Sequence-Specific Free Energy Changes in DNA/RNA Induced by a Single LNA-T Modification in Antisense Oligonucleotides
Abstract
1. Introduction
2. Results
2.1. Two State Transitions of DNA/RNA Duplexes
2.2. Thermodynamic Parameters from Melting Experiments
3. Discussion
3.1. ΔG°, ΔH°, and ΔS° Are More Relevant than Tm to Evaluate the Thermodynamic Stability of Nucleic Acid Double-Strands Containing a Single LNA Residue
= [ΔH°(two LNAs)/{ΔS°(two LNAs) − Rln(4/Ct)}] + [ΔH°(no LNA)/{ΔS°(no LNAs) − Rln(4/Ct)}]
= [(ΔH°(no LNA) + 2a)/{ΔS°(no LNA) +2b − Rln(4/Ct)} + ΔH° (no LNA)/{ΔS° (no LNA) − Rln(4/Ct)}]
2ΔG°37 (single LNA) = ΔG°37 (two LNAs) + ΔG°37 (no LNA),
= {ΔH° (no LNA) + 2a − T(ΔS° (no LNA) + 2b)}+ ΔH° (no LNA) − T(ΔS° (no LNA))
3.2. The Thermodynamic Stabilization Mechanism of LNA Substitution for DNA/RNA Duplexes May Be Sequence-Specific
3.3. The Thermodynamic Stabilization Effect Brought by LNA-T Introduction Should Be Sequence-Dependent
4. Materials and Methods
4.1. Oligonucleotide Synthesis
4.2. Circular Dichroism
4.3. Melting Experiments
4.4. Calculation of the Stabilization Effect of LNA
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Inoue, H.; Hayase, Y.; Imura, A.; Iwai, S.; Miura, K.; Ohtsuka, E. Synthesis and hybridization studies on two complementary nona(2’-O-methyl)ribonucleotides. Nucleic Acids Res. 1987, 15, 6131–6148. [Google Scholar] [CrossRef] [PubMed]
- Freier, S.M.; Altmann, K. The ups and downs of nucleic acid duplex stability: Structure-stability studies on chemically-modified DNA:RNA duplexes. Nucleic Acids Res. 1997, 25, 4429–4443. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, A.M.; Casper, M.D.; Freier, S.M.; Lesnik, E.A.; Zounes, M.C.; Cummins, L.L.; Gonzalez, C.; Cook, P.D. Uniformly modified 2’-deoxy-2’-fluoro phosphorothioate oligonucleotides as nuclease-resistant antisense compounds with high affinity and specificity for RNA targets. J. Med. Chem. 1993, 36, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Obika, S.; Nanbu, D.; Hari, Y.; Morio, K.; In, Y.; Ishida, T.; Imanishi, T. Synthesis of 2’-O,4’-C-methyleneuridine and -cytidine. Novel bicyclic nucleosides having a fixed C3’-endo sugar puckering. Tetrahedron Lett. 1997, 38, 8735–8738. [Google Scholar] [CrossRef]
