β-Cell Deletion of Hypoxia-Inducible Factor 1α (HIF-1α) Increases Pancreatic β-Cell Susceptibility to Streptozotocin
Abstract
1. Introduction
2. Results
2.1. Optimal Multiple Low-Dose Streptozotocin (MLDS) Dosing
2.2. BIN (Beta-Cell Specific HIF1α Null) Mice Have Increased Diabetes Incidence After MLDS
2.3. Decreased β-Cell Mass and Lower Serum Insulin in BIN Mice Post-MLDS
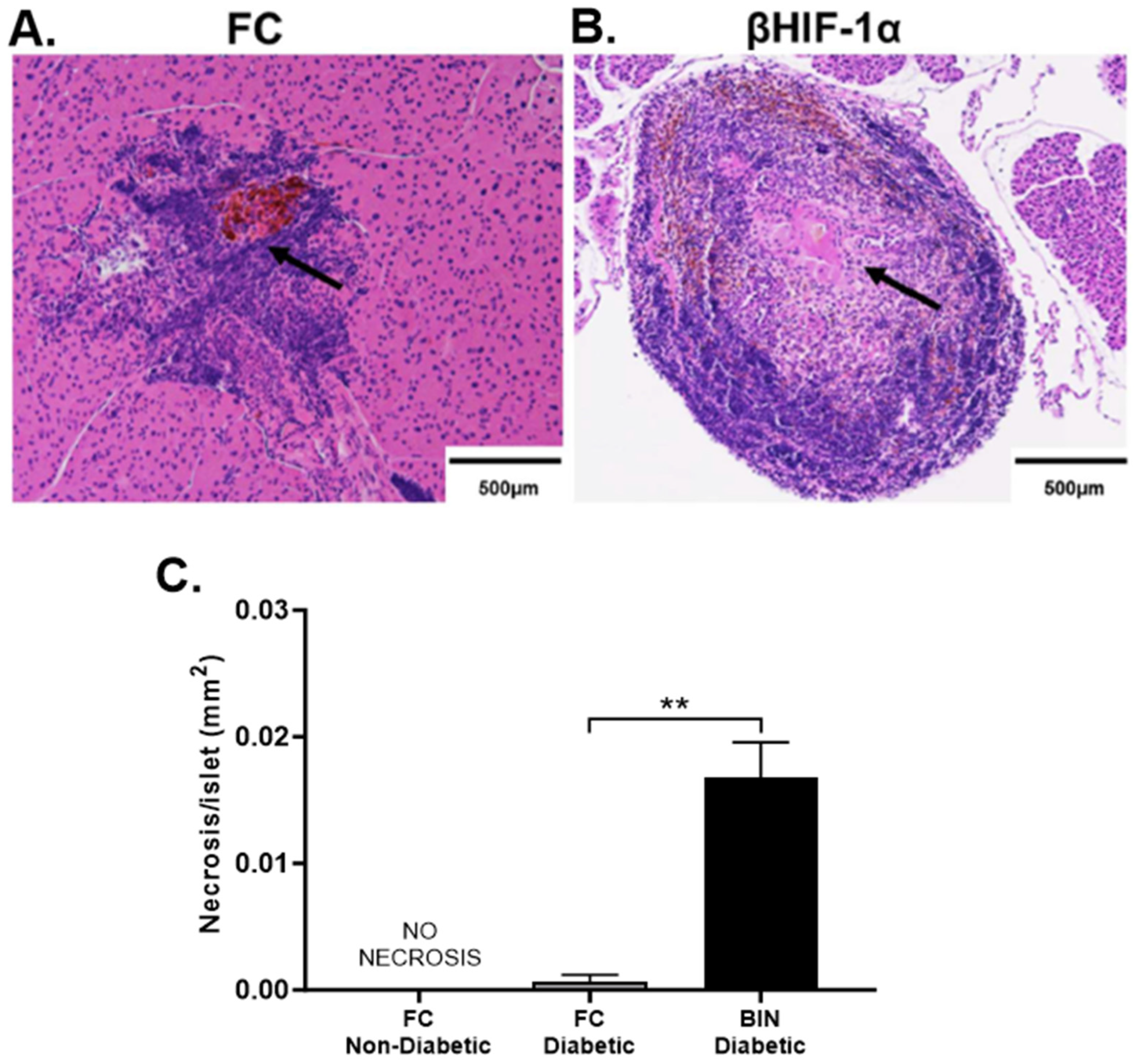
2.4. MLDS Induces Necrosis in Islets of BIN Mice
2.5. Confirmation of Auto-Immune T1D in BIN Mice
3. Discussion
4. Materials and Methods
4.1. Animal Experiments
4.2. Glucose Monitoring and Glucose Tolerance Test (GTT)
4.3. Histology
4.4. Quantification of β-Cell Mass
4.5. Adoptive Transfer
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Diabetes. Type 1 Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes#:~:text=Type%201%20diabetes%20(previously%20known,live%20in%20high%2Dincome%20countries.2023 (accessed on 28 November 2024).
