Preeclampsia Treatment Aspirin/Clampsilin: Oxidative Stress, sFlt-1/PIGF Soluble Tyrosine Kinase 1, and Placental Growth Factor Monitoring
Abstract
1. Introduction
2. Results
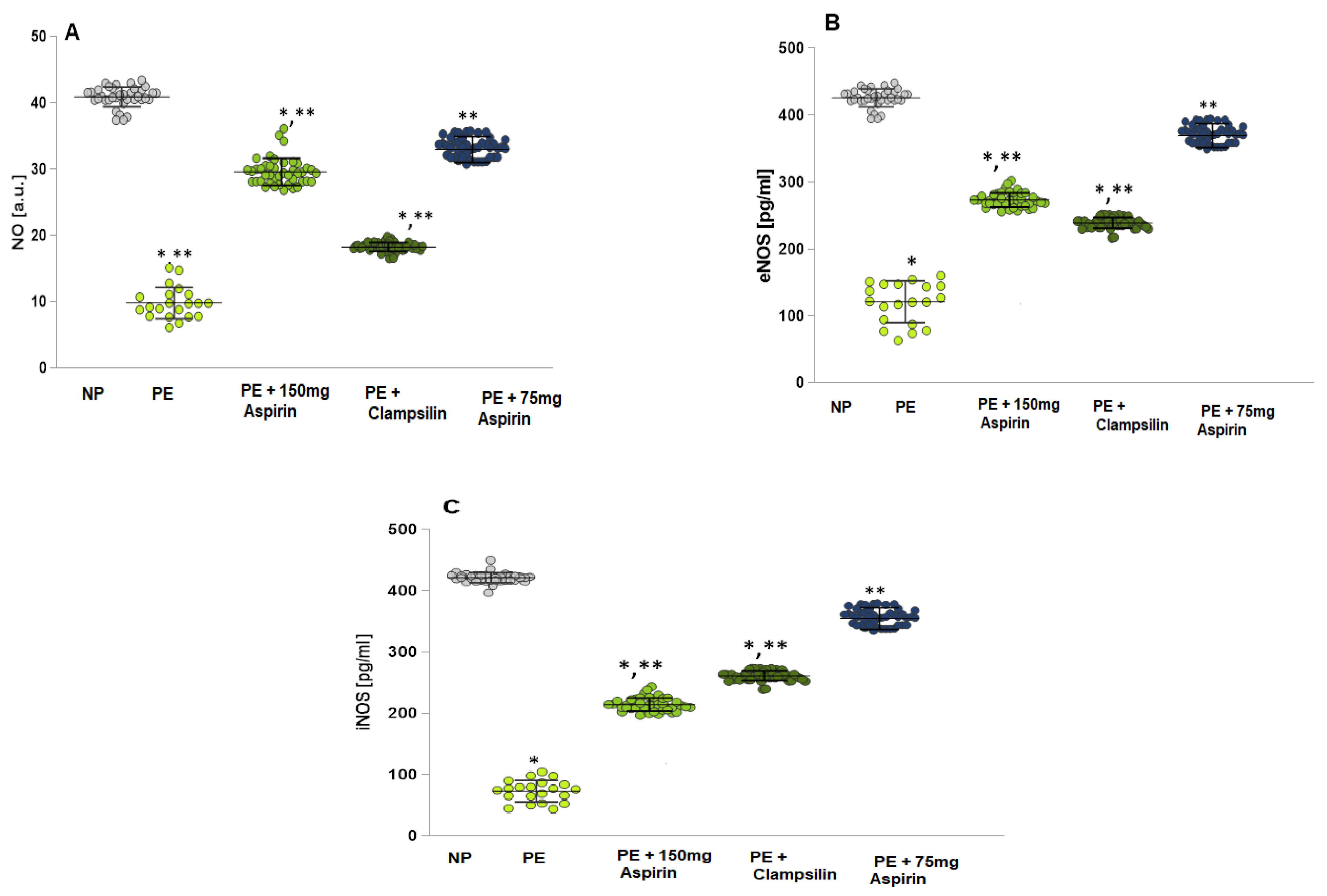
2.1. Levels of NO, eNOS, and iNOS
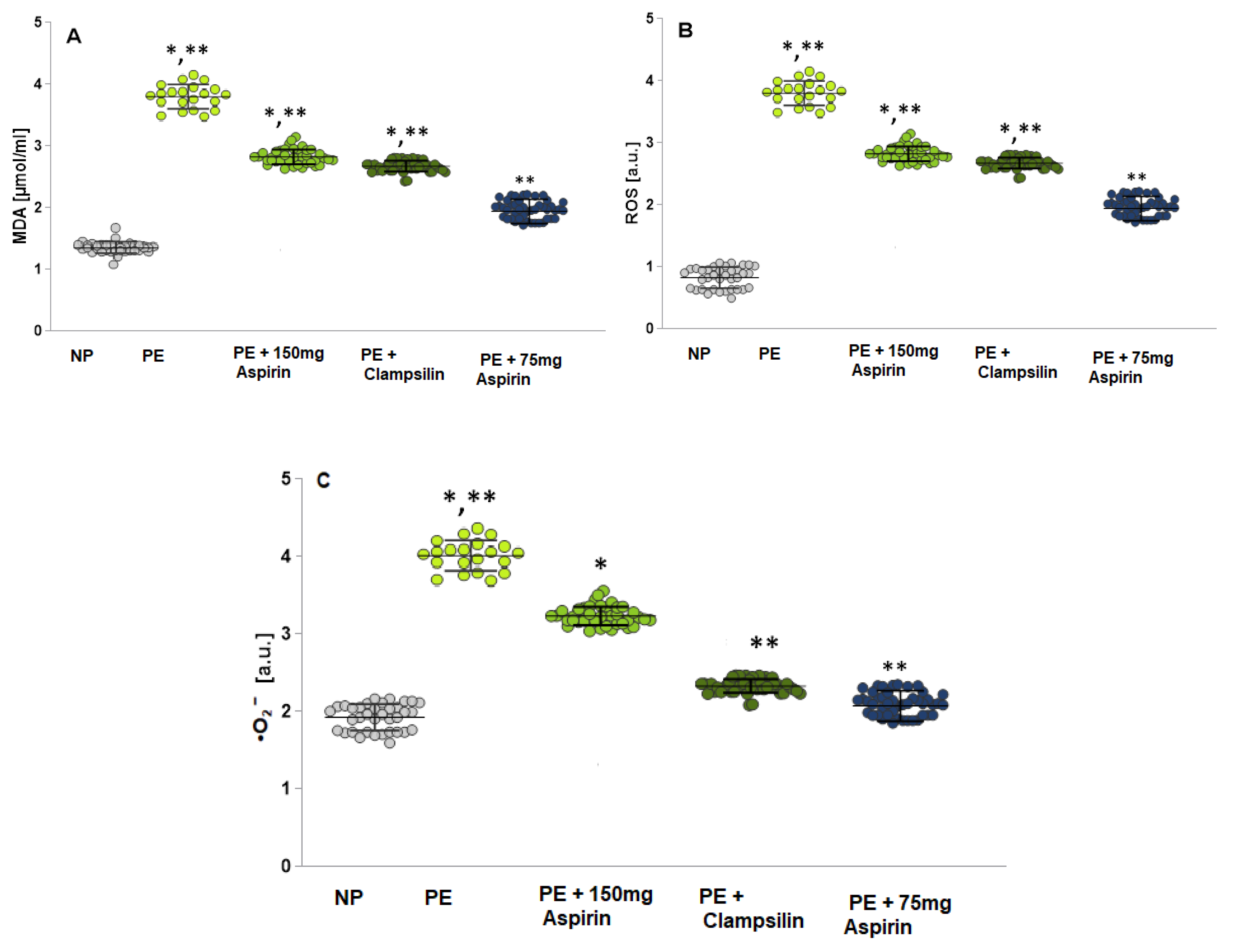
2.2. Oxidative Stress Assessment by Measuring the MDA, ROS and •O2−
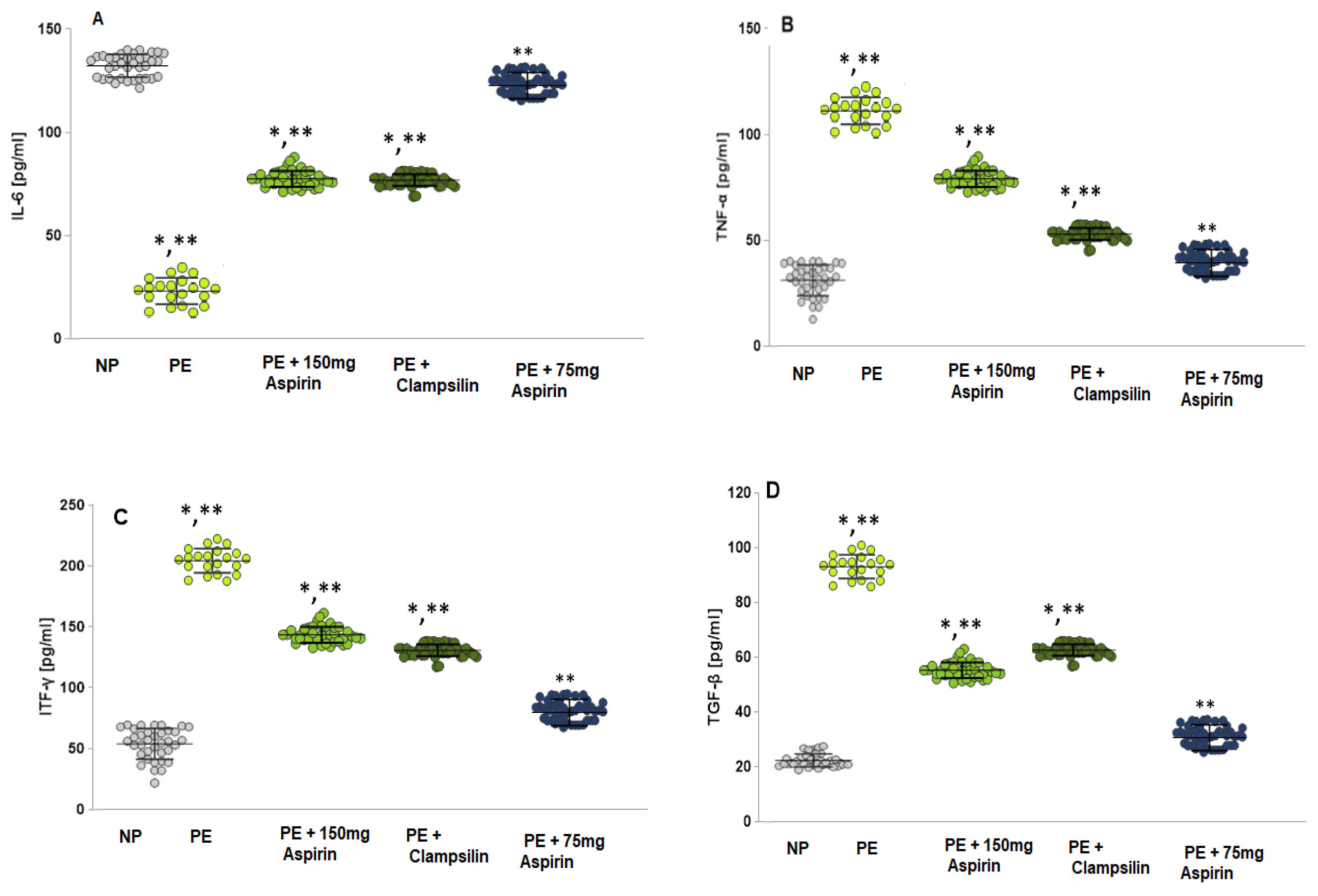
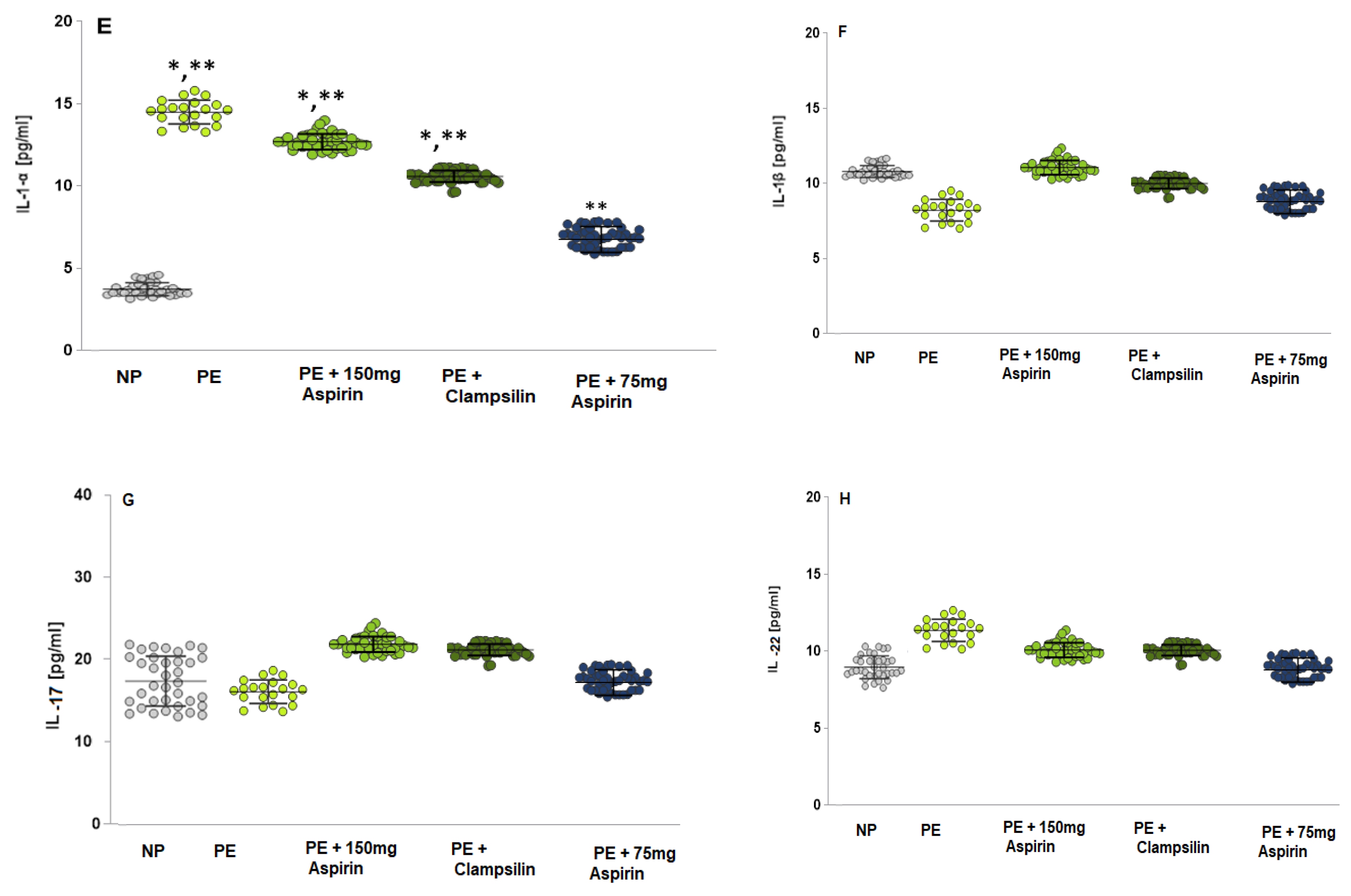
2.3. The Levels of Pro-Inflammatory Cytokines
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Diagnostic Criteria for Outcomes
4.3. Prediction of Preeclampsia
4.4. Patients
4.5. Blood Samples Preparation
4.6. Electron Paramagnetic Resonance (EPR) Study
4.7. Evaluation of the ROS Product Levels
4.8. Evaluation of the •NO Radical Levels
4.9. Superoxide Anion Radical •O2−
4.10. Enzyme-Linked Immunosorbent Assay
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ridder, A.; Giorgione, V.; Khalil, A.; Thilaganathan, B. Preeclampsia: The Relationship between Uterine Artery Blood Flow and Trophoblast Function. Int. J. Mol. Sci. 2019, 20, 3263. [Google Scholar] [CrossRef]
- Jena, M.K.; Sharma, N.R.; Petitt, M.; Maulik, D.; Nayak, N.R. Pathogenesis of Preeclampsia and Therapeutic Approaches Targeting the Placenta. Biomolecules 2020, 10, 953. [Google Scholar] [CrossRef] [PubMed]
- Dawid, M.; Mlyczyńska, E.; Jurek, M.; Respekta, N.; Pich, K.; Kurowska, P.; Gieras, W.; Milewicz, T.; Kotula-Balak, M.; Rak, A. Apelin, APJ, and ELABELA: Role in Placental Function, Pregnancy, and Foetal Development—An Overview. Cells 2022, 11, 99. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, O.; García-Montero, C.; Sáez, M.A.; Álvarez-Mon, M.A.; Torres-Carranza, D.; Álvarez-Mon, M.; Bujan, J.; García-Honduvilla, N.; Bravo, C.; et al. The pivotal role of the placenta in normal and pathological pregnancies: A focus on preeclampsia, fetal growth restriction, and maternal chronic venous disease. Cells 2022, 11, 568. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.; O’Brien, C.L.; Yeoh, C.; Gersh, F.L.; Brennecke, S. Reducing the risk of pre-eclampsia in women with polycystic ovary syndrome using a combination of pregnancy screening, lifestyle, and medical management strategies. J. Clin. Med. 2024, 13, 1774. [Google Scholar] [CrossRef] [PubMed]
- Pankiewicz, K.; Fijałkowska, A.; Issat, T.; Maciejewski, T.M. Insight into the Key Points of Preeclampsia Pathophysiology: Uterine Artery Remodeling and the Role of MicroRNAs. Int. J. Mol. Sci. 2021, 22, 3132. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, K.; Higaki, T.; Matsubara, Y.; Nawa, A. Nitric Oxide and Reactive Oxygen Species in the Pathogenesis of Preeclampsia. Int. J. Mol. Sci. 2015, 16, 4600–4614. [Google Scholar] [CrossRef] [PubMed]
- Ponnampalam, E.N.; Kiani, A.; Santhiravel, S.; Holman, B.W.B.; Lauridsen, C.; Dunshea, F.R. The Importance of Dietary Antioxidants on Oxidative Stress, Meat and Milk Production, and Their Preservative Aspects in Farm Animals: Antioxidant Action, Animal Health, and Product Quality—Invited Review. Animals 2022, 12, 3279. [Google Scholar] [CrossRef] [PubMed]
- Charlton, N.C.; Mastyugin, M.; Török, B.; Török, M. Structural Features of Small Molecule Antioxidants and Strategic Modifications to Improve Potential Bioactivity. Molecules 2023, 28, 1057. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, X.; Obore, N.; Ding, H.; Wu, C.; Yu, H. Aspirin for the prevention of preeclampsia: A systematic review and meta-analysis of randomized controlled studies. Front. Cardiovasc. Med. 2022, 9, 936560. [Google Scholar] [CrossRef]
