Endothelial Dysfunction: Molecular Mechanisms and Therapeutic Strategies in Kawasaki Disease
Abstract
1. Introduction
2. Methods
3. Discussion
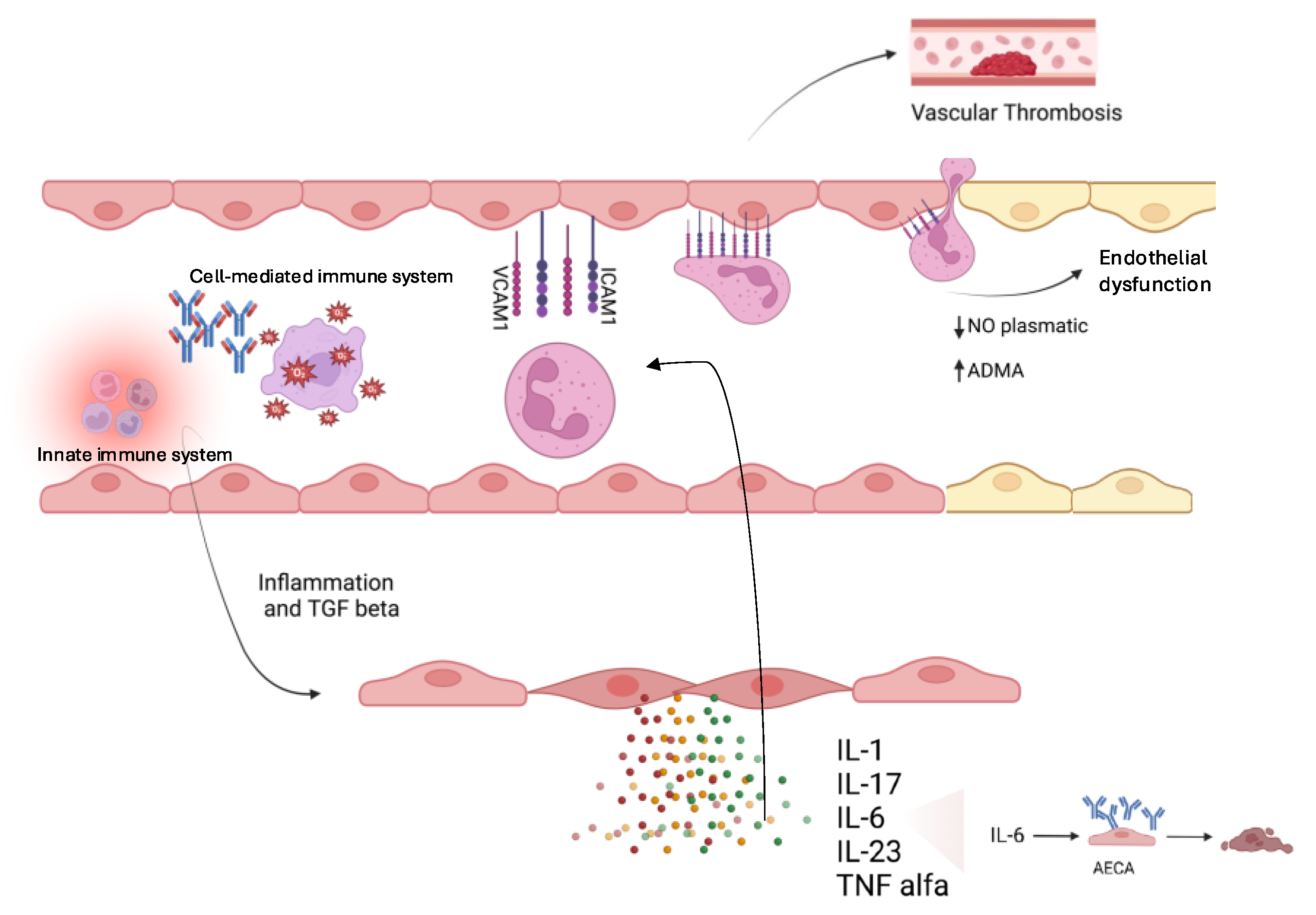
3.1. Molecular Mechanisms of Endothelial Dysfunction in KD
3.2. Therapeutic Strategies in KD
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial function and dysfunction: Testing and clinical relevance. Circulation 2007, 115, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Farrah, T.E.; Melville, V.; Czopek, A.; Fok, H.; Bruce, L.; Mills, N.L.; Bailey, M.A.; Webb, D.J.; Dear, J.W.; Dhaun, N. Arterial stiffness, endothelial dysfunction and impaired fibrinolysis are pathogenic mechanisms contributing to cardiovascular risk in ANCA-associated vasculitis. Kidney Int. 2022, 102, 1115–1126. [Google Scholar] [CrossRef]
- Flammer, A.J.; Anderson, T.; Celermajer, D.S.; Creager, M.A.; Deanfield, J.; Ganz, P.; Hamburg, N.M.; Lüscher, T.F.; Shechter, M.; Taddei, S.; et al. The assessment of endothelial function: From research into clinical practice. Circulation 2012, 126, 753–767. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gimbrone, M.A., Jr. Vascular endothelium, hemodynamic forces, and atherogenesis. Am. J. Pathol. 1999, 155, 1–5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tejero, J.; Shiva, S.; Gladwin, M.T. Sources of Vascular Nitric Oxide and Reactive Oxygen Species and Their Regulation. Physiol. Rev. 2019, 99, 311–379. [Google Scholar] [CrossRef]
- Edwards, G.; Feletou, M.; Weston, A.H. Endothelium-derived hyperpolarizing factors and associated pathways: A synopsis. Pflugers Arch. 2010, 459, 863–879. [Google Scholar] [CrossRef]
- O’Riordan, E.; Chen, J.; Brodsky, S.V.; Smirnova, I.; Li, H.; Goligorsky, M.S. Endothelial cell dysfunction: The syndrome in making. Kidney Int. 2005, 67, 1654–1658. [Google Scholar] [CrossRef]
- Lin, Y.S.; Lin, C.F.; Lei, H.Y.; Liu, H.S.; Yeh, T.M.; Chen, S.H.; Liu, C.C. Antibody-mediated endothelial cell damage via nitric oxide. Curr. Pharm. Des. 2004, 10, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef] [PubMed]
- Holve, T.J.; Patel, A.; Chau, Q.; Marks, A.R.; Meadows, A.; Zaroff, J.G. Long-term cardiovascular outcomes in survivors of Kawasaki disease. Pediatrics 2014, 133, e305–e311. [Google Scholar] [CrossRef]
- Vita, J.A.; Keaney, J.F. Endothelial function: A barometer for cardiovascular risk? Circulation 2022, 106, 640–642. [Google Scholar] [CrossRef]
- Bacon, P.A. Endothelial cell dysfunction in systemic vasculitis: New developments and therapeutic prospects. Curr. Opin. Rheumatol. 2005, 17, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Agrawal, D.K. Kawasaki disease: Etiopathogenesis and novel treatment strategies. Expert. Rev. Clin. Immunol. 2017, 13, 247–258. [Google Scholar] [CrossRef]
- Watanabe, T. Kidney and urinary tract involvement in kawasaki disease. Int. J. Pediatr. 2013, 2013, 831834. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- McCrindle, B.W.; Rowley, A.H.; Newburger, J.W.; Burns, J.C.; Bolger, A.F.; Gewitz, M.; Baker, A.L.; Jackson, M.A.; Takahashi, M.; Shah, P.B.; et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation 2017, 135, e927–e999, Erratum in Circulation 2019, 140, e181–e184. [Google Scholar] [CrossRef]
