Magnetic Isolation of the Linear Trinuclear Anion in [Cu(Him)6] {Cu(Him)4[Cu(μ-EDTA)(Him)]2}·6H2O (1) as the Novel Imidazolium(+) Salt (H2im)2[Cu(Him)4{(µ-EDTA)Cu(Him)}2]·2H2O (2)—A Comparative Look to Their Crystal Structures, Thermal, Spectral and Magnetic Properties and DFT Calculations
Abstract
1. Introduction
2. Results and Discussion
2.1. Strategy for the Synthesis of Compound 1 and 2, Using Basic Copper(II) Carbonates
2.2. Crystal Structures of 1 and 2
2.2.1. Crystal Structure of Compound 1
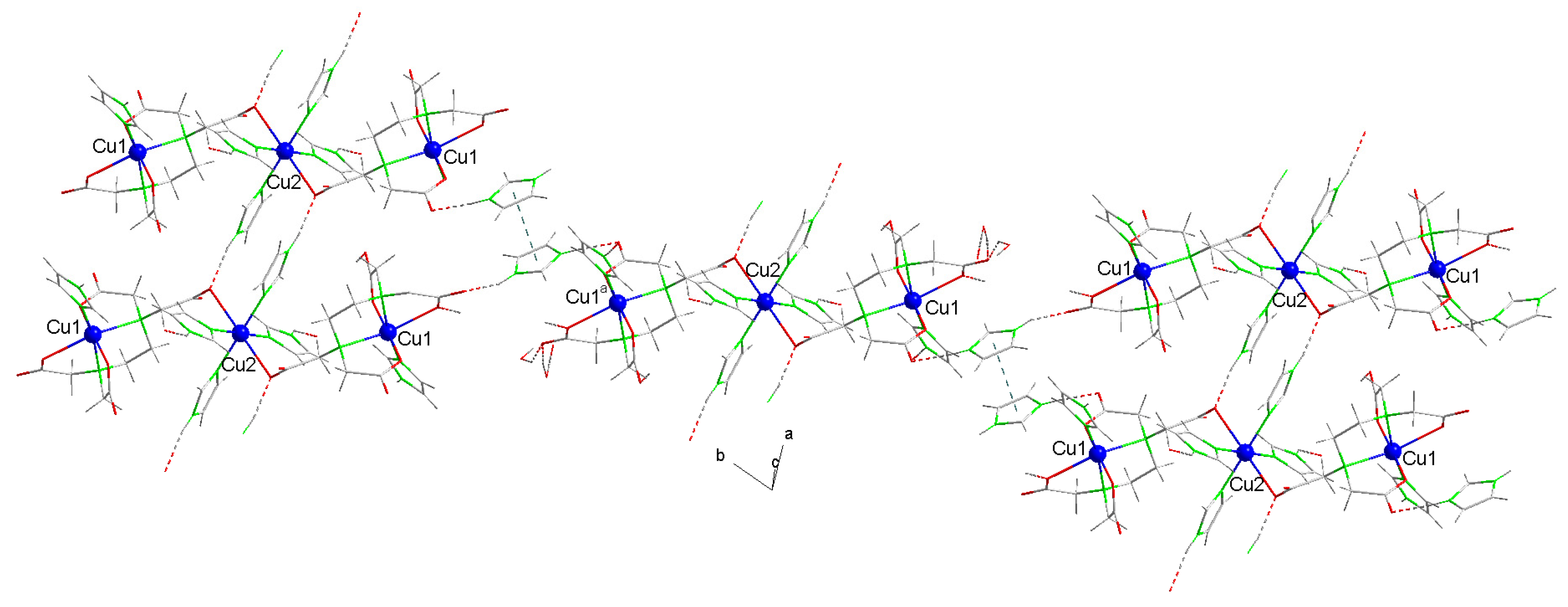
2.2.2. Crystal Structure of Compound 2
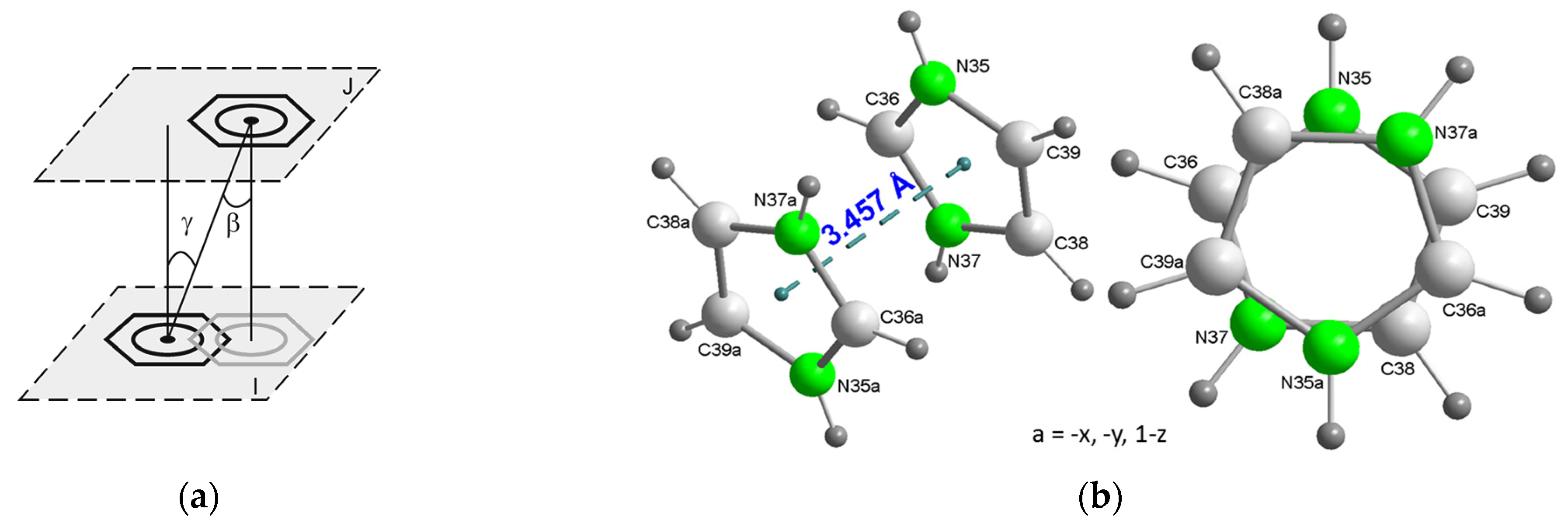
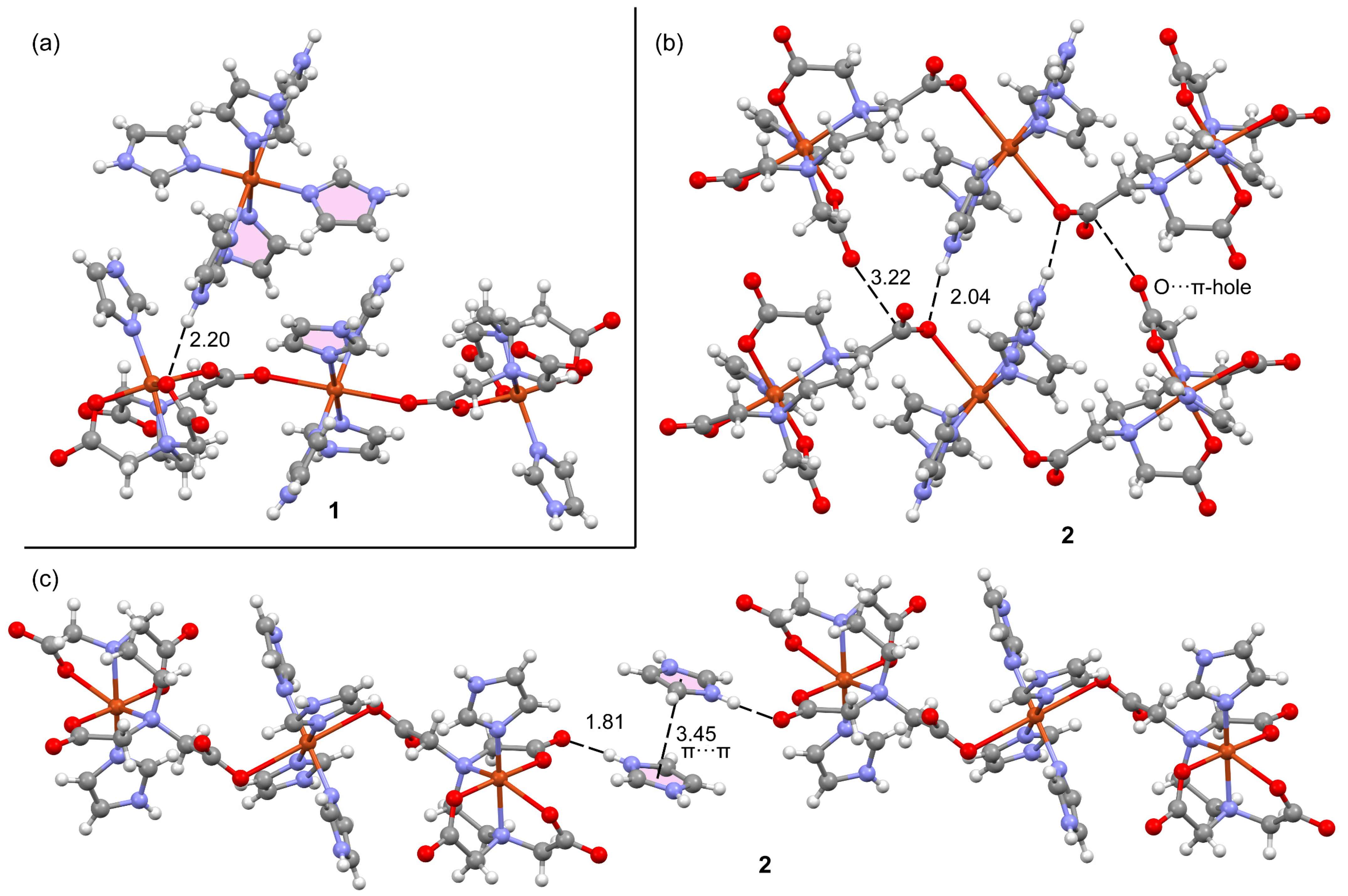
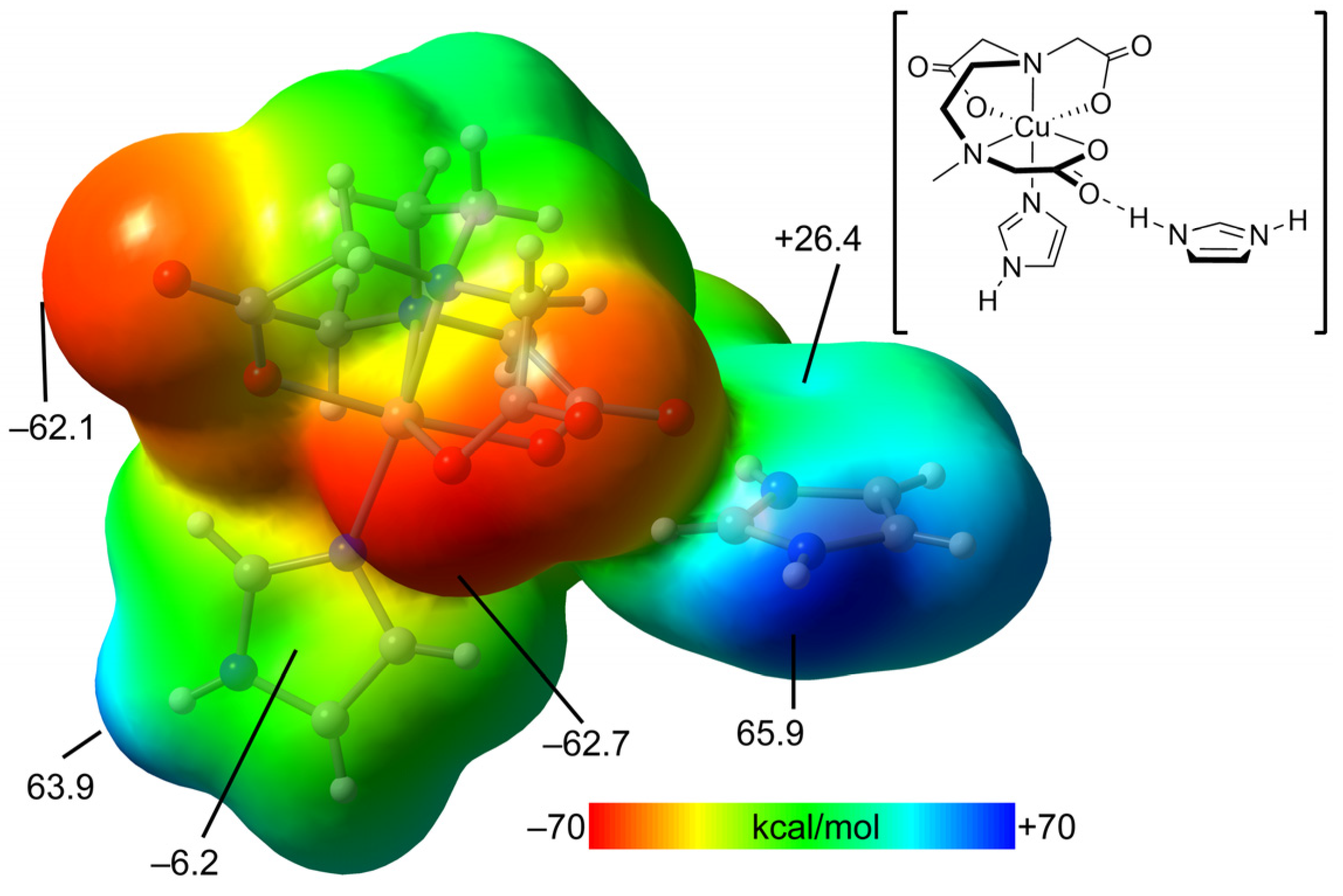
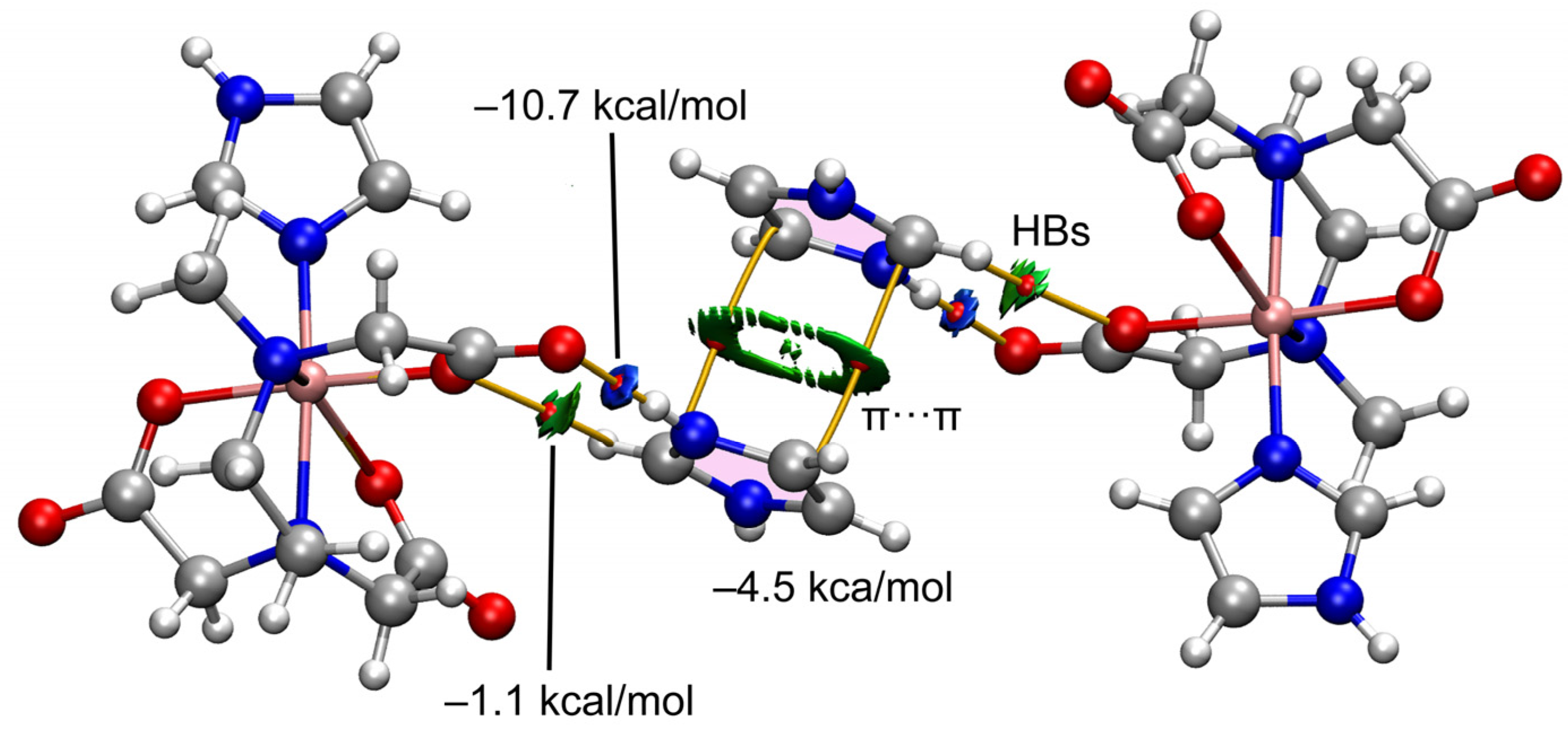
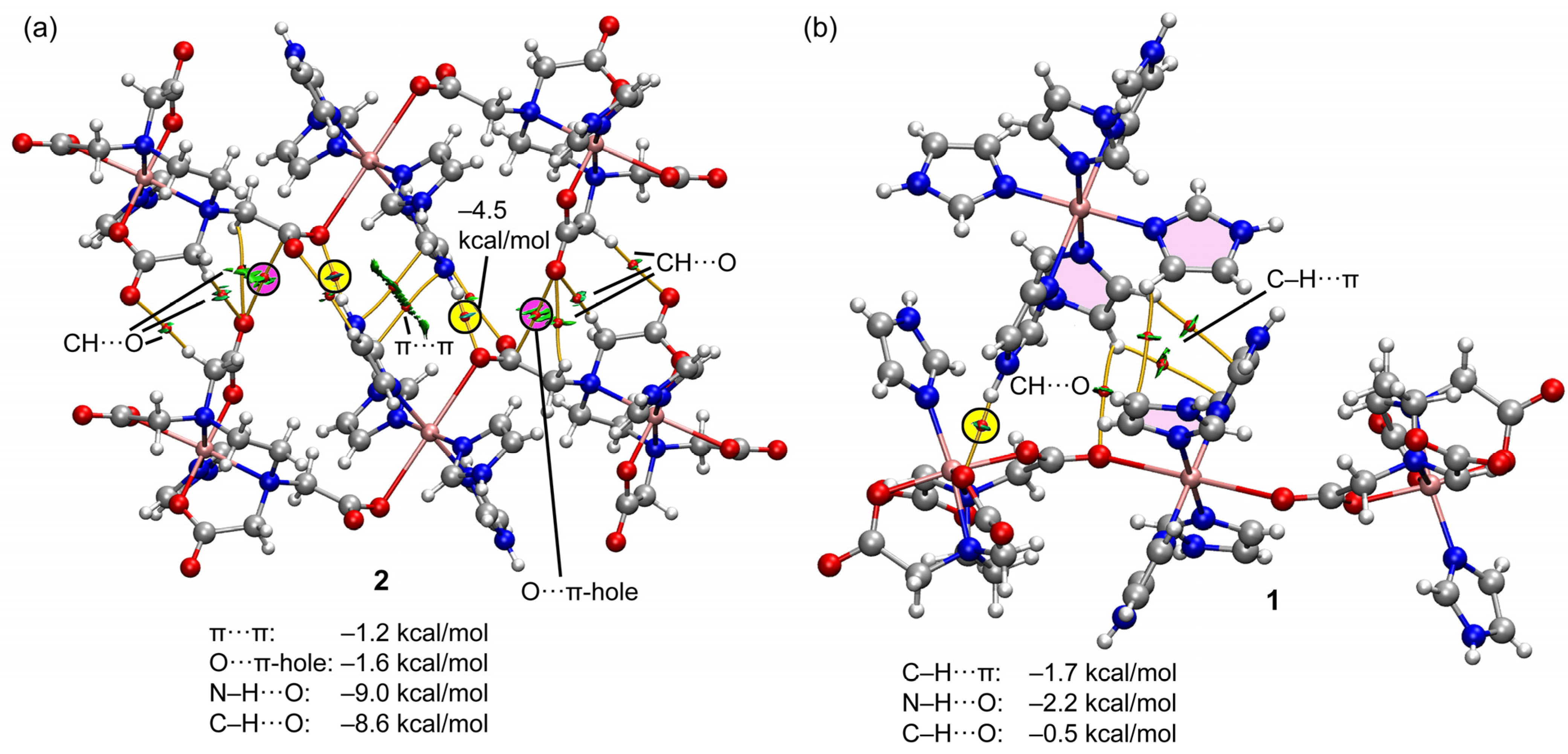
2.2.3. Structural Impact of Using Imidazolium(1+) Ions to Achieve Magnetic Isolation of the Linear Trinuclear Anion in Compound 1
2.3. Physical Properties
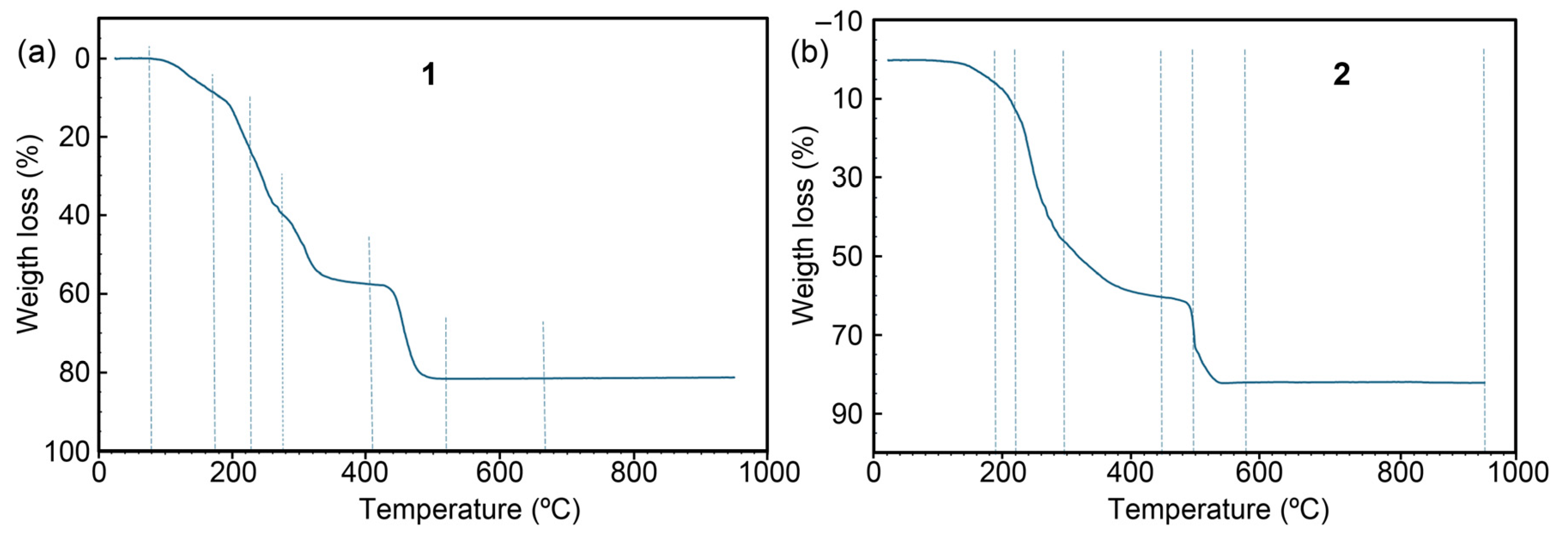
2.3.1. Thermogravimetric Studies, with Identification of Evolved Gases and Estimation of the CuO as Final Residues
2.3.2. Infrared (FT–IR) Spectra of Polycrystalline Samples
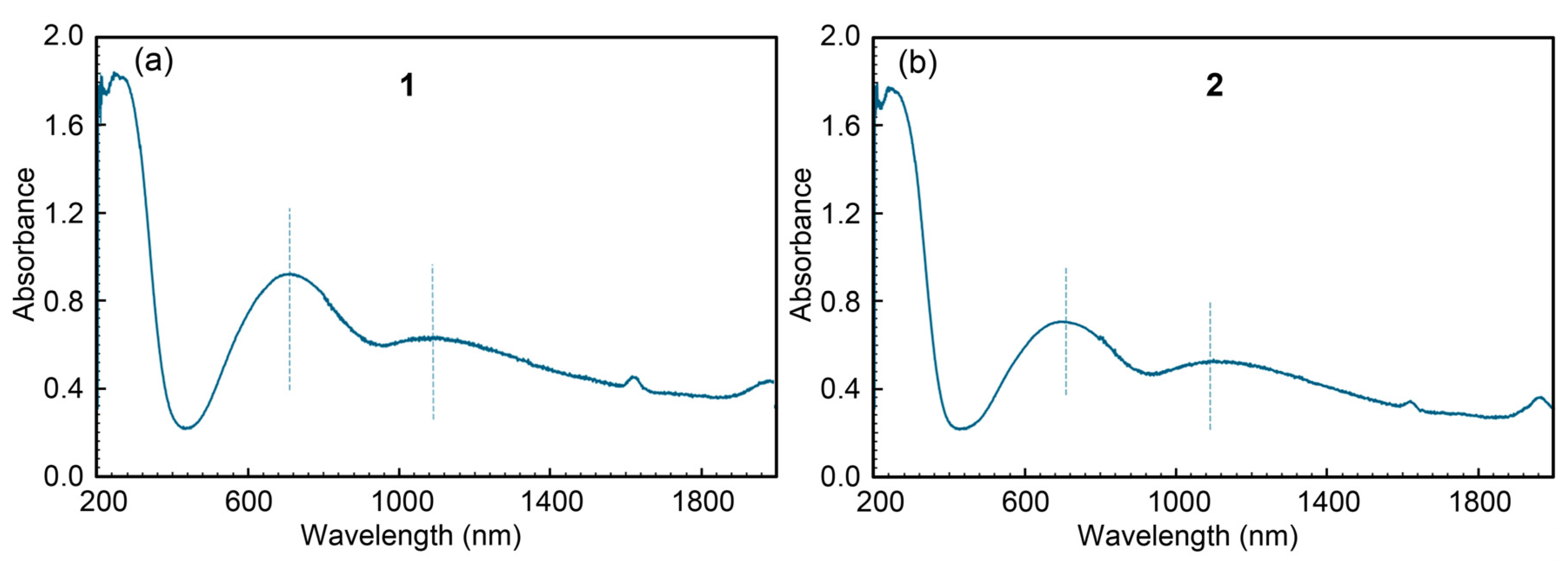
2.3.3. Electronic (Diffuse Reflectance) Spectra
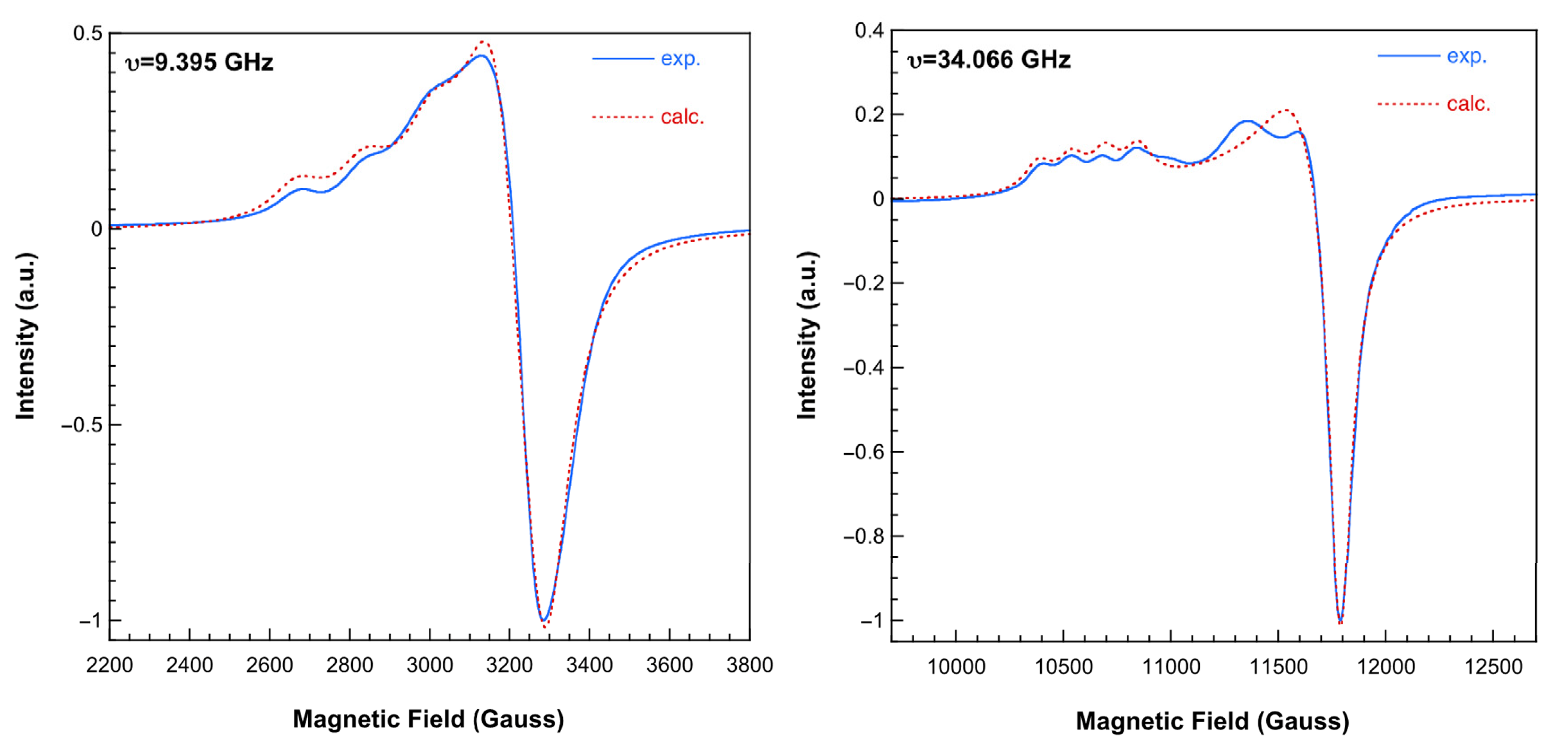
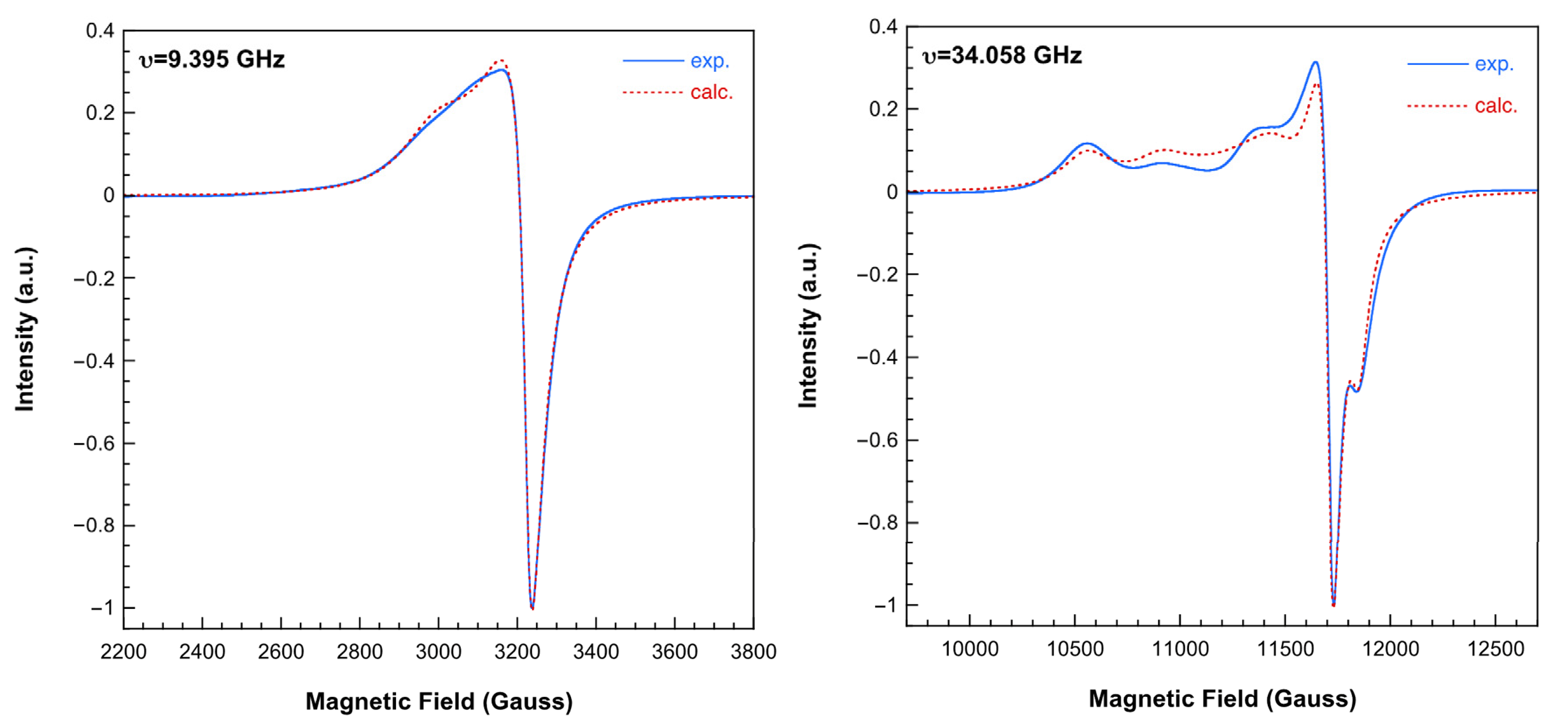
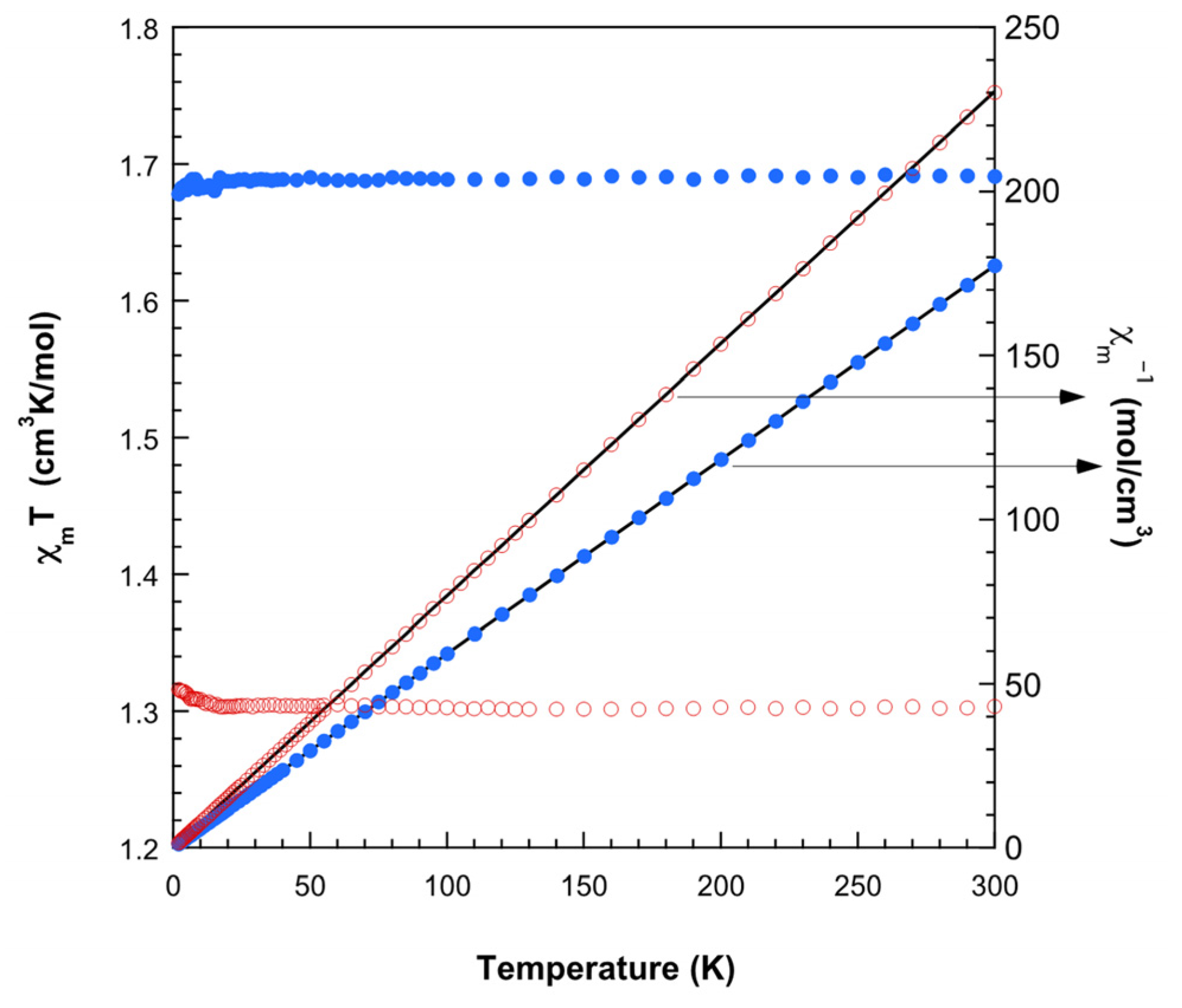
2.3.4. Electron Spin Resonance (ESR) Spectra and Magnetic Properties
2.4. Theoretical DFT Studies
3. Materials and Methods
3.1. Strategy and Procedure for the Synthesis of Both Studied Compounds
3.1.1. Synthesis of [Cu(Him)6]{μ-Cu(Him)4[Cu(EDTA)(Him)]2}·6H2O (1), with Relevant Vis-UV and FTIR Spectral Data