- Mkhize, P.Z.; Phoswa, W.N.; Khaliq, O.P.; Dorsamy, V.; Moodley, J. Aspirin in the prevention of preeclampsia: A protocol for systematic review and meta-analysis. Medicine 2021, 100, e27916. [Google Scholar] [CrossRef] [PubMed]
- Tousty, P.; Fraszczyk-Tousty, M.; Dzidek, S.; Jasiak-Jóźwik, H.; Michalczyk, K.; Kwiatkowska, E.; Cymbaluk-Płoska, A.; Torbé, A.; Kwiatkowski, S. Low-Dose Aspirin after ASPRE—More Questions Than Answers? Current International Approach after PE Screening in the First Trimester. Biomedicines 2023, 11, 1495. [Google Scholar] [CrossRef]
- Tousty, P.; Fraszczyk-Tousty, M.; Golara, A.; Zahorowska, A.; Sławiński, M.; Dzidek, S.; Jasiak-Jóźwik, H.; Nawceniak-Balczerska, M.; Kordek, A.; Kwiatkowska, E.; et al. Screening for Preeclampsia and Fetal Growth Restriction in the First Trimester in Women without Chronic Hypertension. J. Clin. Med. 2023, 12, 5582. [Google Scholar] [CrossRef]
- Ghesquiere, L.; Guerby, P.; Marchant, I.; Kumar, N.; Zare, M.; Foisy, M.-A.; Roberge, S.; Bujold, E. Comparing aspirin 75 to 81 mg vs 150 to 162 mg for prevention of preterm preeclampsia: Systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2023, 5, 101000. [Google Scholar] [CrossRef] [PubMed]
- Antonijevic, N.; Gosnjic, N.; Marjanovic, M.; Antonijevic, J.; Culafic, M.; Starcevic, J.; Plavsic, M.; Mostic Stanisic, D.; Uscumlic, A.; Lekovic, Z.; et al. Antiplatelet Drugs Use in Pregnancy—Review of the Current Practice and Future Implications. J. Pers. Med. 2024, 14, 560. [Google Scholar] [CrossRef] [PubMed]
- Wallenburg, H.C.S.; Makovitz, J.W.; Dekker, G.A.; Rotmans, P. Low-dose aspirin prevents pregnancy-induced hypertension and pre-eclampsia in angiotensin-sensitive primigravidae. Lancet 1986, 327, 1–3. [Google Scholar] [CrossRef]
