Synergistic Effects of Photobiomodulation and Differentiation Inducers on Osteogenic Differentiation of Adipose-Derived Stem Cells in Three-Dimensional Culture
Abstract
1. Introduction
2. Results
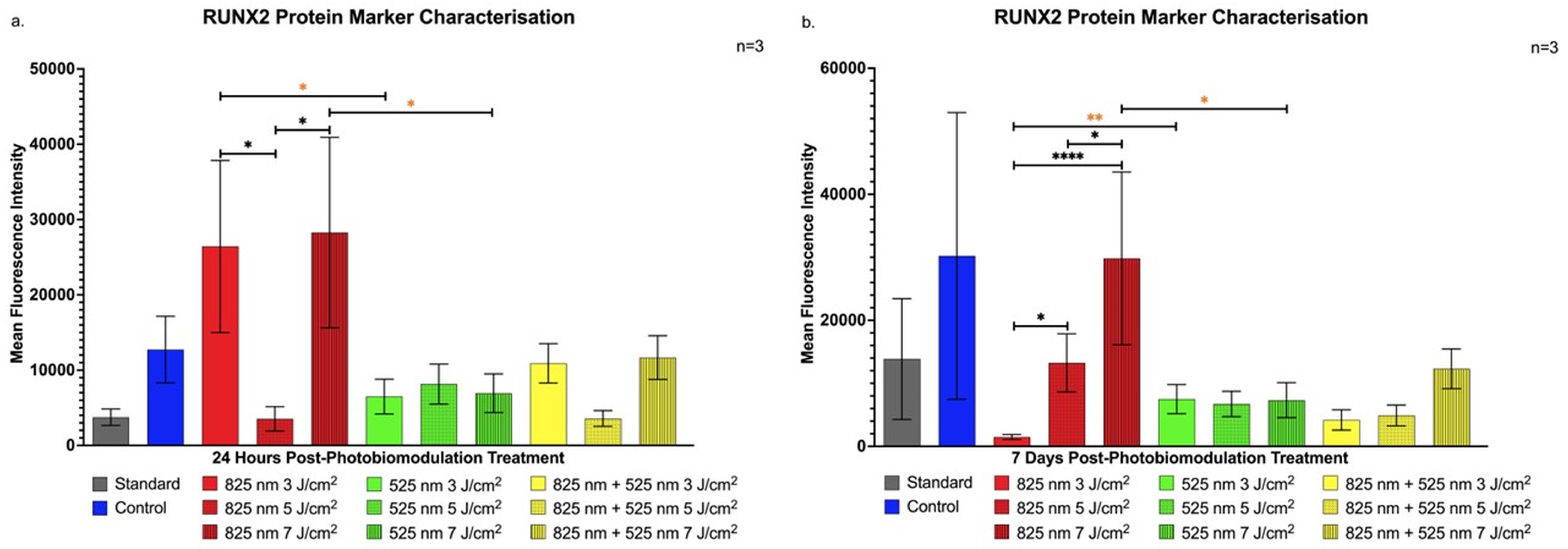
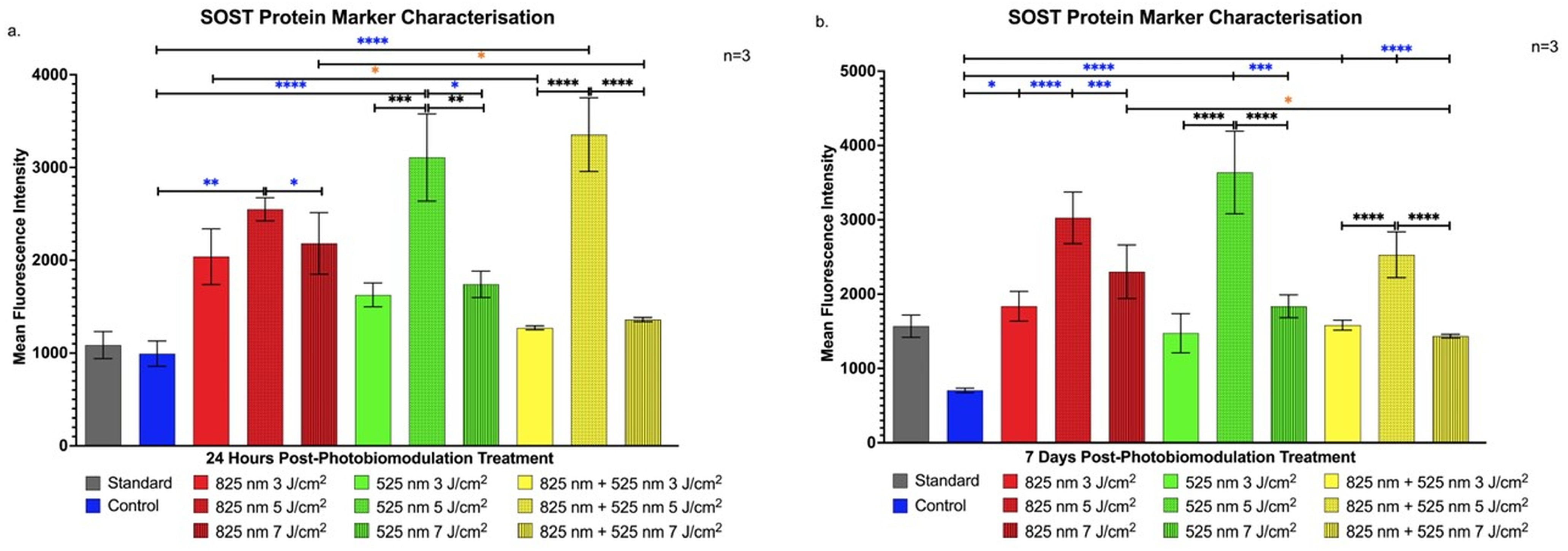
2.1. Characterisation of Protein Markers in Osteoblast-like Differentiation of Immortalised Adipose-Derived Stem Cells
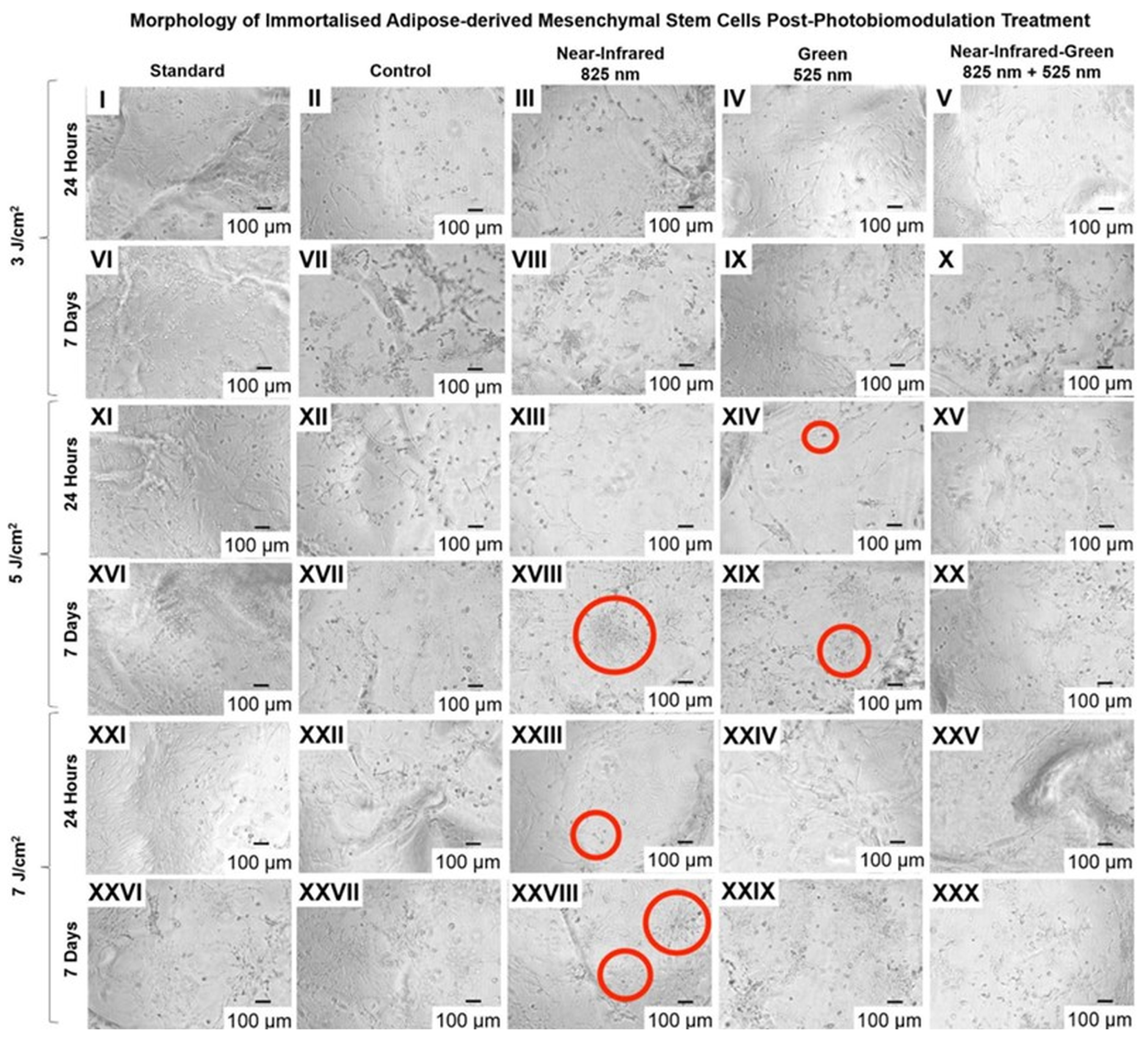
2.2. Cell Configuration
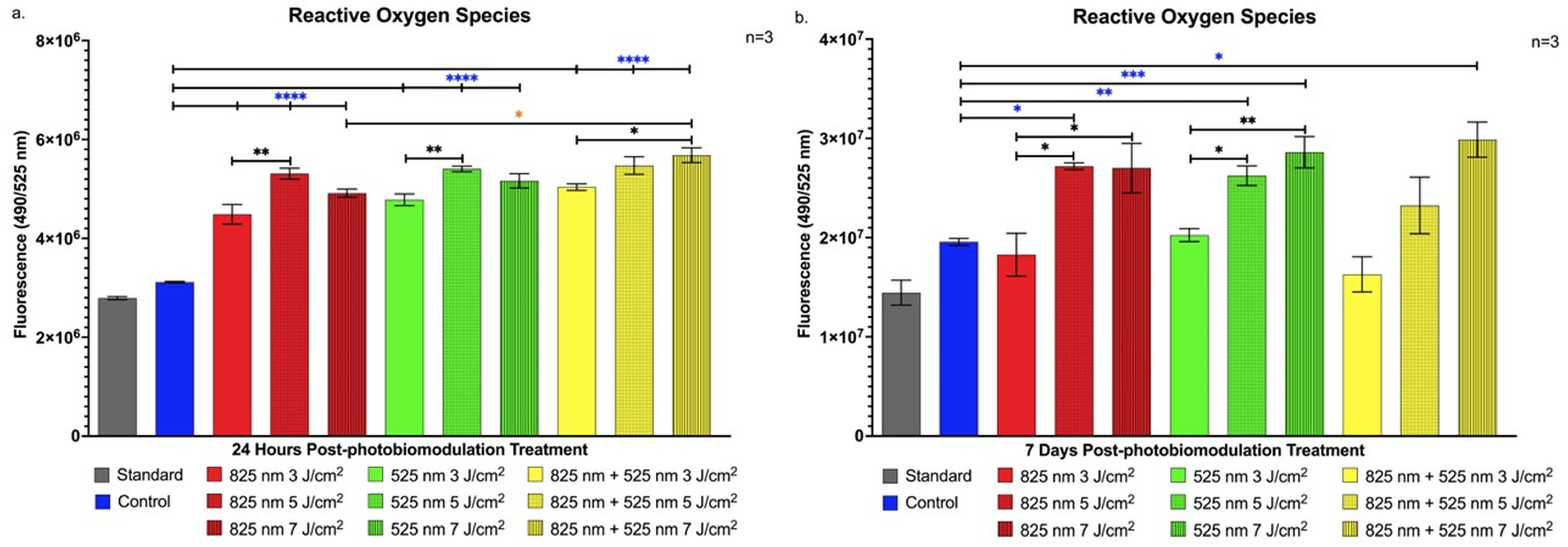
2.3. Detection of Cellular Stress via Reactive Oxygen Species
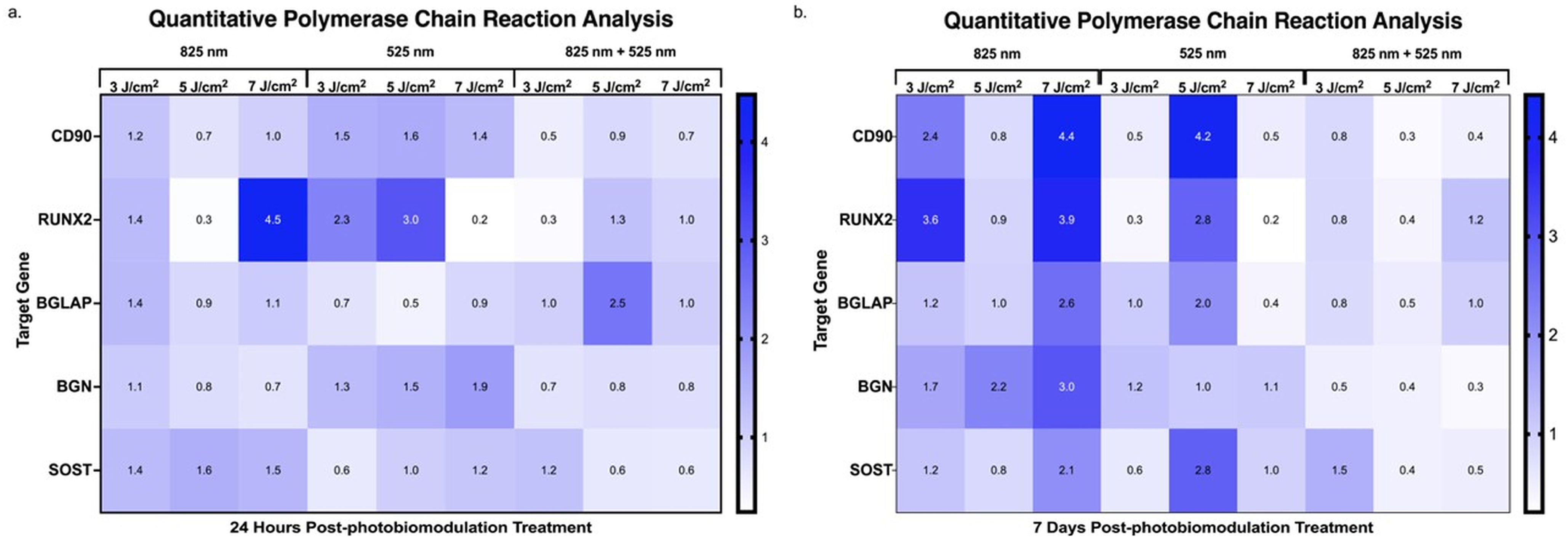
2.4. Assessment of Genetic Expression via Quantitative Polymerase Chain Reaction During the Differentiation of Adipose-Derived Stem Cells
3. Discussion
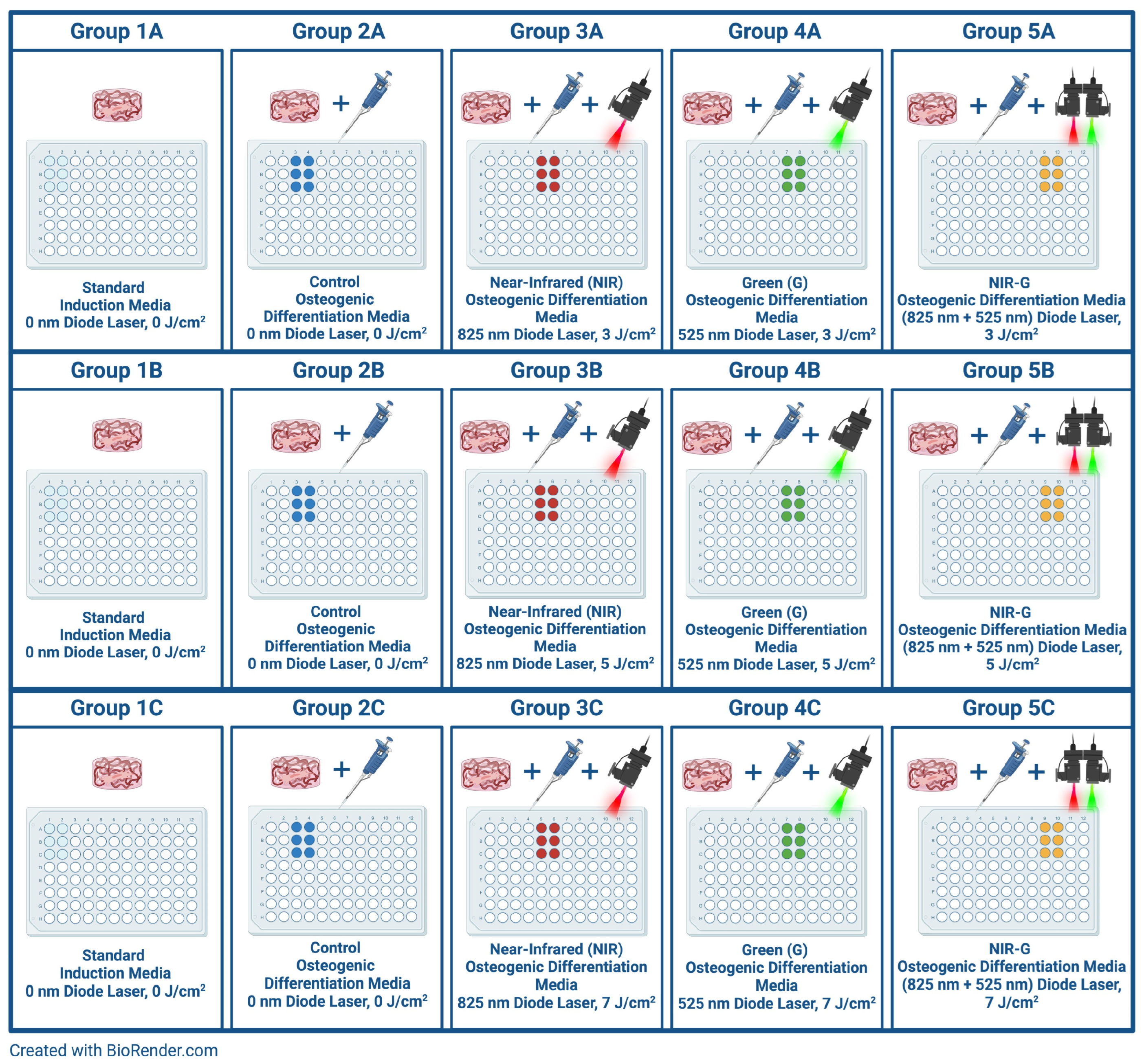
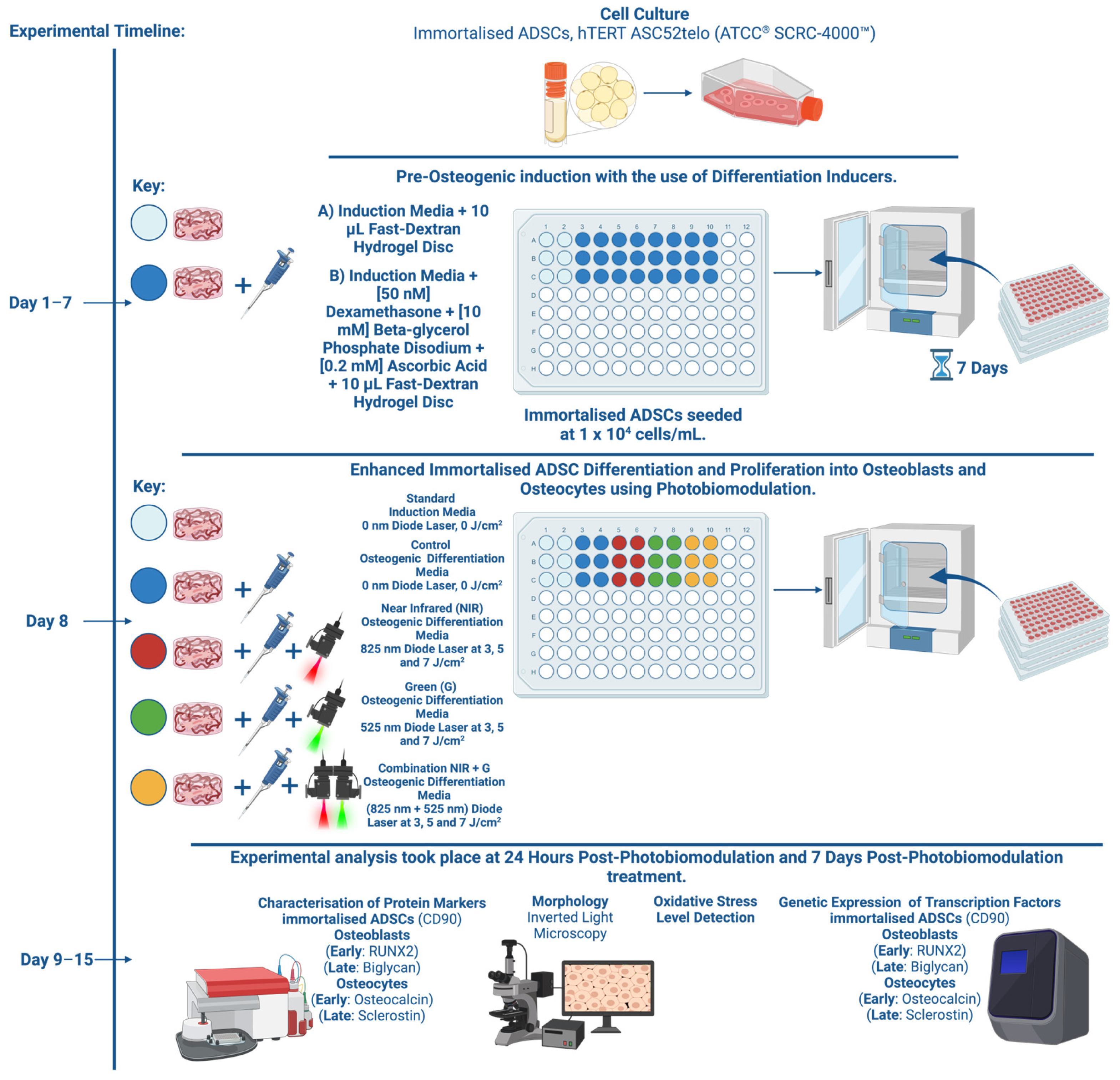
4. Materials and Methods
4.1. Cellular Cultivation
4.2. Osteogenic Differentiation of Adipose-Derived Stem Cells Using Slow-Dextran Hydrogel Matrices and Differentiation Inducer Cocktail
4.3. Photobiomodulation for Enhanced Differentiation
4.4. Characterisation of Protein Markers in the Osteogenic Differentiation of Immortalised Adipose-Derived Stem Cells
Protein Marker Profiling via Flow Cytometry
4.5. Cell Morphology Analysis Using Inverted Light Microscopy
4.6. Detection of Oxidative Stress Levels
4.7. Quantitative Polymerase Chain Reaction Analysis of Gene Expression in Differentiating Adipose-Derived Stem Cells
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sozen, T.; Ozisik, L.; Calik Basaran, N. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Cauley, J.A. Public health impact of osteoporosis. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 68, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, U.; Cariati, I.; Greggi, C.; Iundusi, R.; Gasbarra, E.; Iolascon, G.; Kurth, A.; Akesson, K.E.; Bouxsein, M.; Tranquilli Leali, P.; et al. Gaps and alternative surgical and non-surgical approaches in the bone fragility management: An updated review. Osteoporos. Int. 2022, 33, 2467–2478. [Google Scholar] [CrossRef]