3.1.2. Synthesis of Compound (H2im)2[Cu(Him)4{(µ-EDTA)Cu(Him)}2]·2H2O (2), with Relevant Vis–UV and FT–IR Spectral Data
3.2. Crystal Structure Determination of (H2im)2[Cu(Him)4{(µ-EDTA)Cu(Him)}2]·2H2O (2)
3.3. Other Physical Measurements
3.4. ESR Spectra and Magnetism
3.5. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hathaway, B.J.; Copper Wilkinson, G.; Gillard, R.D.; McCleverty, J.A. (Eds.) Comprehensive Coordination Chemistry; Pergamon Press: Brighton, UK, 1987; Volume 5, pp. 534–540. [Google Scholar]
- Karlin, K.D.; Tyeklár, Z. (Eds.) Bioinorganic Chemistry of Copper; Chapman & Hall: New York, NY, USA; London, UK, 1993. [Google Scholar]
- Choquesillo Lazarte, D.; Sánchez Moreno, M.J.; Bugella Altamirano, E.; Moyano Gallego, M.J.; Martín Ramos, J.D.; González Pérez, J.M.; Carballo, R.; Niclós Gutiérrez, J.; Synthesis, J. Crystal Structure and Properties of [Cu(phen)3][Cu(pheida)2]·10H2O and [(phen)2Cu(μ-BAAP)Cu(m-BAAP)Cu(phen)2] [Cu(BAAP)2]·8.5H2O (H2pheida = N-phenethyl-iminodiacetic acid, H2BAAP = N-benzylaminoacetic-2-propionic acid). Z. Anorg. Allg. Chem. 2003, 629, 291–296. [Google Scholar] [CrossRef]
- Driessen, W.L.; Chang, L.; Finazzo, C.; Gorte, S.; Rehorst, D.; Reedijk, J.; Lutz, M.; Spek, A.L. Two Pyrazolato-Bridged, Linear Trinuclear Cu(II) Complexes. Crystal Structures and Magnetic Properties. Inorg. Chim. Acta 2003, 350, 25–31. [Google Scholar] [CrossRef]
- Dienga, M.; Thiama, I.; Gaye, M.; Salam Salla, A.; Barry, A.H. Synthesis, Crystal Structures and Spectroscopic Properties of a Trinuclear [Cu3(HL)2(NO3)2](H2O)(CH2CH2OH) Complex and a [Mn(HL)(CH3COO)]n Polymer with H3L = N,N′-(2-hydroxypropane-1,3-diyl)-bis-(salicylaldimine). Acta Chim. Slov. 2006, 53, 417–423. [Google Scholar]