- Huo, L.; Harding, J.L.; Peeters, A.; Shaw, J.E.; Magliano, D.J. Life expectancy of type 1 diabetic patients during 1997–2010: A national Australian registry-based cohort study. Diabetologia 2016, 59, 1177–1185. [Google Scholar] [CrossRef]
- Petrie, D.; Lung, T.W.C.; Rawshani, A.; Palmer, A.J.; Svensson, A.-M.; Eliasson, B.; Clarke, P. Recent trends in life expectancy for people with type 1 diabetes in Sweden. Diabetologia 2016, 59, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Bluestone, J.A.; Herold, K.; Eisenbarth, G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 2010, 464, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Hakola, L.; Miettinen, M.E.; Syrjälä, E.; Åkerlund, M.; Takkinen, H.-M.; Korhonen, T.E.; Ahonen, S.; Ilonen, J.; Toppari, J.; Veijola, R.; et al. Association of Cereal, Gluten, and Dietary Fiber Intake With Islet Autoimmunity and Type 1 Diabetes. JAMA Pediatr. 2019, 173, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L.; Nejfelt, M.K.; Chi, S.M.; Antonarakis, S.E. Hypoxia-inducible nuclear factors bind to an enhancer element located 3′ to the human erythropoietin gene. Proc. Natl. Acad. Sci. USA 1991, 88, 5680–5684. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef] [PubMed]
- McGettrick, A.F.; O’Neill, L.A.J. The Role of HIF in Immunity and Inflammation. Cell Metab. 2020, 32, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Gunton, J.E. Hypoxia-inducible factors and diabetes. J. Clin. Investig. 2020, 130, 5063–5073. [Google Scholar] [CrossRef] [PubMed]
- Jaakkola, P.; Mole, D.R.; Tian, Y.-M.; Wilson, M.I.; Gielbert, J.; Gaskell, S.J.; Kriegsheim, A.v.; Hebestreit, H.F.; Mukherji, M.; Schofield, C.J.; et al. Targeting of HIF-alpha to the von Hippel-Lindau Ubiquitylation Complex by O2-Regulated Prolyl Hydroxylation. Science 2001, 292, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Lando, D.; Peet, D.J.; Whelan, D.A.; Gorman, J.J.; Whitelaw, M.L. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science 2002, 295, 858–861. [Google Scholar] [CrossRef] [PubMed]
- Hansson, L.O.; Friedler, A.; Freund, S.; Rudiger, S.; Fersht, A.R. Two sequence motifs from HIF-1alpha bind to the DNA-binding site of p53. Proc. Natl. Acad. Sci. USA 2002, 99, 10305–10309. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, P.H.; Wiesener, M.S.; Chang, G.W.; Clifford, S.C.; Vaux, E.C.; Cockman, M.E.; Wykoff, C.C.; Pugh, C.W.; Maher, E.R.; Ratcliffe, P.J. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 1999, 399, 271–275. [Google Scholar] [CrossRef]
- Ohh, M.; Park, C.W.; Ivan, M.; Hoffman, M.A.; Kim, T.Y.; Huang, L.E.; Pavletich, N.; Chau, V.; Kaelin, W.G. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat. Cell Biol. 2000, 2, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Mahon, P.C.; Hirota, K.; Semenza, G.L. FIH-1: A novel protein that interacts with HIF-1α and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001, 15, 2675–2686. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Pavlovic, D.; Chen, M.C.; Flodstrom, M.; Sandler, S.; Eizirik, D.L. Cytokines induce apoptosis in beta-cells isolated from mice lacking the inducible isoform of nitric oxide synthase (iNOS−/−). Diabetes 2000, 49, 1116–1122. [Google Scholar] [CrossRef]
