First-Principles Study of the Consequences of Cerium Doping on the Photocatalytic Activity of Zinc Oxide
Abstract
1. Introduction
2. Results and Discussion
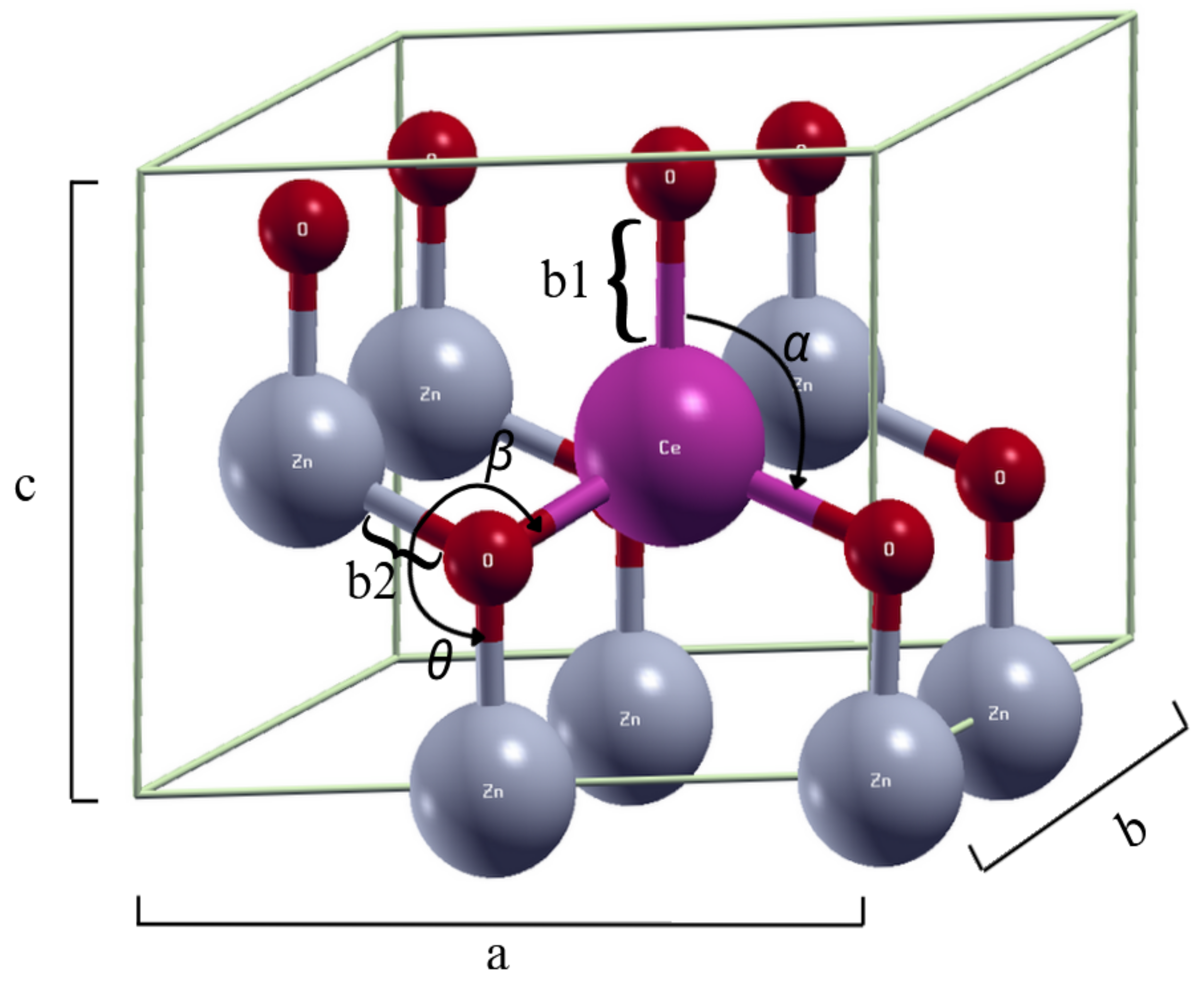
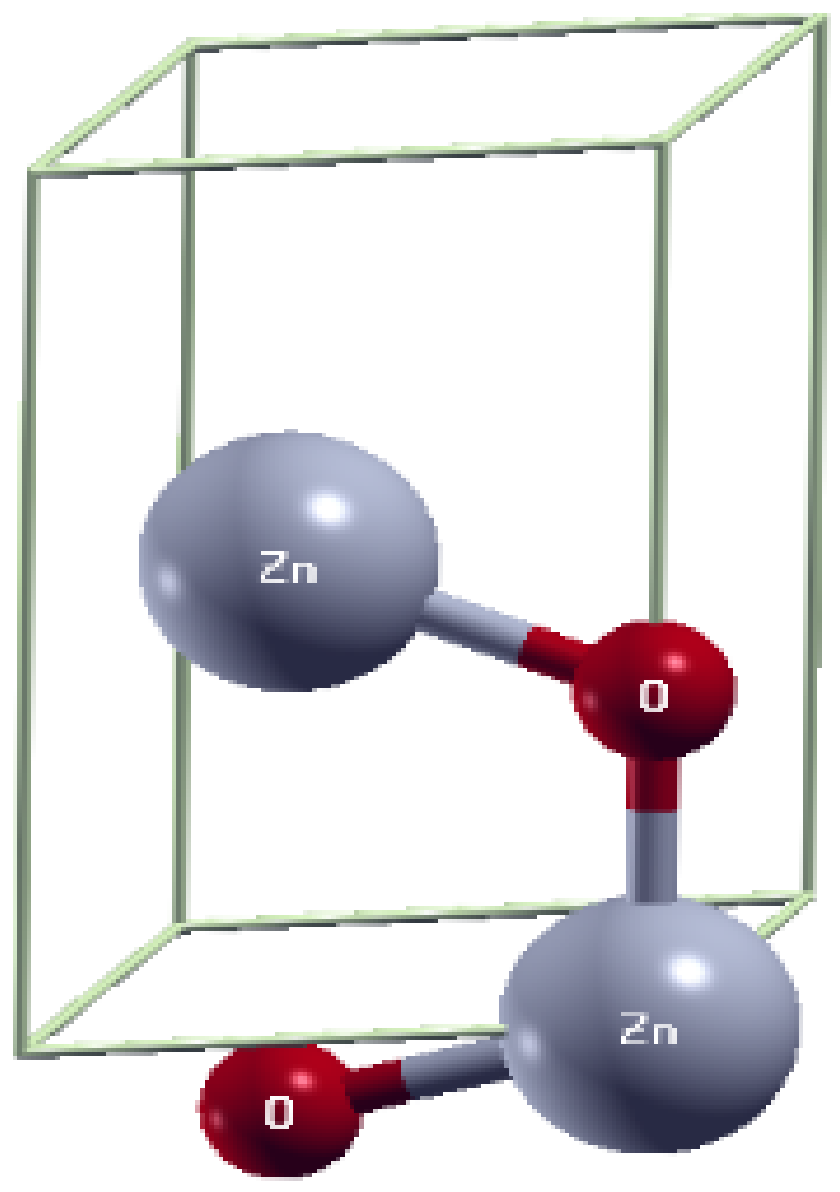
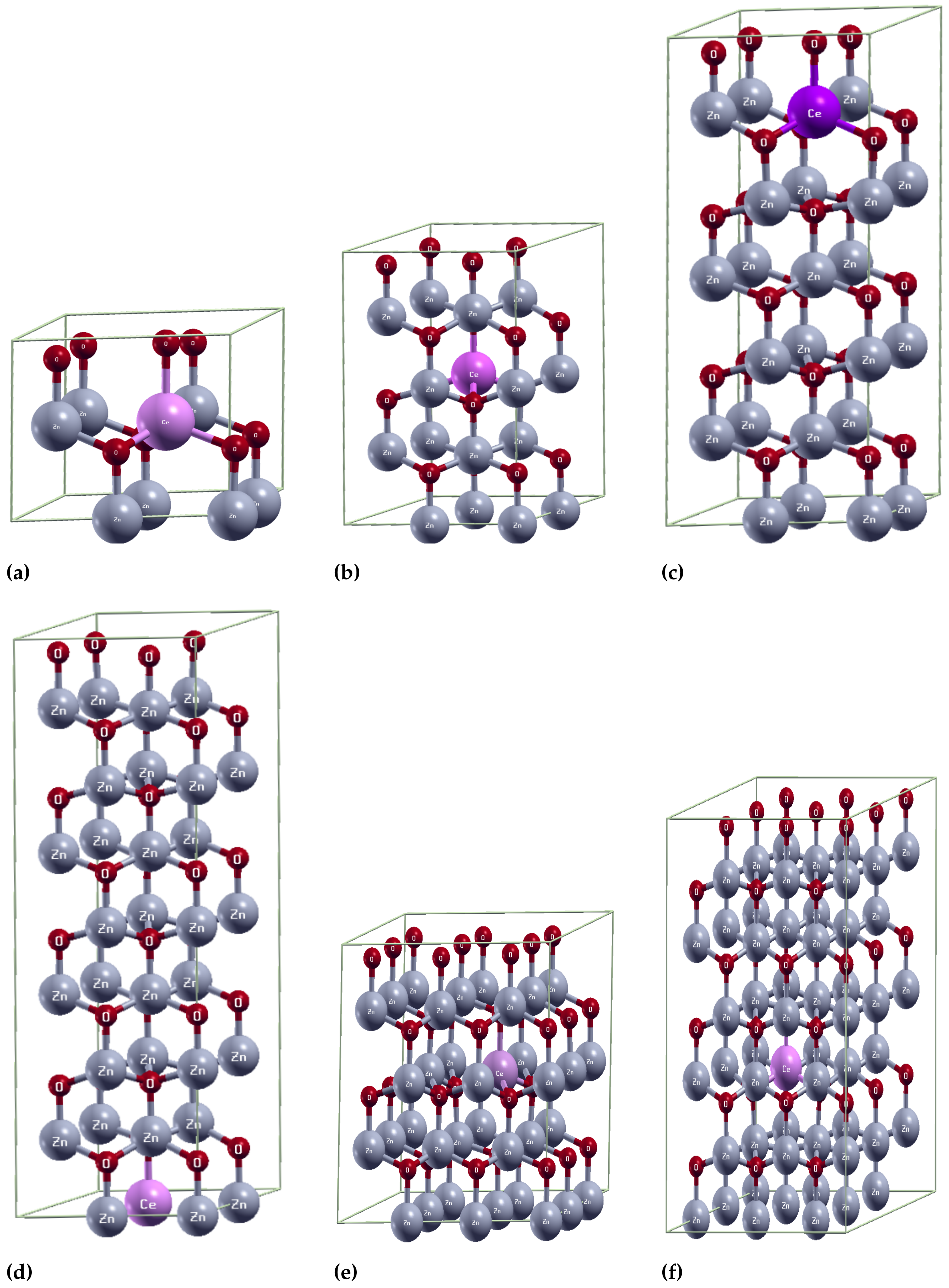
2.1. Structural Properties
| Reference | |||||||
|---|---|---|---|---|---|---|---|
| - | 1.991 | - | 108.662 | - | 3.267 | 5.273 | |
| 2.267 | 1.997 | 108.203 | 113.323 | 105.080 | 3.329 | 5.268 | |
| 2.270 | 1.999 | 108.168 | 113.274 | 105.136 | 3.290 | 5.273 | |