- Antsyshkina, A.S.; Sadikov, G.G.; Poznyak, A.L.; Sergienko, V.S. Crystal Structure of [Cu2(Edta)(Py)2(H2O)2]·2H2O and [Cu(Im)6]{Cu(Im)4[Cu(Edta)(Im)]2}·6H2O, Products of the Interaction of (Ethylenediaminetetraacetato)diaquadicopper(II). Russ. J. Inorg. Chem. 2006, 51, 241–252. [Google Scholar] [CrossRef]
- Zhang, H.-P.; Zhou, H.; Pan, Z.-Q.; Meng, X.-G.; Song, Y. Synthesis, Crystal Structure and Magnetic Characterization of a Novel Linear Trinuclear Copper(II) Complex Bridged by Phenoxy and Benzyloxy Oxygen Atom. Trans. Met. Chem. 2008, 33, 55–60. [Google Scholar] [CrossRef]
- Botana, L.; Ruiz, J.; Seco, J.M.; Mota, A.J.; Rodríguez-Diéguez, A.; Sillanpaa, R.; Colacio, E. Influence of the Anions on the Structure and Magnetic Properties of a series of Bis(l-diphenoxo)-bridged Linear Trinuclear Copper(II) Complexes: An Experimental and Theoretical Study. Dalton Trans. 2011, 40, 12462–12471. [Google Scholar] [CrossRef]
- Seppälä, P.; Colacio, E.; Mota, A.J.; Sillanpää, R. Influence of the Anions on the Structure and Magnetic Properties of a Series of Bis(μ-diphenoxo)-bridged Linear Trinuclear Copper(II) Complexes: An Experimental and Theoretical study. Dalton Trans. 2012, 41, 2648–2658. [Google Scholar] [CrossRef]
- Thatipamula, K.C.; Bhargavi, G.; Rajasekharan, M.V. Synthesis, Crystal Structures and Magnetic Properties of a Linear Trinuclear Cu(II) Complex [Cu3(ClCH2COO)6(bpy)2] and a Dinuclear Complex [Cu2(bpy)2(μ-OH)(μ-OH2)(μ-ClCH2COO)](ClO4)2. ChemistrySelect 2019, 4, 3344–3350. [Google Scholar] [CrossRef]