- Hoang, D.M.; Pham, P.T.; Bach, T.Q.; Ngo, A.T.; Nguyen, Q.T.; Phan, T.T.; Nguyen, G.H.; Le, P.T.; Hoang, V.T.; Forsyth, N.R.; et al. Stem cell-based therapy for human diseases. Signal Transduct. Target. Ther. 2022, 7, 272. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef]
- Lau, C.S.; Park, S.Y.; Ethiraj, L.P.; Singh, P.; Raj, G.; Quek, J.; Prasadh, S.; Choo, Y.; Goh, B.T. Role of Adipose-Derived Mesenchymal Stem Cells in Bone Regeneration. Int. J. Mol. Sci. 2024, 25, 6805. [Google Scholar] [CrossRef]
- Tsuji, W. Adipose-derived stem cells: Implications in tissue regeneration. World J. Stem Cells 2014, 6, 312. [Google Scholar] [CrossRef]
- Hu, L.; Yin, C.; Zhao, F.; Ali, A.; Ma, J.; Qian, A. Mesenchymal stem cells: Cell fate decision to osteoblast or adipocyte and application in osteoporosis treatment. Int. J. Mol. Sci. 2018, 19, 360. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, Y.; Cheng, J.; Wang, Q.; Li, Z.; Wang, Y.; Wang, D.; Wang, H.; Zhang, W.; Ye, J.; et al. Pharmacological activation of TAZ enhances osteogenic differentiation and bone formation of adipose-derived stem cells. Stem Cell Res. Ther. 2018, 9, 53. [Google Scholar] [CrossRef]
- Hutchings, G.; Janowicz, K.; Moncrieff, L.; Dompe, C.; Strauss, E.; Kocherova, I.; Nawrocki, M.J.; Kruszyna, Ł.; Wąsiatycz, G.; Antosik, P.; et al. The proliferation and differentiation of adipose-derived stem cells in neovascularization and angiogenesis. Int. J. Mol. Sci. 2020, 21, 3790. [Google Scholar] [CrossRef]
