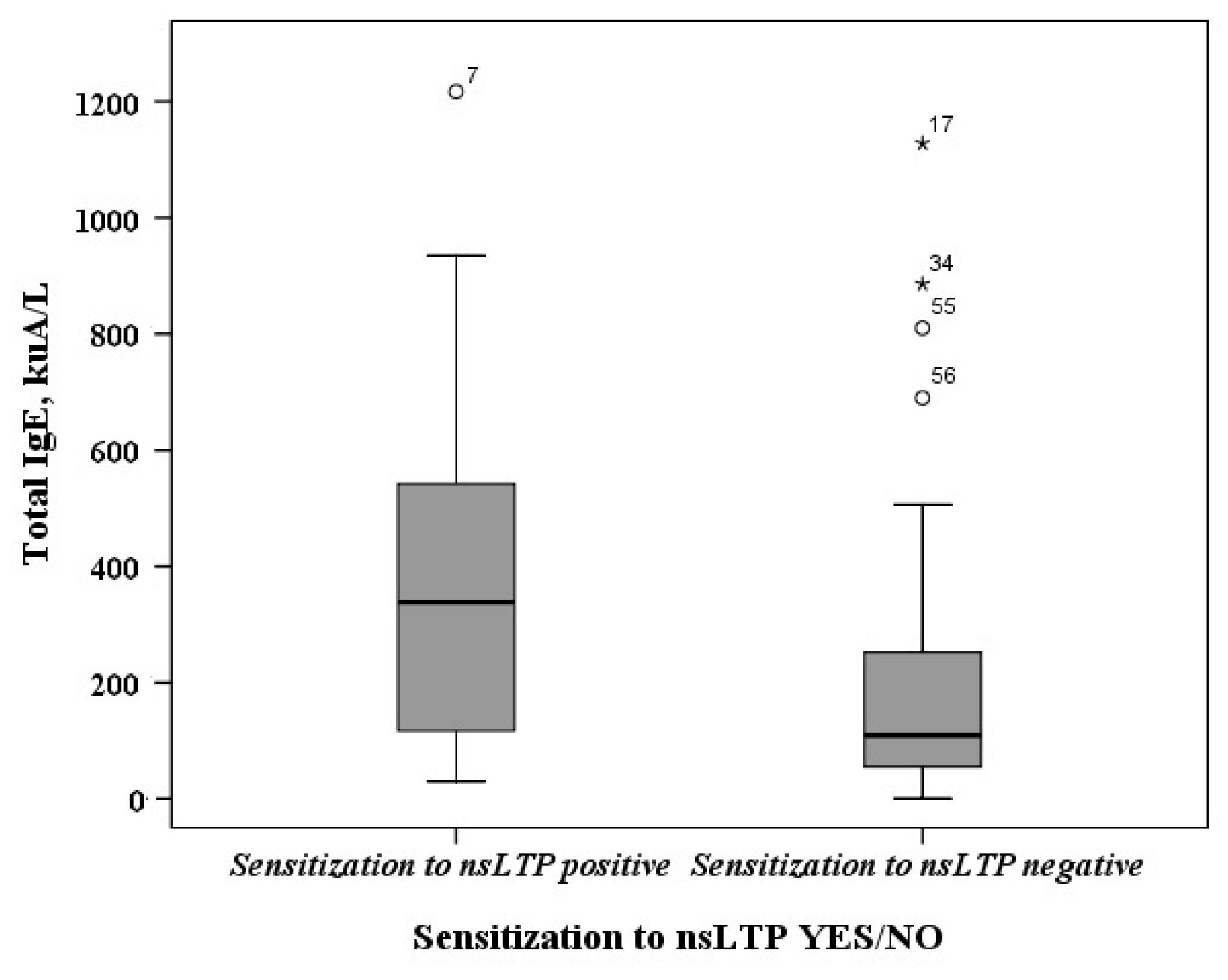
2.2. Sensitization to nsLTP-Containing Allergen Sources
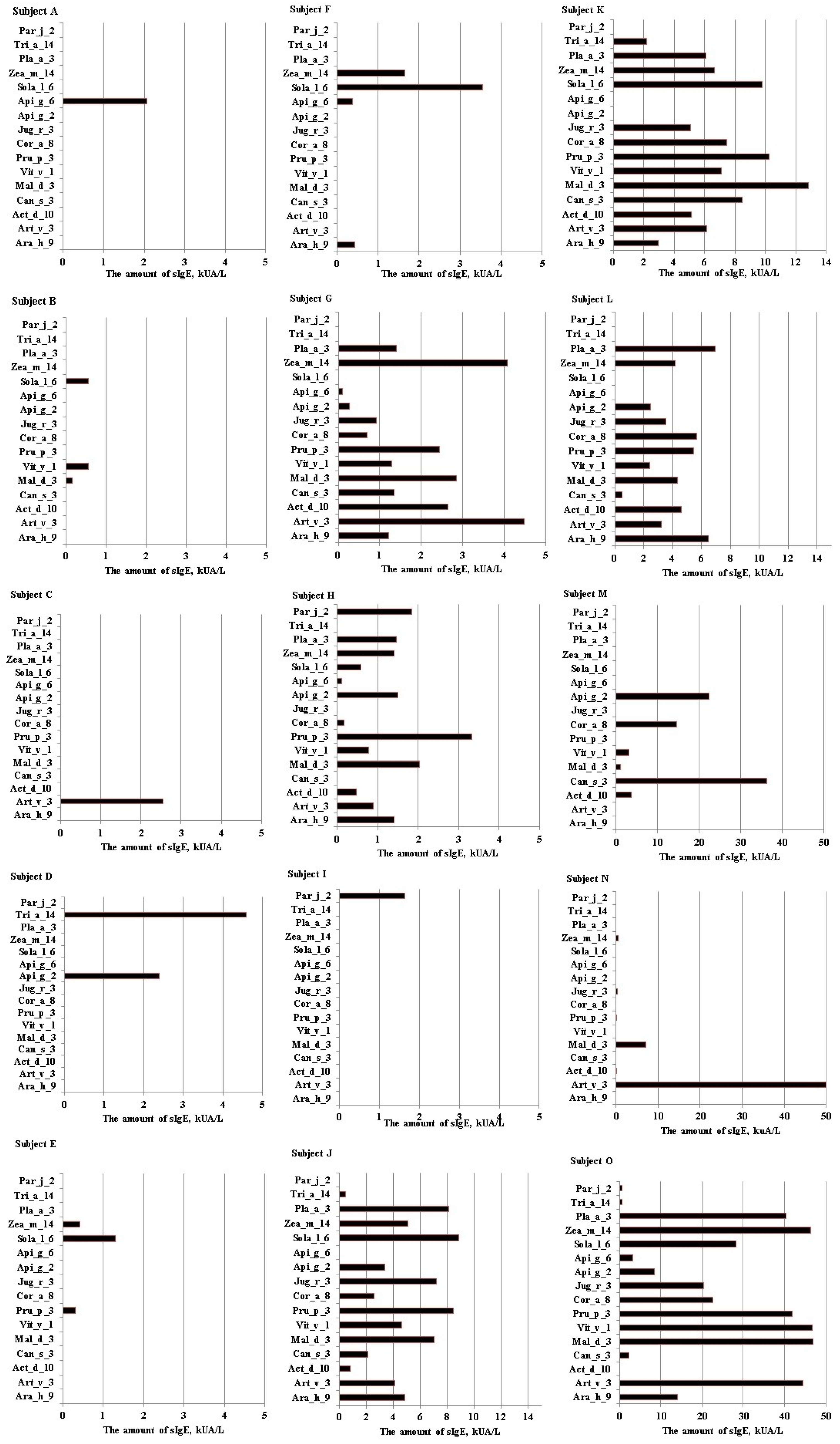
2.3. The Determination of Sensitization Profiles to nsLTPs
2.4. The Comparison of Allergen Extracts and Component Analysis Results in Determining Sensitization to nsLTPs
3. Discussion
4. Materials and Methods
Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seastedt, H.; Nadeau, K. Factors by Which Global Warming Worsens Allergic Disease. Ann. Allergy Asthma Immunol. 2023, 131, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Bonini, S. News From the European Academy of Allergology and Clinical Immunology. Allergy Clin. Immunol. Int. J. World Allergy Organ. 2000, 12, 0186–0187. [Google Scholar] [CrossRef]
- Pawankar, R. Allergic Diseases and Asthma: A Global Public Health Concern and a Call to Action. World Allergy Organ. J. 2014, 7, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Skypala, I.J.; De Jong, N.W.; Angier, E.; Gardner, J.; Kull, I.; Ryan, D.; Venter, C.; Vlieg-Boerstra, B.J.; Grimshaw, K. Promoting and Achieving Excellence in the Delivery of Integrated Allergy Care: The European Academy of Allergy & Clinical Immunology Competencies for Allied Health Professionals Working in Allergy. Clin. Transl. Allergy 2018, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Van Der Valk, J.P.M.; Gerth Van Wijk, R.; Hoorn, E.; Groenendijk, L.; Groenendijk, I.M.; De Jong, N.W. Measurement and Interpretation of Skin Prick Test Results. Clin. Transl. Allergy 2016, 6, 8. [Google Scholar] [CrossRef]
- Ansotegui, I.J.; Melioli, G.; Canonica, G.W.; Caraballo, L.; Villa, E.; Ebisawa, M.; Passalacqua, G.; Savi, E.; Ebo, D.; Gómez, R.M.; et al. IgE Allergy Diagnostics and Other Relevant Tests in Allergy, a World Allergy Organization Position Paper. World Allergy Organ. J. 2020, 13, 100080. [Google Scholar] [CrossRef]
- Lis, K.; Bartuzi, Z. Selected Technical Aspects of Molecular Allergy Diagnostics. Curr. Issues Mol. Biol. 2023, 45, 5481–5493. [Google Scholar] [CrossRef]
- Luengo, O.; Labrador-Horrillo, M. Molecular Allergy Diagnosis in Clinical Practice: Frequently Asked Questions. J. Investig. Allergol. Clin. Immunol. 2022, 32, 1–12. [Google Scholar] [CrossRef]