- Bujold, E.; Roberge, S.; Lacasse, Y.; Bureau, M.; Audibert, F.; Marcoux, S.; Forest, J.-C.; Giguere, Y. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: A meta-analysis. Obstet. Gynecol. 2010, 116 Pt 1, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Loussert, L.; Vidal, F.; Parant, O.; Hamdi, S.M.; Vayssiere, C.; Guerby, P. Aspirin for prevention of preeclampsia and fetal growth restriction. Prenat. Diagn. 2020, 40, 519–527. [Google Scholar] [CrossRef]
- Roberge, S.; Bujold, E.; Nicolaides, K.H. Aspirin for the prevention of preterm and term preeclampsia: Systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2018, 218, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Roberge, S.; Villa, P.; Nicolaides, K.; Giguère, Y.; Vainio, M.; Bakthi, A.; Ebrashy, A.; Bujold, E. Early administration of low-dose aspirin for the prevention of preterm and term preeclampsia: A systematic review and meta-analysis. Fetal Diagn. Ther. 2012, 31, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Roberge, S.; Sibai, B.; McCaw-Binns, A.; Bujold, E. Low-dose aspirin in early gestation for prevention of preeclampsia and small-for-gestational-age neonates: Meta-analysis of large randomized trials. Am. J. Perinatol. 2016, 33, 781–785. [Google Scholar] [CrossRef]
- Opichka, M.A.; Rappelt, M.W.; Gutterman, D.D.; Grobe, J.L.; McIntosh, J.J. Vascular Dysfunction in Preeclampsia. Cells 2021, 10, 3055. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.-T.; Seow, K.-M.; Chen, K.-H. The Pathophysiological, Genetic, and Hormonal Changes in Preeclampsia: A Systematic Review of the Molecular Mechanisms. Int. J. Mol. Sci. 2024, 25, 4532. [Google Scholar] [CrossRef] [PubMed]
