Long-Term Yo-Yo Dieting Exaggerates Liver Steatosis and Lesions but Preserves Muscle Performance in Male Zebrafish †
Abstract
1. Introduction
2. Results
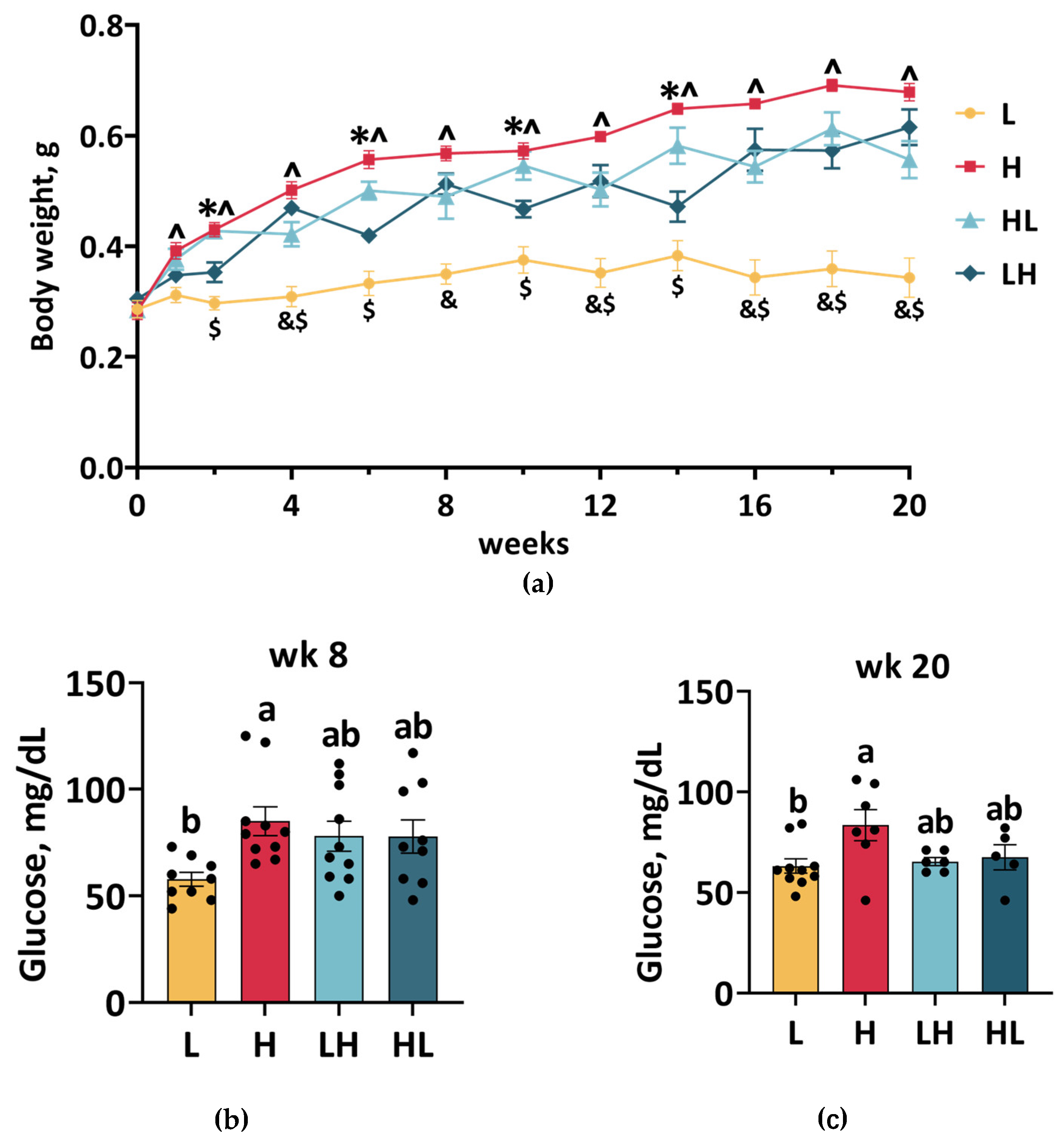
2.1. Dietary Switch Caused Weight Cycling Without Affecting Blood Glucose Levels
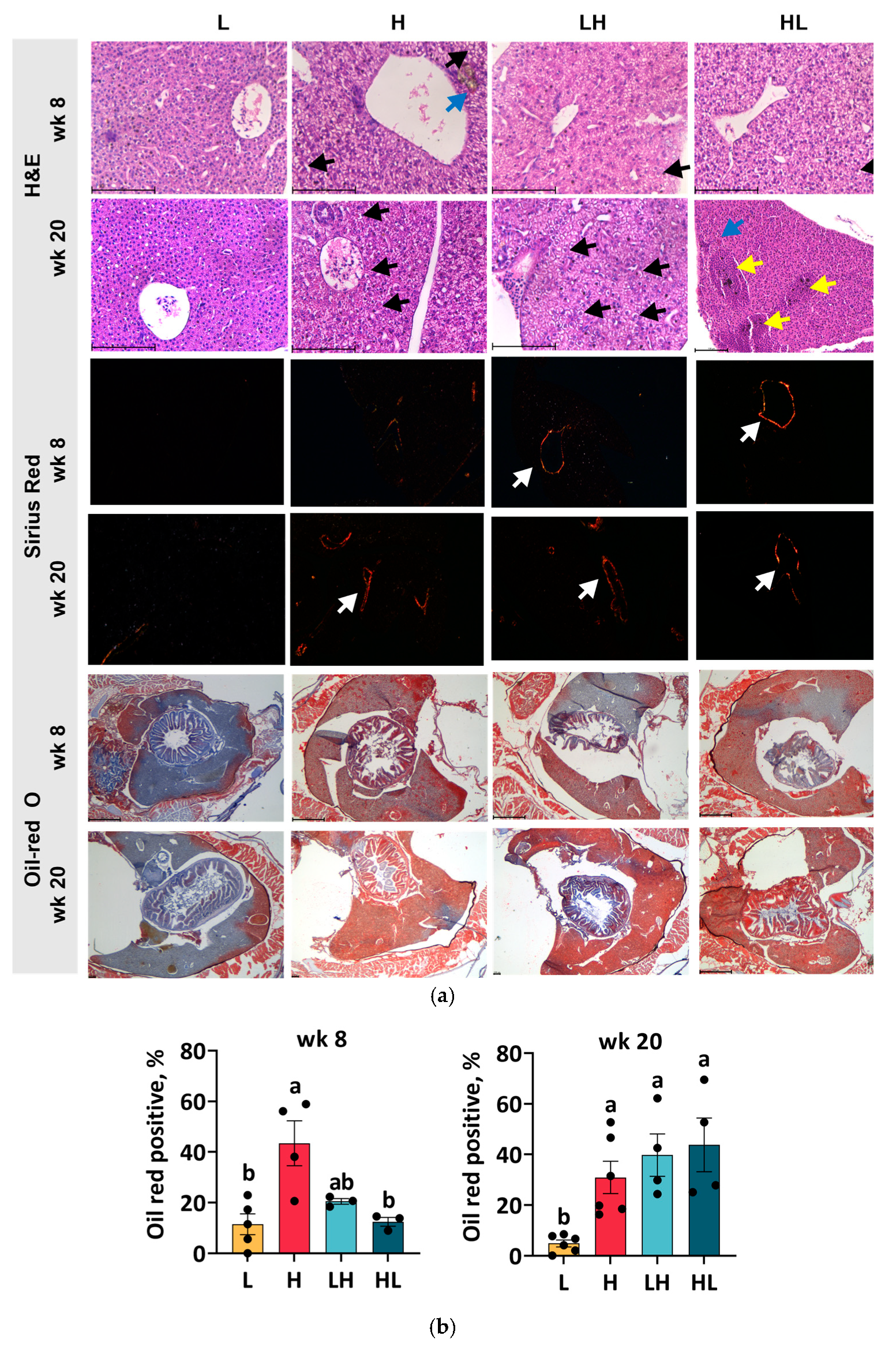
2.2. Yo-Yo Dieting Caused Steatosis and Lesions in the Liver
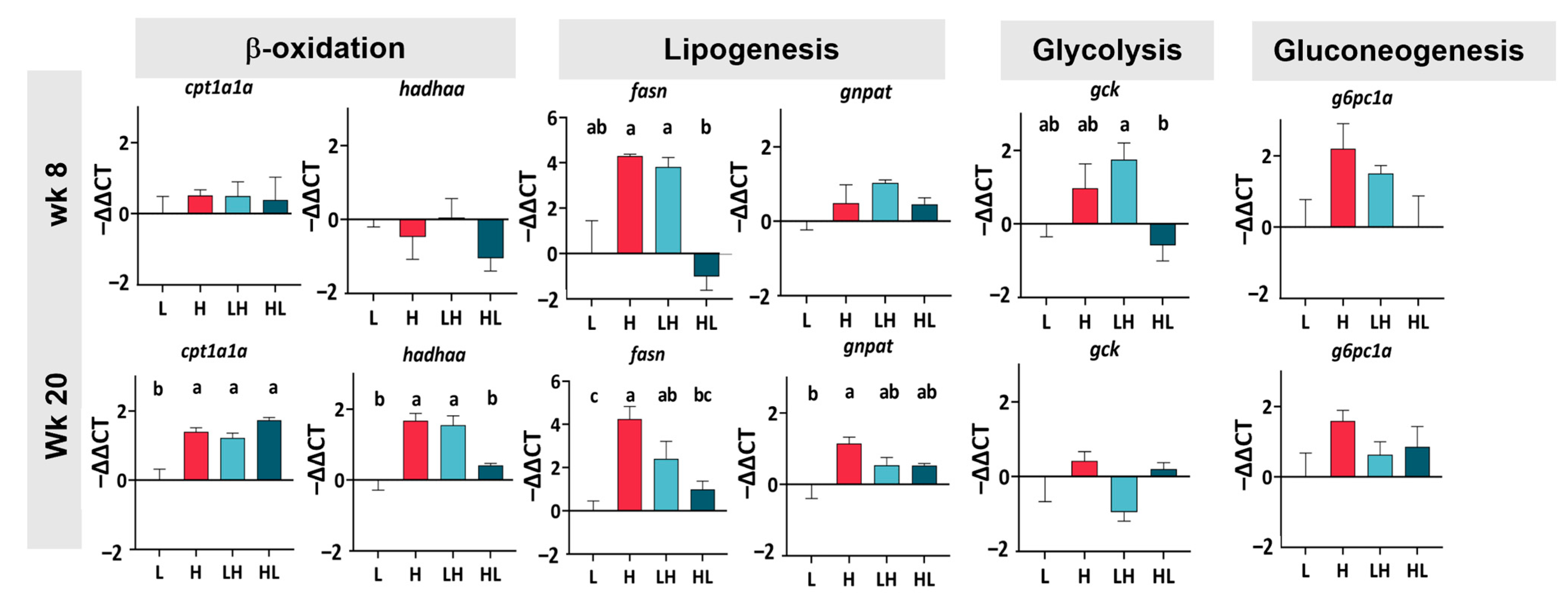
2.3. Long-Term Yo-Yo Dieting Changed the Gene Expression of Lipid Metabolism in the Liver
2.4. Long-Term Yo-Yo Dieting Strengthened Muscle Performance
2.5. Yo-Yo Dieting Ending in a High-Calorie Phase Induced Muscular Hypertrophy
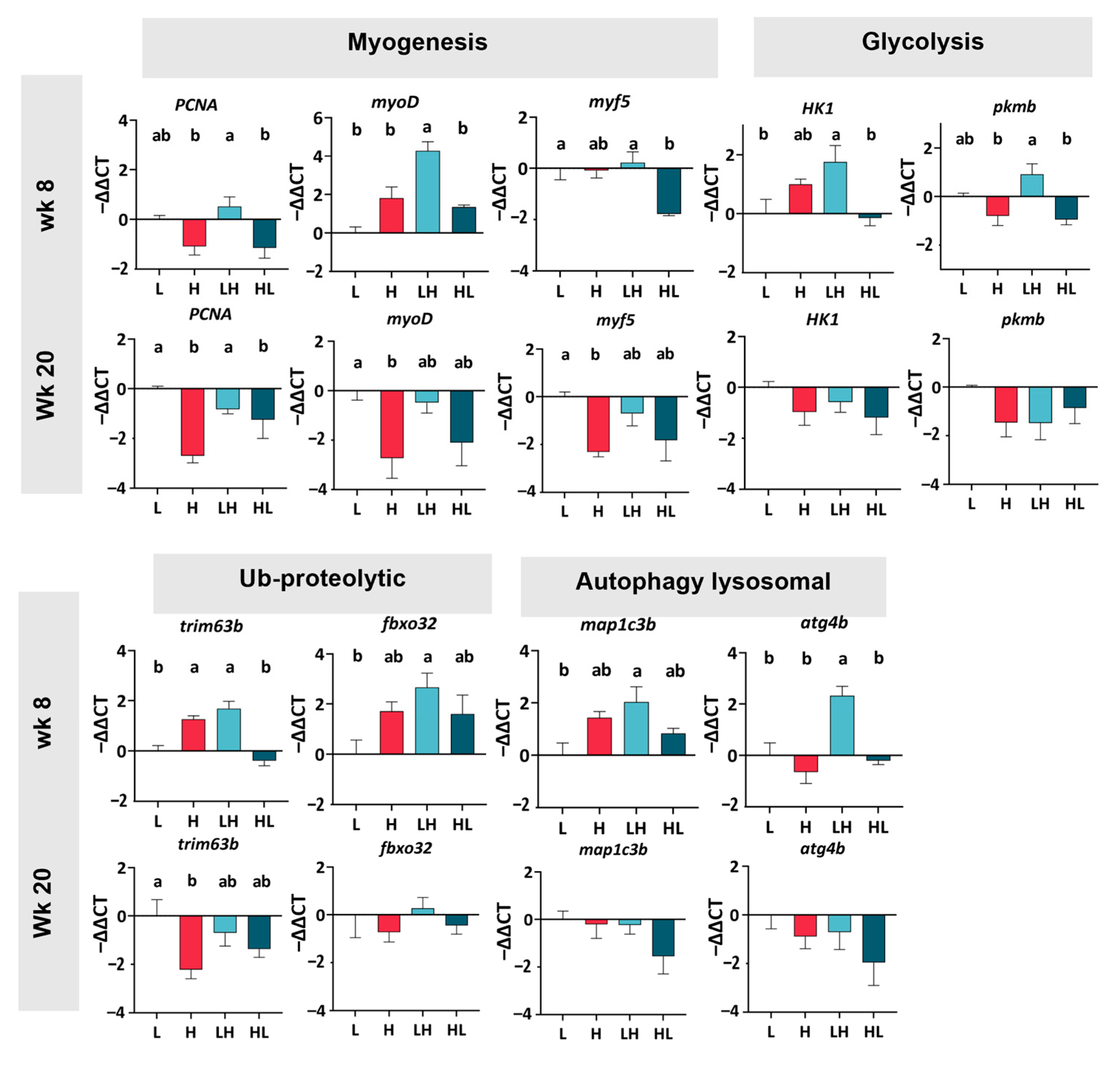
2.6. The Effect of Yo-Yo Dieting on Gene Expression in Fast-Twitch Muscles
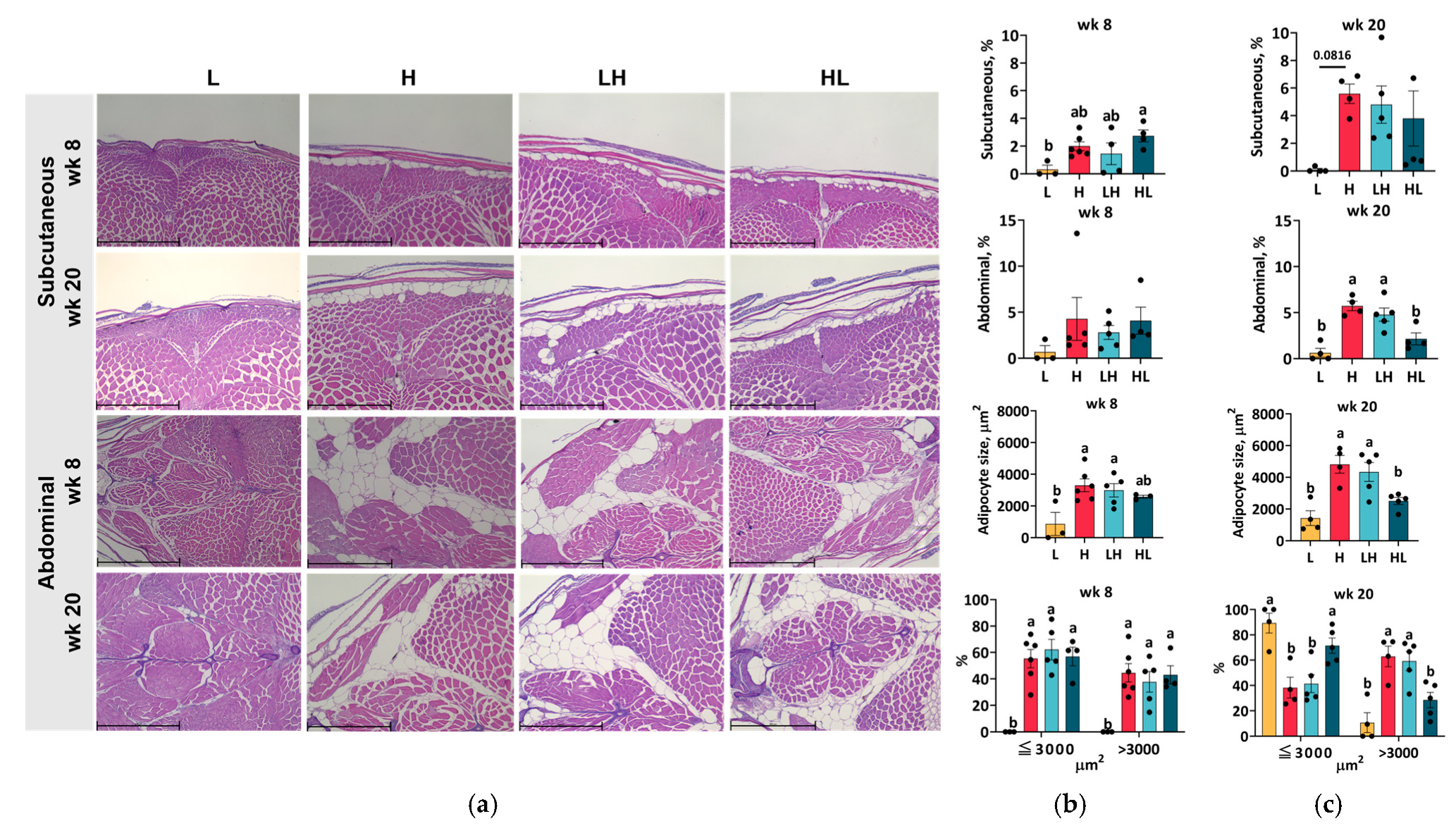
2.7. Yo-Yo Dieting Ending in a High-Calorie Phase Increased Abdominal Adipose Tissue
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Yo-Yo Dieting Model Establishment
4.3. Biochemical Analysis
4.4. Histology
4.5. Swimming Test
4.5.1. Burst Swimming Test
4.5.2. Ramp-Ucrit Swimming Test
4.6. RNA Extraction and RT-PCR Analysis
4.7. Statistics Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colditz, G.A.; Willett, W.C.; Rotnitzky, A.; Manson, J.E. Weight-gain as a risk factor for clinical diabetes-mellitus in women. Ann. Intern. Med. 1995, 122, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Hubert, H.B.; Feinleib, M.; Mcnamara, P.M.; Castelli, W.P. Obesity as an independent risk factor for cardiovascular-disease—A 26-year follow-up of participants in the Framingham heart study. Circulation 1983, 67, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, A.; Nystrom, M.; Noworolski, S.M.; Chu, P.; Mohanty, A.; Merriman, R. MRI steatosis grading: Development and initial validation of a color mapping system. Am. J. Roentgenol. 