- Robinson, C.; Chanchlani, R.; Gayowsky, A.; Brar, S.; Darling, E.; Demers, C.; Mondal, T.; Parekh, R.; Seow, H.; Batthish, M. Cardiovascular outcomes in children with Kawasaki disease: A population-based cohort study. Pediatr. Res. 2023, 93, 1267–1275. [Google Scholar] [CrossRef]
- Maruhashi, T.; Soga, J.; Fujimura, N.; Idei, N.; Mikami, S.; Iwamoto, Y.; Kajikawa, M.; Matsumoto, T.; Hidaka, T.; Kihara, Y.; et al. Nitroglycerine-induced vasodilation for assessment of vascular function: A comparison with flow-mediated vasodilation. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1401–1408. [Google Scholar] [CrossRef]
- Mućka, S.; Miodońska, M.; Jakubiak, G.K.; Starzak, M.; Cieślar, G.; Stanek, A. Endothelial Function Assessment by Flow-Mediated Dilation Method: A Valuable Tool in the Evaluation of the Cardiovascular System. Int. J. Environ. Res. Public. Health 2022, 19, 11242. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuvin, J.T.; Patel, A.R.; Sliney, K.A.; Pandian, N.G.; Sheffy, J.; Schnall, R.P.; Karas, R.H.; Udelson, J.E. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am. Heart J. 2003, 146, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Pettersson-Pablo, P.; Cao, Y.; Breimer, L.H.; Nilsson, T.K.; Hurtig-Wennlöf, A. Pulse wave velocity, augmentation index, and carotid intima-media thickness are each associated with different inflammatory protein signatures in young healthy adults: The lifestyle, biomarkers and atherosclerosis study. Atherosclerosis 2020, 313, 150–155. [Google Scholar] [CrossRef] [PubMed]
- AlHuzaimi, A.; Al Mashham, Y.; Potts, J.E.; De Souza, A.M.; Sandor, G.G. Echo-Doppler assessment of arterial stiffness in pediatric patients with Kawasaki disease. J. Am. Soc. Echocardiogr. 2013, 26, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Milan, A.; Zocaro, G.; Leone, D.; Tosello, F.; Buraioli, I.; Schiavone, D.; Veglio, F. Current assessment of pulse wave velocity: Comprehensive review of validation studies. J. Hypertens. 2019, 37, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Wautier, J.L.; Wautier, M.P. Vascular Permeability in Diseases. Int. J. Mol. Sci. 2022, 23, 3645. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takahashi, K.; Oharaseki, T.; Yokouchi, Y. Update on etio and immunopathogenesis of Kawasaki disease. Curr. Opin. Rheumatol. 2014, 26, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Oharaseki, T.; Yokouchi, Y.; Naoe, S.; Saji, T. Kawasaki disease: Basic and pathological findings. Clin. Exp. Nephrol. 2013, 17, 690–693. [Google Scholar] [CrossRef]
- Burgner, D.; Davila, S.; Breunis, W.B.; Ng, S.B.; Li, Y.; Bonnard, C.; Ling, L.; Wright, V.J.; Thalamuthu, A.; Odam, M.; et al. A genome-wide association study identifies novel and functionally related susceptibility Loci for Kawasaki disease. PLoS Genet. 2009, 5, e1000319. [Google Scholar] [CrossRef] [PubMed]
