Severity-Dependent Long-Term Post-Traumatic Changes in the Circulating Oxylipin Profile
Abstract
1. Introduction
2. Results
2.1. Patient Groups Differ in Their Clinico-Pathological and Laboratory Parameters
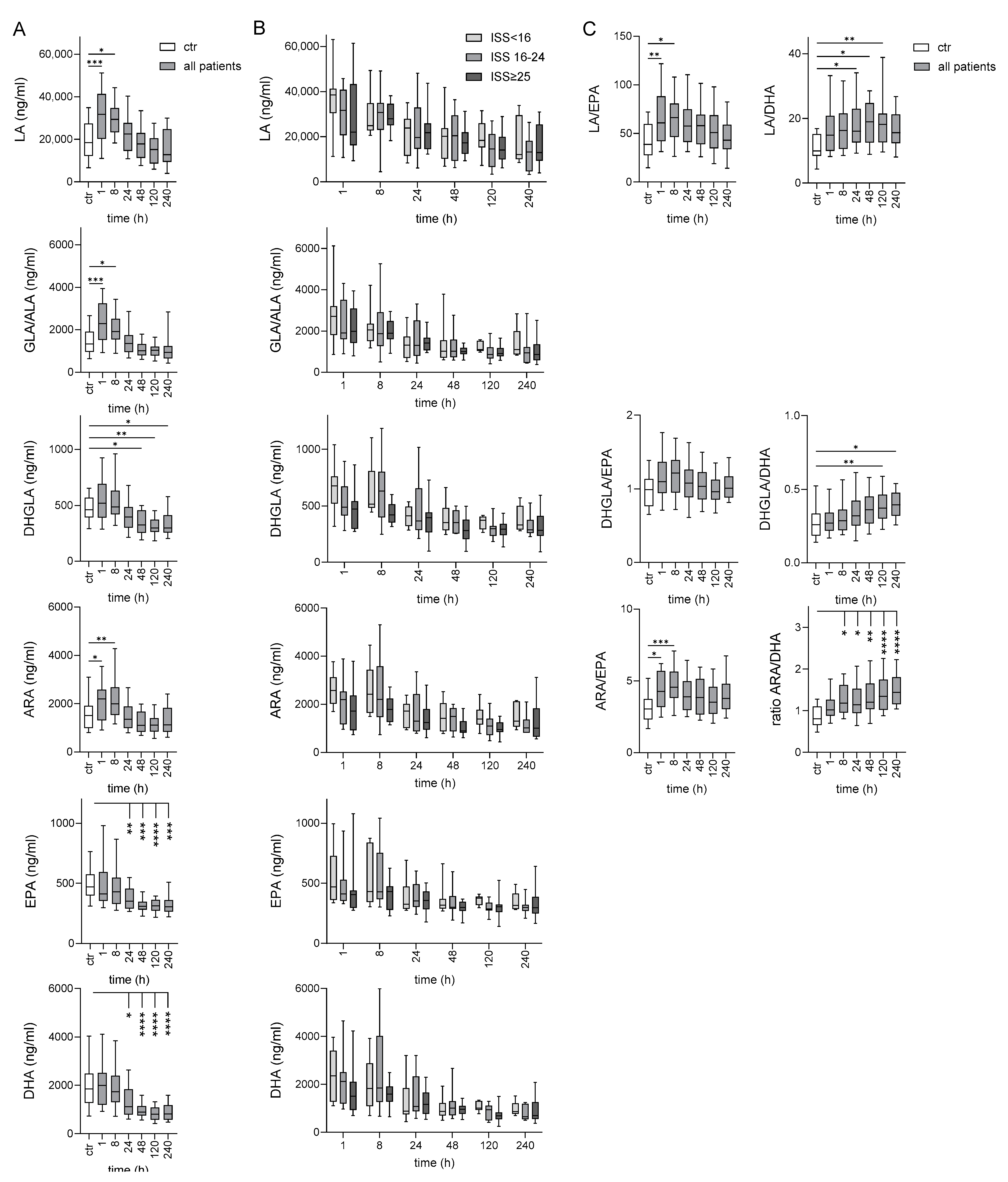
2.2. Long-Term Deficiency of ω-3 PUFAs in Traumatized Patients Compared to Controls
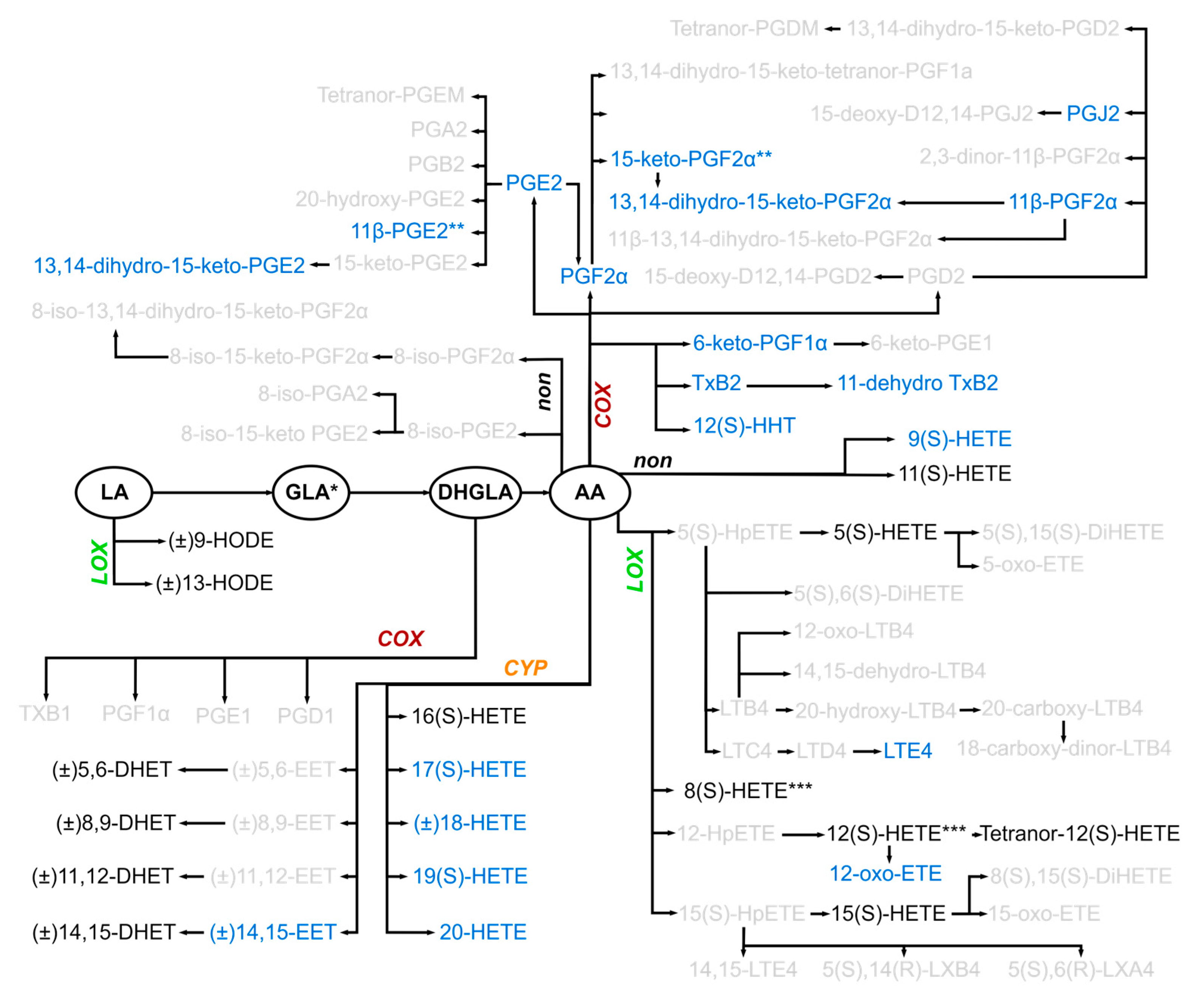
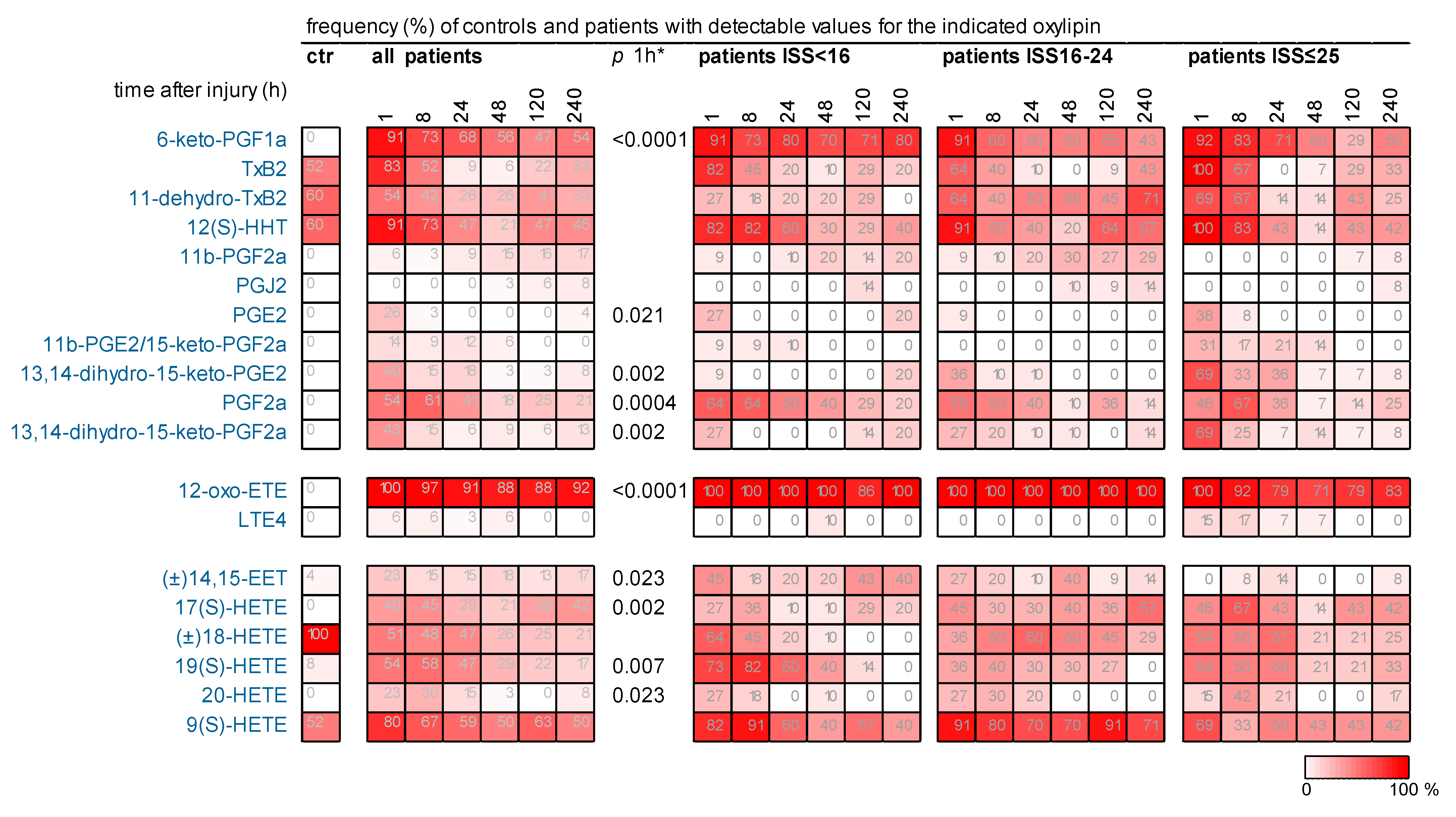
2.3. Many Oxylipins, Present Transiently Immediately After Injury in Some Patients, Were Much Less Detectable or Not Detected at All in Controls
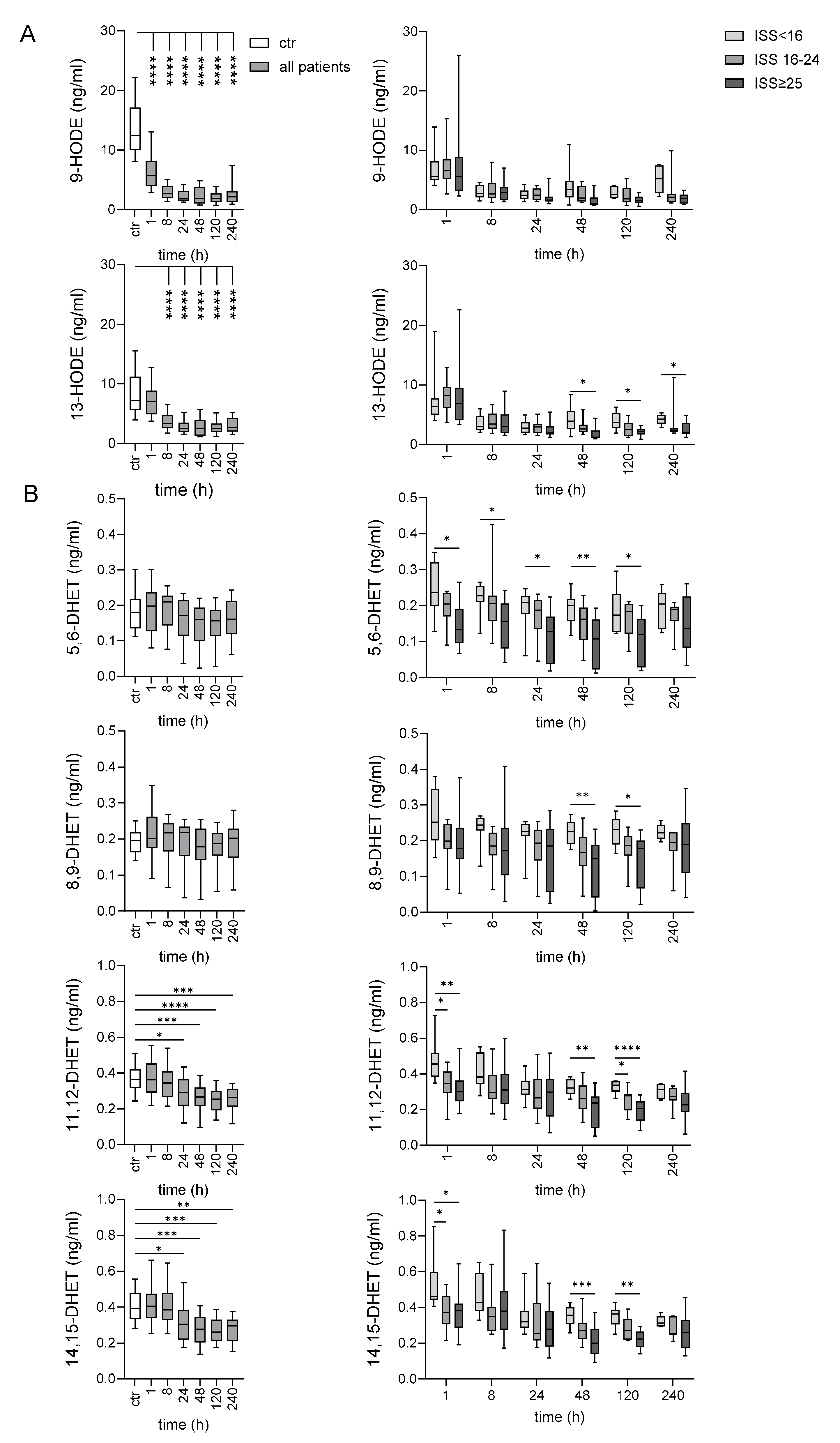
2.4. Late Decrease of LA-Derived 9- and 13-HODE After Very Severe Injury
2.5. Several Oxylipins of the COX-Generated Prostaglandin and Thromboxane Families Appeared Immediately After Injury
2.6. Early Increase in LOX-Generated ARA-Derived Oxylipins After Injury
2.7. Oxylipins Generated by CYP Activity: Lower Levels of Several DHETs After Very Severe Injury
2.8. 11(S)-HETE Increased Immediately After Injury
2.9. Several Oxylipins Correlate to Clinical Parameters in the Posttraumatic Course
3. Discussion
Limitation of the Study
4. Materials and Methods
4.1. Clinical Study on Traumatized Patients and Controls
4.2. Blood Sample Preparation
4.3. LC-MS/MS of PUFAs and Oxylipins
4.4. Quantitation of Further Parameters
4.5. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Huber-Lang, M.; Lambris, J.D.; Ward, P.A. Innate immune responses to trauma. Nat. Immunol. 2018, 19, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Norris, P.C.; Gosselin, D.; Reichart, D.; Glass, C.K.; Dennis, E.A. Phospholipase A2 regulates eicosanoid class switching during inflammasome activation. Proc. Natl. Acad. Sci. USA 2014, 111, 12746–12751. [Google Scholar] [CrossRef] [PubMed]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; Da Costa Souza, F.; Dalli, J.; Durand, T.; Galano, J.-M.; Lein, P.J.; et al. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: Recent advances in the understanding of their biosynthesis, structures, and functions. Prog. Lipid Res. 2022, 86, 101165. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef]
