Fabricating a SFMA/BAChol/PAA/ZnCl2 Hydrogel with Excellent Versatile Comprehensive Properties and Stable Sensitive Freezing-Tolerant Conductivity for Wearable Sensors
Abstract
1. Introduction
2. Results
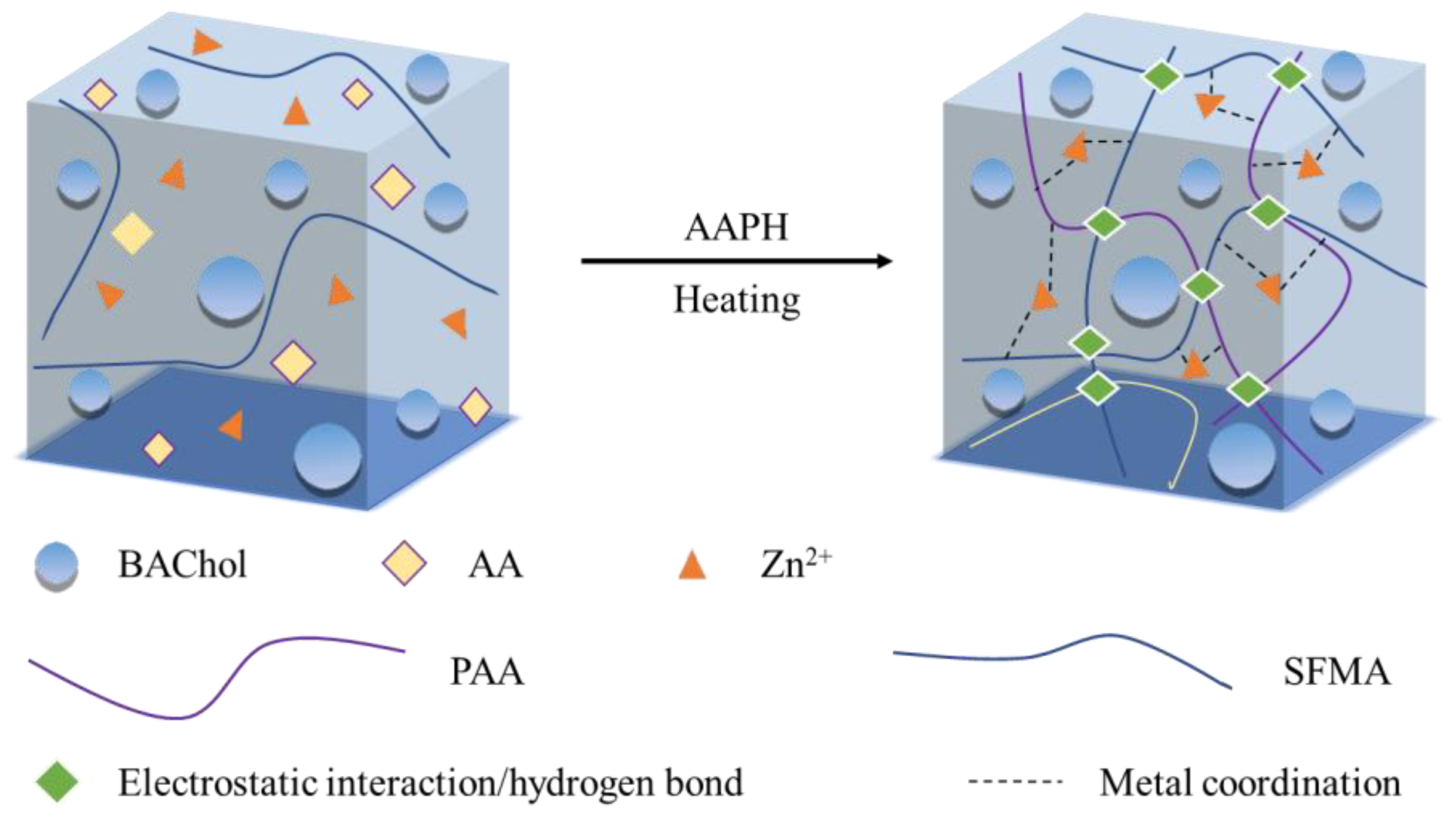
2.1. Effect of the Preparation Conditions on the Hydrogel Mechanical Strength
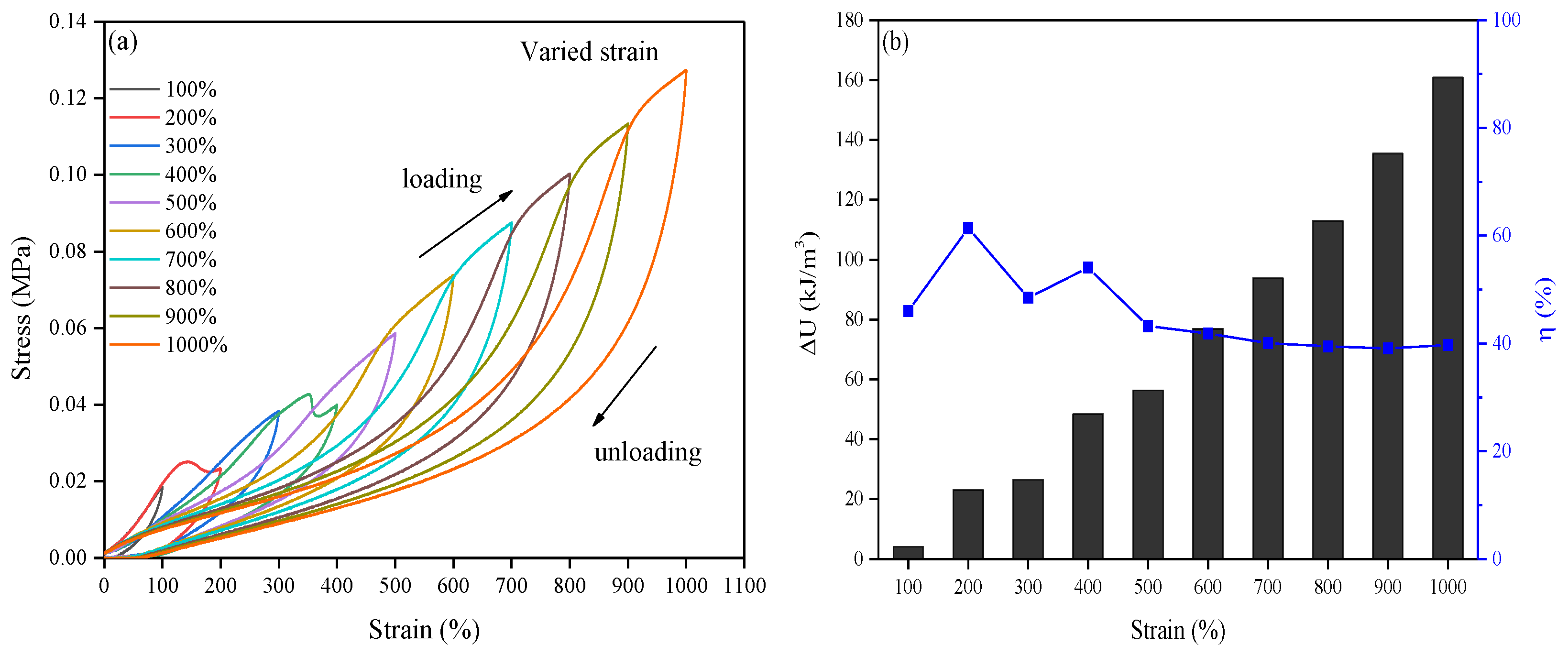
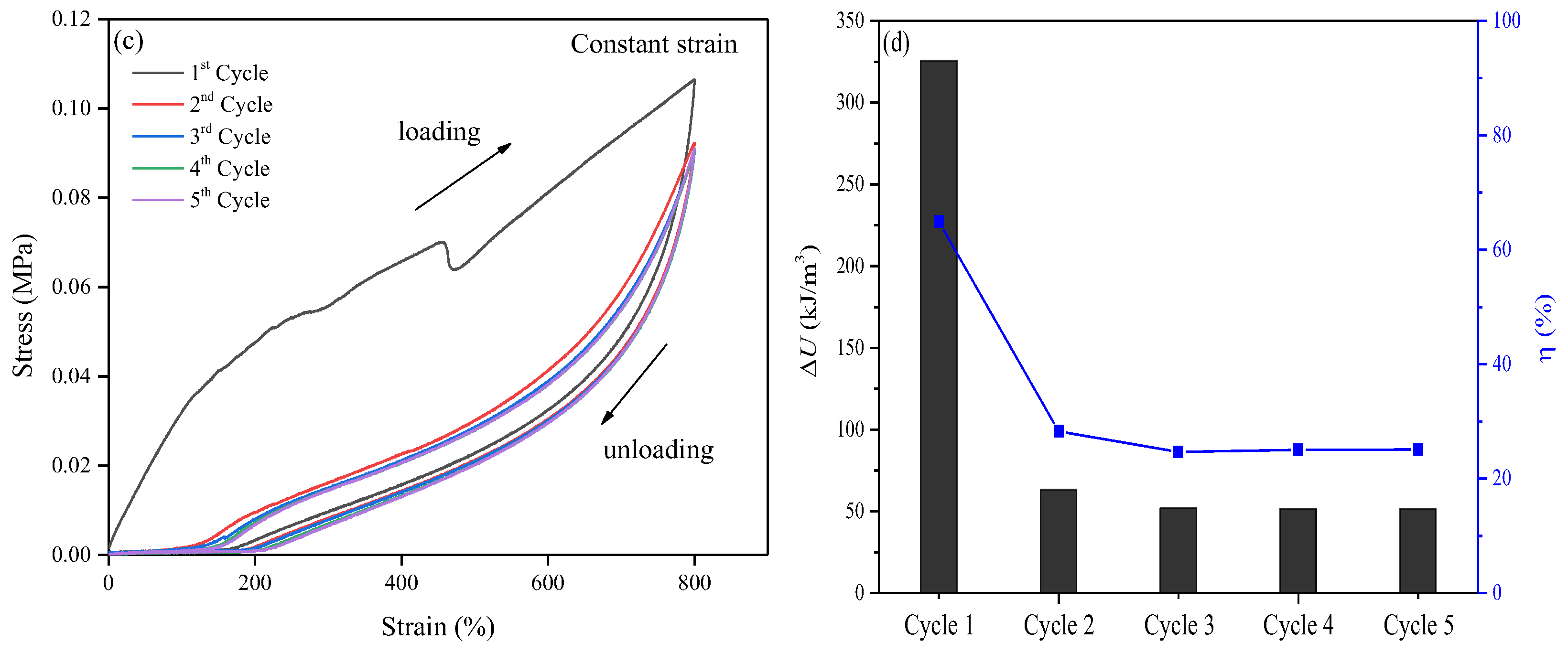
2.2. Toughness and Fatigue Resistance of the Obtained Hydrogel
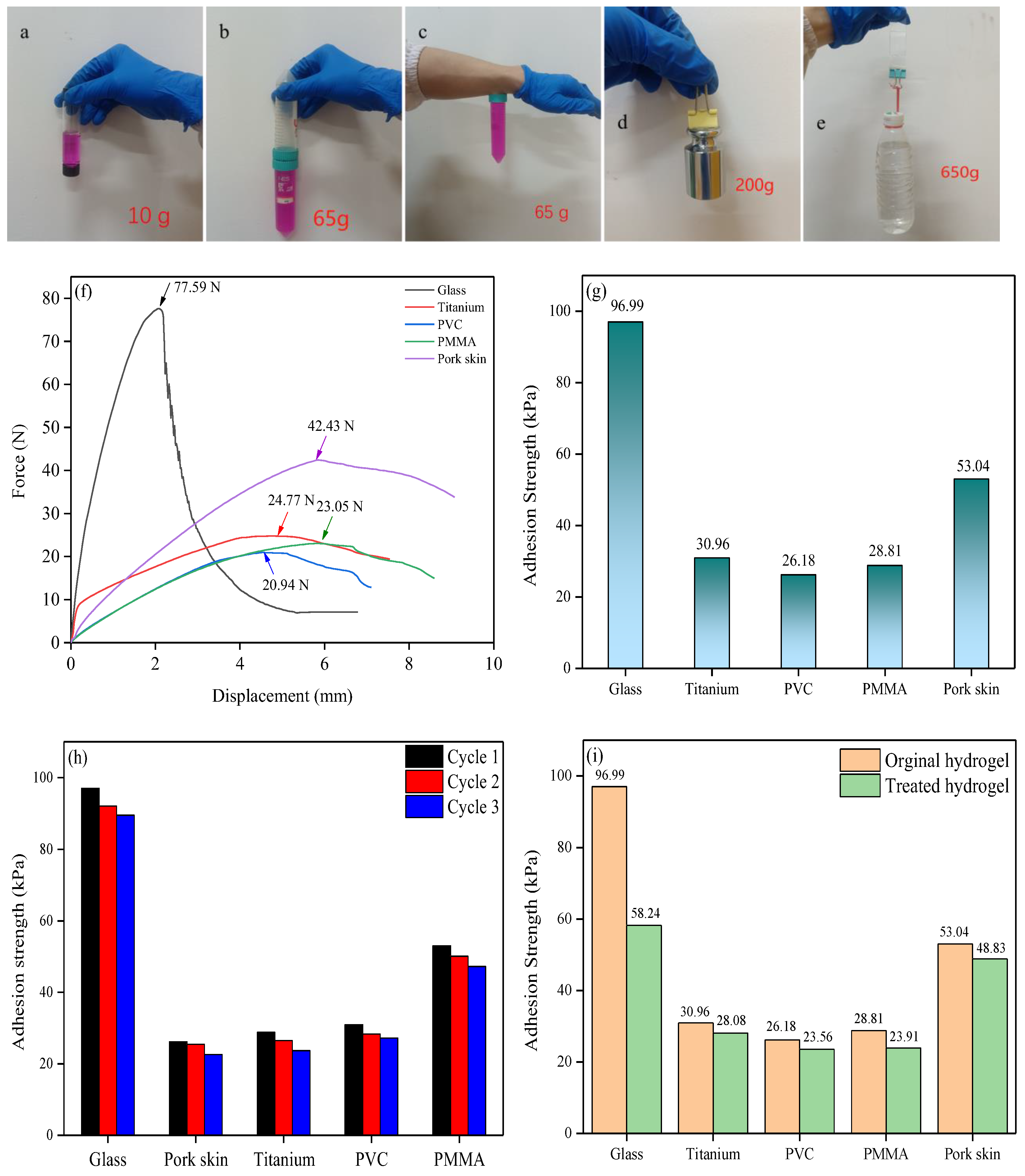
2.3. Adhesive Performance of the Obtained Hydrogel
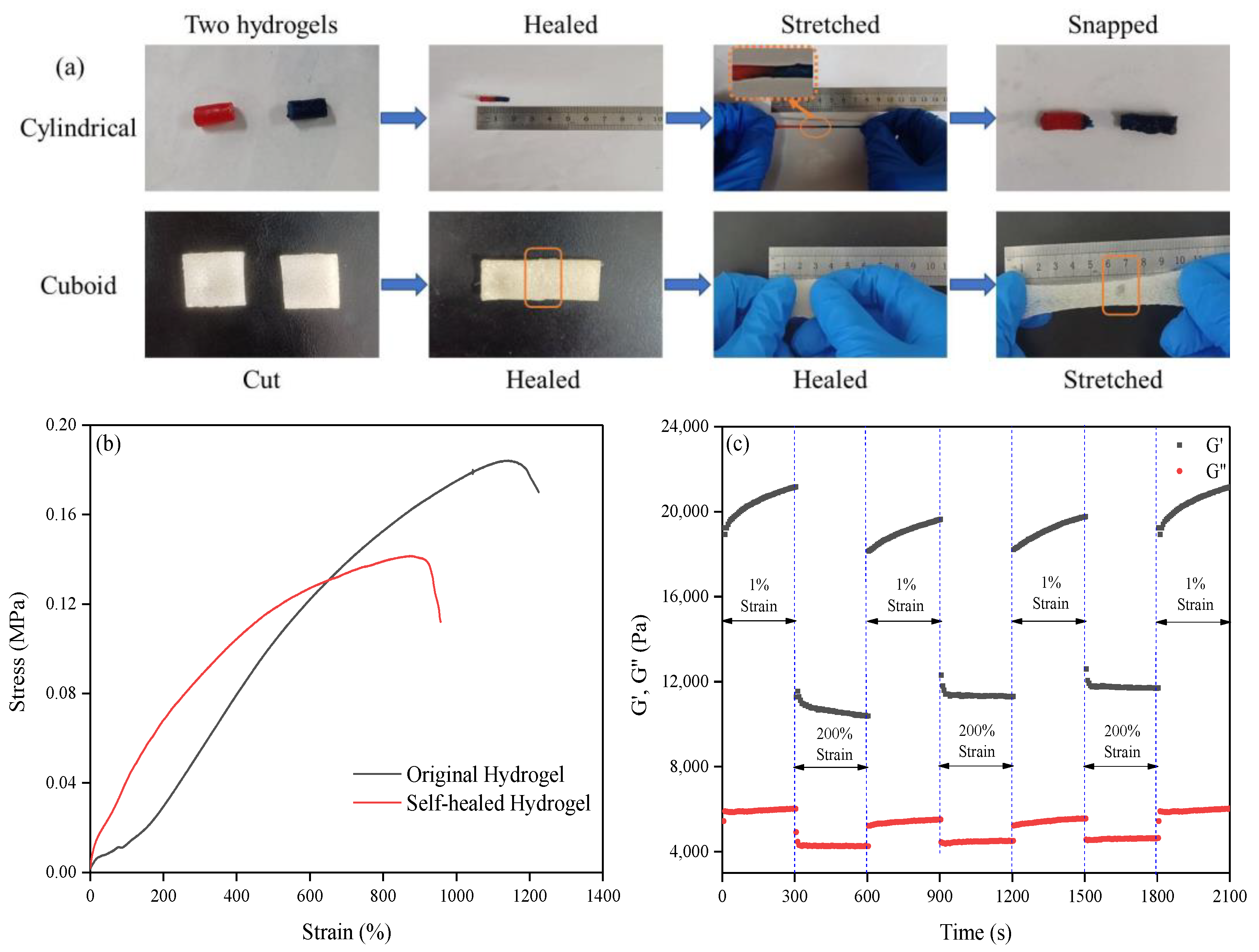
2.4. Self-Healing Performance of the Obtained Hydrogel
2.5. Swelling and De-Swelling Ability, Moisture Retention Ability, Hemolytic Activity, and Antibacterial Activity
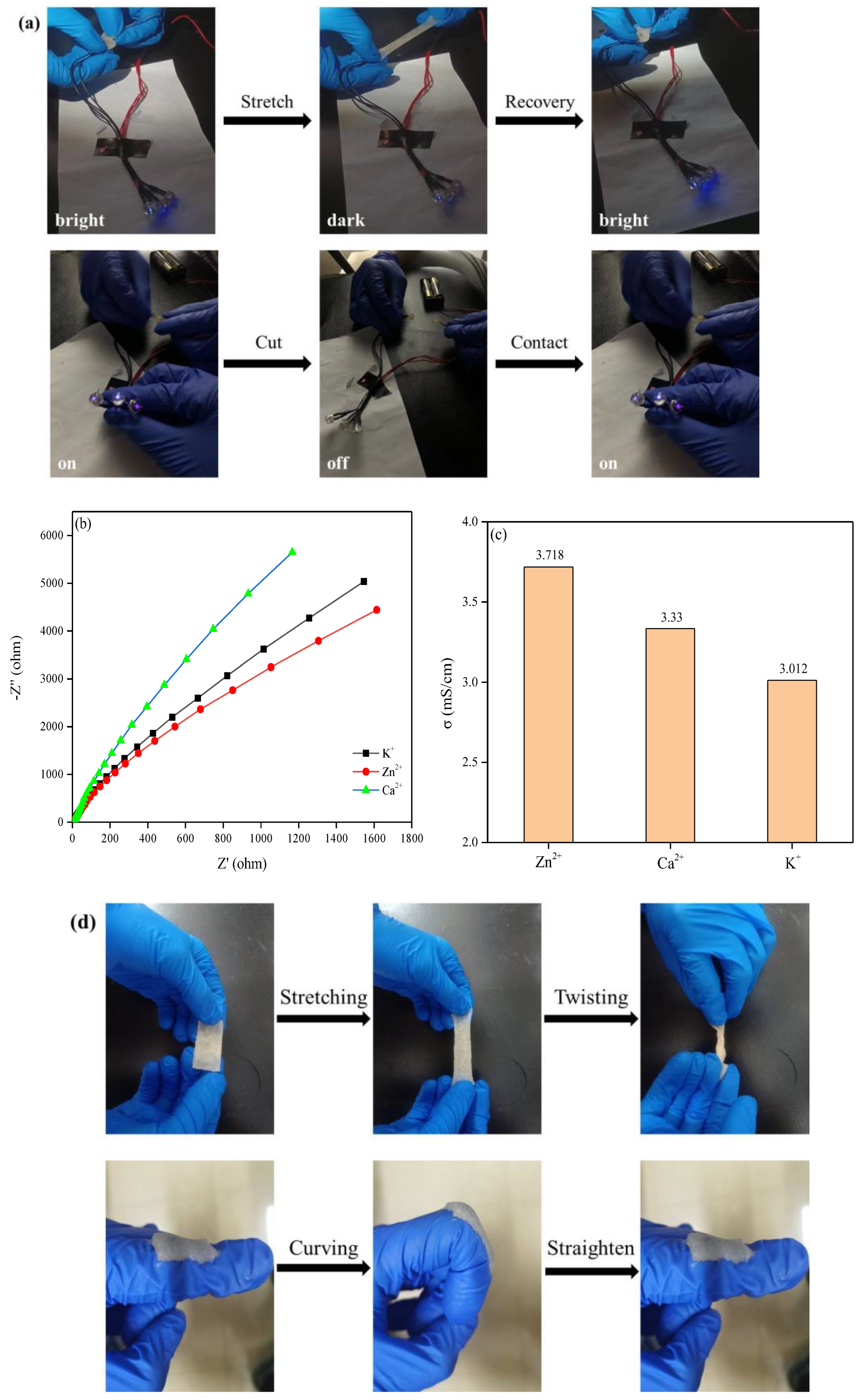
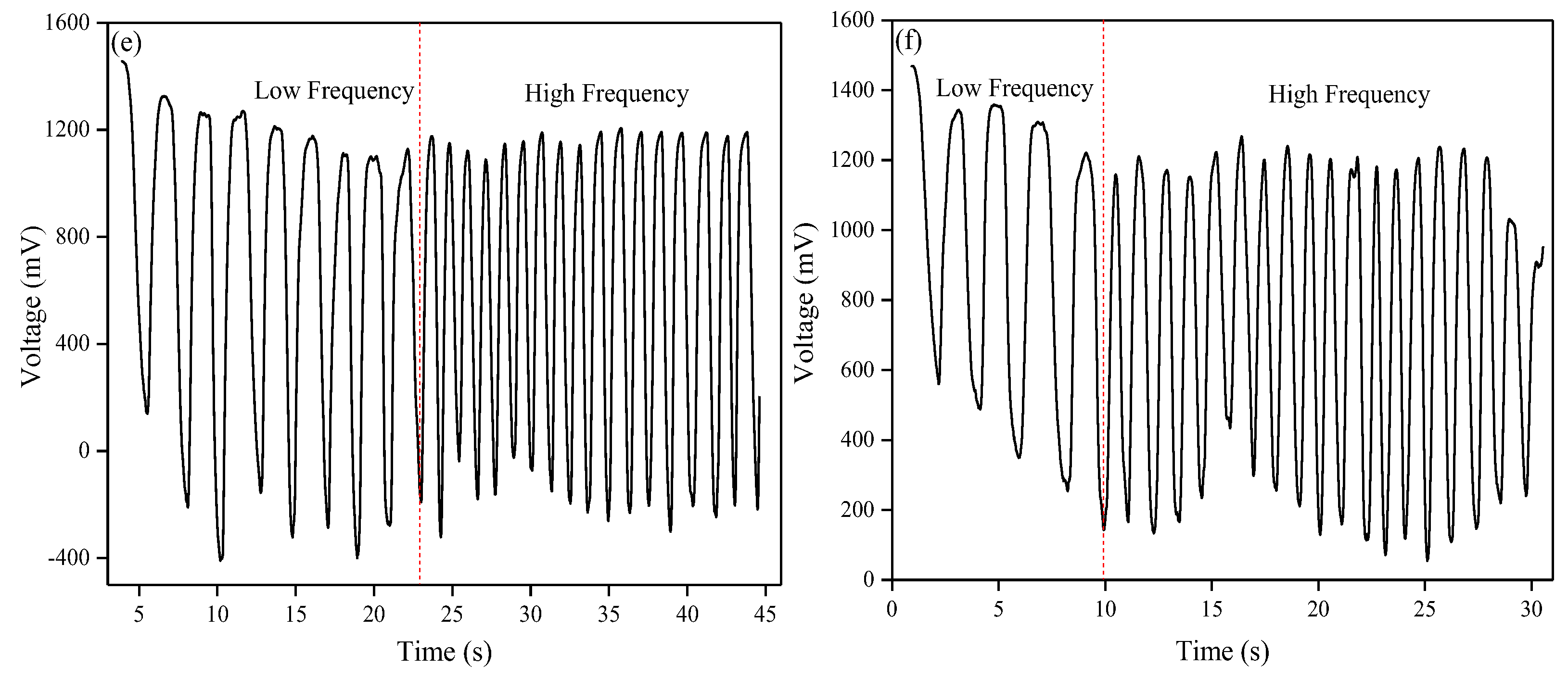
2.6. Electrical Conduction Performance of the Obtained Hydrogel
3. Discussion
4. Methods and Materials
4.1. Materials and Reagents
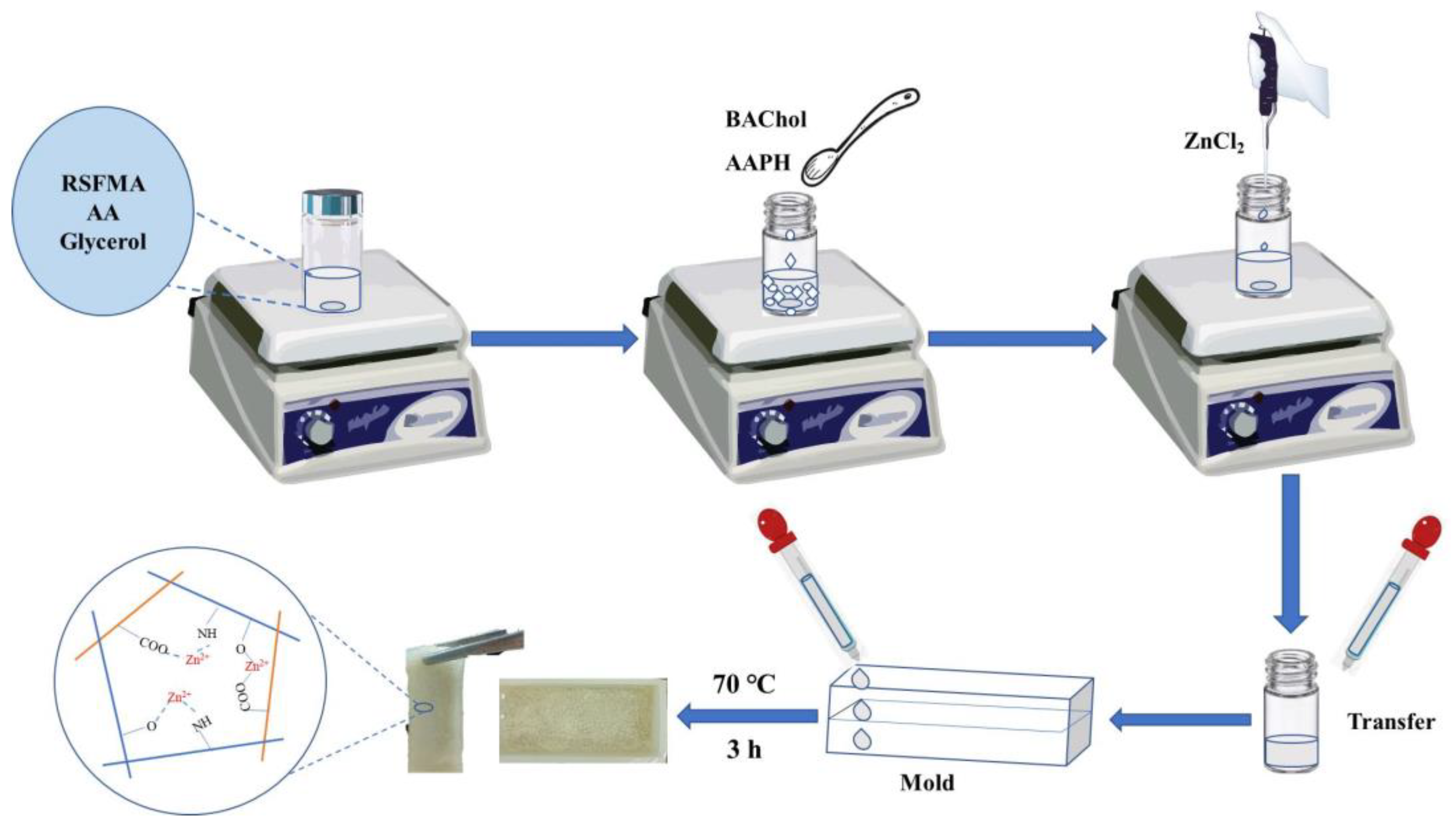
4.2. Preparation of the SFMA/BAChol/PAA/ZnCl2 Hydrogel
4.3. Cell Culture
4.4. Informed Consent
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Y.; Luo, Y.; Cuthbert, T.J.; Shokurov, A.V.; Chu, P.K.; Feng, S.-P.; Menon, C. Hydrogels as Soft Ionic Conductors in Flexible and Wearable Triboelectric Nanogenerators. Adv. Sci. 2022, 9, 2106008. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Chee, P.L.; Sugiarto, S.; Yu, Y.; Shi, C.; Yan, R.; Yao, Z.; Shi, X.; Zhi, J.; Kai, D.; et al. Hydrogel-Based Flexible Electronics. Adv. Mater. 2023, 35, 2205326. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Xie, A.-Q.; Cao, P.; Yang, J.; Ong, W.L.; Zhang, K.-Q.; Ho, G.W. Structuring and Shaping of Mechanically Robust and Functional Hydrogels toward Wearable and Implantable Applications. Adv. Mater. 2024, 36, 2309952. [Google Scholar] [CrossRef] [PubMed]
- Qin, R.; Nong, J.; Wang, K.; Liu, Y.; Zhou, S.; Hu, M.; Zhao, H.; Shan, G. Recent Advances in Flexible Pressure Sensors Based on MXene Materials. Adv. Mater. 2024, 36, 2312761. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Gasik, M. Smart Hydrogels for Advanced Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 3665. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yin, L.; Lv, J.; Gross, A.J.; Le, M.; Gutierrez, N.G.; Li, Y.; Jeerapan, I.; Giroud, F.; Berezovska, A.; et al. Stretchable and Flexible Buckypaper-Based Lactate Biofuel Cell for Wearable Electronics. Adv. Funct. Mater. 2019, 29, 1905785. [Google Scholar] [CrossRef]