- Orenstein, J.M.; Shulman, S.T.; Fox, L.M.; Baker, S.C.; Takahashi, M.; Bhatti, T.R.; Russo, P.A.; Mierau, G.W.; de Chadarévian, J.P.; Perlman, E.J.; et al. Three linked vasculopathic processes characterize Kawasaki disease: A light and transmission electron microscopic study. PLoS ONE 2012, 7, e38998. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fabi, M.; Petrovic, B.; Andreozzi, L.; Corinaldesi, E.; Filice, E.; Biagi, C.; Rizzello, A.; Mattesini, B.E.; Bugani, S.; Lanari, M. Circulating Endothelial Cells: A New Possible Marker of Endothelial Damage in Kawasaki Disease, Multisystem Inflammatory Syndrome in Children and Acute SARS-CoV-2 Infection. Int. J. Mol. Sci. 2022, 23, 10106. [Google Scholar] [CrossRef] [PubMed]
- Conti, F.; Moratti, M.; Leonardi, L.; Catelli, A.; Bortolamedi, E.; Filice, E.; Fetta, A.; Fabi, M.; Facchini, E.; Cantarini, M.E.; et al. Anti-Inflammatory and Immunomodulatory Effect of High-Dose Immunoglobulins in Children: From Approved Indications to Off-Label Use. Cells 2023, 12, 2417. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Woywodt, A.; Bahlmann, F.H.; De Groot, K.; Haller, H.; Haubitz, M. Circulating endothelial cells: Life, death, detachment and repair of the endothelial cell layer. Nephrol. Dial. Transplant. 2002, 17, 1728–1730. [Google Scholar] [CrossRef]
- Feng, S.; Chen, J.W.; Shu, X.Y.; Aihemaiti, M.; Quan, J.W.; Lu, L.; Zhang, R.Y.; Yang, C.D.; Wang, X.Q. Endothelial microparticles: A mechanosensitive regulator of vascular homeostasis and injury under shear stress. Front. Cell Dev. Biol. 2022, 10, 980112. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kevil, C.G.; Bullard, D.C. Roles of leukocyte/endothelial cell adhesion molecules in the pathogenesis of vasculitis. Am. J. Med. 1999, 106, 677–687. [Google Scholar] [CrossRef]
- Singh, V.; Kaur, R.; Kumari, P.; Pasricha, C.; Singh, R. ICAM-1 and VCAM-1: Gatekeepers in various inflammatory and cardiovascular disorders. Clin. Chim. Acta 2023, 548, 117487. [Google Scholar] [CrossRef] [PubMed]
- Turkcapar, N.; Sak, S.D.; Saatci, M.; Duman, M.; Olmez, U. Vasculitis and expression of vascular cell adhesion molecule-1, intercellular adhesion molecule-1, and E-selectin in salivary glands of patients with Sjögren’s syndrome. J. Rheumatol. 2005, 32, 1063–1070. [Google Scholar] [PubMed]
- Oh, J.H.; Han, J.W.; Lee, S.J.; Lee, K.Y.; Suh, B.K.; Koh, D.K.; Lee, J.S.; Oh, C.K.; Kim, T.G.; Choi, H.B. Polymorphisms of human leukocyte antigen genes in korean children with Kawasaki disease. Pediatr. Cardiol. 2008, 29, 402–408. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kato, S.; Kimura, M.; Tsuji, K.; Kusakawa, S.; Asai, T.; Juji, T.; Kawasaki, T. HLA antigens in Kawasaki disease. Pediatrics 1978, 61, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X.; Zhang, D.; Chen, R.; Alifu, A. Whole-exome sequencing reveals Kawasaki disease susceptibility genes and their association with coronary artery lesion. Front. Pediatr. 2024, 12, 1400123. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Uittenbogaard, P.; Netea, S.A.; Tanck, M.W.; Geissler, J.; Buda, P.; Kowalczyk-Domagała, M.; Okarska-Napierała, M.; van Stijn, D.; Tacke, C.E.; US Kawasaki Disease Genetics Consortium; et al. FCGR2/3 polymorphisms are associated with susceptibility to Kawasaki disease but do not predict intravenous immunoglobulin resistance and coronary artery aneurysms. Front. Immunol. 2024, 15, 1323171. [Google Scholar] [CrossRef]