- Brennan, E.; Kantharidis, P.; Cooper, M.E.; Godson, C. Pro-resolving lipid mediators: Regulators of inflammation, metabolism and kidney function. Nat. Rev. Nephrol. 2021, 17, 725–739. [Google Scholar] [CrossRef]
- Mehrotra, P.; Maschalidi, S.; Boeckaerts, L.; Maueröder, C.; Tixeira, R.; Pinney, J.; Burgoa Cardás, J.; Sukhov, V.; Incik, Y.; Anderson, C.J.; et al. Oxylipins and metabolites from pyroptotic cells act as promoters of tissue repair. Nature 2024, 631, 207–215. [Google Scholar] [CrossRef]
- Dorow, J.; Becker, S.; Kortz, L.; Thiery, J.; Hauschildt, S.; Ceglarek, U. Preanalytical investigation of polyunsaturated fatty acids and eicosanoids in human plasma by liquid chromatography-tandem mass spectrometry. Biopreserv. Biobank. 2016, 14, 107–113. [Google Scholar] [CrossRef]
- Kortz, L.; Dorow, J.; Becker, S.; Thiery, J.; Ceglarek, U. Fast liquid chromatography-quadrupole linear ion trap-mass spectrometry analysis of polyunsaturated fatty acids and eicosanoids in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2013, 927, 209–213. [Google Scholar] [CrossRef]
- Heiling, S.; Knutti, N.; Scherr, F.; Geiger, J.; Weikert, J.; Rose, M.; Jahns, R.; Ceglarek, U.; Scherag, A.; Kiehntopf, M. Metabolite ratios as quality indicators for pre-analytical variation in serum and EDTA plasma. Metabolites 2021, 11, 638. [Google Scholar] [CrossRef]
- VanDerHeyden, N.; Cox, T.B. Trauma scoring. In Current Therapy of Trauma and Surgical Critical Care; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Liu, D.; Namas, R.A.; Vodovotz, Y.; Peitzman, A.B.; Simmons, R.L.; Yuan, H.; Mi, Q.; Billiar, T.R. Unsupervised clustering analysis based on MODS severity identifies four distinct organ dysfunction patterns in severely injured blunt trauma patients. Front. Med. 2020, 7, 46. [Google Scholar] [CrossRef]