- Da Silva, D.; Crous, A.; Abrahamse, H. Enhancing osteogenic differentiation in adipose-derived mesenchymal stem cells with Near Infra-Red and Green Photobiomodulation. Regen. Ther. 2023, 24, 602–616. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.R.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L.; Pandit, A. Macromolecular modulation of a 3D hydrogel construct differentially regulates human stem cell tissue-to-tissue interface. Biomater. Adv. 2022, 133, 112611. [Google Scholar] [CrossRef] [PubMed]
- Hoarau-Véchot, J.; Rafii, A.; Touboul, C.; Pasquier, J. Halfway between 2D and animal models: Are 3D cultures the ideal tool to study cancer-microenvironment interactions? Int. J. Mol. Sci. 2018, 19, 181. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, Y.Y.; Wang, Y.; Lyu, P.; Hamblin, M.R. Red (660 nm) or near-infrared (810 nm) photobiomodulation stimulates, while blue (415 nm), green (540 nm) light inhibits proliferation in human adipose-derived stem cells. Sci. Rep. 2017, 7, 7781. [Google Scholar] [CrossRef]
- George, S.; Hamblin, M.R.; Abrahamse, H. Effect of red light and near infrared laser on the generation of reactive oxygen species in primary dermal fibroblasts. J. Photochem. Photobiol. B 2018, 188, 60–68. [Google Scholar] [CrossRef]
- Serrage, H.; Heiskanen, V.; Palin, W.M.; Cooper, P.R.; Milward, M.R.; Hadis, M.; Hamblin, M.R. Under the spotlight: Mechanisms of photobiomodulation concentrating on blue and green light. Photochem. Photobiol. Sci. 2019, 18, 1877–1909. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.Y.; Wang, Y.; Lyu, P.; Hamblin, M.R. Photobiomodulation (blue and green light) encourages osteoblastic-differentiation of human adipose-derived stem cells: Role of intracellular calcium and light-gated ion channels. Sci. Rep. 2016, 6, 33719. [Google Scholar] [CrossRef]
- Chen, J.; Sang, Y.; Li, J.; Zhao, T.; Liu, B.; Xie, S.; Sun, W. Low-level controllable blue LEDs irradiation enhances human dental pulp stem cells osteogenic differentiation via transient receptor potential vanilloid 1. J. Photochem. Photobiol. B. 2022, 233, 112472. [Google Scholar] [CrossRef]