- Nagelkerke, S.Q.; Tacke, C.E.; Breunis, W.B.; Tanck, M.W.T.; Geissler, J.; Png, E.; Hoang, L.T.; van der Heijden, J.; Naim, A.N.M.; Yeung, R.S.M.; et al. Extensive Ethnic Variation and Linkage Disequilibrium at the FCGR2/3 Locus: Different Genetic Associations Revealed in Kawasaki Disease. Front. Immunol. 2019, 10, 185. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khor, C.C.; Davila, S.; Breunis, W.B.; Lee, Y.C.; Shimizu, C.; Wright, V.J.; Yeung, R.S.; Tan, D.E.; Sim, K.S.; Wang, J.J.; et al. Genome-wide association study identifies FCGR2A as a susceptibility locus for Kawasaki disease. Nat. Genet. 2011, 43, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.C.; Shimizu, C.; Gonzalez, E.; Kulkarni, H.; Patel, S.; Shike, H.; Sundel, R.S.; Newburger, J.W.; Ahuja, S.K. Genetic variations in the receptor-ligand pair CCR5 and CCL3L1 are important determinants of susceptibility to Kawasaki disease. J. Infect. Dis. 2005, 192, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Mamtani, M.; Matsubara, T.; Shimizu, C.; Furukawa, S.; Akagi, T.; Onouchi, Y.; Hata, A.; Fujino, A.; He, W.; Ahuja, S.K.; et al. Association of CCR2-CCR5 haplotypes and CCL3L1 copy number with Kawasaki Disease, coronary artery lesions, and IVIG responses in Japanese children. PLoS ONE 2010, 5, e11458. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shrestha, S.; Wiener, H.; Shendre, A.; Kaslow, R.A.; Wu, J.; Olson, A.; Bowles, N.E.; Patel, H.; Edberg, J.C.; Portman, M.A. Role of activating FcγR gene polymorphisms in Kawasaki disease susceptibility and intravenous immunoglobulin response. Circ. Cardiovasc. Genet. 2012, 5, 309–316. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Onouchi, Y.; Fukazawa, R.; Yamamura, K.; Suzuki, H.; Kakimoto, N.; Ebata, R.; Higashi, K.; Tanaka, T. Variations in ORAI1 gene associated with Kawasaki disease. PLoS ONE 2016, 11, e0145486. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, J.; Liu, J.; Geng, Z.; Tao, Y.; Zheng, F.; Wang, Y.; Fu, S.; Wang, W.; Xie, C.; et al. The role of Ca2+/NFAT in Dysfunction and Inflammation of Human Coronary Endothelial Cells induced by Sera from patients with Kawasaki disease. Sci. Rep. 2020, 10, 4706. [Google Scholar] [CrossRef]
- Lv, Y.W.; Wang, J.; Sun, L.; Zhang, J.M.; Cao, L.; Ding, Y.Y.; Chen, Y.; Dou, J.J.; Huang, J.; Tang, Y.F.; et al. Understanding the pathogenesis of Kawasaki disease by network and pathway analysis. Comput. Math. Methods Med. 2013, 2013, 989307. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jia, S.; Li, C.; Wang, G.; Yang, J.; Zu, Y. The T helper type 17/regulatory T cell imbalance in patients with acute Kawasaki disease. Clin. Exp. Immunol. 