- Hamaguchi, M.; Wu, H.N.; Tanaka, M.; Tsuda, N.; Tantengco, O.A.G.; Matsushima, T.; Nakao, T.; Ishibe, T.; Sakata, I.; Yanagihara, I. A case series of the dynamics of lipid mediators in patients with sepsis. Acute Med. Surg. 2019, 6, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Tintle, N.L.; Imamura, F.; Qian, F.; Korat, A.V.A.; Marklund, M.; Djoussé, L.; Bassett, J.K.; Carmichael, P.-H.; Chen, Y.-Y.; et al. Blood n-3 fatty acid levels and total and cause-specific mortality from 17 prospective studies. Nat. Commun. 2021, 12, 2329. [Google Scholar] [CrossRef] [PubMed]
- Strassburg, K.; Huijbrechts, A.M.L.; Kortekaas, K.A.; Lindeman, J.H.; Pedersen, T.L.; Dane, A.; Berger, R.; Brenkman, A.; Hankemeier, T.; van Duynhoven, J.; et al. Quantitative profiling of oxylipins through comprehensive LC-MS/MS analysis: Application in cardiac surgery. Ana Bioanal. Chem. 2012, 404, 1413–1426. [Google Scholar] [CrossRef] [PubMed]
- Vangaveti, V.; Baune, B.T.; Kennedy, R.L. Hydroxyoctadecadienoic acids: Novel regulators of macrophage differentiation and atherogenesis. Ther. Adv. Endocrinol. Metab. 2010, 1, 51–60. [Google Scholar] [CrossRef]
- Morisseau, C.; Hammock, B.D. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 37–58. [Google Scholar] [CrossRef]
- Spector, A.A.; Norris, A.W. Action of epoxyeicosatrienoic acids on cellular function. Am. J. Physiol. Cell Physiol. 2007, 292, C996–C1012. [Google Scholar] [CrossRef]
- Thomson, S.J.; Askari, A.; Bishop-Bailey, D. Anti-inflammatory effects of epoxyeicosatrienoic acids. Int. J. Vasc. Med. 2012, 2012, 605101. [Google Scholar] [CrossRef]
- Tacconelli, S.; Patrignani, P. Inside epoxyeicosatrienoic acids and cardiovascular disease. Front. Pharmacol. 2014, 5, 239. [Google Scholar] [CrossRef]
- Sommer, K.; Jakob, H.; Lettenmeier, T.; Henrich, D.; Sterz, J.; Marzi, I.; Frank, J. Various effects of 11,12 EET rescue wound healing in a combined model of diabetes and ischemia. Sci. Rep. 2023, 13, 6519. [Google Scholar] [CrossRef]
- Bergmann, C.B.; Hammock, B.D.; Wan, D.; Gogolla, F.; Goetzman, H.; Caldwell, C.C.; Supp, D.M. TPPU treatment of burned mice dampens inflammation and generation of bioactive DHET which impairs neutrophil function. Sci. Rep. 2021, 11, 16555. [Google Scholar] [CrossRef]