- Ruan, Y.; Kato, H.; Taguchi, Y.; Yamauchi, N.; Umeda, M. Irradiation by high-intensity red light-emitting diode enhances human bone marrow mesenchymal stem cells osteogenic differentiation and mineralization through Wnt/β-catenin signaling pathway. Lasers Med. Sci. 2021, 36, 55–65. [Google Scholar] [CrossRef]
- Moraes, D.A.; Sibov, T.T.; Pavon, L.F.; Alvim, P.Q.; Bonadio, R.S.; Da Silva, J.R.; Pic-Taylor, A.; Toledo, O.A.; Marti, L.C.; Azevedo, R.B.; et al. A reduction in CD90 (THY-1) expression results in increased differentiation of mesenchymal stromal cells. Stem Cell Res. Ther. 2016, 7, 97. [Google Scholar] [CrossRef]
- Wiesmann, A.; Bühring, H.-J.; Mentrup, C.; Wiesmann, H.-P. Decreased CD90 expression in human mesenchymal stem cells by applying mechanical stimulation. Head Face Med. 2006, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.D.; Qian, H.Y.; Neff, L.; Satomura, K.; Horowitz, M.C. Thy-1 antigen expression by cells in the osteoblast lineage. J. Bone Miner. Res. 1999, 14, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Regulation of proliferation, differentiation and functions of osteoblasts by runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, M.; Miller, S.; Mitra, K. Model development and experimental validation for analyzing initial transients of irradiation of tissues during thermal therapy using short pulse lasers. Lasers Surg. Med. 2015, 47, 711–722. [Google Scholar] [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The mtt assay: Utility, limitations, pitfalls, and interpretation in bulk and single-cell analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef]
- Wang, J.S.; Mazur, C.M.; Wein, M.N. Sclerostin and Osteocalcin: Candidate Bone-Produced Hormones. Front. Endocrinol. 2021, 12, 584147. [Google Scholar] [CrossRef]