- Cruz, C.; Audebrand, N.; Páez-Hernández, D.; Paredes-García, V. Novel linear trinuclear CuII compound with trapped chiral hemiaminal ligand: Magnetostructural study. Magnetochemistry 2023, 9, 175. [Google Scholar] [CrossRef]
- Meenakumari, S.; Tiwary, S.K.; Chakravarty, A.R. Synthesis, Crystal Structure, and Magnetic Properties of a Ferromagnetically Coupled Angular Trinuclear Copper(II) Complex [Cu3(O2CMe)4(bpy)3(H2O)](PF6)2. Inorg. Chem. 1994, 33, 2085–2289. [Google Scholar] [CrossRef]
- Gupta, S.; Mukherjee, A.; Nethaji, M.; Chakravarty, A.R. An angular trinuclear copper (II) complex as a model for the active site of multicopper oxidases. Polyhedron 2004, 23, 643–647. [Google Scholar] [CrossRef]
- Grenda, S.; Beau, M.; Luneau, D. Synthesis, Crystal Structure and Magnetic Properties of a Trinuclear Copper(II) Complex based on p-Cresol-substituted Bis(α-nitronyl nitroxide) Biradical. Magnetochemistry 2023, 9, 175. [Google Scholar] [CrossRef]
- Zheng, J.; Lu, Z.; Wu, K.; Ning, G.H.; Li, D. Coinage-metal-based Cyclic Trinuclear Complexes with Metal−metal Interactions: Theories to Experiments and Structures to Functions. Chem. Rev. 2020, 120, 9675–9742. [Google Scholar] [CrossRef] [PubMed]
- Agnus, Y.; Louis, R.; Metz, B.; Boudon, C.; Gisselbrecht, J.P.; Gross, M. Trinuclear Copper(II) Hydroxo and Hexanuclear Copper(II) Oxo Complexes with the Ligand 3-(Benzylimino)butanone 2-Oxime. Syntheses and Spectral, Structural, and Redox Characteristics. Inorg. Chem. 1991, 30, 3155–3161. [Google Scholar] [CrossRef]