2010, 162, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.M.; Tseng, W.N.; Ko, C.H.; Pan, H.M.; Hsieh, K.S.; Kuo, H.C. Th17- and Treg-related cytokine and mRNA expression are associated with acute and resolving Kawasaki disease. Allergy 2015, 70, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Fujimaru, T.; Ito, S.; Masuda, H.; Oana, S.; Kamei, K.; Ishiguro, A.; Kato, H.; Abe, J. Decreased levels of inflammatory cytokines in immunoglobulin-resistant Kawasaki disease after plasma exchange. Cytokine 2014, 70, 156–160. [Google Scholar] [CrossRef]
- Onouchi, Y.; Onoue, S.; Tamari, M.; Wakui, K.; Fukushima, Y.; Yashiro, M.; Nakamura, Y.; Yanagawa, H.; Kishi, F.; Ouchi, K.; et al. CD40 ligand gene and Kawasaki disease. Eur. J. Hum. Genet. 2004, 12, 1062–1068. [Google Scholar] [CrossRef]
- Jhang, W.K.; Kang, M.-J.; Jin, H.-S.; Yu, J.; Kim, B.-J.; Kim, B.S.; Lee, J.-K.; Seo, E.-J.; Yoo, H.-W.; Park, I.S.; et al. The CCR5 (-2135C/T) polymorphism may be associated with the development of Kawasaki disease in Korean children. J. Clin. Immunol. 2009, 29, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-E.; Kim, J.-J.; Han, M.K.; Lee, K.-Y.; Song, M.S.; Lee, H.-D.; Kim, D.S.; Yu, J.J.; Park, I.-S.; Yun, S.W.; et al. Variations in the number of CCL3L1 gene copies and Kawasaki disease in Korean children. Pediatr. Cardiol. 2012, 33, 1259–1263. [Google Scholar] [CrossRef] [PubMed]
- Praprotnik, S.; Blank, M.; Levy, Y.; Tavor, S.; Boffa, M.C.; Weksler, B.; Eldor, A.; Shoenfeld, Y. Anti-endothelial cell antibodies from patients with thrombotic thrombocytopenic purpura specifically activate small vessel endothelial cells. Int. Immunol. 2001, 13, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.H.; Wang, X.L.; Wilcken, D.E. Nitric oxide induces and inhibits apoptosis through different pathways. FEBS Lett. 1998, 433, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Nicotera, P.; Brune, B.; Bagetta, G. Nitric oxide: Inducer or suppressor of apoptosis? Trends Pharmacol. Sci. 1997, 18, 189–190. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.L. Update of genetic susceptibility in patients with Kawasaki disease. Korean J. Pediatr. 2015, 58, 84–88. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tsuge, M.; Uda, K.; Eitoku, T.; Matsumoto, N.; Yorifuji, T.; Tsukahara, H. Roles of Oxidative Injury and Nitric Oxide System Derangements in Kawasaki Disease Pathogenesis: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 15450. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dhaun, N.; Goddard, J.; Webb, D.J. The endothelin system and its antagonism in chronic kidney disease. J. Am. Soc. Nephrol. 2006, 17, 943–955. [Google Scholar] [CrossRef]
- Di Luozzo, G.; Bhargava, J.; Powell, R.J. Vascular smooth muscle cell effect on endothelial cell endothelin-1 production. J. Vasc. Surg. 2000, 31, 781–789. [Google Scholar] [CrossRef]