- Dubey, N.K.; Mishra, V.K.; Dubey, R.; Deng, Y.H.; Tsai, F.C.; Deng, W.P. Revisiting the advances in isolation, characterization and secretome of adipose-derived stromal/stem cells. Int. J. Mol. Sci. 2018, 19, 2200. [Google Scholar] [CrossRef]
- Liu, X.; Kumagai, G.; Wada, K.; Tanaka, T.; Asari, T.; Oishi, K.; Fujita, T.; Mizukami, H.; Furukawa, K.I.; Ishibashi, Y. High Osteogenic Potential of Adipose-and Muscle-derived Mesenchymal Stem Cells in Spinal-Ossification Model Mice. Spine 2017, 42, E1342–E1349. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Chen, Y.; Yuan, L.; Liu, H.; Wang, J.; Liu, Q.; Zhang, Y. Adipose-Derived Stem Cells: Current Applications and Future Directions in the Regeneration of Multiple Tissues. Stem Cells Int. 2020, 2020, 8810813. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Chen, A.C.H.; Carroll, J.D.; Hamblin, M.R. Biphasic dose response in low level lightherapy. Dose-Response 2009, 7, 358–383. [Google Scholar] [CrossRef]
- Tsao, Y.T.; Huang, Y.J.; Wu, H.H.; Liu, Y.A.; Liu, Y.S.; Lee, O.K. Osteocalcin mediates biomineralization during osteogenic maturation in human mesenchymal stromal cells. Int. J. Mol. Sci. 2017, 18, 159. [Google Scholar] [CrossRef] [PubMed]
- Nasello, G.; Alamán-Díez, P.; Schiavi, J.; Pérez, M.Á.; McNamara, L.; García-Aznar, J.M. Primary Human Osteoblasts Cultured in a 3D Microenvironment Create a Unique Representative Model of Their Differentiation Into Osteocytes. Front. Bioeng. Biotechnol. 2020, 8, 336. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Li, S.H.; Xie, G.H.; Xie, S.; Xiao, L.L.; Song, J.X.; Liu, H.W. Preconditioning With Low-Level Laser Irradiation Enhances the Therapeutic Potential of Human Adipose-derived Stem Cells in a Mouse Model of Photoaged Skin. Photochem. Photobiol. 2018, 94, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Su, J.; Wei, J.; Jiang, N.; Ge, X. Recent Advances in Three-Dimensional Stem Cell Culture Systems and Applications. Stem Cells Int. 2021, 2021, 9477332. [Google Scholar] [CrossRef]