- Ferrer, S.; Lloret, F.; Bertomeu, I.; Alzuet, G.; Borrás, J.; García-Granda, S.; Liu-González, M.; Haasnoot, J.G. Cyclic Trinuclear and Chain of Cyclic Trinuclear Copper(II) Complexes Containing a Pyramidal Cu3O(H) Core. Crystal Structures and Magnetic Properties of [Cu3(µ3-OH)(aaat)3(H2O)3](NO3)2·H2O [aaat = 3-Acetylamino-5-amino-1,2,4-triazolate] and {[Cu3(µ3-OH)(aat)3 (µ3-SO4)]·6H2O}n [aat = 3-Cetylamino-1,2,4-triazolate]: New Cases of Spin-frustrated Systems. Inorg. Chem. 2002, 41, 5821–5830. [Google Scholar] [CrossRef]
- Casarin, M.; Corvaja, C.; di Nicola, C.; Falcomer, D.; Franco, L.; Monari, M.; Pandolfo, L.; Pettinari, C.; Piccinelli, F.; Tagliatesta, P. Spontaneous Self-assembly of an Unsymmetric Trinuclear Triangular Copper(II) Pyrazolate Complex, [Cu3(µ3-OH)(µ-pz)3(MeCOO)2(Hpz)] (Hpz = pyrazole). Synthesis, Experimental and Theoretical Characterization, Reactivity, and Catalytic Activity. Inorg. Chem. 2004, 43, 5865–5876. [Google Scholar] [CrossRef]
- Yang, L.; Yuan, Z.; He, L.; Han, L.; Li, B.; Xu, Y. Polyoxometalate Functionalized Cyclic Trinuclear Copper Compounds for Bifunctional Electrochemical Detection and Photocatalytic Reduction of Cr(VI). Inorg. Chem. 2024, 63, 12564–12571. [Google Scholar] [CrossRef]
- El Bakkali, H.; Choquesillo-Lazarte, D.; Domínguez-Martín, A.; Pérez-Toro, M.I.; Lezama, L.; González-Pérez, J.M.; Castiñeiras, A.; Niclós-Gutiérrez, J. Cocrystallization of Mononuclear and Trinuclear Metallacycle Molecules from an Aqueous Mixed-ligand Copper(II) Solution. Cryst. Growth Des. 2014, 14, 889–892. [Google Scholar] [CrossRef]
- Yao, Z.S.; Guan, H.; Shiota, Y.; He, C.T.; Wang, X.L.; Wu, S.Q.; Zheng, X.; Su, S.-Q.; Yoshizawa, K.; Kong, X.; et al. Giant Anisotropic Thermal Expansion Actuated by Thermodynamically Assisted Reorientation of Imidazoliums in a Single Crystal. Nat. Commun. 2019, 10, 4805. [Google Scholar] [CrossRef]