- Ives, C.W.; Sinkey, R.; Rajapreyar, I.; Tita, A.T.; Oparil, S. Preeclampsia—Pathophysiology and clinical presentations: JACC state-of-the-art review. J. Am. Coll. Card. 2020, 76, 1690–1702. [Google Scholar] [CrossRef] [PubMed]
- Ergashev, U.; Yu, M.; Luo, L.; Tang, J.; Han, Y. The Key Targets of NO-Mediated Post-Translation Modification (PTM) Highlighting the Dynamic Metabolism of ROS and RNS in Peroxisomes. Int. J. Mol. Sci. 2024, 25, 8873. [Google Scholar] [CrossRef]
- Chiarello, D.I.; Abad, C.; Rojas, D.; Toledo, F.; Vázquez, C.M.; Mate, A.; Marín, R. Oxidative stress: Normal pregnancy versus preeclampsia. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165354. [Google Scholar] [CrossRef] [PubMed]
- Grzeszczak, K.; Łanocha-Arendarczyk, N.; Malinowski, W.; Ziętek, P.; Kosik-Bogacka, D. Oxidative Stress in Pregnancy. Biomolecules 2023, 13, 1768. [Google Scholar] [CrossRef]
- Vangrieken, P.; Al-Nasiry, S.; Bast, A.; Leermakers, P.A.; Tulen, C.B.; Janssen, G.M.; Remels, A.H. Hypoxia-induced mitochondrial abnormalities in cells of the placenta. PLoS ONE 2021, 16, e0245155. [Google Scholar] [CrossRef]
- Phoswa, W.N.; Khaliq, O.P. The role of oxidative stress in hypertensive disorders of pregnancy (preeclampsia, gestational hypertension) and metabolic disorder of pregnancy (gestational diabetes mellitus). Oxidative Med. Cell. Longev. 2021, 5581570. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Powell, M.; Cromartie, W.; Smith, S.; Jones, K.; Castillo, A.; Cunningham, M. Intrauterine growth-restricted pregnant rats, from placental ischemic dams, displays preeclamptic-like symptoms: A new rat model of preeclampsia. Physiol. Rep. 2024, 12, e70112. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Aranguren, L.C.; Prada, C.E.; Riaño-Medina, C.E.; Lopez, M. Endothelial dysfunction and preeclampsia: Role of oxidative stress. Front. Physiol. 2014, 5, 372. [Google Scholar] [CrossRef] [PubMed]