- Kaur, G.; Valarmathi, M.T.; Potts, J.D.; Jabbari, E.; Sabo-Attwood, T.; Wang, Q. Regulation of osteogenic differentiation of rat bone marrow stromal cells on 2D nanorod substrates. Biomaterials 2010, 31, 1732–1741. [Google Scholar] [CrossRef]
- Choi, J.U.A.; Kijas, A.W.; Lauko, J.; Rowan, A.E. The Mechanosensory Role of Osteocytes and Implications for Bone Health and Disease States. Front. Cell Dev. Biol. 2022, 9, 770143. [Google Scholar] [CrossRef]
- Wu, Z.; Li, W.; Jiang, K.; Lin, Z.; Qian, C.; Wu, M.; Xia, Y.; Li, N.; Zhang, H.; Xiao, H.; et al. Regulation of bone homeostasis: Signaling pathways and therapeutic targets. MedComm 2024, 5, e657. [Google Scholar] [CrossRef]
- Peng, F.; Wu, H.; Zheng, Y.; Xu, X.; Yu, J. The effect of noncoherent red light irradiation on proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells. Lasers Med. Sci. 2012, 27, 645–653. [Google Scholar] [CrossRef]
- Oliveira, N.K.; Salles, T.H.C.; Pedroni, A.C.; Miguita, L.; D’Ávila, M.A.; Marques, M.M.; Deboni, M.C.Z. Osteogenic potential of human dental pulp stem cells cultured onto poly-ε-caprolactone/poly (rotaxane) scaffolds. Dent. Mater. 2019, 35, 1740–1749. [Google Scholar] [CrossRef]
- Cui, Y.; Zeng, F.; Zhu, Z.; Huang, F.; Chen, J.; He, C.; Li, Y.; Chen, Z.; Yang, Z.; Zu, X.; et al. Suppression of osteogenic-like differentiation in human renal interstitial fibroblasts by miRNA-410-3p through MSX2. Transl. Androl. Urol. 2020, 9, 2082–2093. [Google Scholar] [CrossRef]
- Xu, J.; Li, Z.; Hou, Y.; Fang, W. Potential mechanisms underlying the Runx2 induced osteogenesis of bone marrow mesenchymal stem cells. Am. J. Transl. Res. 2015, 7, 2527–2535. [Google Scholar] [PubMed]