- Werfel, T.; Asero, R.; Ballmer-Weber, B.K.; Beyer, K.; Enrique, E.; Knulst, A.C.; Mari, A.; Muraro, A.; Ollert, M.; Poulsen, L.K.; et al. Position Paper of the EAACI: Food Allergy Due to Immunological Cross-Reactions with Common Inhalant Allergens. Allergy 2015, 70, 1079–1090. [Google Scholar] [CrossRef]
- Passalacqua, G.; Melioli, G.; Bonifazi, F.; Bonini, S.; Maggi, E.; Senna, G.; Triggiani, M.; Nettis, E.; Rossi, R.E.; Vacca, A.; et al. The Additional Values of Microarray Allergen Assay in the Management of Polysensitized Patients with Respiratory Allergy. Allergy 2013, 68, 1029–1033. [Google Scholar] [CrossRef]
- Kato, Y.; Morikawa, T.; Fujieda, S. Comprehensive Review of Pollen-Food Allergy Syndrome: Pathogenesis, Epidemiology, and Treatment Approaches. Allergol. Int. 2024; in press. [Google Scholar] [CrossRef] [PubMed]
- Carlson, G.; Coop, C. Pollen Food Allergy Syndrome (PFAS): A Review of Current Available Literature. Ann. Allergy Asthma Immunol. 2019, 123, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Urisu, A. Oral Allergy Syndrome. Allergol. Int. 2009, 58, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Wąsik, J.; Likońska, A.; Kurowski, M. IgE-Mediated Allergy and Asymptomatic Sensitization to Cannabis Allergens—Review of Current Knowledge and Presentation of Six Cases. Medicina 2024, 60, 954. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Li, L.; Lyu, P.C.; Chang, J.Y. Distinct Unfolding and Refolding Pathways of Lipid Transfer Proteins LTP1 and LTP2. Protein J. 2004, 23, 553–566. [Google Scholar] [CrossRef]
- Skypala, I.J.; Asero, R.; Barber, D.; Cecchi, L.; Diaz Perales, A.; Hoffmann-Sommergruber, K.; Pastorello, E.A.; Swoboda, I.; Bartra, J.; Ebo, D.G.; et al. Non-specific Lipid-transfer Proteins: Allergen Structure and Function, Cross-reactivity, Sensitization, and Epidemiology. Clin. Transl. Allergy 2021, 11, 19. [Google Scholar] [CrossRef]
- Romano, A.; Scala, E.; Rumi, G.; Gaeta, F.; Caruso, C.; Alonzi, C.; Maggioletti, M.; Ferrara, R.; Palazzo, P.; Palmieri, V.; et al. Lipid Transfer Proteins: The Most Frequent Sensitizer in Italian Subjects with Food-Dependent Exercise-Induced Anaphylaxis. Clin. Exp. Allergy 2012, 42, 1643–1653. [Google Scholar] [CrossRef]
