One Change, Many Benefits: A Glycine-Modified Bacteriochlorin with NIR Absorption and a Type I Photochemical Mechanism for Versatile Photodynamic Therapy
Abstract
1. Introduction
2. Results
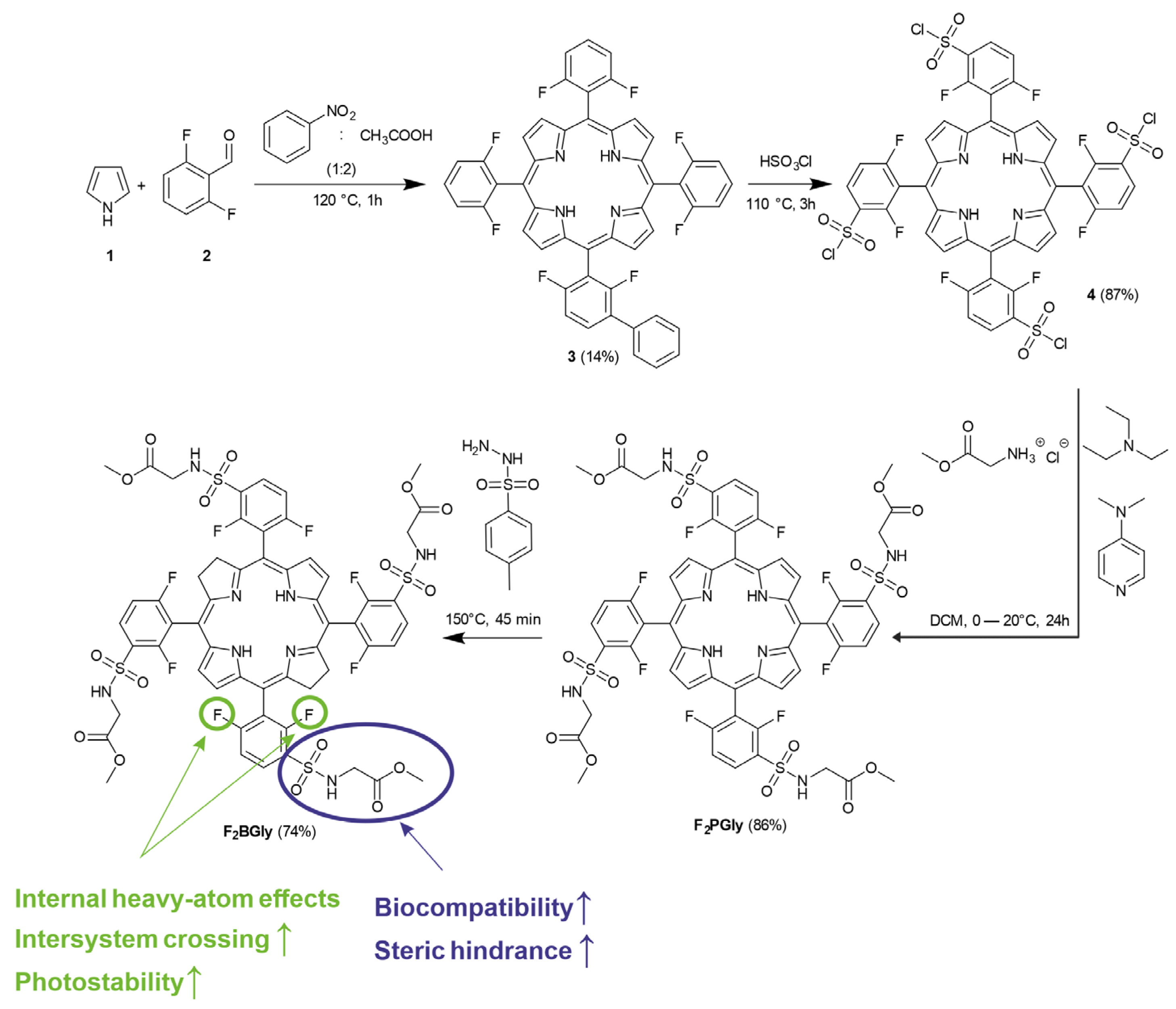
2.1. Synthesis of 5,10,15,20-Tetrakis[2,6-difluoro-3-(methoxycarbonylmethylsulfamido)-phenyl] Bacteriochlorin
2.2. Optical and Photophysical Properties
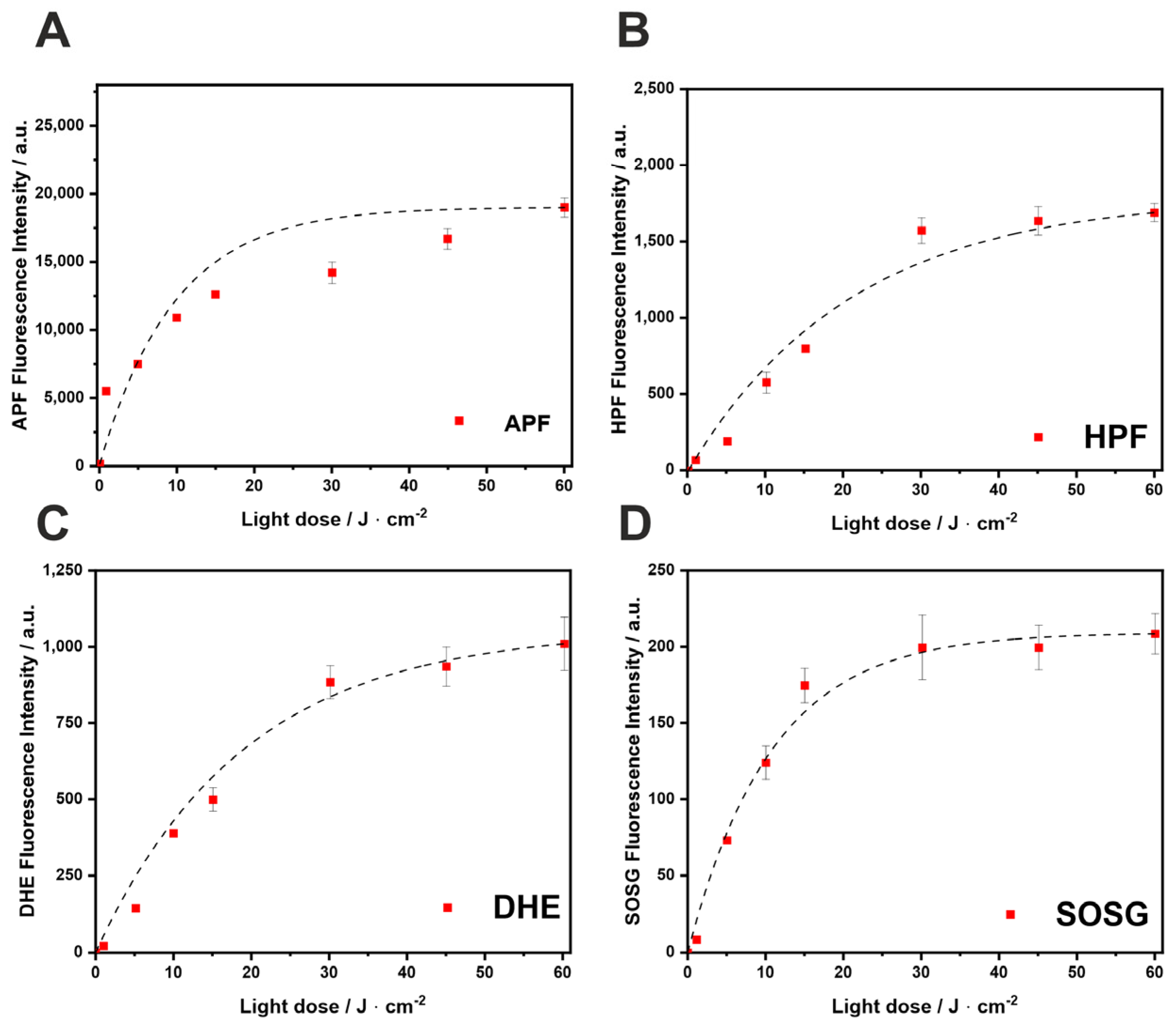
2.3. ROS Detection by Fluorescent Probes
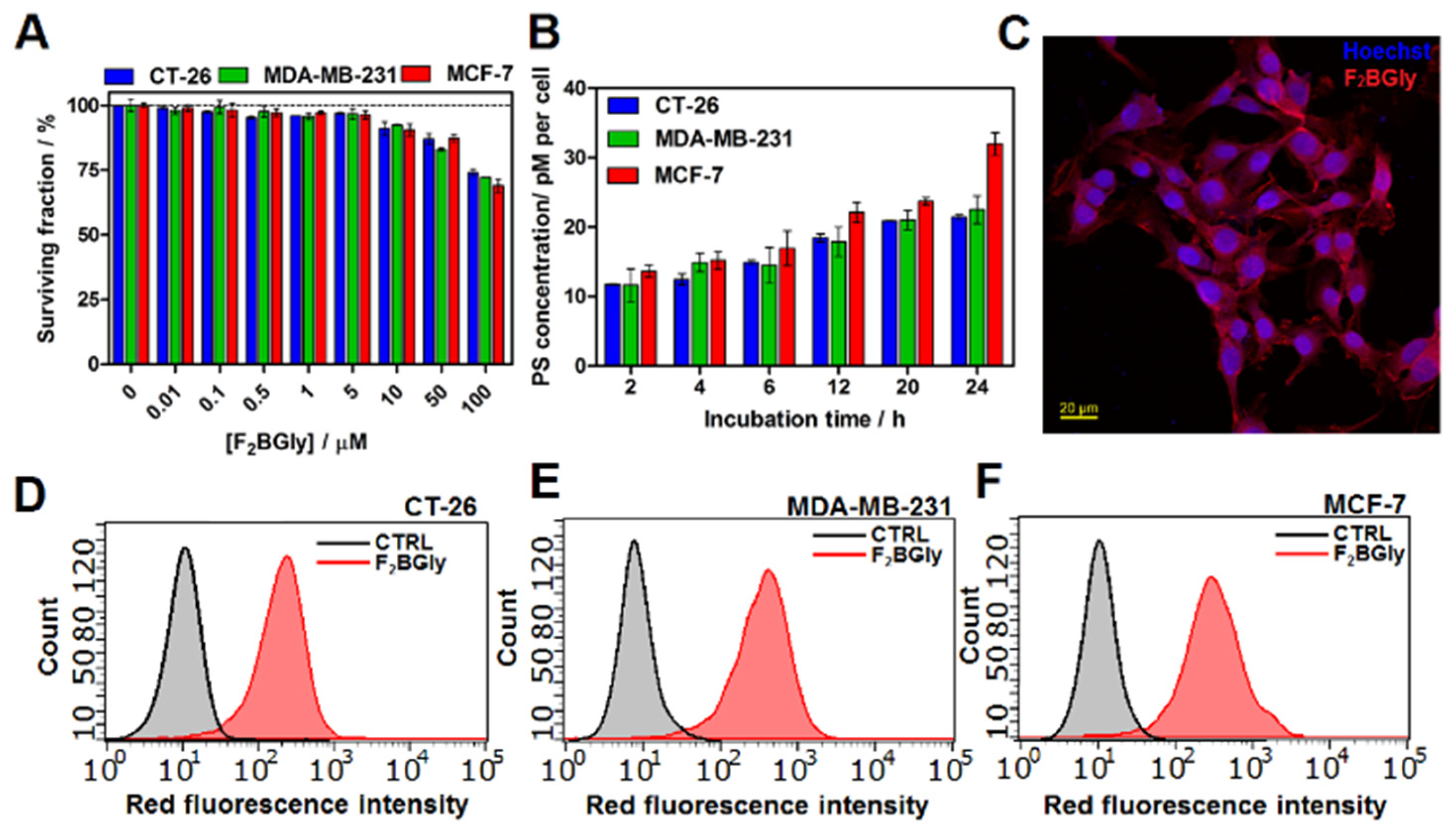
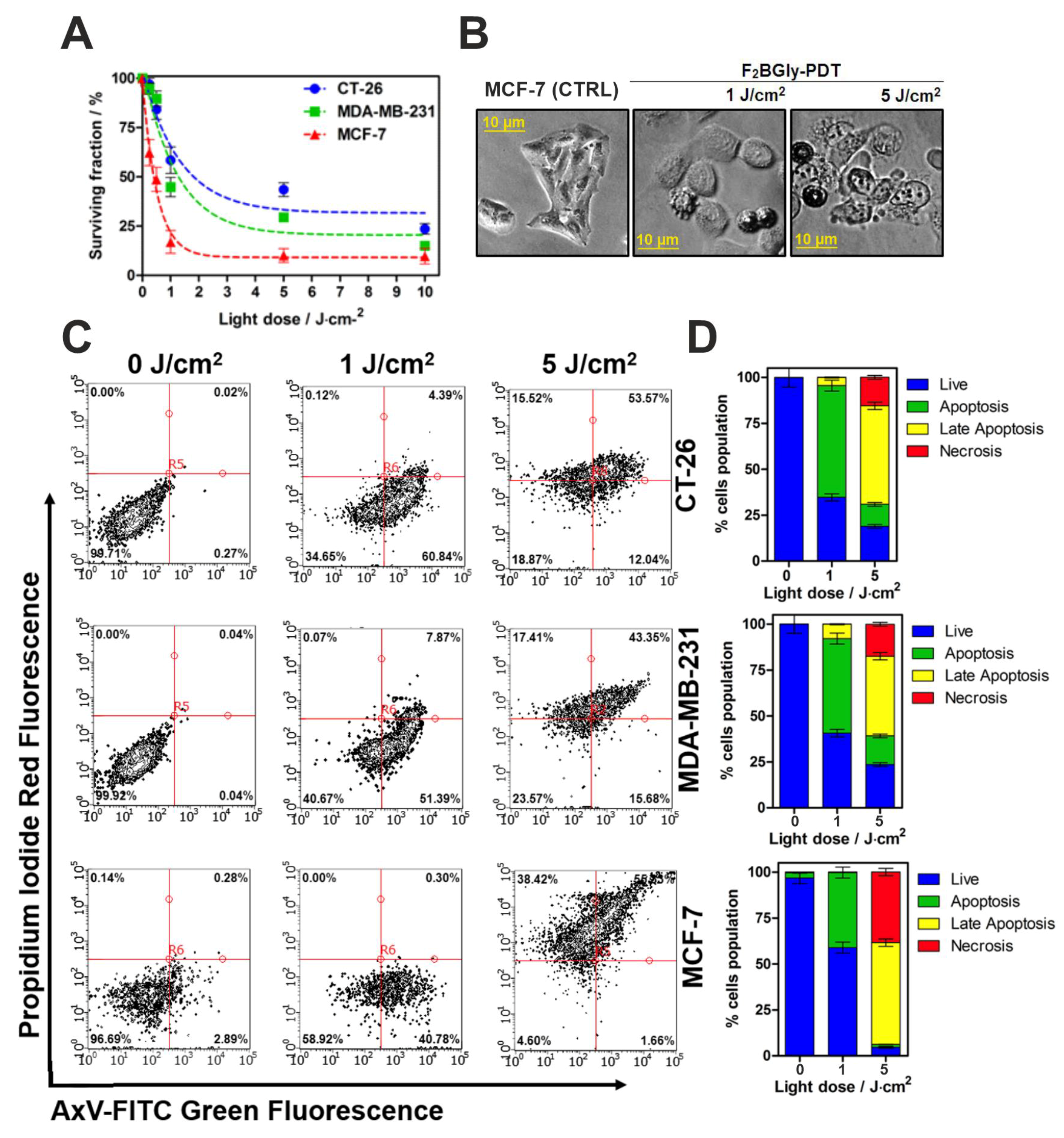
2.4. Biological Studies In Vitro
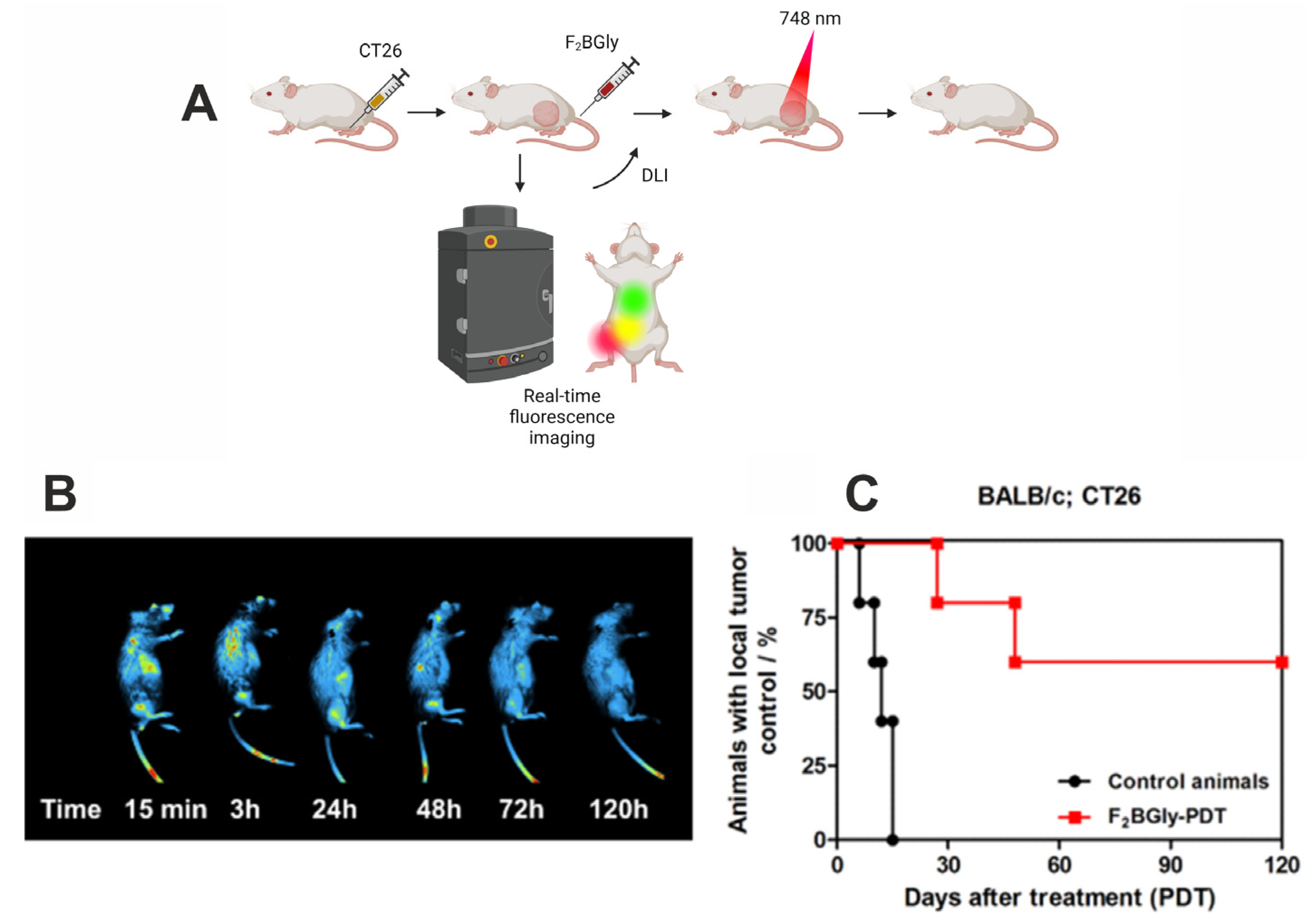
2.5. Fluorescence Imaging and Photodynamic Therapy In Vivo
3. Discussion
4. Materials and Methods
4.1. Synthesis of 5,10,15,20-Tetrakis[2,6-difluoro-3-(methoxycarbonylmethylsulfamido)-phenyl] Bacteriochlorin
4.2. Optical Properties
4.3. Fluorescence Quantum Yield Measurements
4.4. LogP Determination
4.5. Detection of Reactive Oxygen Species (ROS) with Fluorescent Probes
4.6. Cell Cultures
4.7. Cellular Uptake
4.8. Detection of Reactive Oxygen Species In Vitro
4.9. Cell Metabolic Activity Assay
4.10. Photodynamic Effect
4.11. Intracellular Accumulation
4.12. Animal Model
4.13. Real-Time Whole-Body Imaging
4.14. PDT In Vivo
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APF | 3′-(p-aminophenyl) fluorescein |
| BW | Body weight |
| CT26 | Murine colon carcinoma cell line derived from a BALB/c mouse |
| DCM | Dichloromethane |
| DHE | Dihydroethidium |
| DLI | Drug-to-light interval |
| DMSO | Dimethyl sulfoxide |
| EGFR | Epidermal growth factor receptor |
| F2BGly | 5,10,15,20-tetrakis[2,6-difluoro-3-(methoxycarbonylmethylsulfamido)-phenyl] bacteriochlorin |
| F2BMet | 5,10,15,20-tetrakis[2,6-difluoro-3-(methylsulfamoyl)-phenyl] bacteriochlorin |
| F2PGly | 5,10,15,20-tetrakis[2,6-difluoro-3-(methoxycarbonylmethylsulfamido)-phenyl] porphyrin |
| FDA | Food and Drug Administration |
| HCl | Hydrochloric acid |
| HPF | Hydroxyphenyl fluorescein |
| MCF-7 | Human breast cancer cell line |
| MDA-MB-231 | Triple negative breast cancer cell line |
| MTT | 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide |
| NMR | Nuclear magnetic resonance |
| PBS | Phosphate-buffered saline |
| PDT | Photodynamic therapy |
| PpIX | Protoporphyrin IX |
| PS | Photosensitizer |
| ROS | Reactive oxygen species |
| SOSG | Singlet Oxygen Sensor Green |
| V-PDT | Vascular-targeted photodynamic therapy |
References
- Warszyńska, M.; Repetowski, P.; Dąbrowski, J.M. Photodynamic therapy combined with immunotherapy: Recent advances and future research directions. Coord. Chem. Rev. 2023, 495, 215350. [Google Scholar] [CrossRef]
- Obaid, G.; Celli, J.P.; Broekgaarden, M.; Bulin, A.-L.; Uusimaa, P.; Pogue, B.; Hasan, T.; Huang, H.-C. Engineering photodynamics for treatment, priming and imaging. Nat. Rev. Bioeng. 2024, 2, 752–769. [Google Scholar] [CrossRef]