- Neve, A.; Corrado, A.; Cantatore, F.P. Osteocalcin: Skeletal and extra-skeletal effects. J. Cell Physiol. 2013, 228, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Pei, W.; Wang, K.; Zhang, S.; Chen, F.; Wu, Y.; Guan, W. In vitro culture and biological properties of broiler adipose-derived stem cells. Exp. Ther. Med. 2018, 16, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Yaralı Çevik, Z.B.; Karaman, O.; Topaloğlu, N. Photobiomodulation therapy at red and near-infrared wavelengths for osteogenic differentiation in the scaffold-free microtissues. J. Photochem. Photobiol. B 2023, 238, 112615. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Mechanisms and Mitochondrial Redox Signaling in Photobiomodulation. Photochem. Photobiol. 2018, 94, 199–212. [Google Scholar] [CrossRef]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive Oxygen Species Signaling and Oxidative Stress: Transcriptional Regulation and Evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef]
- Marahleh, A.; Kitaura, H.; Ohori, F.; Noguchi, T.; Mizoguchi, I. The osteocyte and its osteoclastogenic potential. Front. Endocrinol. 2023, 14, 1121727. [Google Scholar] [CrossRef]
- Lewiecki, E.M. Role of sclerostin in bone and cartilage and its potential as a therapeutic target in bone diseases. Ther. Adv. Musculoskelet. Dis. 2014, 6, 48–57. [Google Scholar] [CrossRef]



| Components | Single Well (μL) |
|---|---|
| Fast Dextran | 0.8 |
| Truegel3d Buffer | 0.8 |
| Water | 5.1 |
| CD Cell-Degradable Crosslinker | 1 |
| Cell Suspension | 2 |
| RGD Peptide | 0.3 |
| Total Volume | 10 |
| Laser Parameters | Near-Infrared (NIR) | Green (G) |
|---|---|---|
| Light Source | Diode Laser | Diode Laser |
| Wavelength (nm) | 825 | 525 |
| Power Output (mW) | 187 | 551 |
| Power Density (mW/cm2) | 20.60 | 60.68 |
| Intensity (W/cm2) | 0.02 | 0.06 |