- Moritz, W.; Meier, F.; Stroka, D.M.; Giuliani, M.; Kugelmeier, P.; Nett, P.C.; Lehmann, R.; Candinas, D.; Gassmann, M.; Weber, M. Apoptosis in hypoxic human pancreatic islets correlated with HIF-1a expression. FASEB J. 2002, 16, 745–747. [Google Scholar] [CrossRef]
- Keshtkar, S.; Kaviani, M.; Jabbarpour, Z.; Geramizadeh, B.; Motevaseli, E.; Nikeghbalian, S.; Shamsaeefar, A.; Motazedian, N.; Al-Abdullah, I.H.; Ghahremani, M.H.; et al. Protective effect of nobiletin on isolated human islets survival and function against hypoxia and oxidative stress-induced apoptosis. Sci. Rep. 2019, 9, 11701. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Ho, K.; Stokes, R.; Scott, C.; Lau, S.M.; Hawthorne, W.J.; O’Connell, P.J.; Loudovaris, T.; Kay, T.; Kulkarni, R.N.; et al. Hypoxia-Inducible Factor-1α Regulates β-Cell Function in Mouse and Human Islets. J. Clin. Investig. 2010, 120, 2171–2183. [Google Scholar] [CrossRef]
- Lalwani, A.; Warren, J.; Liuwantara, D.; Hawthorne, W.J.; O’Connell, P.J.; Gonzalez, F.J.; Stokes, R.A.; Chen, J.; Laybutt, D.R.; Craig, M.E.; et al. β Cell Hypoxia-Inducible Factor-1α Is Required for the Prevention of Type 1 Diabetes. Cell Rep. 2019, 27, 2370–2384.e6. [Google Scholar] [CrossRef]
- Stokes, R.A.; Cheng, K.; Deters, N.; Lau, S.M.; Hawthorne, W.J.; O’Connell, P.J.; Stolp, J.; Grey, S.; Loudovaris, T.; Kay, T.W.; et al. Hypoxia-inducible factor 1α (HIF-1α) potentiates β-cell survival after islet transplantation of human and mouse islets. Cell Transplant. 2013, 22, 253–266. [Google Scholar] [CrossRef]
- Bento, C.F.; Pereira, P. Regulation of hypoxia-inducible factor 1 and the loss of the cellular response to hypoxia in diabetes. Diabetologia 2011, 54, 1946–1956. [Google Scholar] [CrossRef] [PubMed]
- Bento, C.F.; Fernandes, R.; Ramalho, J.; Marques, C.; Shang, F.; Taylor, A.; Pereira, P. The chaperone-dependent ubiquitin ligase CHIP targets HIF-1alpha for degradation in the presence of methylglyoxal. PLoS ONE 2010, 5, e15062. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Semenza, G.L. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc. Natl. Acad. Sci. USA 1993, 90, 4304–4308. [Google Scholar] [CrossRef]
- Carrero, J.A.; Benshoff, N.D.; Nalley, K.; Unanue, E.R. Type I and II Interferon Receptors Differentially Regulate Type 1 Diabetes Susceptibility in Male Versus Female NOD Mice. Diabetes 2018, 67, 1830–1835. [Google Scholar] [CrossRef]
- Catrina, S.B.; Zheng, X. Hypoxia and hypoxia-inducible factors in diabetes and its complications. Diabetologia 2021, 64, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Botusan, I.R.; Sunkari, V.G.; Savu, O.; Catrina, A.I.; Grünler, J.; Lindberg, S.; Pereira, T.; Ylä-Herttuala, S.; Poellinger, L.; Brismar, K.; et al. Stabilization of HIF-1a is critical to improve wound healing in diabetic mice. Proc. Natl. Acad. Sci. USA 2008, 105, 19426–19431. [Google Scholar] [CrossRef]
- Catrina, S.-B.; Okamoto, K.; Pereira, T.; Brismar, K.; Poellinger, L. Hyperglycemia Regulates Hypoxia-Inducible Factor-1a Protein Stability and Function. Diabetes 2004, 53, 3226–3232. [Google Scholar] [CrossRef]