- Asero, R.; Pravettoni, V.; Villalta, D.; Cecchi, L.; Scala, E. IgE-Mediated Reactivity to Non-Specific Lipid Transfer Protein (NsLTP): Clinical Implications and Management—A Consensus Document of the Association of Italian Territorial and Hospital Allergists and Immunologists (AAIITO). Eur. Ann. Allergy Clin. Immunol. 2024, 56, 176–182. [Google Scholar] [CrossRef]
- Faber, M.A.; Van Gasse, A.L.; Decuyper, I.I.; Uyttebroek, A.; Sabato, V.; Hagendorens, M.M.; Bridts, C.H.; De Clerck, L.S.; Fernandez-Rivas, M.; Pascal, M.; et al. IgE-Reactivity Profiles to Nonspecific Lipid Transfer Proteins in a Northwestern European Country. J. Allergy Clin. Immunol. 2017, 139, 679–682.e5. [Google Scholar] [CrossRef]
- Scheurer, S.; van Ree, R.; Vieths, S. The Role of Lipid Transfer Proteins as Food and Pollen Allergens Outside the Mediterranean Area. Curr. Allergy Asthma Rep. 2021, 21, 7. [Google Scholar] [CrossRef]
- Ciardiello, M.A.; Giangrieco, I.; Tuppo, L.; Tamburrini, M.; Buccheri, M.; Palazzo, P.; Bernardi, M.L.; Ferrara, R.; Mari, A. Influence of the Natural Ripening Stage, Cold Storage, and Ethylene Treatment on the Protein and Ige-Binding Profiles of Green and Gold Kiwi Fruit Extracts. J. Agric. Food Chem. 2009, 57, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Skypala, I.J.; Bartra, J.; Ebo, D.G.; Antje Faber, M.; Fernández-Rivas, M.; Gomez, F.; Luengo, O.; Till, S.J.; Asero, R.; Barber, D.; et al. The Diagnosis and Management of Allergic Reactions in Patients Sensitized to Non-Specific Lipid Transfer Proteins. Allergy 2021, 76, 2433–2446. [Google Scholar] [CrossRef] [PubMed]
- Sonneveld, L.J.H.; Emons, J.A.M.; Arends, N.J.T.; Landzaat, L.J.; Veenbergen, S.; Schreurs, M.W.J. ALEX versus ISAC Multiplex Array in Analyzing Food Allergy in Atopic Children. Clin. Mol. Allergy 2022, 20, 10. [Google Scholar] [CrossRef] [PubMed]
- Poziomkowska-Gęsicka, I. Idiopathic Anaphylaxis? Analysis of Data from the Anaphylaxis Registry for West Pomerania Province, Poland. Int. J. Environ. Res. Public Health 2022, 19, 16716. [Google Scholar] [CrossRef]
- Quan, P.L.; Sabaté-Brescó, M.; D’amelio, C.M.; Pascal, M.; García, B.E.; Gastaminza, G.; Blanca-López, N.; Alvarado, M.I.; Fernández, J.; Moya, C.; et al. Validation of a Commercial Allergen Microarray Platform for Specific Immunoglobulin E Detection of Respiratory and Plant Food Allergens. Ann. Allergy Asthma Immunol. 2022, 128, 290. [Google Scholar] [CrossRef]