- Li, M.; Pan, G.; Zhang, H.; Guo, B. Hydrogel adhesives for generalized wound treatment: Design and applications. J. Polym. Sci. 2022, 60, 1328–1359. [Google Scholar] [CrossRef]
- Chen, Y.; Wan, X.; Li, G.; Ye, J.; Gao, J.; Wen, D. Metal Hydrogel-Based Integrated Wearable Biofuel Cell for Self-Powered Epidermal Sweat Biomarker Monitoring. Adv. Funct. Mater. 2024, 34, 2404329. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Yang, W.; Xu, Z.; Dai, H.; He, F.; Yan, S.; Shi, X. In Situ-Sprayed Bioinspired Adhesive Conductive Hydrogels for Cavernous Nerve Repair. Adv. Mater. 2024, 36, 2311264. [Google Scholar] [CrossRef]
- Xie, C.; Wang, X.; He, H.; Ding, Y.; Lu, X. Mussel-Inspired Hydrogels for Self-Adhesive Bioelectronics. Adv. Funct. Mater. 2020, 30, 1909954. [Google Scholar] [CrossRef]
- Han, S.; Hu, Y.; Wei, J.; Li, S.; Yang, P.; Mi, H.; Liu, C.; Shen, C. A Semi-Interpenetrating Poly(Ionic Liquid) Network-Driven Low Hysteresis and Transparent Hydrogel as a Self-Powered Multifunctional Sensor. Adv. Funct. Mater. 2024, 34, 2401607. [Google Scholar] [CrossRef]
- Yang, W.; Kang, X.; Gao, X.; Zhuang, Y.; Fan, C.; Shen, H.; Chen, Y.; Dai, J. Biomimetic Natural Biopolymer-Based Wet-Tissue Adhesive for Tough Adhesion, Seamless Sealed, Emergency/Nonpressing Hemostasis, and Promoted Wound Healing. Adv. Funct. Mater. 2023, 33, 2211340. [Google Scholar] [CrossRef]
- Yu, H.C.; Zheng, S.Y.; Fang, L.; Ying, Z.; Du, M.; Wang, J.; Ren, K.-F.; Wu, Z.L.; Zheng, Q. Reversibly Transforming a Highly Swollen Polyelectrolyte Hydrogel to an Extremely Tough One and its Application as a Tubular Grasper. Adv. Mater. 2020, 32, 2005171. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shan, Y.; Wang, X.; Li, H.; Yang, K.; Cui, Y. Self-healing flexible sensor based on metal-ligand coordination. Chem. Eng. J. 2020, 394, 124932. [Google Scholar] [CrossRef]
- Wang, X.-H.; Song, F.; Qian, D.; He, Y.-D.; Nie, W.-C.; Wang, X.-L.; Wang, Y.-Z. Strong and tough fully physically crosslinked double network hydrogels with tunable mechanics and high self-healing performance. Chem. Eng. J. 2018, 349, 588–594. [Google Scholar] [CrossRef]