- Wang, X.; Xue, J.; Sun, X.; Zhao, Y.; Wu, S.; Yao, Z.; Tao, J. Giant Single-crystal Shape Transformation with Wide Thermal Hysteresis Actuated by Synergistic Motions of Molecular Cations and Anions. Chem. Eur. J. 2020, 26, 6778–6783. [Google Scholar] [CrossRef]
- Belmont-Sánchez, J.C.; García-Rubiño, M.E.; Frontera, A.; González-Pérez, J.M.; Castiñeiras, A.; Niclós-Gutiérrez, J. H-bonds, π-Stacking and (Water)O-H/π Interactions in (µ4-EDTA)bis(Imidazole) Dicopper(II) Dihydrate. Crystals 2021, 11, 48. [Google Scholar] [CrossRef]
- Filippova, T.V.; Polynova, T.N.; Porai-Koshits, M.A.; Novozhilova, N.V.; Martynenko, L.I. The X-ray Crystal Structure of Copper Ethylenediaminetetraacetate, Cu2[C2N2H4(CH2COO)4]·4H2O. Russ. J. Struct. Chem. 1973, 14, 249–253. [Google Scholar] [CrossRef]
- García-Rubiño, M.E.; Matilla-Hernández, A.; Frontera, A.; Lezama, L.; Niclós-Gutiérrez, J.; Choquesillo-Lazarte, D. Dicopper(II)-EDTA Chelate as a Bicephalic Receptor Model for a Synthetic Adenine Nucleoside. Pharmaceuticals 2021, 14, 426. [Google Scholar] [CrossRef]
- Bonomo, R.P.; Riggi, F.; Di Bilio, A.J. EPR Reinvestigation of the Copper(II)-Imidazole System. Inorg. Chem. 1988, 27, 2510–2512. [Google Scholar] [CrossRef]
- Krishna, R.M.; Gupta, S.K. Electron paramagnetic resonance investigations of the Cu2+ ion in a variety of host lattices—A review. Bull. Magn. Reson. 1994, 16, 239–291. [Google Scholar]
- Kahn, O. Molecular Magnetism; VCH-Verlag: Weinheim, Germany; New York, NY, USA, 1993. [Google Scholar]
- Bruker. APEX3 Software, v2018.7-2; Bruker AXS Inc.: Madison, WI, USA, 2018.