2012, 198, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.C.; Galuska, D.A.; Kettel Khan, L.; Gillespie, C.; Serdula, M.K. Weight regain in U.S. adults who experienced substantial weight loss, 1999–2002. Am. J. Prev. Med. 2007, 33, 34–40. [Google Scholar] [CrossRef]
- Wing, R.R.; Phelan, S. Long-term weight loss maintenance. Am. J. Clin. Nutr. 2005, 82 (Suppl. 1), 222S–225S. [Google Scholar] [CrossRef]
- Dulloo, A.G. Physiology of weight regain: Lessons from the classic Minnesota Starvation Experiment on human body composition regulation. Obes. Rev. 2021, 22, e13189. [Google Scholar] [CrossRef]
- Rossi, A.P.; Rubele, S.; Calugi, S.; Caliari, C.; Pedelini, F.; Soave, F.; Chignola, E.; Bazzani, P.V.; Mazzali, G.; Dalle Grave, R.; et al. Weight cycling as a risk factor for low muscle mass and strength in a population of males and females with obesity. Obesity 2019, 27, 1068–1075. [Google Scholar] [CrossRef]
- Cho, E.J.; Yu, S.J.; Jung, G.C.; Kwak, M.S.; Yang, J.I.; Yim, J.Y.; Chung, G.E. Body weight gain rather than body weight variability associated with increased risk of nonalcoholic fatty liver disease. Sci. Rep. 2021, 11, 14428. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Yi, J.Y.; Guo, J.J.; Ren, X.P. Weigh change across adulthood is related to the presence of NAFLD: Results from NHANES III. J. Transl. Med. 2023, 21, 142. [Google Scholar] [CrossRef]