- Singh, S.K.; Nielsen, P.; Koshkin, A.A.; Wengel, J. LNA (locked nucleic acids): Synthesis and high-affinity nucleic acid recognition. Chem. Commun. 1998, 4, 455–456. [Google Scholar] [CrossRef]
- Morita, K.; Hasegawa, C.; Kaneko, M.; Tsutsumi, S.; Sone, J.; Ishikawa, T.; Imanishi, T.; Koizumi, M. 2’-O,4’-C-ethylene-bridged nucleic acids (ENA): Highly nuclease-resistant and thermodynamically stable oligonucleotides for antisense drug. Bioorg Med. Chem. Lett. 2002, 12, 73–76. [Google Scholar] [CrossRef]
- Kurreck, J.; Wyszko, E.; Gillen, C.; Erdmann, V.A. Design of antisense oligonucleotides stabilized by locked nucleic acids. Nucleic Acids Res. 2002, 30, 1911–1918. [Google Scholar] [CrossRef]
- Bondensgaard, K.; Petersen, M.; Singh, S.K.; Rajwanshi, V.K.; Kumar, R.; Wengel, J.; Jacobsen, J.P. Structural studies of LNA:RNA duplexes by NMR: Conformations and implications for RNase H activity. Chem. Eur. J. 2000, 6, 2687–2695. [Google Scholar] [CrossRef]
- Dauksaite, V.; Tas, A.; Wachowius, F.; Spruit, A.; van Hemert, M.J.; Snijder, E.J.; van der Veer, E.P.; van Zonneveld, A.J. Highly potent antisense oligonucleotides locked nucleic acid gapmers targeting the SARS-CoV-2 RNA genome. Nucleic Acid. Ther. 2023, 6, 381–385. [Google Scholar] [CrossRef]
- Hu, J.; Gong, X.; Fan, Y.; Aguilar, S.; Rigo, F.; Prakash, T.P.; Corey, D.R.; Mootha, V.V. Modulation of gene expression in the eye with antisense oligonucleotides. Nucleic Acid. Ther. 2023, 6, 339–347. [Google Scholar] [CrossRef]
- Mata-Ventosa, A.; Vila-Planas, A.; Solsona-Pujol, A.; Dueña, J.; Torrents, M.; Izquierdo-García, E.; Pastor-Anglada, M.; Pérez-Torras, S.; Terrazas, M. RNase H-sensitive multifunctional ASO-based constructs as promising tools for the treatment of multifactorial complex pathologies. Bioorg Chem. 2024, 150, 107595. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Maeta, K.; Suzuki, H.; Kurosawa, R.; Takenouchi, T.; Awaya, T.; Ajiro, M.; Takeuchi, A.; Nishio, H.; Hagiwara, M.; et al. Successful skipping of abnormal pseudoexon by antisense oligonucleotides in vitro for a patient with beta-propeller protein-associated neurodegeneration. Sci. Rep. 2024, 14, 6506. [Google Scholar] [CrossRef] [PubMed]