- Nath, S.; Obaid, G.; Hasan, T. The Course of Immune Stimulation by Photodynamic Therapy: Bridging Fundamentals of Photochemically Induced Immunogenic Cell Death to the Enrichment of T-Cell Repertoire. Photochem. Photobiol. 2019, 95, 1288–1305. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA A Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic microenvironment in cancer: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef]
- Penetra, M.; Arnaut, L.G.; Gomes-da-Silva, L.C. Trial watch: An update of clinical advances in photodynamic therapy and its immunoadjuvant properties for cancer treatment. Oncoimmunology 2023, 12, 2226535. [Google Scholar] [CrossRef]
- Lobo, A.C.S.; Gomes-da-Silva, L.C.; Rodrigues-Santos, P.; Cabrita, A.; Santos-Rosa, M.; Arnaut, L.G. Immune Responses after Vascular Photodynamic Therapy with Redaporfin. J. Clin. Med. 2019, 9, 104. [Google Scholar] [CrossRef]
- Xiong, H.; Xu, Y.; Kim, B.; Rha, H.; Zhang, B.; Li, M.; Yang, G.-F.; Kim, J.S. Photo-controllable biochemistry: Exploiting the photocages in phototherapeutic window. Chem 2023, 9, 29–64. [Google Scholar] [CrossRef]
- Algorri, J.F.; Ochoa, M.; Roldán-Varona, P.; Rodríguez-Cobo, L.; López-Higuera, J.M. Light Technology for Efficient and Effective Photodynamic Therapy: A Critical Review. Cancers 2021, 13, 3483. [Google Scholar] [CrossRef]
- Warszyńska, M.; Pucelik, B.; Vinagreiro, C.S.; Repetowski, P.; Barzowska, A.; Barczyk, D.; Schaberle, F.A.; Duque-Prata, A.; Arnaut, L.G.; Pereira, M.M.; et al. Better in the Near Infrared: Sulfonamide Perfluorinated-Phenyl Photosensitizers for Improved Simultaneous Targeted Photodynamic Therapy and Real-Time Fluorescence Imaging. ACS Appl. Mater. Interfaces 2024, 16, 50389–50406. [Google Scholar] [CrossRef]
- Mallidi, S.; Anbil, S.; Bulin, A.L.; Obaid, G.; Ichikawa, M.; Hasan, T. Beyond the Barriers of Light Penetration: Strategies, Perspectives and Possibilities for Photodynamic Therapy. Theranostics 2016, 6, 2458–2487. [Google Scholar] [CrossRef]
- Dąbrowski, J.M. Reactive Oxygen Species in Photodynamic Therapy: Mechanisms of Their Generation and Potentiation. In Advances in Inorganic Chemistry; van Eldik, R., Hubbard, C.D., Eds.; Academic Press: Cambrigde, MA, USA, 2017; Volume 70, pp. 343–394. [Google Scholar]
- Li, D.; Liu, P.; Tan, Y.; Zhang, Z.; Kang, M.; Wang, D.; Tang, B.Z. Type I Photosensitizers Based on Aggregation-Induced Emission: A Rising Star in Photodynamic Therapy. Biosensors 2022, 12, 722. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, P.J.; Boni, L.D.; Neto, N.M.B.; Rodrigues, J.J.; Zílio, S.C.; Borissevitch, I.E. Effect of protonation on the photophysical properties of meso-tetra (sulfonatophenyl) porphyrin. Chem. Phys. Lett. 2005, 407, 236–241. [Google Scholar] [CrossRef]