| Area (cm2) | 9.52 | 9.52 |
| Emission | Continuous Wave | Continuous Wave |
| Fluence (J/cm2) | 3, 5 and 7 | 3, 5 and 7 |
| Time of irradiation (s) | 145, 242 and 339 | 49, 82 and 115 |
| Target Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Thy-1 | CCAAGGACGAGGGGACATAC | AGCAGCCATGAGGTGTTCTG |
| Runt-Related Transcription Factor-2 | TCTTAGAACAAATTCTGCCCTTT | TGCTTTGGTCTTGAAATCACA |
| Biglycan | CTCGTCCTGGTGAACAACAA | CAGGTGGTTCTTGGAGATGTAG |
| Osteocalcin | AGCAAAGGTGCAGCCTTTGT | GCGCCTGGGTCTCTTCACT |
| Sclerostin | GGGCAACTGTAGATGTGGTT | GTCCCGAAGGAGAATTGTGTA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Da Silva, D.; Crous, A.; Abrahamse, H. Synergistic Effects of Photobiomodulation and Differentiation Inducers on Osteogenic Differentiation of Adipose-Derived Stem Cells in Three-Dimensional Culture. Int. J. Mol. Sci. 2024, 25, 13350. https://doi.org/10.3390/ijms252413350
Da Silva D, Crous A, Abrahamse H. Synergistic Effects of Photobiomodulation and Differentiation Inducers on Osteogenic Differentiation of Adipose-Derived Stem Cells in Three-Dimensional Culture. International Journal of Molecular Sciences. 2024; 25(24):13350. https://doi.org/10.3390/ijms252413350
Chicago/Turabian StyleDa Silva, Daniella, Anine Crous, and Heidi Abrahamse. 2024. "Synergistic Effects of Photobiomodulation and Differentiation Inducers on Osteogenic Differentiation of Adipose-Derived Stem Cells in Three-Dimensional Culture" International Journal of Molecular Sciences 25, no. 24: 13350. https://doi.org/10.3390/ijms252413350
APA StyleDa Silva, D., Crous, A., & Abrahamse, H. (2024). Synergistic Effects of Photobiomodulation and Differentiation Inducers on Osteogenic Differentiation of Adipose-Derived Stem Cells in Three-Dimensional Culture. International Journal of Molecular Sciences, 25(24), 13350. https://doi.org/10.3390/ijms252413350