- Engelbeen, S.; O’Reilly, D.; Van De Vijver, D.; Verhaart, I.; van Putten, M.; Hariharan, V.; Hassler, M.; Khvorova, A.; Damha, M.J.; Aartsma-Rus, A. Challenges of assessing exon 53 skipping of the human DMD transcript with locked nucleic acid-modified antisense oligonucleotides in a mouse model for Duchenne muscular dystrophy. Nucleic Acid. Ther. 2023, 33, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Grainok, J.; Pitout, I.L.; Chen, F.K.; McLenachan, S.; Heath Jeffery, R.C.; Mitrpant, C.; Fletcher, S. A precision therapy approach for retinitis pigmentosa 11 using splice-switching antisense oligonucleotides to restore the open reading frame of PRPF31. Int. J. Mol. Sci. 2024, 25, 3391. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Fujii, N.; Yasuhara, H.; Wada, S.; Wada, F.; Shigesada, N.; Harada-Shiba, M.; Obika, S. Evaluation of multiple-turnover capability of locked nucleic acid antisense oligonucleotides in cell-free RNase H-mediated antisense reaction and in mice. Nucleic Acid. Ther. 2014, 24, 283–290. [Google Scholar] [CrossRef]
- Pedersen, L.; Hagedorn, P.H.; Lindholm, M.W.; Lindow, M. A kinetic model explains why shorter and less affine enzyme-recruiting oligonucleotides can be more potent. Mol. Ther. Nucleic Acids 2014, 3, e139. [Google Scholar] [CrossRef]
- Shimo, T.; Tachibana, K.; Saito, K.; Yoshida, T.; Tomita, E.; Waki, R.; Yamamoto, T.; Doi, T.; Inoue, T.; Kawakami, J.; et al. Design and evaluation of locked nucleic acid-based splice-switching oligonucleotides in vitro. Nucleic Acids Res. 2014, 42, 8174–8187. [Google Scholar] [CrossRef]
- Breslauer, K.J.; Frank, R.; Blöcker, H.; Marky, L.A. Predicting DNA duplex stability from the base sequence. Proc. Natl. Acad. Sci. USA 1986, 83, 3746–3750. [Google Scholar] [CrossRef]