- Vallance, P.; Leiper, J. Cardiovascular biology of the asymmetric dimethylarginine: Dimethylarginine dimethylaminohydrolase pathway. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Chen, Z.; Martin, M.; Zhang, J.; Sangwung, P.; Woo, B.; Tremoulet, A.H.; Shimizu, C.; Jain, M.K.; Burns, J.C.; et al. miR-483 Targeting of CTGF Suppresses Endothelial-to-Mesenchymal Transition: Therapeutic Implications in Kawasaki Disease. Circ. Res. 2017, 120, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, C.; Jain, S.; Davila, S.; Hibberd, M.L.; Lin, K.O.; Molkara, D.; Frazer, J.R.; Sun, S.; Baker, A.L.; Newburger, J.W.; et al. Transforming growth factor-beta signaling pathway in patients with Kawasaki disease. Circ. Cardiovasc. Genet. 2011, 4, 16–25, Erratum in Circ. Cardiovasc. Genet. 2011, 4, e9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peng, Q.; Deng, Y.; Yang, X.; Leng, X.; Yang, Y.; Liu, H. Genetic variants of ADAM17 are implicated in the pathological process of Kawasaki disease and secondary coronary artery lesions via the TGF-β/SMAD3 signaling pathway. Eur. J. Pediatr. 2016, 175, 705–713. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, N.; Prattichizzo, F.; Martino, E.; Anastasio, C.; Mele, L.; La Grotta, R.; Sardu, C.; Ceriello, A.; Marfella, R.; Paolisso, G.; et al. MiR-27b attenuates mitochondrial oxidative stress and inflammation in endothelial cells. Redox Biol. 2023, 62, 102681. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rong, X.; Ge, D.; Shen, D.; Chen, X.; Wang, X.; Zhang, L.; Jia, C.; Zeng, J.; He, Y.; Qiu, H.; et al. miR-27b Suppresses Endothelial Cell Proliferation and Migration by Targeting Smad7 in Kawasaki Disease. Cell Physiol. Biochem. 2018, 48, 1804–1814. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.G.; Men, L.N.; Zhao, C.Y.; Zhao, X.; Wang, Y.X.; Meng, X.C.; Shen, D.R.; Meng, B.Y.; Zhang, Q.; Wang, T. The number and function of circulating endothelial progenitor cells in patients with Kawasaki disease. Eur. J. Pediatr. 2010, 169, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Yuan, Y.; Chen, S.; Chen, Y.; Chen, T.X. Plasma Endothelial Microparticles, TNF-a and IL-6 in Kawasaki Disease. Indian Pediatr. 2013, 50, 501–503. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Smith, L.A.; Harrington, S.; Nath, K.A.; Caplice, N.M.; Katusic, Z.S. Transplantation of circulating endothelial progenitor cells restores endothelial function of denuded rabbit carotid arteries. Stroke 2004, 35, 2378–2384. [Google Scholar] [CrossRef]
- Shimizu, C.; Oharaseki, T.; Takahashi, K.; Kottek, A.; Franco, A.; Burns, J.C. The role of TGF-β and myofibroblasts in the arteritis of Kawasaki disease. Hum. Pathol. 2013, 44, 189–198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoder, M.C. Human endothelial progenitor cells. Cold Spring Harb. Perspect. Med. 2012, 2, a006692. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tian, J.; Lv, H.T.; An, X.J.; Ling, N.; Xu, F. Endothelial microparticles induce vascular endothelial cell injury in children with Kawasaki disease. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 1814–1818. [Google Scholar] [PubMed]