- Marfella, R.; D’Amico, M.; Di Filippo, C.; Piegari, E.; Nappo, F.; Esposito, K.; Berrino, L.; Rossi, F.; Giugliano, D. Myocardial infarction in diabetic rats: Role of hyperglycaemia on infarct size and early expression of hypoxia-inducible factor 1. Diabetologia 2002, 45, 1172–1181. [Google Scholar] [CrossRef]
- Forrest, J.M.; Menser, M.A.; Burgess, J.A. High frequency of diabetes mellitus in young adults with congenital rubella. Lancet 1971, 2, 332–334. [Google Scholar] [CrossRef]
- Ginsberg-Fellner, F.; Witt, M.; Yagihashi, S.; Dobersen, M.; Taub, F.; Fedun, B.; McEvoy, R.; Roman, S.H.; Davies, T.; Cooper, L. Congenital rubella syndrome as a model for type 1 (insulin-dependent) diabetes mellitus: Increased prevalence of islet cell surface antibodies. Diabetologia 1984, 27, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Clements, G.B.; Galbraith, D.N.; Taylor, K.W. Coxsackie-B Virus-Infection and Onset of Childhood Diabetes. Lancet 1995, 346, 221–223. [Google Scholar] [CrossRef]
- Cudworth, A.G.; Woodrow, J.C.; Gamble, D.R. Coxsackie-B4 Virus-Infection and Diabetes. Lancet 1975, 306, 29. [Google Scholar] [CrossRef]
- Deeds, M.C.; Anderson, J.M.; Armstrong, A.S.; Gastineau, D.A.; Hiddinga, H.J.; Jahangir, A.; Eberhardt, N.L.; Kudva, Y.C. Single dose streptozotocin-induced diabetes: Considerations for study design in islet transplantation models. Lab. Anim. 2011, 45, 131–140. [Google Scholar] [CrossRef]
- Sreenan, S.; Pick, A.J.; Levisetti, M.; Baldwin, A.C.; Pugh, W.; Polonsky, K.S. Increased beta-cell proliferation and reduced mass before diabetes onset in the nonobese diabetic mouse. Diabetes 1999, 48, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Cnop, M.; Welsh, N.; Jonas, J.C.; Jorns, A.; Lenzen, S.; Eizirik, D.L. Mechanisms of Pancreatic beta-Cell Death in Type 1 and Type 2 Diabetes: Many Differences, Few Similarities. Diabetes 2005, 54 (Suppl. S2), S97–S107. [Google Scholar] [CrossRef]
- Horwitz, M.S.; Ilic, A.; Fine, C.; Rodriguez, E.; Sarvetnick, N. Presented antigen from damaged pancreatic beta cells activates autoreactive T cells in virus-mediated autoimmune diabetes. J. Clin. Investig. 2002, 109, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, M.S.; Bradley, L.M.; Harbertson, J.; Krahl, T.; Lee, J.; Sarvetnick, N. Diabetes induced by Coxsackie virus: Initiation by bystander damage and not molecular mimicry. Nat. Med. 1998, 4, 781–785. [Google Scholar] [CrossRef]
- Nekoua, M.P.; Alidjinou, E.K.; Hober, D. Persistent coxsackievirus B infection and pathogenesis of type 1 diabetes mellitus. Nat. Rev. Endocrinol. 2022, 18, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Serreze, D.V.; Ottendorfer, E.W.; Ellis, T.M.; Gauntt, C.J.; Atkinson, M.A. Acceleration of type 1 diabetes by a coxsackievirus infection requires a preexisting critical mass of autoreactive T-cells in pancreatic islets. Diabetes 2000, 49, 708–711. [Google Scholar] [CrossRef] [PubMed]
- Aoki, C.A.; Borchers, A.T.; Ridgway, W.M.; Keen, C.L.; Ansari, A.A.; Gershwin, M.E. NOD mice and autoimmunity. Autoimmun. Rev. 2005, 4, 373–379. [Google Scholar] [CrossRef]