- Aebisher, D.; Woźnicki, P.; Bartusik-Aebisher, D. Photodynamic Therapy and Adaptive Immunity Induced by Reactive Oxygen Species: Recent Reports. Cancers 2024, 16, 967. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, N.; Sevilla, A. Current Advances in Photodynamic Therapy (PDT) and the Future Potential of PDT-Combinatorial Cancer Therapies. Int. J. Mol. Sci. 2024, 25, 1023. [Google Scholar] [CrossRef] [PubMed]
- Pucelik, B.; Arnaut, L.G.; Dąbrowski, J.M. Lipophilicity of Bacteriochlorin-Based Photosensitizers as a Determinant for PDT Optimization through the Modulation of the Inflammatory Mediators. J. Clin. Med. 2019, 9, 8. [Google Scholar] [CrossRef]
- Pucelik, B.; Sułek, A.; Dąbrowski, J.M. Bacteriochlorins and their metal complexes as NIR-absorbing photosensitizers: Properties, mechanisms, and applications. Coord. Chem. Rev. 2020, 416, 213340. [Google Scholar] [CrossRef]
- Plotnikova, E.; Abramova, O.; Ostroverkhov, P.; Vinokurova, A.; Medvedev, D.; Tihonov, S.; Usachev, M.; Shelyagina, A.; Efremenko, A.; Feofanov, A.; et al. Conjugate of Natural Bacteriochlorin with Doxorubicin for Combined Photodynamic and Chemotherapy. Int. J. Mol. Sci. 2024, 25, 7210. [Google Scholar] [CrossRef]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef]
- Mokoena, D.R.; George, B.P.; Abrahamse, H. Photodynamic Therapy Induced Cell Death Mechanisms in Breast Cancer. Int. J. Mol. Sci. 2021, 22, 10506. [Google Scholar] [CrossRef]
- Udrea, A.M.; Smarandache, A.; Dinache, A.; Mares, C.; Nistorescu, S.; Avram, S.; Staicu, A. Photosensitizers-Loaded Nanocarriers for Enhancement of Photodynamic Therapy in Melanoma Treatment. Pharmaceutics 2023, 15, 2124. [Google Scholar] [CrossRef]
- Repetowski, P.; Warszyńska, M.; Kostecka, A.; Pucelik, B.; Barzowska, A.; Emami, A.; İşci, Ü.; Dumoulin, F.; Dąbrowski, J.M. Synthesis, Photo-Characterizations, and Pre-Clinical Studies on Advanced Cellular and Animal Models of Zinc(II) and Platinum(II) Sulfonyl-Substituted Phthalocyanines for Enhanced Vascular-Targeted Photodynamic Therapy. ACS Appl. Mater. Interfaces 2024, 16, 48937–48954. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowski, J.M.; Pucelik, B.; Pereira, M.M.; Arnaut, L.G.; Macyk, W.; Stochel, G. New hybrid materials based on halogenated metalloporphyrins for enhanced visible light photocatalysis. RSC Adv. 2015, 5, 93252–93261. [Google Scholar] [CrossRef]
- Malec, D.; Warszyńska, M.; Repetowski, P.; Siomchen, A.; Dąbrowski, J.M. Enhancing Visible-Light Photocatalysis with Pd(II) Porphyrin-Based TiO2 Hybrid Nanomaterials: Preparation, Characterization, ROS Generation, and Photocatalytic Activity. Molecules 2023, 28, 7819. [Google Scholar] [CrossRef] [PubMed]
- Sułek, A.; Pucelik, B.; Kuncewicz, J.; Dubin, G.; Dąbrowski, J.M. Sensitization of TiO2 by halogenated porphyrin derivatives for visible light biomedical and environmental photocatalysis. Catal. Today 2019, 335, 538–549. [Google Scholar] [CrossRef]
- Obaid, G.; Broekgaarden, M.; Bulin, A.-L.; Huang, H.-C.; Kuriakose, J.; Liu, J.; Hasan, T. Photonanomedicine: A convergence of photodynamic therapy and nanotechnology. Nanoscale 2016, 8, 12471–12503. [Google Scholar] [CrossRef]