- Gkaliagkousi, E.; Corrigall, V.; Becker, S.; de Winter, P.; Shah, A.; Zamboulis, C.; Ritter, J.; Ferro, A. Decreased platelet nitric oxide contributes to increased circulating monocyte-platelet aggregates in hypertension. Eur. Heart J. 2009, 30, 3048–3054. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, K.; Yoshimoto, S.; Asai, O.; Sakan, H.; Terada, M.; Saito, Y.; Nose, M.; Iwano, M.; Konishi, N. Enhanced expression of the soluble form of E-selectin attenuates progression of lupus nephritis and vasculitis in MRL/lpr mice. Immun. Inflamm. Dis. 2013, 1, 37–46. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bordea, M.A.; Costache, C.; Grama, A.; Florian, A.I.; Lupan, I.; Samasca, G.; Deleanu, D.; Makovicky, P.; Makovicky, P.; Rimarova, K. Cytokine cascade in Kawasaki disease versus Kawasaki-like syndrome. Physiol. Res. 2022, 71, 17–27. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakatani, K.; Takeshita, S.; Tsujimoto, H.; Kawamura, Y.; Tokutomi, T.; Sekine, I. Circulating endothelial cells in Kawasaki disease. Clin. Exp. Immunol. 2003, 131, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Yang, H.W.; Lin, T.Y.; Yang, K.D. Perspective of Immunopathogenesis and Immunotherapies for Kawasaki Disease. Front. Pediatr. 2021, 9, 697632. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kone-Paut, I.; Cimaz, R.; Herberg, J.; Bates, O.; Carbasse, A.; Saulnier, J.P.; Maggio, M.C.; Anton, J.; Piram, M. The use of interleukin 1 receptor antagonist (anakinra) in Kawasaki disease: A retrospective cases series. Autoimmun. Rev. 2018, 17, 768–774. [Google Scholar] [CrossRef]
- Millar, J.K.; Salmon, M.; Nasser, E.; Malik, S.; Kolli, P.; Lu, G.; Pinteaux, E.; Hawkins, R.B.; Ailawadi, G. Endothelial to mesenchymal transition in the interleukin-1 pathway during aortic aneurysm formation. J. Thorac. Cardiovasc. Surg. 2024, 167, e146–e158. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, F.C.; Kuo, H.C.; Huang, Y.H.; Yu, H.R.; Li, S.C.; Kuo, H.C. Anti-inflammatory effect of resveratrol in human coronary arterial endothelial cells via induction of autophagy: Implication for the treatment of Kawasaki disease. BMC Pharmacol. Toxicol. 2017, 18, 3. [Google Scholar] [CrossRef]
- D’Onofrio, N.; Sardu, C.; Paolisso, P.; Minicucci, F.; Gragnano, F.; Ferraraccio, F.; Panarese, I.; Scisciola, L.; Mauro, C.; Rizzo, M.R.; et al. MicroRNA-33 and SIRT1 influence the coronary thrombus burden in hyperglycemic STEMI patients. J. Cell Physiol. 2020, 235, 1438–1452. [Google Scholar] [CrossRef] [PubMed]
- Speck, D.; Kleinau, G.; Szczepek, M.; Kwiatkowski, D.; Catar, R.; Philippe, A.; Scheerer, P. Angiotensin and Endothelin Receptor Structures With Implications for Signaling Regulation and Pharmacological Targeting. Front. Endocrinol. 2022, 13, 880002. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goddard, J.; Johnston, N.R.; Hand, M.F.; Cumming, A.D.; Rabelink, T.J.; Rankin, A.J.; Webb, D.J. Endothelin-A receptor antagonism reduces blood pressure and increases renal blood flow in hypertensive patients with chronic renal failure: A comparison of selective and combined endothelin receptor blockade. Circulation 2004, 109, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Dong, J.; Jiang, J.; Yang, F.; Zheng, Y.; Wang, S.; Wang, N.; Ma, J.; Hou, M.; Ding, Y.; et al. The role of FOXO4/NFAT2 signaling pathway in dysfunction of human coronary endothelial cells and inflammatory infiltration of vasculitis in Kawasaki disease. Front. Immunol. 