- Barshes, N.R.; Wyllie, S.; Goss, J.A. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: Implications for intrahepatic grafts. J. Leukoc. Biol. 2005, 77, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Bottino, R.; Balamurugan, A.N.; Tse, H.; Thirunavukkarasu, C.; Ge, X.; Profozich, J.; Milton, M.; Ziegenfuss, A.; Trucco, M.; Piganelli, J.D. Response of human islets to isolation stress and the effect of antioxidant treatment. Diabetes 2004, 53, 2559–2568. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Storling, J.; Maedler, K.; Mandrup-Poulsen, T. Inflammatory mediators and islet beta-cell failure: A link between type 1 and type 2 diabetes. J. Mol. Med. 2003, 81, 455–470. [Google Scholar] [CrossRef]
- Wek, R.C.; Anthony, T.G. EXtENDINg beta cell survival by UPRegulating ATF4 translation. Cell Metab. 2006, 4, 333–334. [Google Scholar] [CrossRef][Green Version]
- Laybutt, D.R.; Preston, A.M.; Åkerfeldt, M.C.; Kench, J.G.; Busch, A.K.; Biankin, A.V.; Biden, T.J. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia 2007, 50, 752–763. [Google Scholar] [CrossRef]
- Faideau, B.a.; Larger, E.; Lepault, F.o.; Carel, J.C.; Boitard, C. Role of β-Cells in Type 1 Diabetes Pathogenesis. Diabetes 2005, 54, S87–S96. [Google Scholar] [CrossRef] [PubMed]
- Bensellam, M.; Maxwell, E.L.; Chan, J.Y.; Luzuriaga, J.; West, P.K.; Jonas, J.-C.; Gunton, J.E.; Laybutt, D.R. Hypoxia reduces ER-to-Golgi protein trafficking and increases cell death by inhibiting the adaptive unfolded protein response in mouse beta cells. Diabetologia 2016, 59, 1492–1502. [Google Scholar] [CrossRef]
- Lalwani, A.; Stokes, R.A.; Lau, S.M.; Gunton, J.E. Deletion of ARNT (Aryl Hydrocarbon Receptor Nuclear Translocator) in β-Cells Causes Islet Transplant Failure with Impaired β-Cell Function. PLoS ONE 2014, 9, e98435. [Google Scholar] [CrossRef] [PubMed]
- Gunton, J.E.; Kulkarni, R.N.; Yim, S.; Okada, T.; Hawthorne, W.J.; Tseng, Y.H.; Roberson, R.S.; Ricordi, C.; O’Connell, P.J.; Gonzalez, F.J.; et al. Loss of ARNT/HIF1beta Mediates Altered Gene Expression and Pancreatic-Islet Dysfunction in Human Type 2 Diabetes. Cell 2005, 122, 337–349. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Lalwani, A.; Gunton, J.E. β-Cell Deletion of Hypoxia-Inducible Factor 1α (HIF-1α) Increases Pancreatic β-Cell Susceptibility to Streptozotocin. Int. J. Mol. Sci. 2024, 25, 13451. https://doi.org/10.3390/ijms252413451
Yu J, Lalwani A, Gunton JE. β-Cell Deletion of Hypoxia-Inducible Factor 1α (HIF-1α) Increases Pancreatic β-Cell Susceptibility to Streptozotocin. International Journal of Molecular Sciences. 2024; 25(24):13451. https://doi.org/10.3390/ijms252413451
Chicago/Turabian StyleYu, Josephine, Amit Lalwani, and Jenny E. Gunton. 2024. "β-Cell Deletion of Hypoxia-Inducible Factor 1α (HIF-1α) Increases Pancreatic β-Cell Susceptibility to Streptozotocin" International Journal of Molecular Sciences 25, no. 24: 13451. https://doi.org/10.3390/ijms252413451
APA StyleYu, J., Lalwani, A., & Gunton, J. E. (2024). β-Cell Deletion of Hypoxia-Inducible Factor 1α (HIF-1α) Increases Pancreatic β-Cell Susceptibility to Streptozotocin. International Journal of Molecular Sciences, 25(24), 13451. https://doi.org/10.3390/ijms252413451