- Dai, Y.; Dong, J.; Wu, Y.; Zhu, M.; Xiong, W.; Li, H.; Zhao, Y.; Hammock, B.D.; Zhu, X. Enhancement of the liver's neuroprotective role ameliorates traumatic brain injury pathology. Proc. Natl. Acad. Sci. USA 2023, 120, e2301360120. [Google Scholar] [CrossRef] [PubMed]
- Imig, J.D. Prospective for cytochrome P450 epoxygenase cardiovascular and renal therapeutics. Pharmacol. Ther. 2018, 192, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Endo, S.; Suganami, A.; Fukushima, K.; Senoo, K.; Araki, Y.; Regan, J.W.; Mashimo, M.; Tamura, Y.; Fujino, H. 15-Keto-PGE(2) acts as a biased/partial agonist to terminate PGE(2)-evoked signaling. J. Biol. Chem. 2020, 295, 13338–13352. [Google Scholar] [CrossRef]
- Kourpa, A.; Kaiser-Graf, D.; Sporbert, A.; Philippe, A.; Catar, R.; Rothe, M.; Mangelsen, E.; Schulz, A.; Bolbrinker, J.; Kreutz, R.; et al. 15-keto-Prostaglandin E(2) exhibits bioactive role by modulating glomerular cytoarchitecture through EP2/EP4 receptors. Life Sci. 2022, 310, 121114. [Google Scholar] [CrossRef]
- Mitsuhashi, T.; Ikata, T.; Morimoto, K.; Tonai, T.; Katoh, S. Increased production of eicosanoids, TXA2, PGI2 and LTC4 in experimental spinal cord injuries. Paraplegia 1994, 32, 524–530. [Google Scholar] [CrossRef]
- Resnick, D.K.; Nguyen, P.; Cechvala, C.F. Regional and temporal changes in prostaglandin E2 and thromboxane B2 concentrations after spinal cord injury. Spine J. 2001, 1, 432–436. [Google Scholar] [CrossRef]
- Aibiki, M.; Maekawa, S.; Yokono, S. Moderate hypothermia improves imbalances of thromboxane A2 and prostaglandin I2 production after traumatic brain injury in humans. Crit. Care Med. 2000, 28, 3902–3906. [Google Scholar] [CrossRef]
- Vassar, M.J.; Weber, C.J.; Holcroft, J.W. Measurement of 6-keto-PGF1 alpha and thromboxane B2 levels in critically ill surgical patients. Prostaglandins Leukot. Essent. Fatty Acids 1988, 33, 129–135. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Ostermann, A.I.; Stork, L.; Fritzsch, S.; Kohrs, H.; Greupner, T.; Hahn, A.; Schebb, N.H. Effect of DHA supplementation on oxylipin levels in plasma and immune cell stimulated blood. Prostaglandins Leukot. Essent. Fatty Acids 2017, 121, 76–87. [Google Scholar] [CrossRef]