- Yang, S.B.; Banik, N.; Han, B.; Lee, D.N.; Park, J. Peptide-Based Bioconjugates and Therapeutics for Targeted Anticancer Therapy. Pharmaceutics 2022, 14, 1378. [Google Scholar] [CrossRef]
- Sun, L.; Liu, H.; Ye, Y.; Lei, Y.; Islam, R.; Tan, S.; Tong, R.; Miao, Y.-B.; Cai, L. Smart nanoparticles for cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 418. [Google Scholar] [CrossRef]
- Fan, D.; Cao, Y.; Cao, M.; Wang, Y.; Cao, Y.; Gong, T. Nanomedicine in cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 293. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, J.; Fan, J.; Chao, H.; Peng, X. Recent progress in photosensitizers for overcoming the challenges of photodynamic therapy: From molecular design to application. Chem. Soc. Rev. 2021, 50, 4185–4219. [Google Scholar] [CrossRef]
- Kue, C.S.; Kamkaew, A.; Lee, H.B.; Chung, L.Y.; Kiew, L.V.; Burgess, K. Targeted PDT Agent Eradicates TrkC Expressing Tumors via Photodynamic Therapy (PDT). Mol. Pharm. 2015, 12, 212–222. [Google Scholar] [CrossRef] [PubMed]
- del Carmen, M.G.; Rizvi, I.; Chang, Y.; Moor, A.C.E.; Oliva, E.; Sherwood, M.; Pogue, B.; Hasan, T. Synergism of Epidermal Growth Factor Receptor–Targeted Immunotherapy With Photodynamic Treatment of Ovarian Cancer In Vivo. J. Natl. Cancer Inst. 2005, 97, 1516–1524. [Google Scholar] [CrossRef] [PubMed]
- Mfouo-Tynga, I.S.; Dias, L.D.; Inada, N.M.; Kurachi, C. Features of third generation photosensitizers used in anticancer photodynamic therapy: Review. Photodiagnosis Photodyn. Ther. 2021, 34, 102091. [Google Scholar] [CrossRef] [PubMed]
- Thomas-Moore, B.A.; Dedola, S.; Russell, D.A.; Field, R.A.; Marín, M.J. Targeted photodynamic therapy for breast cancer: The potential of glyconanoparticles. Nanoscale Adv. 2023, 5, 6501–6513. [Google Scholar] [CrossRef]
- Wang, X.; Luo, D.; Basilion, J.P. Photodynamic Therapy: Targeting Cancer Biomarkers for the Treatment of Cancers. Cancers 2021, 13, 2992. [Google Scholar] [CrossRef]
- Dhaini, B.; Kenzhebayeva, B.; Ben-Mihoub, A.; Gries, M.; Acherar, S.; Baros, F.; Thomas, N.; Daouk, J.; Schohn, H.; Hamieh, T.; et al. Peptide-conjugated nanoparticles for targeted photodynamic therapy. Nanophotonics 2021, 10, 3089–3134. [Google Scholar] [CrossRef]
- Ulfo, L.; Costantini, P.E.; Di Giosia, M.; Danielli, A.; Calvaresi, M. EGFR-Targeted Photodynamic Therapy. Pharmaceutics 2022, 14, 241. [Google Scholar] [CrossRef]
- Li, S.-Y.; Cheng, H.; Qiu, W.-X.; Liu, L.-H.; Chen, S.; Hu, Y.; Xie, B.-R.; Li, B.; Zhang, X.-Z. Protease-Activable Cell-Penetrating Peptide–Protoporphyrin Conjugate for Targeted Photodynamic Therapy in Vivo. ACS Appl. Mater. Interfaces 2015, 7, 28319–28329. [Google Scholar] [CrossRef]
- Meng, Z.; Yu, B.; Han, G.; Liu, M.; Shan, B.; Dong, G.; Miao, Z.; Jia, N.; Tan, Z.; Li, B.; et al. Chlorin p6-Based Water-Soluble Amino Acid Derivatives as Potent Photosensitizers for Photodynamic Therapy. J. Med. Chem. 2016, 59, 4999–5010. [Google Scholar] [CrossRef]