- Freire, V.A.F.; de Melo, A.D.; de Lima Santos, H.; Barros-Pinheiro, M. Evaluation of oxidative stress markers in subtypes of preeclampsia: A systematic review and meta-analysis. Placenta 2023, 132, 55–67. [Google Scholar] [CrossRef]
- Taravati, A.; Tohidi, F. Comprehensive analysis of oxidative stress markers and antioxidants status in preeclampsia. Taiwan J. Obstet. Gynecol. 2018, 57, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Magee, L.A.; Smith, G.N.; Bloch, C.; Côté, A.M.; Jain, V.; Nerenberg, K.; Rey, E. Guideline No. 426: Hypertensive disorders of pregnancy: Diagnosis, prediction, prevention, and management. J. Obstet. Gynaecol. Can. 2022, 44, 547–571. [Google Scholar] [CrossRef]
- Webster, K.; Fishburn, S.; Maresh, M.; Findlay, S.C.; Chappell, L.C. Diagnosis and management of hypertension in pregnancy: Summary of updated NICE guidance. BMJ 2019, 366. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Sui, Y.; Wang, X.; Luo, Y.; Ji, L. Hydroxyl radical production and oxidative damage induced by cadmium and naphthalene in liver of Carassius auratus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2005, 140, 115–121. [Google Scholar] [CrossRef]
- Yokoyama, K.; Hashiba, K.; Wakabayashi, H.; Hashimoto, K.; Satoh, K.; Kurihara, T.; Motohashi, N.; Sakagami, H. Inhibition of LPS-stimulated NO production in mouse macrophage-like cells by tropolones. Anticancer Res. 2004, 24, 3917–3922. [Google Scholar]
- Yoshioka, T.; Iwamoto, N.; Lto, K. An application of Electron Paramagnetic Resonance to evaluate nitric oxide and its quenchers. J. Am. Soc. Nephrol. 1996, 7, 961–965. [Google Scholar] [CrossRef]