- Janssen, C.I.F.; Kiliaan, A.J. Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: The influence of LCPUFA on neural development, aging, and neurodegeneration. Prog. Lipid Res. 2014, 53, 1–17. [Google Scholar] [CrossRef]
- Anthonymuthu, T.S.; Kenny, E.M.; Amoscato, A.A.; Lewis, J.; Kochanek, P.M.; Kagan, V.E.; Bayır, H. Global assessment of oxidized free fatty acids in brain reveals an enzymatic predominance to oxidative signaling after trauma. Biochim. Biophys. Acta 2017, 1863, 2601–2613. [Google Scholar] [CrossRef] [PubMed]
- Mallah, K.; Quanico, J.; Trede, D.; Kobeissy, F.; Zibara, K.; Salzet, M.; Fournier, I. Lipid changes associated with traumatic brain injury revealed by 3D MALDI-MSI. Anal. Chem. 2018, 90, 10568–10576. [Google Scholar] [CrossRef]
- Farias, S.E.; Heidenreich, K.A.; Wohlauer, M.V.; Murphy, R.C.; Moore, E.E. Lipid mediators in cerebral spinal fluid of traumatic brain injured patients. J. Trauma. 2011, 71, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Orr, S.K.; Butler, K.L.; Hayden, D.; Tompkins, R.G.; Serhan, C.N.; Irimia, D. Gene expression of proresolving lipid mediator pathways is associated with clinical outcomes in trauma patients. Crit. Care Med. 2015, 43, 2642–2650. [Google Scholar] [CrossRef] [PubMed]
- Basil, M.C.; Levy, B.D. Specialized pro-resolving mediators: Endogenous regulators of infection and inflammation. Nat. Rev. Immunol. 2016, 16, 51–67. [Google Scholar] [CrossRef]
- Chiang, N.; Serhan, C.N. Specialized pro-resolving mediator network: An update on production and actions. Essays Biochem. 2020, 64, 443–462. [Google Scholar] [CrossRef]
- Schebb, N.H.; Kühn, H.; Kahnt, A.S.; Rund, K.M.; O'Donnell, V.B.; Flamand, N.; Peters-Golden, M.; Jakobsson, P.-J.; Weylandt, K.H.; Rohwer, N.; et al. Formation, signaling and occurrence of specialized pro-resolving lipid mediators-What is the evidence so far? Front. Pharmacol. 2022, 13, 838782. [Google Scholar] [CrossRef]
- Calder, P.C. Eicosapentaenoic and docosahexaenoic acid derived specialised pro-resolving mediators: Concentrations in humans and the effects of age, sex, disease and increased omega-3 fatty acid intake. Biochimie 2020, 178, 105–123. [Google Scholar] [CrossRef]