- Bao, Y.; Yu, H.; Zhang, Y.; Chen, L. Comparative study of two poly(amino acid)-based photosensitizer-delivery systems for photodynamic therapy. Int. J. Biol. Macromol. 2021, 169, 153–160. [Google Scholar] [CrossRef]
- Silva, J.N.; Haigle, J.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Mazière, J.-C.; Mazière, C.; Santus, R.; Cavaleiro, J.A.S.; Filipe, P.; et al. Enhancement of the photodynamic activity of tri-cationic porphyrins towards proliferating keratinocytes by conjugation to poly-S-lysine. Photochem. Photobiol. Sci. 2006, 5, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Du, E.; Hu, X.; Roy, S.; Wang, P.; Deasy, K.; Mochizuki, T.; Zhang, Y. Taurine-modified Ru(ii)-complex targets cancerous brain cells for photodynamic therapy. Chem. Commun. 2017, 53, 6033–6036. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chang, L.; Gao, H.; Yu, C.; Gao, Y.; Peng, Q. Nanomaterials-based advanced systems for photothermal/photodynamic therapy of oral cancer. Eur. J. Med. Chem. 2024, 272, 116508. [Google Scholar] [CrossRef]
- Vale, N.; Ramos, R.; Cruz, I.; Pereira, M. Application of Peptide-Conjugated Photosensitizers for Photodynamic Cancer Therapy: A Review. Organics 2024, 5, 429–442. [Google Scholar] [CrossRef]
- Wang, J.T.W.; Giuntini, F.; Eggleston, I.M.; Bown, S.G.; MacRobert, A.J. Photochemical internalisation of a macromolecular protein toxin using a cell penetrating peptide-photosensitiser conjugate. J. Control. Release 2012, 157, 305–313. [Google Scholar] [CrossRef]
- Yang, S.; Wang, M.; Wang, T.; Sun, M.; Huang, H.; Shi, X.; Duan, S.; Wu, Y.; Zhu, J.; Liu, F. Self-assembled short peptides: Recent advances and strategies for potential pharmaceutical applications. Mater. Today Bio 2023, 20, 100644. [Google Scholar] [CrossRef]
- Pereira, M.M.; Monteiro, C.J.P.; Simões, A.V.C.; Pinto, S.M.A.; Abreu, A.R.; Sá, G.F.F.; Silva, E.F.F.; Rocha, L.B.; Dąbrowski, J.M.; Formosinho, S.J.; et al. Synthesis and photophysical characterization of a library of photostable halogenated bacteriochlorins: An access to near infrared chemistry. Tetrahedron 2010, 66, 9545–9551. [Google Scholar] [CrossRef]
- Jones, H.J.; Vernon, D.I.; Brown, S.B. Photodynamic therapy effect of m-THPC (Foscan®) in vivo: Correlation with pharmacokinetics. Br. J. Cancer 2003, 89, 398–404. [Google Scholar] [CrossRef]
- Bonnett, R.; Djelal, B.D.; Hamilton, P.A.; Martinez, G.; Wierrani, F. Photobleaching of 5,10,15,20-tetrakis(m-hydroxyphenyl)porphyrin (m-THPP) and the corresponding chlorin (m-THPC) and bacteriochlorin(m-THPBC). A comparative study. J. Photochem. Photobiol. B Biol. 1999, 53, 136–143. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Balasubramanian, T.; Yang, E.; Luo, D.; Diers, J.R.; Bocian, D.F.; Lindsey, J.S.; Holten, D.; Hamblin, M.R. Stable Synthetic Bacteriochlorins for Photodynamic Therapy: Role of Dicyano Peripheral Groups, Central Metal Substitution (2H, Zn, Pd), and Cremophor EL Delivery. ChemMedChem 2012, 7, 2155–2167. [Google Scholar] [CrossRef]