- Simenauer, A.M. HIV Tat Mediation of Redox Sensitive Transcription Factors in Pulmonary Vascular Cells; University of Colorado Denver, Anschutz Medical Campus: Aurora, CO, USA, 2020. [Google Scholar] [CrossRef]
| Variables | Normotensive Pregnant (NP) n = 34 | PE Before Therapy (n = 77) | PE with Aspirine Acard (n = 34) 150 mg/day | PE with Clampsilin Therapy (n = 21) 2 Sachets Daily | PE with Aspirine Acard (n = 22) 75 mg/day |
|---|---|---|---|---|---|
| Age at delivery (years) | 27.33 ±9.28 | 29.17 ± 10.01 | 30.17 ± 10.01 | 28.17 ± 9.25 | 28.11 ± 10.36 |
| BMI (kg/m2) mean ± SD | 24.5 (21.9–28.4) | 27.1 (23.5–32.3) | 33.56 (31.4 ± 36.4) | 33.76 (31.6 ± 36.1) | 33.61 (31.4 ± 36.9) |
| Gestational age at sampling (weeks) | 11–13+6 | 11–13+6 | 20–34 | 20–34 | 20–34 |
| Blood shugar mmol/L, mean ±SD | 4.98 ± 0.51 | 5.12 ± 2.35 | 5.32 ± 4.89 | 4.85 ± 5.41 | 4.98 ± 5.02 |
| Chronic hypertension | no | no | no | no | no |
| HbA1C% | 5.69 ± 0.44 | 5.08 ± 0.52 | 5.16 ± 0.27 | 5.27 ± 0.11 | 5.11 ± 0.18 |
| Diabetes Mellitus (DM) Status | no | no | no | no | no |
| sFlT-1/PIGF | 5.03 ± 4.62 | 87.61 ± 14.11 | 70.85 ± 10.04 | 40.07 ± 9.33 | 72.01± 6.07 |
| sFlT-1 (soluble thyrosinkinase) | 991 ± 1.17 | 5005 ± 123.22 | 4002 ± 111.34 | 4208 ± 174.01 | 4199 ± 169.22 |
| PIGF (placental growth factor) | 197 ± 15.05 | 90 ± 12.85 | 103 ± 18.01 | 105 ± 14.15 | 98 ± 10.01 |
| blood pressure | 110/60 | 145/100 | 120/85 | 115/80 | 115/80 |
| proteinuria (UPCR) mg/mmol | <30 | >45 | <40 | <40 | <40 |
| Superoxide dismutase SOD (U/gHb) | 97.13 ± 12.78 | 29.41 ± 2.99 | 59.77 ± 7.81 | 61.01 ± 8.05 | 58.55 ± 10.13 |
| Catalase CAT (U/gHb) | 42.71 ± 9.09 | 113.21 ± 21.12 | 72.66 ± 13.45 | 74.68 ± 13.55 | 65.47 ± 5.16 |