- Freier, S.M.; Kierzek, R.; Jaeger, J.A.; Sugimoto, N.; Caruthers, M.H.; Neilson, T.; Turner, D.H. Improved free-energy parameters for predictions of RNA duplex stability. Proc. Natl. Acad. Sci. USA 1986, 83, 9373–9377. [Google Scholar] [CrossRef]
- Sugimoto, N.; Nakano, S.; Katoh, M.; Matsumura, A.; Nakamuta, H.; Ohmichi, T.; Yoneyama, M.; Sasaki, M. Thermodynamic parameters to predict stability of RNA/DNA hybrid duplexes. Biochemistry 1995, 34, 11211–11216. [Google Scholar] [CrossRef]
- McTigue, P.M.; Peterson, R.J.; Kahn, J.D. Sequence-dependent thermodynamic parameters for locked nucleic acid (LNA)-DNA duplex formation. Biochemistry 2004, 43, 5388–5405. [Google Scholar] [CrossRef] [PubMed]
- Kierzek, E.; Ciesielska, A.; Pasternak, K.; Mathews, D.H.; Turner, D.H.; Kierzek, R. The influence of locked nucleic acid residues on the thermodynamic properties of 2’-O-methyl RNA/RNA heteroduplexes. Nucleic Acids Res. 2005, 33, 5082–5093. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Wengel, J.; Maiti, S. Thermodynamics of DNA-RNA heteroduplex formation: Effect of locked nucleic acid nucleotides incorporated into the DNA strand. Biochemistry 2008, 47, 1218–1227. [Google Scholar] [CrossRef]
- Kaur, H.; Arora, A.; Wengel, J.; Maiti, S. Thermodynamic, counterion, and hydration effects for the incorporation of locked nucleic acid nucleotides into DNA duplexes. Biochemistry 2006, 45, 7347–7355. [Google Scholar] [CrossRef] [PubMed]
- Owczarzy, R.; You, Y.; Groth, C.L.; Tataurov, A.V. Stability and mismatch discrimination of locked nucleic acid-DNA duplexes. Biochemistry 2011, 50, 9352–9367. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, J.; Maiti, S.; Muhuri, S.; Nakano, S.; Miyoshi, D.; Sugimoto, N. Effect of locked nucleic acid modification on the thermal stability of noncanonical DNA structure. Biochemistry 2011, 50, 7414–7425. [Google Scholar] [CrossRef]
- Hughesman, C.B.; Turner, R.F.B.; Haynes, C.A. Role of the heat capacity change in understanding and modeling melting thermodynamics of complementary duplexes containing standard and nucleobase-modified LNA. Biochemistry 2011, 50, 5354–5368. [Google Scholar] [CrossRef]