- Ashur, I.; Goldschmidt, R.; Pinkas, I.; Salomon, Y.; Szewczyk, G.; Sarna, T.; Scherz, A. Photocatalytic Generation of Oxygen Radicals by the Water-Soluble Bacteriochlorophyll Derivative WST11, Noncovalently Bound to Serum Albumin. J. Phys. Chem. A 2009, 113, 8027–8037. [Google Scholar] [CrossRef]
- Vakrat-Haglili, Y.; Weiner, L.; Brumfeld, V.; Brandis, A.; Salomon, Y.; McLlroy, B.; Wilson, B.C.; Pawlak, A.; Rozanowska, M.; Sarna, T.; et al. The Microenvironment Effect on the Generation of Reactive Oxygen Species by Pd−Bacteriopheophorbide. J. Am. Chem. Soc. 2005, 127, 6487–6497. [Google Scholar] [CrossRef] [PubMed]
- De Boni, L.; Monteiro, C.; Mendonça, C.R.; Zílio, S.C.; Gonçalves, P. Influence of halogen atoms and protonation on the photophysical properties of sulfonated porphyrins. Chem. Phys. Lett. 2015, 633, 146–151. [Google Scholar] [CrossRef]
- Morozova, N.B.; Pavlova, M.A.; Plyutinskaya, A.D.; Pankratov, A.A.; Efendiev, K.T.; Semkina, A.S.; Pritmov, D.A.; Mironov, A.F.; Panchenko, P.A.; Fedorova, O.A. Photodiagnosis and photodynamic effects of bacteriochlorin-naphthalimide conjugates on tumor cells and mouse model. J. Photochem. Photobiol. B Biol. 2021, 223, 112294. [Google Scholar] [CrossRef]
- Wu, M.; Liu, Z.; Zhang, W. An ultra-stable bio-inspired bacteriochlorin analogue for hypoxia-tolerant photodynamic therapy. Chem. Sci. 2021, 12, 1295–1301. [Google Scholar] [CrossRef]
- Luo, T.; Ni, K.; Culbert, A.; Lan, G.; Li, Z.; Jiang, X.; Kaufmann, M.; Lin, W. Nanoscale Metal–Organic Frameworks Stabilize Bacteriochlorins for Type I and Type II Photodynamic Therapy. J. Am. Chem. Soc. 2020, 142, 7334–7339. [Google Scholar] [CrossRef]
- Arnaut, L.; Lara-Santos, L. Redaporfin: Clinical Validation of a New Approach to Head and Neck Cancer. Photodiagnosis Photodyn. Ther. 2023, 41, 103401. [Google Scholar] [CrossRef]
- Hamblin, M.R. Photodynamic Therapy for Cancer: What’s Past is Prologue. Photochem. Photobiol. 2020, 96, 506–516. [Google Scholar] [CrossRef]
- Santos, L.L.; Oliveira, J.; Monteiro, E.; Santos, J.; Sarmento, C. Treatment of Head and Neck Cancer with Photodynamic Therapy with Redaporfin: A Clinical Case Report. Case Rep. Oncol. 2018, 11, 769–776. [Google Scholar] [CrossRef]
- Rocha, L.B.; Schaberle, F.; Dąbrowski, J.M.; Simões, S.; Arnaut, L.G. Intravenous Single-Dose Toxicity of Redaporfin-Based Photodynamic Therapy in Rodents. Int. J. Mol. Sci. 2015, 16, 29236–29249. [Google Scholar] [CrossRef]
- Nakanishi, T.; Ross, D.D. Breast cancer resistance protein (BCRP/ABCG2): Its role in multidrug resistance and regulation of its gene expression. Chin. J. Cancer 2012, 31, 73–99. [Google Scholar] [CrossRef] [PubMed]