- Chiang, C.H.; Zhang, T.R.; Hsu, P.S.; Lin, S.P.; Chen, C.Y. Weight regain, but not weight loss exacerbates hepatic fibrosis during multiple weight cycling events in male mice. Eur. J. Nutr. 2024, 63, 965–976. [Google Scholar] [CrossRef]
- Barbosa-da-Silva, S.; da Silva, N.C.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Liver damage is not reversed during the lean period in diet-induced weight cycling in mice. Hepatol. Res. 2014, 44, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Mackie, G.M.; Samocha-Bonet, D.; Tam, C.S. Does weight cycling promote obesity and metabolic risk factors? Obes. Res. Clin. Pract. 2017, 11, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.; Koo, D.J.; Lee, M.Y.; Moon, S.J.; Kwon, H.; Park, S.E.; Rhee, E.J.; Lee, W.Y. Increased risk of nonalcoholic fatty liver disease in individuals with high weight variability. Endocrinol. Metab. 2021, 36, 845–854. [Google Scholar] [CrossRef]
- Lahti-Koski, M.; Männistö, S.; Pietinen, P.; Vartiainen, E. Prevalence of weight cycling and its relation to health indicators in Finland. Obes. Res. 2005, 13, 333–341. [Google Scholar] [CrossRef]
- Stevens, V.L.; Jacobs, E.J.; Sun, J.Z.; Patel, A.V.; McCullough, M.L.; Teras, L.R.; Gapstur, S.M. Weight cycling and mortality in a large prospective US study. Am. J. Epidemiol. 2012, 175, 785–792. [Google Scholar] [CrossRef]
- Barbosa-da-Silva, S.; Fraulob-Aquino, J.C.; Lopes, J.R.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Weight cycling enhances adipose tissue inflammatory responses in male mice. PLoS ONE 2012, 7, e39837. [Google Scholar] [CrossRef] [PubMed]