- Sheldrick, G.M. SADABS: Program for Empirical Absorption Correction of Area Detector Data; University of Goettingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Wilson, A.J.C. International Tables for Crystallography, Volume C; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1995. [Google Scholar]
- Spek, A.L. Structure Validation in Chemical Crystallography. Acta Crystallogr. 2009, D65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Ahlrichs, R.; Bar, M.; Haser, M.; Horn, H.; Kolmel, C. Electronic Structure Calculations on Workstation Computers: The Programs Turbomole. Chem. Phys. Lett. 1989, 162, 165–169. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate ab initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting Basis Sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Adamo, C.; Barone, V. Toward Reliable Density Functional Methods without Adjustable Parameters: The PBE0 Model. J. Chem. Phys. 1999, 110, 6158–6169. [Google Scholar] [CrossRef]
- Contreras-García, J.; Johnson, E.R.; Keinan, S.; Chaudret, R.; Piquemal, J.-P.; Beratan, D.N.; Yang, W. NCIPLOT: A Program for Plotting Non-covalent Interaction Regions. J. Chem. Theory Comput. 2011, 7, 625–632. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.S.; Hoard, J.L. The Structure of Dihydrogen Ethylenediaminetetraacetato-aquonickel(II). J. Am. Chem. Soc. 1959, 81, 556–561. [Google Scholar] [CrossRef]
- Kocanova, I.; Kuchar, J.; Dankovicova, V.; Cernak, J. Redetermination of aqua-(dihydrogen ethyl-enediamine-tetra-acetato-κO,O′,N,N′,O″)nickel(II). Acta Crystallogr. Sect. E 2010, 66, m196–m1967. [Google Scholar] [CrossRef]
- Garvan, F.L. Chapter 7: Metal Chelates of Ethylenediaminotetraacetic acid and Related Substances. In Chelating Agents and Metal Chelates; Dwyer, F.P., Mellor, D.P., Eds.; Academic Press Inc.: Cambridge, MA, USA, 1964. [Google Scholar]
- Von Zelewsky, A. Chapter 5: Topographical Stereochemistry of Mononuclear Coordination, Units. In Stereochemistry of Coordination Compounds; John Wiley & Son Ltd.: New York, NY, USA, 1996; pp. 116–128. [Google Scholar]
- Hawkins, C.J. Absolute Configuration of Metal Complexes; John Wiley & Son Ltd.: New York, NY, USA, 1971; Chapters 2 (Notation of Absolute Configuration) and 3 (Conformational Analysis). [Google Scholar]
- Bellamy, L.J. The Infrared Spectra of Complex Molecules, 3rd ed.; Chapman and Hall: London, UK, 1975; Chapters 13 (Amino-acids, their Hydrochlorides and Salts, and Amido-acids), 14 (Amines and Imines), 15 (Unsaturated Nitrogen Compounds). [Google Scholar]
- Nakanishi, K. Infrared Absorption Spectroscopy Practical; Holden-Day, Inc.: San Francisco, CA, USA; Nankodo Co., Ltd.: Tokyo, Japan, 1962; Chapter 2. Tables of characteristic frequencies. The Table 7. Amines and Ammonium Salts). See, in particular, Table 7d (Amine Salts) in pag. 37. Band(s) or group of relatively sharp band at 2700–2250 cm−1. [Google Scholar]
- Nakanoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 6th ed.; Part B: Applications in Coordination, Organometallic and Bioinorganic Chemistry; Wiley & Sons: New York, NY, USA, 2009; pp. 67–74, Part 1 (Applications in Coordination Chemistry). 1.10. Complexes of Amino acids, EDTA and Related Ligands. [Google Scholar]
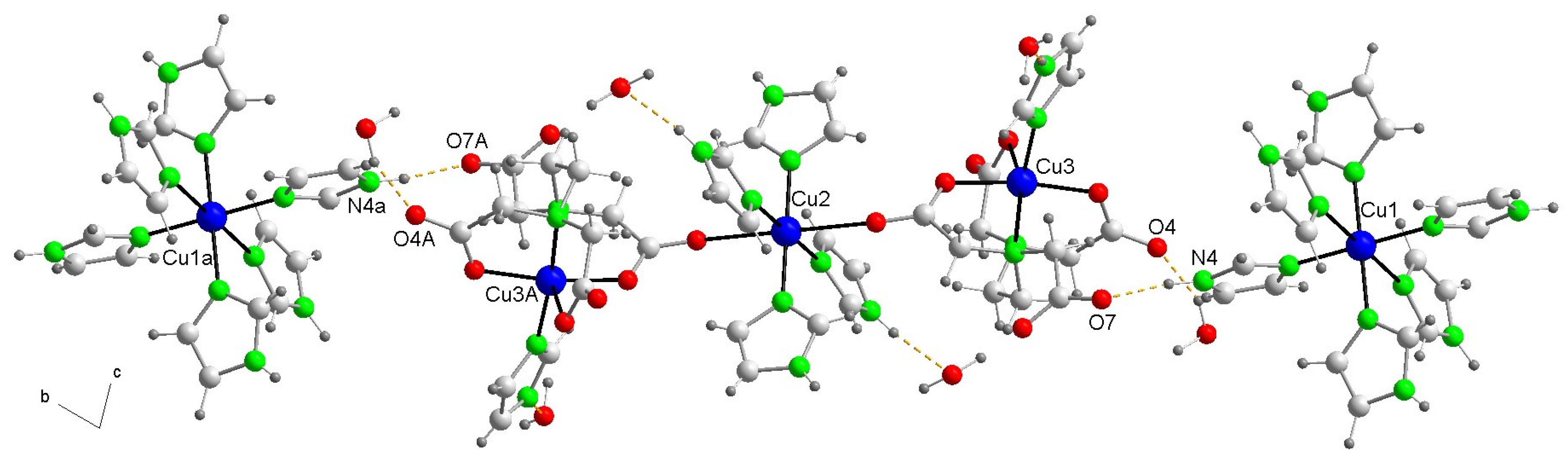
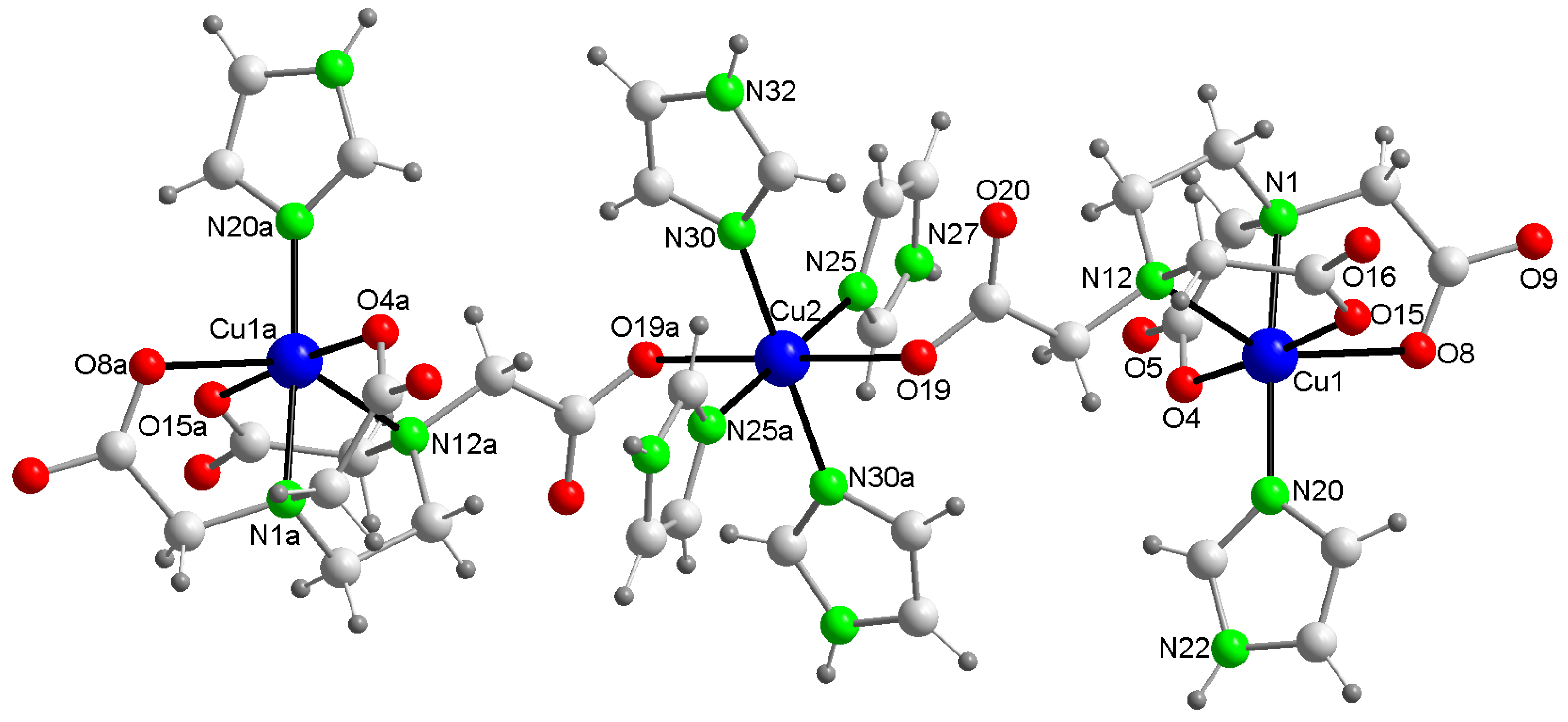
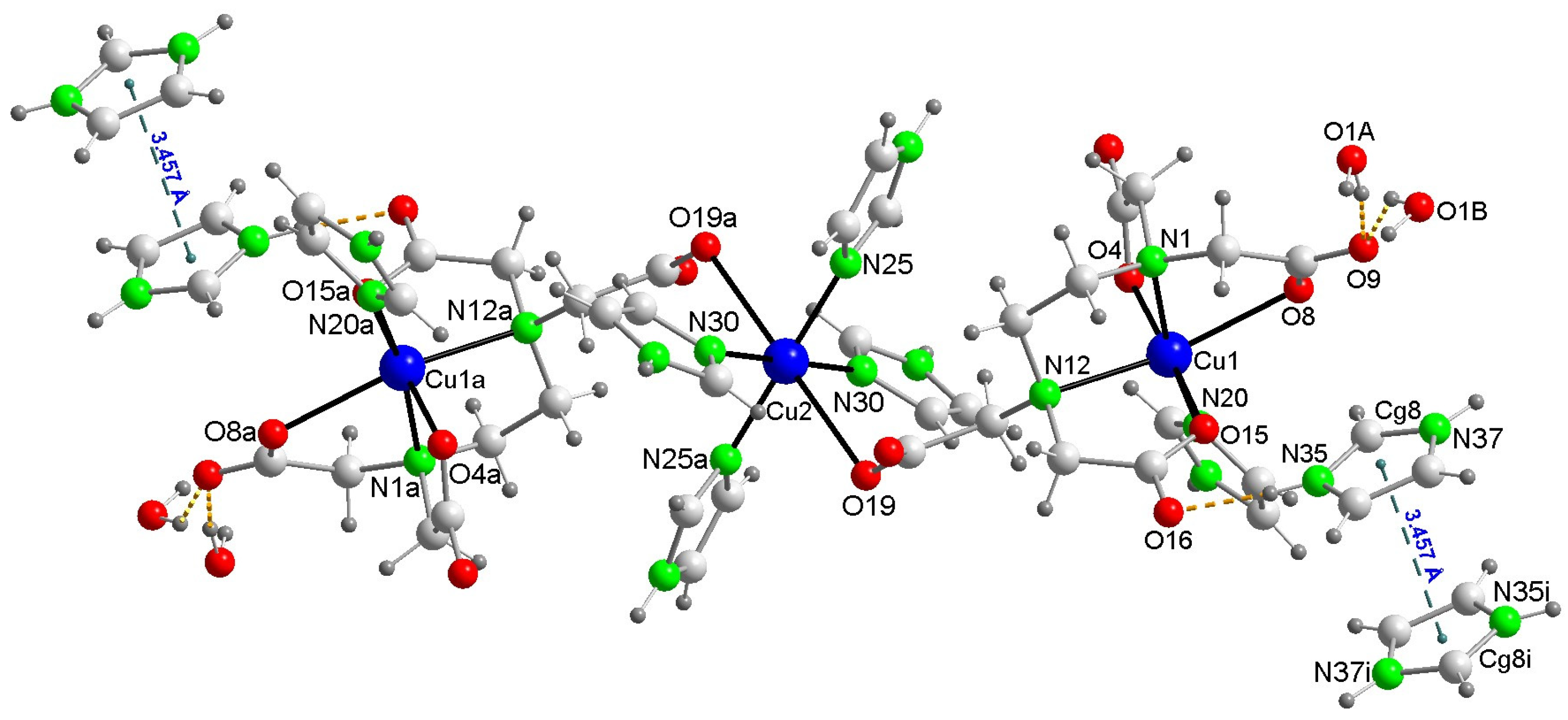
| Bond or Intermetallic | Distance (Å) | Trans-Angles (°) | |
|---|---|---|---|
| Cu(1)-O(4) | 1.950(2) | O(4)-Cu(1)-O(15) | 175.75(8) |
| Cu(1)-O(15) | 1.974(2) | ||
| Cu(1)-N(20) | 1.988(2) | N(20)-Cu(1)-N(1) | 168.44(9) |