- O'Donnell, V.B.; Schebb, N.H.; Milne, G.L.; Murphy, M.P.; Thomas, C.P.; Steinhilber, D.; Gelhaus, S.L.; Kühn, H.; Gelb, M.H.; Jakobsson, P.-J.; et al. Failure to apply standard limit-of-detection or limit-of-quantitation criteria to specialized pro-resolving mediator analysis incorrectly characterizes their presence in biological samples. Nat. Commun. 2023, 14, 7172. [Google Scholar] [CrossRef]
- Chaves-Filho, A.B.; Diniz, L.S.; Santos, R.S.; Lima, R.S.; Oreliana, H.; Pinto, I.F.D.; Dantas, L.S.; Inague, A.; Faria, R.L.; Medeiros, M.H.G.; et al. Plasma oxylipin profiling by high resolution mass spectrometry reveal signatures of inflammation and hypermetabolism in amyotrophic lateral sclerosis. Free Radic. Biol. Med. 2023, 208, 285–298. [Google Scholar] [CrossRef]
- Pauls, S.D.; Du, Y.; Clair, L.; Winter, T.; Aukema, H.M.; Taylor, C.G.; Zahradka, P. Impact of age, menopause, and obesity on oxylipins linked to vascular health. Arterioscler.Thromb.Vasc.Biol. 2021, 41, 883–897. [Google Scholar] [CrossRef] [PubMed]
- Beutner, F.; Teupser, D.; Gielen, S.; Holdt, L.M.; Scholz, M.; Boudriot, E.; Schuler, G.; Thiery, J. Rationale and design of the Leipzig (LIFE) Heart Study: Phenotyping and cardiovascular characteristics of patients with coronary artery disease. PLoS ONE 2011, 6, e29070. [Google Scholar] [CrossRef] [PubMed]
- Weissgerber, A.; Scholz, M.; Teren, A.; Sandri, M.; Teupser, D.; Gielen, S.; Thiery, J.; Schuler, G.; Beutner, F. The value of noncoronary atherosclerosis for identifying coronary artery disease: Results of the Leipzig LIFE Heart Study. Clin. Res. Cardiol. 2016, 105, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Yang, J.; Shao, Y.; Li, P.; Guo, H.; Wu, J.; Zhu, Y.; Zhang, H.; Zhang, X.; Du, J.; et al. Therapeutic and prognostic significance of arachidonic acid in heart failure. Circ. Res. 2022, 130, 1056–1071. [Google Scholar] [CrossRef]

| Traumatized Patients | p Value * | |||||
|---|---|---|---|---|---|---|
| Group 1, ISS < 16 | Group 2, ISS 16–24 | Group 3, ISS ≥ 25 | Comparing Group | |||