- Kim, S.; Regitsky, A.U.; Song, J.; Ilavsky, J.; McKinley, G.H.; Holten-Andersen, N. In situ mechanical reinforcement of polymer hydrogels via metal-coordinated crosslink mineralization. Nat. Commun. 2021, 12, 667. [Google Scholar] [CrossRef]
- Ghosh, A.; Pandit, S.; Kumar, S.; Ganguly, D.; Chattopadhyay, S.; Pradhan, D.; Das, R.K. Designing dynamic metal-coordinated hydrophobically associated mechanically robust and stretchable hydrogels for versatile, multifunctional applications in strain sensing, actuation and flexible supercapacitors. Chem. Eng. J. 2023, 475, 146160. [Google Scholar] [CrossRef]
- He, F.; You, X.; Gong, H.; Yang, Y.; Bai, T.; Wang, W.; Guo, W.; Liu, X.; Ye, M. Stretchable, Biocompatible, and Multifunctional Silk Fibroin-Based Hydrogels toward Wearable Strain/Pressure Sensors and Triboelectric Nanogenerators. ACS Appl. Mater. Interfaces 2020, 12, 6442–6450. [Google Scholar] [CrossRef]
- Kim, S.H.; Yeon, Y.K.; Lee, J.M.; Chao, J.R.; Lee, Y.J.; Seo, Y.B.; Sultan, M.T.; Lee, O.J.; Lee, J.S.; Yoon, S.-i.; et al. Precisely printable and biocompatible silk fibroin bioink for digital light processing 3D printing. Nat. Commun. 2018, 9, 1620. [Google Scholar] [CrossRef]
- Fan, J.-P.; Tian, J.-M.; Zhong, H.; Chen, H.-Q.; Xie, C.-F.; Chen, H.-P.; Peng, H.-L.; Liu, Y.-D. Synthesis of a porous hollow magnetic molecularly imprinted microsphere by O/W/O composite emulsion polymerization for specifically recognizing bovine serum albumin. Sep. Purif. Technol. 2024, 329, 125197. [Google Scholar] [CrossRef]
- Fan, J.-P.; Lai, Z.-T.; Mao, D.-Y.; Xie, C.-F.; Chen, H.-P.; Peng, H.-L. Preparation of a silk fibroin/gelatin composite hydrogel for high-selectively adsorbing bovine hemoglobin. Colloids Surf. A Physicochem. Eng. Asp. 2023, 660, 130869. [Google Scholar] [CrossRef]
- Sennakesavan, G.; Mostakhdemin, M.; Dkhar, L.K.; Seyfoddin, A.; Fatihhi, S.J. Acrylic acid/acrylamide based hydrogels and its properties—A review. Polym. Degrad. Stab. 2020, 180, 109308. [Google Scholar] [CrossRef]