- Zattoni, I.F.; Delabio, L.C.; Dutra, J.d.P.; Kita, D.H.; Scheiffer, G.; Hembecker, M.; Pereira, G.d.S.; Moure, V.R.; Valdameri, G. Targeting breast cancer resistance protein (BCRP/ABCG2): Functional inhibitors and expression modulators. Eur. J. Med. Chem. 2022, 237, 114346. [Google Scholar] [CrossRef] [PubMed]
- Szafraniec, M.J.; Szczygieł, M.; Urbanska, K.; Fiedor, L. Determinants of the activity and substrate recognition of breast cancer resistance protein (ABCG2). Drug Metab. Rev. 2014, 46, 459–474. [Google Scholar] [CrossRef]
- Mao, Q.; Unadkat, J.D. Role of the Breast Cancer Resistance Protein (BCRP/ABCG2) in Drug Transport—An Update. AAPS J. 2015, 17, 65–82. [Google Scholar] [CrossRef]
- Ogbodu, R.O.; Nyokong, T. The effect of ascorbic acid on the photophysical properties and photodynamic therapy activities of zinc phthalocyanine-single walled carbon nanotube conjugate on MCF-7 cancer cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 151, 174–183. [Google Scholar] [CrossRef]
- Aniogo, E.C.; George, B.P.; Abrahamse, H. Response of MCF-7 Breast Cancer Cells Overexpressed with P-Glycoprotein to Apoptotic Induction after Photodynamic Therapy. Molecules 2021, 26, 7412. [Google Scholar] [CrossRef]
- Mao, C.; Qu, P.; Miley, M.J.; Zhao, Y.; Li, Z.; Ming, X. P-glycoprotein targeted photodynamic therapy of chemoresistant tumors using recombinant Fab fragment conjugates. Biomater. Sci. 2018, 6, 3063–3074. [Google Scholar] [CrossRef]
- Dyson, J.; Foll, F.L.; Magal, P.; Noussair, A.; Pasquier, J. Direct and indirect P-glycoprotein transfers in MCF7 breast cancer cells. J. Theor. Biol. 2019, 461, 239–253. [Google Scholar] [CrossRef]
- Ogbodu, R.O.; Limson, J.L.; Prinsloo, E.; Nyokong, T. Photophysical properties and photodynamic therapy effect of zinc phthalocyanine-spermine-single walled carbon nanotube conjugate on MCF-7 breast cancer cell line. Synth. Met. 2015, 204, 122–132. [Google Scholar] [CrossRef]
- Pocasap, P.; Weerapreeyakul, N.; Timonen, J.; Järvinen, J.; Leppänen, J.; Kärkkäinen, J.; Rautio, J. Tyrosine–Chlorambucil Conjugates Facilitate Cellular Uptake through L-Type Amino Acid Transporter 1 (LAT1) in Human Breast Cancer Cell Line MCF-7. Int. J. Mol. Sci. 2020, 21, 2132. [Google Scholar] [CrossRef]
- Xue, Q.; Zhang, J.; Jiao, J.; Qin, W.; Yang, X. Photodynamic therapy for prostate cancer: Recent advances, challenges and opportunities. Front. Oncol. 2022, 12, 980239. [Google Scholar] [CrossRef] [PubMed]
- Karges, J. Clinical Development of Metal Complexes as Photosensitizers for Photodynamic Therapy of Cancer. Angew. Chem. Int. Ed. 2022, 61, e202112236. [Google Scholar] [CrossRef] [PubMed]
- Koudinova, N.V.; Pinthus, J.H.; Brandis, A.; Brenner, O.; Bendel, P.; Ramon, J.; Eshhar, Z.; Scherz, A.; Salomon, Y. Photodynamic therapy with Pd-bacteriopheophorbide (TOOKAD): Successful in vivo treatment of human prostatic small cell carcinoma xenografts. Int. J. Cancer 2003, 104, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Diaz, M.; Huang, Y.-Y.; Hamblin, M.R. Use of fluorescent probes for ROS to tease apart Type I and Type II photochemical pathways in photodynamic therapy. Methods 2016, 109, 158–166. [Google Scholar] [CrossRef]