- Gut, P.; Reischauer, S.; Stainier, D.Y.R.; Arnaout, R. Little fish, big data: Zebrafish as a model for cardiovascular and metabolic disease. Physiol. Rev. 2017, 97, 889–938. [Google Scholar] [CrossRef]
- Hsu, H.C.; Chiang, C.H.; Liu, I.H.; Chen, C.Y. The distinct effect of yoyo dieting on liver and muscle performance in a male zebrafish model. In Proceedings of the 58th Annual Scientific Meeting of the European Society of Clinical Investigation, Barcelona, Spain, 5–7 June 2024. [Google Scholar]
- Tallis, J.; James, R.S.; Seebacher, F. The effects of obesity on skeletal muscle contractile function. J. Exp. Biol. 2018, 221 Pt 13, jeb163840. [Google Scholar] [CrossRef]
- Aksoy, Y.A.; Nguyen, D.T.; Chow, S.; Chung, R.S.; Guillemin, G.J.; Cole, N.J.; Hesselson, D. Chemical reprogramming enhances homology-directed genome editing in zebrafish embryos. Commun. Biol. 2019, 2, 198. [Google Scholar] [CrossRef]
- McKenzie, D. The Energetics of Fish Swimming; Elsevier Inc.: Amsterdam, The Netherlands, 2011; pp. 1075–1080. [Google Scholar]
- Hickey, M.S.; Carey, J.O.; Azevedo, J.L.; Houmard, J.A.; Pories, W.J.; Israel, R.G.; Dohm, G.L. Skeletal-muscle fiber composition is related to adiposity and in-vitro glucose-transport rate in humans. Am. J. Physiol.-Endoc. Metab. 1995, 268, E453–E457. [Google Scholar] [CrossRef]
- Tanner, C.J.; Barakat, H.A.; Dohm, G.L.; Pories, W.J.; MacDonald, K.G.; Cunningham, P.R.G.; Swanson, M.S.; Houmard, J.A. Muscle fiber type is associated with obesity and weight loss. Am. J. Physiol.-Endoc. Metab. 2002, 282, E1191–E1196. [Google Scholar] [CrossRef] [PubMed]