- Yin, M.-J.; Yao, M.; Gao, S.; Zhang, A.P.; Tam, H.-Y.; Wai, P.-K.A. Rapid 3D Patterning of Poly(acrylic acid) Ionic Hydrogel for Miniature pH Sensors. Adv. Mater. 2016, 28, 1394–1399. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Zhang, X.; Zamora, J.; McCloy, J.; Song, M.-K. Silk fibroin-based biopolymer composite binders with gradient binding energy and strong adhesion force for high-performance micro-sized silicon anodes. J. Energy Chem. 2023, 80, 442–451. [Google Scholar] [CrossRef]
- Li, H.; Sun, J.; Qin, S.; Song, Y.; Liu, Z.; Yang, P.; Li, S.; Liu, C.; Shen, C. Zwitterion Functionalized Graphene Oxide/Polyacrylamide/Polyacrylic Acid Hydrogels with Photothermal Conversion and Antibacterial Properties for Highly Efficient Uranium Extraction from Seawater. Adv. Funct. Mater. 2023, 33, 2301773. [Google Scholar] [CrossRef]
- An, H.; Zhang, M.; Huang, Z.; Xu, Y.; Ji, S.; Gu, Z.; Zhang, P.; Wen, Y. Hydrophobic Cross-Linked Chains Regulate High Wet Tissue Adhesion Hydrogel with Toughness, Anti-hydration for Dynamic Tissue Repair. Adv. Mater. 2024, 36, 2310164. [Google Scholar] [CrossRef]
- Peng, X.; Xia, X.; Xu, X.; Yang, X.; Yang, B.; Zhao, P.; Yuan, W.; Chiu, P.W.Y.; Bian, L. Ultrafast self-gelling powder mediates robust wet adhesion to promote healing of gastrointestinal perforations. Sci. Adv. 2024, 7, eabe8739. [Google Scholar] [CrossRef]
- Li, W.; Li, L.; Zheng, S.; Liu, Z.; Zou, X.; Sun, Z.; Guo, J.; Yan, F. Recyclable, Healable, and Tough Ionogels Insensitive to Crack Propagation. Adv. Mater. 2022, 34, 2203049. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, G.; Xue, Y.; Duan, Q.; Liang, X.; Lin, T.; Wu, Z.; Tan, Y.; Zhao, Q.; Zheng, W.; et al. Fatigue-Resistant Conducting Polymer Hydrogels as Strain Sensor for Underwater Robotics. Adv. Funct. Mater. 2023, 33, 2305705. [Google Scholar] [CrossRef]
- Lu, Y.; Yue, Y.; Ding, Q.; Mei, C.; Xu, X.; Wu, Q.; Xiao, H.; Han, J. Self-Recovery, Fatigue-Resistant, and Multifunctional Sensor Assembled by a Nanocellulose/Carbon Nanotube Nanocomplex-Mediated Hydrogel. ACS Appl. Mater. Interfaces 2021, 13, 50281–50297. [Google Scholar] [CrossRef]
- Yang, T.; Lu, S.-H.; Zhu, H.; Patetsos, A.; McDonald, E.; Mellor, M.D.; Luo, Y.; Rusling, J.F.; Wang, X.; He, J. Tough and Elastic Cellulose Composite Hydrogels/Films for Flexible Wearable Sensors. ACS Appl. Mater. Interfaces 2024, 16, 40018–40029. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, D.; Bai, P.; Mao, Y.; Li, Q.; Guo, J.; Fang, Y.; Ma, R. Strong Tough Thermogalvanic Hydrogel Thermocell with Extraordinarily High Thermoelectric Performance. Adv. Mater. 2023, 35, 2300696. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Wang, W.; Yang, W.; Chen, J.; Peng, Q.; Wang, T.; Yang, D.; Wang, J.; Zhang, H.; Zeng, H. Stretchable, compressible, and conductive hydrogel for sensitive wearable soft sensors. J. Colloid Interface Sci. 2022, 618, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Qiao, Y.; Qiu, D. Coordinatively Stiffen and Toughen Hydrogels with Adaptable Crystal-Domain Cross-Linking. Adv. Mater. 2023, 35, 2209913. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, R.; Chaudhari, U.; Anand, S. How to Select Phase Change Materials for Tuning Condensation and Frosting? Adv. Funct. Mater. 2023, 33, 2206301. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, H.; Wang, Y.; Fan, X.; Li, Z.; Zhang, X.; Liu, T. Highly Stretchable, Ultra-Soft, and Fast Self-Healable Conductive Hydrogels Based on Polyaniline Nanoparticles for Sensitive Flexible Sensors. Adv. Funct. Mater. 2022, 32, 2204366. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, D.; Liu, D.; Han, X.; Liu, X.; Torun, H.; Guo, Z.; Duan, S.; He, X.; Zhang, X.; et al. A Universal Interfacial Strategy Enabling Ultra-Robust Gel Hybrids for Extreme Epidermal Bio-Monitoring. Adv. Funct. Mater. 2023, 33, 2301117. [Google Scholar] [CrossRef]
- Li, T.; Li, X.; Yang, J.; Sun, H.; Sun, J. Healable Ionic Conductors with Extremely Low-Hysteresis and High Mechanical Strength Enabled by Hydrophobic Domain-Locked Reversible Interactions. Adv. Mater. 2023, 35, 2307990. [Google Scholar] [CrossRef]
- GB/T 7124-2008; Adhesives, Determination of Tensile Lap-Shear Strength of Rigid-to-Rigid Bonded Assemblies. Standards Press of China: Beijing, China, 2008.
- Qiao, H.; Qi, P.; Zhang, X.; Wang, L.; Tan, Y.; Luan, Z.; Xia, Y.; Li, Y.; Sui, K. Multiple Weak H-Bonds Lead to Highly Sensitive, Stretchable, Self-Adhesive, and Self-Healing Ionic Sensors. ACS Appl. Mater. Interfaces 2019, 11, 7755–7763. [Google Scholar] [CrossRef]
- Oloyede, H.O.B.; Ajiboye, H.O.; Salawu, M.O.; Ajiboye, T.O. Influence of oxidative stress on the antibacterial activity of betulin, betulinic acid and ursolic acid. Microb. Pathog. 2017, 111, 338–344. [Google Scholar] [CrossRef]