- Koshkin, A.A.; Poul, N.; Meldgaard, M.; Rajwanshi, V.K.; Singh, S.K.; Wengel, J. LNA (locked nucleic acid): An RNA mimic forming exceedingly stable LNA:LNA duplexes. J. Am. Chem. Soc. 1998, 120, 13252–13253. [Google Scholar] [CrossRef]
- Christensen, U.; Jacovsen, N.; Rajwanshi, V.K.; Wengel, J.; Koch, T. Stopped-flow kinetics of locked nucleic acid (LNA)-oligonucleotide duplex formation: Studies of LNA-DNA and DNA-DNA interactions. Biochem. J. 2001, 354, 481–484. [Google Scholar] [CrossRef][Green Version]
- Bruylants, G.; Boccongelli, M.; Snoussi, K.; Bartik, K. Comparison of the thermodynamics and base-pair dynamics of a full LNA:DNA duplex and of the isosequential DNA:DNA duplex. Biochemistry 2009, 48, 8473–8482. [Google Scholar] [CrossRef][Green Version]
- Fakhfakf, K.; Marais, O.; Cheng, X.B.J.; Castañeda, J.R.; Hughesman, C.B.; Haynes, C. Molecular thermodynamics of LNA:LNA base pairs and the hyperstabilizing effect of 5’-proximal LNA:DNA base pairs. AIChE J. 2015, 61, 2711–2731. [Google Scholar] [CrossRef]
- Kumar, S.; Mapa, K.; Maiti, S. Understanding the effect of locked nucleic acid and 2’-O-methyl modification on the hybridization thermodynamics of a miRNA-mRNA pair in the presence and absence of AfPiwi protein. Biochemistry 2014, 53, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Kierzek, E.; Pasternak, A.; Pasternak, K.; Gdaniec, Z.; Yildirim, I.; Turner, D.H.; Kierzek, R. Contributions of stacking, preorganization, and hydrogen bonding to the thermodynamic stability of duplexes between RNA and 2’-O-methyl RNA with locked nucleic acids. Biochemistry 2009, 48, 4377–4387. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Nguyen, M.; Overacre, A.; Seaton, S.; Schroeder, S.J. Effects of salt, polyethylene glycol, and locked nucleic acids on the thermodynamic stabilities of consecutive terminal adenosine mismatches in RNA duplexes. J. Phys. Chem. 2013, 117, 3531–3540. [Google Scholar] [CrossRef]