- Devoto, S.H.; Melancon, E.; Eisen, J.S.; Westerfield, M. Identification of separate slow and fast muscle precursor cells in vivo, prior to somite formation. Development 1996, 122, 3371–3380. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, D.J.; Erskine, R.M.; Morse, C.I.; Winwood, K.; Onambélé-Pearson, G. The impact of obesity on skeletal muscle strength and structure through adolescence to old age. Biogerontology 2016, 17, 467–483. [Google Scholar] [CrossRef] [PubMed]
- Oppert, J.M.; Ciangura, C.; Bellicha, A. Physical activity and exercise for weight loss and maintenance in people living with obesity. Rev. Endocr. Metab. Dis. 2023, 24, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Poggiogalle, E.; Rossignon, F.; Carayon, A.; Capel, F.; Rigaudière, J.P.; De Saint Vincent, S.; Le-Bacquer, O.; Salles, J.; Giraudet, C.; Patrac, V.; et al. Deleterious effect of high-fat diet on skeletal muscle performance is prevented by high-protein intake in adult rats but not in old Rats. Front. Physiol. 2022, 12, 749049. [Google Scholar] [CrossRef]
- Byrne, N.M.; Weinsier, R.L.; Hunter, G.R.; Desmond, R.; Patterson, M.A.; Darnell, B.E.; Zuckerman, P.A. Influence of distribution of lean body mass on resting metabolic rate after weight loss and weight regain: Comparison of responses in white and black women. Am. J. Clin. Nutr. 2003, 77, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Bosy-Westphal, A.; Schautz, B.; Lagerpusch, M.; Pourhassan, M.; Braun, W.; Goele, K.; Heller, M.; Gluer, C.C.; Muller, M.J. Effect of weight loss and regain on adipose tissue distribution, composition of lean mass and resting energy expenditure in young overweight and obese adults. Int. J. Obes. 2013, 37, 1371–1377. [Google Scholar] [CrossRef]