| Cu(1)-N(1) | 2.102(2) | ||
| Cu(1)-N(12) | 2.326(2) | N(12)-Cu(1)-O(8) | 150.93(7) |
| Cu(1)-O(8) | 2.456(2) | ||
| Cu(2)-N(30) | 1.996(2) | N(30)-Cu(2)-N(30)#1 | 180.0(1) |
| Cu(2)-N(30)#1 | 1.996(2) | ||
| Cu(2)-N(25) | 2.033(2) | N(25)-Cu(2)-N(25)#1 | 180.0 |
| Cu(2)-N(25)#1 | 2.033(2) | ||
| Cu(2)-O(19) | 2.720(3) | O(19)#1-Cu(2)-O(19) | 180.0 |
| Cu(2)-O(19)#1 | 2.720(3) | ||
| Cu(1)···Cu(2) | 6.803(1) | ||
| Step or R | Temp. (°C) | Time (min) | Weight (%) Exp. | Weight (%) Cal. | Evolved Gases or Residue (R) |
|---|---|---|---|---|---|
| 1 | 80–175 | 3–15 | 8.903 | >6.157 | 6 H2O, CO2 (t) |
| 2 | 175–260 | 15–24 | 28.909 | - | CO2, H2O |
| 3 | 260–270 | 24–26 | CO2, H2O, CO | ||
| 4 | 270–485 | 26–38 | 18.109 | - | CO2, H2O, CO, N2O (t) |
| 5 | 485–515 | 38–50 | 23.913 | - | CO2, H2O, N2O, NO, NO2, X |
| R | 565 | 57 | 18.341 | 18.123 | CuO |
| Step or R | Temp. (°C) | Time (min) | Weight (%) Exp. | Weight (%) Cal. | Evolved Gases or Residue (R) |
|---|---|---|---|---|---|
| 1 | 50–190 | 2–17 | 7.220 | >>2.669 | 2 H2O (N), CO2 |
| 2 | 190–220 | 17–20 | 7.565 | - | CO2, H2O |
| 3 | 220–295 | 20–25 | 32.015 | CO2, H2O, CO (t) | |
| 4 | 295–455 | 25–44 | 13.743 | CO2, H2O, CO, N2O | |
| 5 | 455–500 | 44–47 | 13.771 | - | CO2, H2O, CO, N2O, NO, CH4 |
| 6 | 500–580 | 47–60 | 7.767 | CO2, H2O, CO, N2O, NO, NO2, CH4, X | |
| R1 | 580 | 60 | 17.896 | 17.680 | CuO (with some impurities) |
| R2 | 950 | 93 | 17.852 | 17.680 | CuO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belmont-Sánchez, J.C.; Choquesillo-Lazarte, D.; Frontera, A.; Lezama, L.; Castiñeiras, A.; Niclós-Gutiérrez, J. Magnetic Isolation of the Linear Trinuclear Anion in [Cu(Him)6] {Cu(Him)4[Cu(μ-EDTA)(Him)]2}·6H2O (1) as the Novel Imidazolium(+) Salt (H2im)2[Cu(Him)4{(µ-EDTA)Cu(Him)}2]·2H2O (2)—A Comparative Look to Their Crystal Structures, Thermal, Spectral and Magnetic Properties and DFT Calculations. Int. J. Mol. Sci. 2024, 25, 13130. https://doi.org/10.3390/ijms252313130
Belmont-Sánchez JC, Choquesillo-Lazarte D, Frontera A, Lezama L, Castiñeiras A, Niclós-Gutiérrez J. Magnetic Isolation of the Linear Trinuclear Anion in [Cu(Him)6] {Cu(Him)4[Cu(μ-EDTA)(Him)]2}·6H2O (1) as the Novel Imidazolium(+) Salt (H2im)2[Cu(Him)4{(µ-EDTA)Cu(Him)}2]·2H2O (2)—A Comparative Look to Their Crystal Structures, Thermal, Spectral and Magnetic Properties and DFT Calculations. International Journal of Molecular Sciences. 2024; 25(23):13130. https://doi.org/10.3390/ijms252313130
Chicago/Turabian StyleBelmont-Sánchez, Jeannette Carolina, Duane Choquesillo-Lazarte, Antonio Frontera, Luis Lezama, Alfonso Castiñeiras, and Juan Niclós-Gutiérrez. 2024. "Magnetic Isolation of the Linear Trinuclear Anion in [Cu(Him)6] {Cu(Him)4[Cu(μ-EDTA)(Him)]2}·6H2O (1) as the Novel Imidazolium(+) Salt (H2im)2[Cu(Him)4{(µ-EDTA)Cu(Him)}2]·2H2O (2)—A Comparative Look to Their Crystal Structures, Thermal, Spectral and Magnetic Properties and DFT Calculations" International Journal of Molecular Sciences 25, no. 23: 13130. https://doi.org/10.3390/ijms252313130
APA StyleBelmont-Sánchez, J. C., Choquesillo-Lazarte, D., Frontera, A., Lezama, L., Castiñeiras, A., & Niclós-Gutiérrez, J. (2024). Magnetic Isolation of the Linear Trinuclear Anion in [Cu(Him)6] {Cu(Him)4[Cu(μ-EDTA)(Him)]2}·6H2O (1) as the Novel Imidazolium(+) Salt (H2im)2[Cu(Him)4{(µ-EDTA)Cu(Him)}2]·2H2O (2)—A Comparative Look to Their Crystal Structures, Thermal, Spectral and Magnetic Properties and DFT Calculations. International Journal of Molecular Sciences, 25(23), 13130. https://doi.org/10.3390/ijms252313130