2023, 13, 1090056. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, N.; Wang, Z.; Zhao, M.; Chen, L.; Shi, Z. Protective role of forsythoside B in Kawasaki disease-induced cardiac injury: Inhibition of pyroptosis via the SIRT1-NF-κB-p65 signaling pathway. Chem. Biol. Interact. 2024, 392, 110953. [Google Scholar] [CrossRef]
- Shimizu, C.; Kim, J.; He, M.; Tremoulet, A.H.; Hoffman, H.M.; Shyy, J.Y.; Burns, J.C. RNA Sequencing Reveals Beneficial Effects of Atorvastatin on Endothelial Cells in Acute Kawasaki Disease. J. Am. Heart Assoc. 2022, 11, e025408. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- D’Onofrio, N.; Servillo, L.; Balestrieri, M.L. SIRT1 and SIRT6 Signaling Pathways in Cardiovascular Disease Protection. Antioxid. Redox Signal 2018, 28, 711–732. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nozawa, K.; Fujishiro, M.; Kawasaki, M.; Yamaguchi, A.; Ikeda, K.; Morimoto, S.; Iwabuchi, K.; Yanagida, M.; Ichinose, S.; Morioka, M.; et al. Inhibition of connective tissue growth factor ameliorates disease in a murine model of rheumatoid arthritis. Arthritis Rheum. 2013, 65, 1477–1486. [Google Scholar] [CrossRef]
- Tremoulet, A.H.; Jain, S.; Jone, P.-N.; Best, B.M.; Duxbury, E.H.; Franco, A.; Printz, B.; Dominguez, S.R.; Heizer, H.; Anderson, M.S.; et al. Phase I/IIa Trial of Atorvastatin in Patients with Acute Kawasaki Disease with Coronary Artery Aneurysm. J. Pediatr. 2019, 215, 107–117.e12. [Google Scholar] [CrossRef]
- Huang, S.M.; Weng, K.P.; Chang, J.S.; Lee, W.Y.; Huang, S.H.; Hsieh, K.S. Effects of statin therapy in children complicated with coronary arterial abnormality late after Kawasaki disease: A pilot study. Circ. J. 2008, 72, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paolini, L.; Guida, F.; Calvaruso, A.; Andreozzi, L.; Pierantoni, L.; Lanari, M.; Fabi, M. Endothelial Dysfunction: Molecular Mechanisms and Therapeutic Strategies in Kawasaki Disease. Int. J. Mol. Sci. 2024, 25, 13322. https://doi.org/10.3390/ijms252413322
Paolini L, Guida F, Calvaruso A, Andreozzi L, Pierantoni L, Lanari M, Fabi M. Endothelial Dysfunction: Molecular Mechanisms and Therapeutic Strategies in Kawasaki Disease. International Journal of Molecular Sciences. 2024; 25(24):13322. https://doi.org/10.3390/ijms252413322
Chicago/Turabian StylePaolini, Lucia, Fiorentina Guida, Antonino Calvaruso, Laura Andreozzi, Luca Pierantoni, Marcello Lanari, and Marianna Fabi. 2024. "Endothelial Dysfunction: Molecular Mechanisms and Therapeutic Strategies in Kawasaki Disease" International Journal of Molecular Sciences 25, no. 24: 13322. https://doi.org/10.3390/ijms252413322
APA StylePaolini, L., Guida, F., Calvaruso, A., Andreozzi, L., Pierantoni, L., Lanari, M., & Fabi, M. (2024). Endothelial Dysfunction: Molecular Mechanisms and Therapeutic Strategies in Kawasaki Disease. International Journal of Molecular Sciences, 25(24), 13322. https://doi.org/10.3390/ijms252413322