- Huo, S.; Liu, S.; Liu, Q.; Xie, E.; Miao, L.; Meng, X.; Xu, Z.; Zhou, C.; Liu, X.; Xu, G. Copper–Zinc-Doped Bilayer Bioactive Glasses Loaded Hydrogel with Spatiotemporal Immunomodulation Supports MRSA-Infected Wound Healing. Adv. Sci. 2024, 11, 2302674. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, R.; Duan, Z.; Zhu, C.; Fan, D. Construction of multifunctional hydrogel based on the tannic acid-metal coating decorated MoS2 dual nanozyme for bacteria-infected wound healing. Bioact. Mater. 2022, 9, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Xiang, L.; Ou, H.; Li, F.; Zhang, Y.; Qian, Y.; Hao, L.; Diao, J.; Zhang, M.; Zhu, P.; et al. MXene-Based Conductive Organohydrogels with Long-Term Environmental Stability and Multifunctionality. Adv. Funct. Mater. 2020, 30, 2005135. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, X.; Wu, J.; Jiang, M.; Zhang, H.; Yan, C. Antifreezing/Antiswelling Hydrogels: Synthesis Strategies and Applications as Flexible Motion Sensors. ACS Appl. Mater. Interfaces 2024, 16, 58100–58120. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yang, K.; Zhu, G.; Ou, C.; Jiang, J.; Zhuravlev, E.; Zhang, Y. Anti-freezing multifunctional conductive hydrogels: From structure design to flexible electronic devices. Mater. Chem. Front. 2024, 8, 381–403. [Google Scholar] [CrossRef]
- Xia, Y.; Wu, Y.; Yu, T.; Xue, S.; Guo, M.; Li, J.; Li, Z. Multifunctional Glycerol–Water Hydrogel for Biomimetic Human Skin with Resistance Memory Function. ACS Appl. Mater. Interfaces 2019, 11, 21117–21125. [Google Scholar] [CrossRef]
- Rong, Q.; Lei, W.; Chen, L.; Yin, Y.; Zhou, J.; Liu, M. Anti-freezing, Conductive Self-healing Organohydrogels with Stable Strain-Sensitivity at Subzero Temperatures. Angew. Chem. Int. Ed. 2017, 56, 14159–14163. [Google Scholar] [CrossRef]
- Lyu, Y.; Guo, R.; Lin, Z.; Zhai, F.; Wu, T.; Jiang, P.; Ji, Z.; Ma, S.; Shi, X.; Wang, X. Ion Clusters-Driven Strong and Acid/Alkali/ Freezing-Tolerant Conductive Hydrogels for Flexible Sensors in Extreme Environments. Adv. Funct. Mater. 2023, 33, 2306300. [Google Scholar] [CrossRef]