- El Ghoch, M.; Calugi, S.; Dalle Grave, R. Weight cycling in adults with severe obesity: A longitudinal study. Nutr. Diet. 2018, 75, 256–262. [Google Scholar] [CrossRef]
- Sugasawa, T.; Komine, R.; Manevich, L.; Tamai, S.; Takekoshi, K.; Kanki, Y. Gene expression profile provides novel insights of fasting-refeeding response in zebrafish skeletal muscle. Nutrients 2022, 14, 2239. [Google Scholar] [CrossRef]
- da Silva-Gomes, R.N.; Gabriel Kuniyoshi, M.L.; Oliveira da Silva Duran, B.; Thomazini Zanella, B.T.; Paccielli Freire, P.; Gutierrez de Paula, T.; de Almeida Fantinatti, B.E.; Simoes Salomao, R.A.; Carvalho, R.F.; Delazari Santos, L.; et al. Prolonged fasting followed by refeeding modifies proteome profile and parvalbumin expression in the fast-twitch muscle of pacu (Piaractus mesopotamicus). PLoS ONE 2019, 14, e0225864. [Google Scholar] [CrossRef]
- Shimizu, N.; Shiraishi, H.; Hanada, T. Zebrafish as a Useful Model System for Human Liver Disease. Cells 2023, 12, 2246. [Google Scholar] [CrossRef] [PubMed]
- Christian, C.J.; Benian, G.M. Animal models of sarcopenia. Aging Cell 2020, 19, e13223. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, W.; Yang, G.; Zhu, L.; Liu, X. The Impact of Weight Cycling on Health and Obesity. Metabolites 2024, 14, 344. [Google Scholar] [CrossRef] [PubMed]
- Zeigler, Z.S.; Birchfield, N.; Moreno, K.; James, D.; Swan, P. Fatness and fluctuating body weight: Effect on central vasculature. BioRes. Open Access 2018, 7, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Wsterfield, M. The Zebrafish Book; A Guide for the Laboratory Use of Zebrafish (Danio rerio), 4th ed.; University of Oregon Press: Eugene, OR, USA, 2000. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Jian, Q.L.; HuangFu, W.C.; Lee, Y.H.; Liu, I.H. Age, but not short-term intensive swimming, affects chondrocyte turnover in zebrafish vertebral cartilage. PeerJ 2018, 6, e5739. [Google Scholar] [CrossRef]
- Tierney, K.B. Swimming performance assessment in fishes. J. Vis. Exp. 2011, 51, 2572. [Google Scholar] [CrossRef]

| Gene Name | bp | Primer Sequence | Template |
| actb1 (ß-actin) | 231 | Forward: TCCTGGGTATGGAATCTTGC | NM_131031.2 |
| Reverse: CACCGATCCAGACGGAGTAT | |||
| g6pc1a | 90 | Forward: TCACAGCGTTGCTTTCAATC | NM_001003512.2 |
| Reverse: AACCCAGAAACATCCACAGC | |||
| cpt1a1a | 98 | Forward: GCATTGATCGGCATCTCTTT | NM_001044854. |
| Reverse: CAGTCTCCAAGGCTCTGACA | |||
| hadhaa | 243 | Forward: GCCATAAACGGCTCCTGTCT | NM_001105276.1 |
| Reverse: CTGATGGACGAGCCCCATTT | |||
| fasn | 157 | Forward: GTTGTGTGTGGTGTCGAAGC | XM_009306806.3 |
| Reverse: CTGCAAGAGTCTGGGGACTG | |||
| gnpat | 160 | Forward: ACTGCTCTGATGTCGCCAAA | XM_002665648.6 |
| Reverse: ACAGAGCCAAATCCGGTGAG | |||
| HK1 | 137 | Forward: ACTTTGGGTGCAATCCTGAC | NM_213252.1P |
| Reverse: AGACGACGCACTGTTTTGTG | |||
| pkmb | 144 | Forward: TGGGCTTATTAAGGGCAGTG | NM_001003488.1 |
| Reverse: TGCACCACCTTTGTGATGTT | |||
| myf5 | 82 | Forward: CCTCCCCAAGGTAGAGATCC | NM_131576.1 |
| Reverse: GTTCTCCACCTGTTCCCTGA | |||
| myoD | 58 | Forward: GGAGCGAATTTCCACAGAGACT | NM_131262.2 |
| Reverse: GTGCCCCTCCGGTACTGA | |||
| PCNA | 91 | Forward: GCCTTGGCACTGGTCTTTG | NM_131404.2 |
| Reverse: TGCCAAGCTGCTCCACATC | |||
| fbxo32 | 101 | Forward: GACTTCTGCAGTGCCATCAA | NM_200917.1 |