- Malik, Z. Fundamentals of 5-aminolevulinic acid photodynamic therapy and diagnosis: An overview. Transl. Biophotonics 2020, 2, e201900022. [Google Scholar] [CrossRef]
- Mahmoudi, K.; Garvey, K.L.; Bouras, A.; Cramer, G.; Stepp, H.; Jesu Raj, J.G.; Bozec, D.; Busch, T.M.; Hadjipanayis, C.G. 5-aminolevulinic acid photodynamic therapy for the treatment of high-grade gliomas. J. Neuro-Oncol. 2019, 141, 595–607. [Google Scholar] [CrossRef]
- Nishinaka, Y.; Arai, T.; Adachi, S.; Takaori-Kondo, A.; Yamashita, K. Singlet oxygen is essential for neutrophil extracellular trap formation. Biochem. Biophys. Res. Commun. 2011, 413, 75–79. [Google Scholar] [CrossRef]
- Dellinger, M. Apoptosis or Necrosis Following Photofrin® Photosensitization: Influence of the Incubation Protocol. Photochem. Photobiol. 1996, 64, 182–187. [Google Scholar] [CrossRef]


| Photosensitizer | Absorption λmax/nm; ε/M−1 cm−1 | Fluorescence λmax/nm | ΦF | logPow | |||||
|---|---|---|---|---|---|---|---|---|---|
| Soret (B) | Q(1–0) | Q(0–0) | |||||||
| Bx | By | Qx | Qy | Qx | Qy | ||||
| F2PGly | 410 - | 505 - | 540 - | 584 - | 639; 7980 | 642, 705 | 0.037 | 1.7 | |
| F2BGly | 346; 70,100 | 373; 76,260 | 505; 36,420 | 746; 66,580 | 764 | 0.096 | 1.9 | ||
| F2BOH [17] | - | - | - | 743; 70,000 | 764 | 0.023 | −1.4 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Werłos, M.; Barzowska-Gogola, A.; Pucelik, B.; Repetowski, P.; Warszyńska, M.; Dąbrowski, J.M. One Change, Many Benefits: A Glycine-Modified Bacteriochlorin with NIR Absorption and a Type I Photochemical Mechanism for Versatile Photodynamic Therapy. Int. J. Mol. Sci. 2024, 25, 13132. https://doi.org/10.3390/ijms252313132
Werłos M, Barzowska-Gogola A, Pucelik B, Repetowski P, Warszyńska M, Dąbrowski JM. One Change, Many Benefits: A Glycine-Modified Bacteriochlorin with NIR Absorption and a Type I Photochemical Mechanism for Versatile Photodynamic Therapy. International Journal of Molecular Sciences. 2024; 25(23):13132. https://doi.org/10.3390/ijms252313132
Chicago/Turabian StyleWerłos, Mateusz, Agata Barzowska-Gogola, Barbara Pucelik, Paweł Repetowski, Marta Warszyńska, and Janusz M. Dąbrowski. 2024. "One Change, Many Benefits: A Glycine-Modified Bacteriochlorin with NIR Absorption and a Type I Photochemical Mechanism for Versatile Photodynamic Therapy" International Journal of Molecular Sciences 25, no. 23: 13132. https://doi.org/10.3390/ijms252313132
APA StyleWerłos, M., Barzowska-Gogola, A., Pucelik, B., Repetowski, P., Warszyńska, M., & Dąbrowski, J. M. (2024). One Change, Many Benefits: A Glycine-Modified Bacteriochlorin with NIR Absorption and a Type I Photochemical Mechanism for Versatile Photodynamic Therapy. International Journal of Molecular Sciences, 25(23), 13132. https://doi.org/10.3390/ijms252313132