- Scheurer, S.; Schülke, S. Interaction of Non-Specific Lipid-Transfer Proteins With Plant-Derived Lipids and Its Impact on Allergic Sensitization. Front. Immunol. 2018, 9, 1389. [Google Scholar] [CrossRef]
- Asero, R.; Pravettoni, V.; Scala, E.; Villalta, D. Lipid Transfer Protein Allergy: A Review of Current Con-468troversies. Clin. Exp. Allergy 2022, 52, 222–230. [Google Scholar] [CrossRef]
- Movérare, R.; Larsson, H.; Carlsson, R.; Holmquist, I. Mugwort-Sensitized Individuals from North Europe, South Europe and North America Show Different IgE Reactivity Patterns. Int. Arch. Allergy Immunol. 2011, 154, 164–172. [Google Scholar] [CrossRef]
- Albert, E.; Walsemann, T.; Behrends, J.; Jappe, U. Lipid Transfer Protein Syndrome in a Northern European Patient: An Unusual Case Report. Front. Med. 2023, 10, 6–11. [Google Scholar] [CrossRef]
- Arkwright, P.D.; Summers, C.W.; Riley, B.J.; Alsediq, N.; Pumphrey, R.S.H. IgE Sensitization to the Nonspecific Lipid-Transfer Protein Ara h 9 and Peanut-Associated Bronchospasm. BioMed Res. Int. 2013, 2013, 746507. [Google Scholar] [CrossRef]
- Adomaite, I.; Gorbikova, E.; Miskinyte, M.; Eidukaite, A.; Miskiniene, A.; Rudzeviciene, O. Molecular Peanut Sensitization Patterns in Lithuanian Children with Suspected Allergic Symptoms. Adv. Dermatol. Allergol. 2023, 40, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Calamelli, E.; Caffarelli, C.; Ricci, G. Peanut Sensitization Profiles in Italian Children and Adolescents with Specific IgE to Peanuts. BioMed Res. Int. 2013, 2013, 170452. [Google Scholar] [CrossRef] [PubMed]
- Mauer, Y.; Do, R.M.T. Managing Adult Asthma: The 2019 GINA Guidelines. Clevel. Clin. J. Med. 2020, 87, 569–575. [Google Scholar] [CrossRef]
- Bousquet, J.; Schünemann, H.J.; Togias, A.; Bachert, C.; Erhola, M.; Hellings, P.W.; Klimek, L.; Pfaar, O.; Wallace, D.; Ansotegui, I.; et al. Next-Generation Allergic Rhinitis and Its Impact on Asthma (ARIA) Guidelines for Allergic Rhinitis Based on Grading of Recommendations Assessment, Development and Evaluation (GRADE) and Real-World Evidence. J. Allergy Clin. Immunol. 2020, 145, 70–80.e3. [Google Scholar] [CrossRef]
- Santos, A.F.; Riggioni, C.; Agache, I.; Akdis, C.A.; Akdis, M.; Alvarez-Perea, A.; Alvaro-Lozano, M.; Ballmer-Weber, B.; Barni, S.; Beyer, K.; et al. EAACI Guidelines on the Diagnosis of IgE-Mediated Food Allergy. Allergy 2023, 78, 3057–3076. [Google Scholar] [CrossRef]