- Petersen, M.; Bondensgaard, K.; Wengel, J.; Jacobsen, J.P. Locked Nucleic Acid (LNA) Recognition of RNA: NMR Solution Structures of LNA:RNA Hybrids. J. Am. Chem. Soc. 2002, 124, 5974–5982. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Tateishi-Karimata, H.; Ohyama, T.; Ghosh, S.; Endoh, T.; Takahashi, S.; Sugimoto, N. Improved nearest-neighbor parameters for the stability of RNA/DNA hybrids under a physiological condition. Nucleic Acids Res. 2020, 48, 12042–12054. [Google Scholar] [CrossRef]
- Nakano, S.; Fujimoto, M.; Hara, H.; Sugimoto, N. Nucleic acid duplex stability: Influence of base composition on cation effects. Nucleic Acids Res. 1999, 27, 2957–2965. [Google Scholar] [CrossRef]
- Cantor, C.R.; Warshaw, M.M.; Shapiro, H. Oligonucleotide interactions. 3. Circular dichroism studies of the conformation of deoxyoligonucleotides. Biopolymers 1970, 9, 1059–1077. [Google Scholar] [CrossRef]

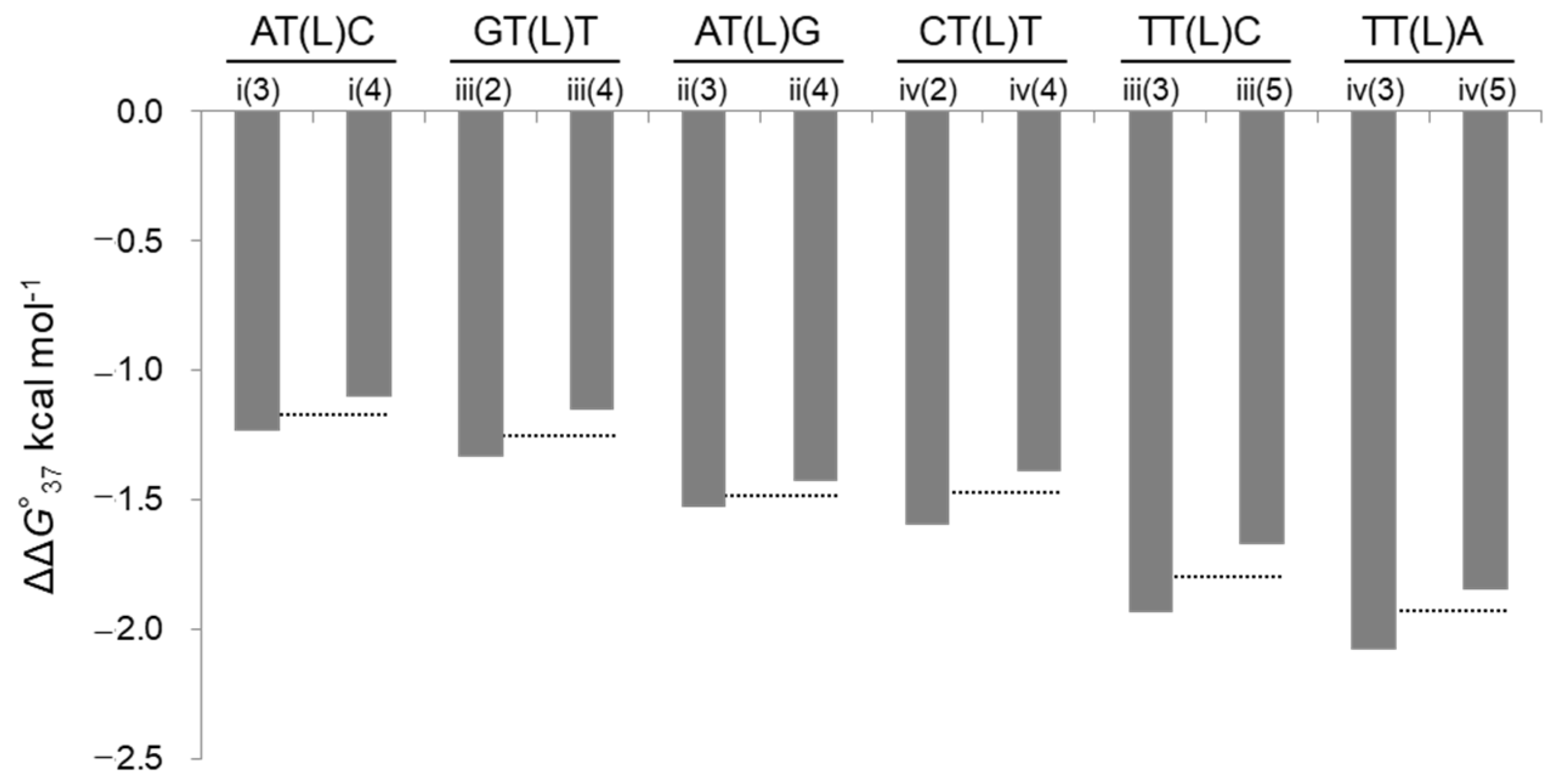
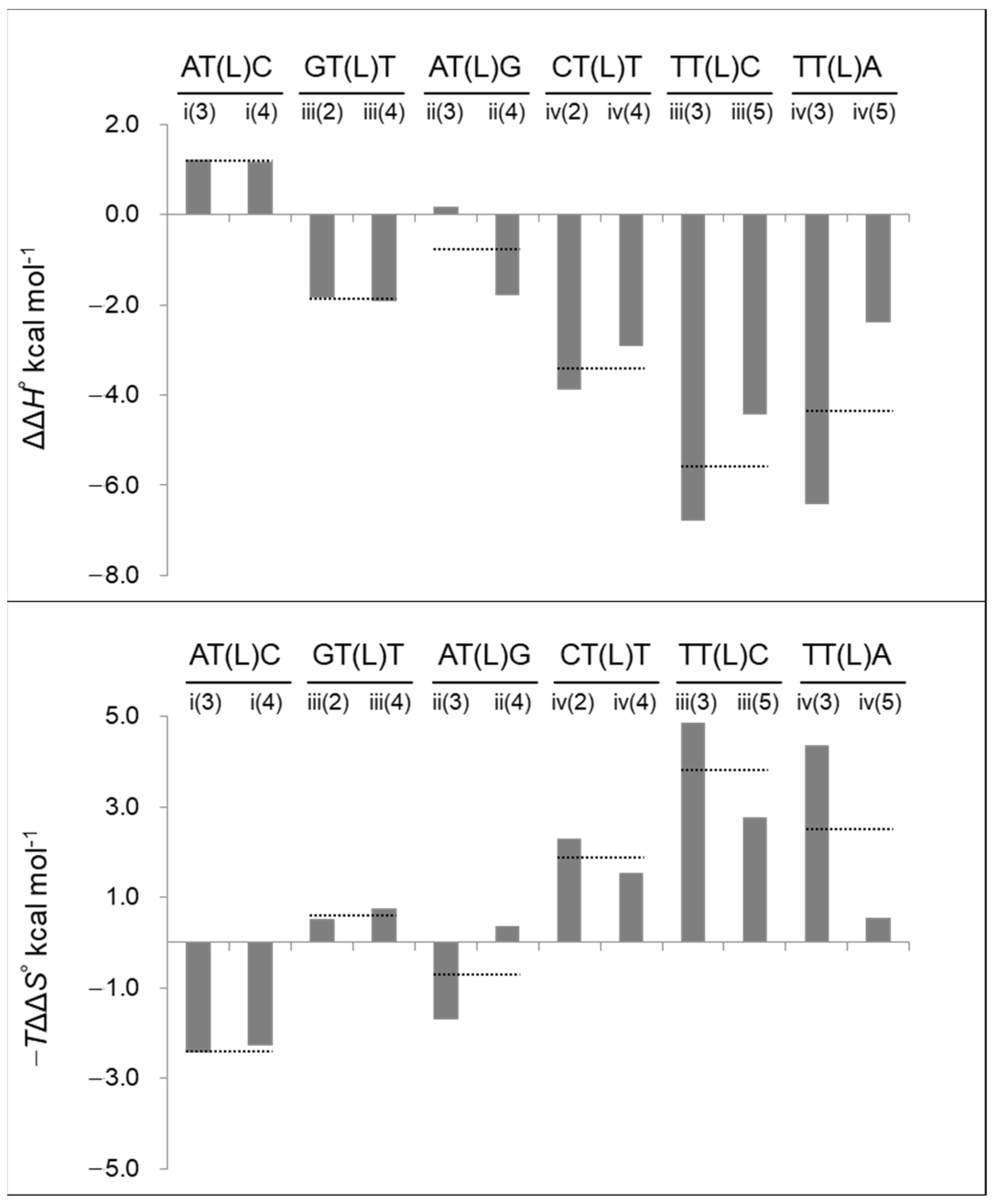

| Oligo Name | Triplet Including LNA | ΔG°37 | ΔH° | ΔS° | −TΔS° * | Tm ** | ΔΔG°37 | ΔΔH° | −TΔΔS° * | ΔTm ** |
|---|---|---|---|---|---|---|---|---|---|---|
| (kcal mol−1) | (kcal mol−1) | (cal mol−1 K−1) | (kcal mol−1) | (°C) | (kcal mol−1) | (kcal mol−1) | (kcal mol−1) | (°C) | ||
| i(DNA) | −8.5 | −85.2 | −247.5 | 76.8 | 41.4 | |||||
| i(3) | AT(L)C | −9.7 | −84.0 | −239.6 | 74.3 | 46.4 | −1.2 | 1.2 | −2.4 | 4.9 |
| i(4) | AT(L)C | −9.6 | −84.1 | −240.2 | 74.5 | 46.1 | −1.1 | 1.2 | −2.3 | 4.6 |
| ii(DNA) | −7.3 | −77.7 | −227.0 | 70.4 | 37.2 | |||||
| ii(3) | AT(L)G | −8.9 | −77.6 | −221.5 | 68.7 | 43.3 | −1.5 | 0.2 | −1.7 | 6.1 |
| ii(4) | AT(L)G | −8.8 | −79.5 | −228.2 | 70.8 | 42.8 | −1.4 | −1.8 | 0.4 | 5.6 |
| iii(DNA) | −12.1 | −89.9 | −250.8 | 77.8 | 54.7 | |||||
| iii(2) | GT(L)T | −13.5 | −91.8 | −252.4 | 78.3 | 59.3 | −1.3 | −1.9 | 0.5 | 4.7 |
| iii(3) | TT(L)C | −14.1 | −96.7 | −266.4 | 82.6 | 60.4 | −1.9 | −6.8 | 4.8 | 5.7 |
| iii(4) | GT(L)T | −13.3 | −91.8 | −253.2 | 78.5 | 58.6 | −1.2 | −1.9 | 0.8 | 3.9 |
| iii(5) | TT(L)C | −13.8 | −94.3 | −259.6 | 80.5 | 60.0 | −1.7 | −4.4 | 2.8 | 5.3 |
| iv(DNA) | −8.4 | −88.6 | −258.7 | 80.2 | 40.7 | |||||
| iv(2) | CT(L)T | −10.0 | −92.5 | −266.1 | 82.5 | 46.3 | −1.6 | −3.9 | 2.3 | 5.5 |