| patients (n) | 11 | 11 | 14 | 1–2 | 2–3 | 1–3 |
| ISS | 12.5 (9.0–13.0) | 20.0 (19.0–21.0) | 41.0 (34.0–50.0) | 0.043 | 0.010 | <0.001 |
| age (years) | 55.5 (32.0–68.0) | 60.0 (35.0–70.0) | 53.0 (34.0–59.0) | 0.849 | 0.599 | 0.455 |
| sex, female (n) | 1 | 3 | 7 | 1 | 1 | 0.126 |
| days at ICU (n) | 2.5 (2.0–3.0) | 2.0 (2.0–8.0) | 15.0 (6.0–34.0) | 1 | 0.004 | 0.002 |
| days in hospital (n) | 12.0 (7.0–16.0) | 14.0 (12.0–17.0) | 23.0 (16.0–42.0) | 0.732 | 0.217 | 0.007 |
| death in hospital (n) | 0 | 0 | 2 | |||
| SOFA score at 24 h | 1.0 (1.0–3.0) | 3.0 (1.0–4.0) | 7.0 (5.0–9.0) | 0.880 | 0.087 | 0.003 |
| PUFA/Metabolite | Present in Controls (n = 25) | PUFA/Metabolite Derived From | 1st; 2nd Pathway | Fatty Acid | Molecular Formula | |
|---|---|---|---|---|---|---|
| 1 | LA | 25 | PUFA | ω 6 | C18H32O2 | |
| 2 | GLA/ALA * | 25 | PUFA | ω 6 | C18H30O2 | |
| 3 | DHGLA | 25 | PUFA | ω 6 | C20H34O2 | |
| 4 | ARA | 25 | PUFA | ω 6 | C20H32O2 | |
| 5 | EPA | 25 | PUFA | ω 3 | C20H30O2 | |
| 6 | DHA | 25 | PUFA | ω 3 | C22H32O2 | |
| 7 | 9-HODE | 25 | LA | LOX; peroxidation | ω 6 | C18H32O3 |
| 8 | 13-HODE | 25 | LA | LOX; peroxidation | ω 6 | C18H32O3 |
| 9 | 6-keto-PGF1a | - | ARA | COX | ω 6 | C20H34O6 |
| 10 | TxB2 | 13/25 | ARA | COX2 | ω 6 | C20H34O6 |
| 11 | 11-dehydro-TxB2 | 15/25 | ARA | COX2 | ω 6 | C20H32O6 |
| 12 | 12(S)-HHT | 13/25 | ARA | COX | ω 6 | C17H28O3 |
| 13 | 11b-PGF2a | - | ARA | COX2 | ω 6 | C20H34O5 |
| 14 | PGJ2 | - | ARA | COX | ω 6 | C20H32O5 |
| 15 | PGE2 | - | ARA | COX | ω 6 | C20H32O5 |
| 16 | 11b-PGE2/15-keto-PGF2a ** | - | ARA | COX2 | ω 6 | C20H32O5 |
| 17 | 13,14-dihydro-15-keto-PGE2 | - | ARA | COX | ω 6 | C20H32O5 |
| 18 | PGF2a | - | ARA | COX2 | ω 6 | C20H34O5 |
| 19 | 13,14-dihydro-15-keto-PGF2a | - | ARA | COX2 | ω 6 | C20H34O5 |
| 20 | 5(S)-HETE | 25 | ARA | LOX; CYP | ω 6 | C20H32O3 |
| 21 | 8(S)-HETE/12(S)-HETE *** | 25 | ARA | LOX; CYP | ω 6 | C20H32O3 |
| 22 | 12-oxo-ETE | - | ARA | LOX | ω 6 | C20H30O3 |
| 23 | tetranor-12(S)-HETE | 25 | ARA | peroxidation; LOX | ω 6 | C16H26O3 |