- Dou, X.; Wang, H.; Yang, F.; Shen, H.; Wang, X.; Wu, D. One-Step Soaking Strategy toward Anti-Swelling Hydrogels with a Stiff “Armor”. Adv. Sci. 2023, 10, 2206242. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Y.; Chang, X.; Pan, D.; Song, G.; Guo, Z.; Naik, N. Recent Progress in Essential Functions of Soft Electronic Skin. Adv. Funct. Mater. 2021, 31, 2104686. [Google Scholar] [CrossRef]
- Yan, Y.; Huang, J.; Qiu, X.; Cui, X.; Xu, S.; Wu, X.; Yao, P.; Huang, C. An ultra-stretchable glycerol-ionic hybrid hydrogel with reversible gelid adhesion. J. Colloid Interface Sci. 2021, 582, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Wei, W.; Zhang, Z.; Liu, B.; Feng, W.; Ma, Y.; Zhang, X. Highly stretchable, self-healing, and adhesive polymeric eutectogel enabled by hydrogen-bond networks for wearable strain sensor. Chem. Eng. J. 2022, 449, 137878. [Google Scholar] [CrossRef]
- Yin, J.; Pan, S.; Wu, L.; Tan, L.; Chen, D.; Huang, S.; Zhang, Y.; He, P. A self-adhesive wearable strain sensor based on a highly stretchable, tough, self-healing and ultra-sensitive ionic hydrogel. J. Mater. Chem. C 2020, 8, 17349–17364. [Google Scholar] [CrossRef]
- Mo, J.; Dai, Y.; Zhang, C.; Zhou, Y.; Li, W.; Song, Y.; Wu, C.; Wang, Z. Design of ultra-stretchable, highly adhesive and self-healable hydrogels via tannic acid-enabled dynamic interactions. Mater. Horiz. 2021, 8, 3409–3416. [Google Scholar] [CrossRef] [PubMed]
- Shui, T.; Pan, M.; Li, A.; Fan, H.; Wu, J.; Liu, Q.; Zeng, H. Poly(vinyl Alcohol) (PVA)-Based Hydrogel Scaffold with Isotropic Ultratoughness Enabled by Dynamic Amine–Catechol Interactions. Chem. Mater. 2022, 34, 8613–8628. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Wu, C.; Pei, X.; Cong, Y.; Zhang, R.; Fu, J. Antibacterial Zwitterionic Polyelectrolyte Hydrogel Adhesives with Adhesion Strength Mediated by Electrostatic Mismatch. ACS Appl. Mater. Interfaces 2020, 12, 46816–46826. [Google Scholar] [CrossRef]
- Xiong, X.; Chen, Y.; Wang, Z.; Liu, H.; Le, M.; Lin, C.; Wu, G.; Wang, L.; Shi, X.; Jia, Y.-G.; et al. Polymerizable rotaxane hydrogels for three-dimensional printing fabrication of wearable sensors. Nat. Commun. 2023, 14, 1331. [Google Scholar] [CrossRef]
- Hu, Y.; Jiang, X.; Ding, Y.; Ge, H.; Yuan, Y.; Yang, C. Synthesis and characterization of chitosan–poly(acrylic acid) nanoparticles. Biomaterials 2002, 23, 3193–3201. [Google Scholar] [CrossRef]


| Articles | Materials | Stress (kPa) | Strain (%) | Adhesion Strength (kPa) |