| iv(3) | TT(L)A | −10.5 | −95.1 | −272.7 | 84.6 | 47.6 | −2.1 | −6.4 | 4.4 | 6.9 |
| iv(4) | CT(L)T | −9.8 | −91.6 | −263.6 | 81.8 | 45.7 | −1.4 | −2.9 | 1.5 | 4.9 |
| iv(5) | TT(L)A | −10.2 | −91.0 | −260.5 | 80.8 | 47.2 | −1.8 | −2.4 | 0.5 | 6.4 |
| Oligo Name | Sequence (5′ to 3′) |
|---|---|
| i(RNA) | AAUGAUGAUGAA |
| i(DNA) | TTCATCATCATT |
| i(3) | TTCAT(L)CATCATT |
| i(4) | TTCATCAT(L)CATT |
| ii(RNA) | AAUCAUCAUCAA |
| ii(DNA) | TTGATGATGATT |
| ii(3) | TTGAT(L)GATGATT |
| ii(4) | TTGATGAT(L)GATT |
| iii(RNA) | ACGAACGAACGA |
| iii(DNA) | TCGTTCGTTCGT |
| iii(2) | TCGT(L)TCGTTCGT |
| iii(3) | TCGTT(L)CGTTCGT |
| iii(4) | TCGTTCGT(L)TCGT |
| iii(5) | TCGTTCGTT(L)CGT |
| iv(RNA) | AGUAAGUAAGUA |
| iv(DNA) | TACTTACTTACT |
| iv(2) | TACT(L)TACTTACT |
| iv(3) | TACTT(L)ACTTACT |
| iv(4) | TACTTACT(L)TACT |
| iv(5) | TACTTACTT(L)ACT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomita-Sudo, E.; Akita, T.; Sakimoto, N.; Tahara-Takamine, S.; Kawakami, J. Sequence-Specific Free Energy Changes in DNA/RNA Induced by a Single LNA-T Modification in Antisense Oligonucleotides. Int. J. Mol. Sci. 2024, 25, 13240. https://doi.org/10.3390/ijms252413240
Tomita-Sudo E, Akita T, Sakimoto N, Tahara-Takamine S, Kawakami J. Sequence-Specific Free Energy Changes in DNA/RNA Induced by a Single LNA-T Modification in Antisense Oligonucleotides. International Journal of Molecular Sciences. 2024; 25(24):13240. https://doi.org/10.3390/ijms252413240
Chicago/Turabian StyleTomita-Sudo, Elisa, Tomoka Akita, Nae Sakimoto, Saori Tahara-Takamine, and Junji Kawakami. 2024. "Sequence-Specific Free Energy Changes in DNA/RNA Induced by a Single LNA-T Modification in Antisense Oligonucleotides" International Journal of Molecular Sciences 25, no. 24: 13240. https://doi.org/10.3390/ijms252413240
APA StyleTomita-Sudo, E., Akita, T., Sakimoto, N., Tahara-Takamine, S., & Kawakami, J. (2024). Sequence-Specific Free Energy Changes in DNA/RNA Induced by a Single LNA-T Modification in Antisense Oligonucleotides. International Journal of Molecular Sciences, 25(24), 13240. https://doi.org/10.3390/ijms252413240