| Gender | The Part of Population (n = 60), % |
|---|---|
| Man | 45.00 |
| Woman | 55.00 |
| Age mean (min–max), m | 33.58 (18–60) |
| Diagnosis | |
| AD + AR | 12.20 |
| AR | 58.50 |
| AA + AR | 29.30 |
| Molecular Component | |||||||
|---|---|---|---|---|---|---|---|
| Protein Family | nsLTP | PR-10 | |||||
| Allergen Source | κ | p-Value | p-Value of McNemar’s Test | κ | p-Value | p-Value of McNemar’s Test | |
| Allergen extracts | Hazelnut | Cor a 8 | Cor a 1.0103 | ||||
| −0.118 | 0.581 | 0.508 | 0.532 | 0.024 | 1 | ||
| Cor a 1.0401 | |||||||
| 0.538 | 0.019 | 0.625 | |||||
| Apple | Mal d 3 | Mal d 1 | |||||
| 0.341 | 0.001 | 0.001 | 0.389 | 0.004 | 1 | ||
| Peanut | Ara h 9 | Ara h 8 | |||||
| 0.542 | 0.001 | 1 | −0.101 | 0.309 | 0.021 | ||
| Celery | Api g 2 | Api g 1 | |||||
| 0.379 | 0.075 | 0.375 | 0.286 | 0.180 | 0.375 | ||
| Api g 6 | |||||||
| 0.174 | 0.179 | 0.031 | |||||
| Kiwi | Act d 10 | - | |||||
| 0.301 | 0.201 | 1 | |||||
| Tomato | Sol l 6 | - | |||||
| 0.612 | 0.004 | 0.5 | |||||
| Mugwort | Art v 3 | - | |||||
| 0.259 | 0.004 | 0.001 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakalauskaite, S.; Pilkyte, L.; Gasiuniene, E.; Gradauskiene, B. Molecular Profiles of Sensitization to Non-Specific Lipid Transfer Proteins in Lithuania: Single Center Experience. Int. J. Mol. Sci. 2024, 25, 13535. https://doi.org/10.3390/ijms252413535
Sakalauskaite S, Pilkyte L, Gasiuniene E, Gradauskiene B. Molecular Profiles of Sensitization to Non-Specific Lipid Transfer Proteins in Lithuania: Single Center Experience. International Journal of Molecular Sciences. 2024; 25(24):13535. https://doi.org/10.3390/ijms252413535
Chicago/Turabian StyleSakalauskaite, Sandra, Ligita Pilkyte, Edita Gasiuniene, and Brigita Gradauskiene. 2024. "Molecular Profiles of Sensitization to Non-Specific Lipid Transfer Proteins in Lithuania: Single Center Experience" International Journal of Molecular Sciences 25, no. 24: 13535. https://doi.org/10.3390/ijms252413535
APA StyleSakalauskaite, S., Pilkyte, L., Gasiuniene, E., & Gradauskiene, B. (2024). Molecular Profiles of Sensitization to Non-Specific Lipid Transfer Proteins in Lithuania: Single Center Experience. International Journal of Molecular Sciences, 25(24), 13535. https://doi.org/10.3390/ijms252413535




.jpg)