| Glutathione peroxidase GSH-Px (U/gHb) | 278.44 ± 31.25 | 101.59 ± 33.55 | 147.13 ± 41.25 | 151.71 ± 31.13 | 189.87 ± 7.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostadinova-Slavova, D.; Petkova-Parlapanska, K.; Koleva, I.; Angelova, M.; Sadi J. Al-Dahwi, R.; Georgieva, E.; Karamalakova, Y.; Nikolova, G. Preeclampsia Treatment Aspirin/Clampsilin: Oxidative Stress, sFlt-1/PIGF Soluble Tyrosine Kinase 1, and Placental Growth Factor Monitoring. Int. J. Mol. Sci. 2024, 25, 13497. https://doi.org/10.3390/ijms252413497
Kostadinova-Slavova D, Petkova-Parlapanska K, Koleva I, Angelova M, Sadi J. Al-Dahwi R, Georgieva E, Karamalakova Y, Nikolova G. Preeclampsia Treatment Aspirin/Clampsilin: Oxidative Stress, sFlt-1/PIGF Soluble Tyrosine Kinase 1, and Placental Growth Factor Monitoring. International Journal of Molecular Sciences. 2024; 25(24):13497. https://doi.org/10.3390/ijms252413497
Chicago/Turabian StyleKostadinova-Slavova, Denitsa, Kamelia Petkova-Parlapanska, Irina Koleva, Mariya Angelova, Rafaah Sadi J. Al-Dahwi, Ekaterina Georgieva, Yanka Karamalakova, and Galina Nikolova. 2024. "Preeclampsia Treatment Aspirin/Clampsilin: Oxidative Stress, sFlt-1/PIGF Soluble Tyrosine Kinase 1, and Placental Growth Factor Monitoring" International Journal of Molecular Sciences 25, no. 24: 13497. https://doi.org/10.3390/ijms252413497
APA StyleKostadinova-Slavova, D., Petkova-Parlapanska, K., Koleva, I., Angelova, M., Sadi J. Al-Dahwi, R., Georgieva, E., Karamalakova, Y., & Nikolova, G. (2024). Preeclampsia Treatment Aspirin/Clampsilin: Oxidative Stress, sFlt-1/PIGF Soluble Tyrosine Kinase 1, and Placental Growth Factor Monitoring. International Journal of Molecular Sciences, 25(24), 13497. https://doi.org/10.3390/ijms252413497