| 24 | 15(S)-HETE | 25 | ARA | 15-LOX-1/2; CYP | ω 6 | C20H32O3 |
| 25 | LTE4 | - | ARA | 5-LOX | ω 6 | C23H37NO5S |
| 26 | 5,6-DHET | 24/25 | ARA | CYP | ω 6 | C20H34O4 |
| 27 | 8,9-DHET | 24/25 | ARA | CYP | ω 6 | C20H34O4 |
| 28 | 11,12-DHET | 25 | ARA | CYP | ω 6 | C20H34O4 |
| 29 | 14,15-EET | 1/25 | ARA | CYP | ω 6 | C20H32O3 |
| 30 | 14,15-DHET | 25 | ARA | CYP | ω 6 | C20H34O4 |
| 31 | 16(S)-HETE | 25 | ARA | CYP | ω 6 | C20H32O3 |
| 32 | 17(S)-HETE | - | ARA | CYP | ω 6 | C20H32O3 |
| 33 | 18-HETE | 25 | ARA | CYP/LOX | ω 6 | C20H32O3 |
| 34 | 19(S)-HETE | 2/25 | ARA | CYP | ω 6 | C20H32O3 |
| 35 | 20-HETE | - | ARA | CYP | ω 6 | C20H32O3 |
| 36 | 9(S)-HETE | 13/25 | ARA | peroxidation; CYP | ω 6 | C20H32O3 |
| 37 | 11(S)-HETE | 25 | ARA | peroxidation; COX, LOX | ω 6 | C20H32O3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reinicke, M.; Zheng, L.; Rang, M.; Fuchs, C.; Weikert, J.; Keß, A.; Kleber, C.; Ceglarek, U.; Osterhoff, G.; Aust, G. Severity-Dependent Long-Term Post-Traumatic Changes in the Circulating Oxylipin Profile. Int. J. Mol. Sci. 2024, 25, 13530. https://doi.org/10.3390/ijms252413530
Reinicke M, Zheng L, Rang M, Fuchs C, Weikert J, Keß A, Kleber C, Ceglarek U, Osterhoff G, Aust G. Severity-Dependent Long-Term Post-Traumatic Changes in the Circulating Oxylipin Profile. International Journal of Molecular Sciences. 2024; 25(24):13530. https://doi.org/10.3390/ijms252413530
Chicago/Turabian StyleReinicke, Madlen, Leyu Zheng, Moujie Rang, Carolin Fuchs, Juliane Weikert, Annette Keß, Christian Kleber, Uta Ceglarek, Georg Osterhoff, and Gabriela Aust. 2024. "Severity-Dependent Long-Term Post-Traumatic Changes in the Circulating Oxylipin Profile" International Journal of Molecular Sciences 25, no. 24: 13530. https://doi.org/10.3390/ijms252413530
APA StyleReinicke, M., Zheng, L., Rang, M., Fuchs, C., Weikert, J., Keß, A., Kleber, C., Ceglarek, U., Osterhoff, G., & Aust, G. (2024). Severity-Dependent Long-Term Post-Traumatic Changes in the Circulating Oxylipin Profile. International Journal of Molecular Sciences, 25(24), 13530. https://doi.org/10.3390/ijms252413530