| Reverse: GCCACTCCACTCAGAGAAGG | |||
| trim63b | 158 | Forward: CACCAACATGGACATTCAGC | NM_201095.1 |
| Reverse: TAGCACATCCTCGACACAGG | |||
| map1c3b | 85 | Forward: GTGGAGGATGTACGGCTGAT | NM_199604.1 |
| Reverse: GCAGTTGCTTCTCTCCCTTG | |||
| atg4b | 112 | Forward: GTCTGGATTTTGGGAAAGCA | NM_001089352.1 |
| Reverse: CACCAATTGGCTGGAAGTTT | |||
| gck | 81 | Forward: GCTGTGAAGTCGGCATGATA | NM_001045385.2 |
| Reverse: CTTCAACCAGCTCCACCTTAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, T.-C.; Chiang, C.-H.; Liu, I.-H.; Wang, C.-Y.; Chen, C.-Y. Long-Term Yo-Yo Dieting Exaggerates Liver Steatosis and Lesions but Preserves Muscle Performance in Male Zebrafish. Int. J. Mol. Sci. 2024, 25, 13225. https://doi.org/10.3390/ijms252313225
Hsu T-C, Chiang C-H, Liu I-H, Wang C-Y, Chen C-Y. Long-Term Yo-Yo Dieting Exaggerates Liver Steatosis and Lesions but Preserves Muscle Performance in Male Zebrafish. International Journal of Molecular Sciences. 2024; 25(23):13225. https://doi.org/10.3390/ijms252313225
Chicago/Turabian StyleHsu, Tzu-Chieh, Chun-Hsien Chiang, I-Hsuan Liu, Chih-Yun Wang, and Ching-Yi Chen. 2024. "Long-Term Yo-Yo Dieting Exaggerates Liver Steatosis and Lesions but Preserves Muscle Performance in Male Zebrafish" International Journal of Molecular Sciences 25, no. 23: 13225. https://doi.org/10.3390/ijms252313225
APA StyleHsu, T.-C., Chiang, C.-H., Liu, I.-H., Wang, C.-Y., & Chen, C.-Y. (2024). Long-Term Yo-Yo Dieting Exaggerates Liver Steatosis and Lesions but Preserves Muscle Performance in Male Zebrafish. International Journal of Molecular Sciences, 25(23), 13225. https://doi.org/10.3390/ijms252313225