|---|---|---|---|---|
| Yan et al. [52] | glycerol-ionic hybrid hydrogel | 5.4 | 15,000 | 6 |
| Fan et al. [53] | poly(1-vinylimidazole)-choline chloride-glycerol eutectogel | 490 | 2310 | 13.6 |
| Yin et al. [54] | chitosan/tannic acid/poly(acrylic acid)-Al3+ ionic hydrogel | 179.3 | 1450 | 7.02 |
| Mo et al. [55] | tannic acid enabled dynamic interactions hydrogel | 54 | 7300 | 50 |
| Shui et al. [56] | oligo-polydopamine-tyrosine- poly(vinyl alcohol) hydrogel | 13,300 | 813 | 42.1 |
| Wang et al. [57] | zwitterionic polyelectrolyte hydrogel | 44.5 | 874 | 3.97 |
| Xiong et al. [58] | polymerizable rotaxane hydrogel | 78.1 | 830 | 4.5 |
| This study | SFMA/BAChol/PAA/ZnCl2 polymer hydrogel | 145 | 1580 | 53.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, J.-P.; Xie, M.-R.; Yuan, C.; Ma, J.; Fu, K.-P.; Huang, C.-H.; Chen, H.-P.; Peng, H.-L.; Xie, C.-F. Fabricating a SFMA/BAChol/PAA/ZnCl2 Hydrogel with Excellent Versatile Comprehensive Properties and Stable Sensitive Freezing-Tolerant Conductivity for Wearable Sensors. Int. J. Mol. Sci. 2024, 25, 13339. https://doi.org/10.3390/ijms252413339
Fan J-P, Xie M-R, Yuan C, Ma J, Fu K-P, Huang C-H, Chen H-P, Peng H-L, Xie C-F. Fabricating a SFMA/BAChol/PAA/ZnCl2 Hydrogel with Excellent Versatile Comprehensive Properties and Stable Sensitive Freezing-Tolerant Conductivity for Wearable Sensors. International Journal of Molecular Sciences. 2024; 25(24):13339. https://doi.org/10.3390/ijms252413339
Chicago/Turabian StyleFan, Jie-Ping, Ming-Ru Xie, Chao Yuan, Jia Ma, Ke-Pu Fu, Chun-Hong Huang, Hui-Ping Chen, Hai-Long Peng, and Chun-Fang Xie. 2024. "Fabricating a SFMA/BAChol/PAA/ZnCl2 Hydrogel with Excellent Versatile Comprehensive Properties and Stable Sensitive Freezing-Tolerant Conductivity for Wearable Sensors" International Journal of Molecular Sciences 25, no. 24: 13339. https://doi.org/10.3390/ijms252413339
APA StyleFan, J.-P., Xie, M.-R., Yuan, C., Ma, J., Fu, K.-P., Huang, C.-H., Chen, H.-P., Peng, H.-L., & Xie, C.-F. (2024). Fabricating a SFMA/BAChol/PAA/ZnCl2 Hydrogel with Excellent Versatile Comprehensive Properties and Stable Sensitive Freezing-Tolerant Conductivity for Wearable Sensors. International Journal of Molecular Sciences, 25(24), 13339. https://doi.org/10.3390/ijms252413339