| 2.275 | 2.001 | 108.117 | 113.195 | 110.790 | 3.297 | 5.284 | |
| 2.268 | 1.976 | 111.109 | 114.590 | 107.785 | 3.294 | 5.305 | |
| 2.269 | 1.978 | 110.540 | 114.379 | 103.322 | 3.302 | 5.317 | |
| 2.275 | 1.984 | 110.402 | 114.143 | 103.702 | 3.319 | 5.339 | |
| 2.342 | 2.004 | 109.054 | 110.987 | 104.773 | 3.373 | 5.394 | |
| Exp [33] | - | - | - | - | - | 3.242 | 5.194 |
| Exp [29] | - | - | - | - | - | 3.254 | 5.211 |
| Exp (3.0%) [29] | - | - | - | - | - | 3.252 | 5.213 |
| Exp (6.0%) [29] | - | - | - | - | - | 3.253 | 5.215 |
| Exp [30] | - | - | - | - | - | 3.249 | 5.204 |
| Exp (3.0%) [30] | - | - | - | - | - | 3.252 | 5.209 |
| Exp (6.0%) [30] | - | - | - | - | - | 3.253 | 5.210 |
| Exp (12.0%) [30] | - | - | - | - | - | 3.255 | 5.214 |
| [31] | - | - | - | - | - | 3.242 | 5.211 |
| [31] | - | - | - | - | - | 3.297 | 5.275 |
| [31] | - | - | - | - | - | 3.301 | 5.314 |
| [31] | - | - | - | - | - | 3.328 | 5.365 |
| [32] | - | - | - | - | - | 3.289 | 5.308 |
| [32] | - | - | - | - | - | 3.315 | 5.344 |
| [32] | - | - | - | - | - | 3.328 | 5.346 |
| [32] | - | - | - | - | - | 3.338 | 5.364 |
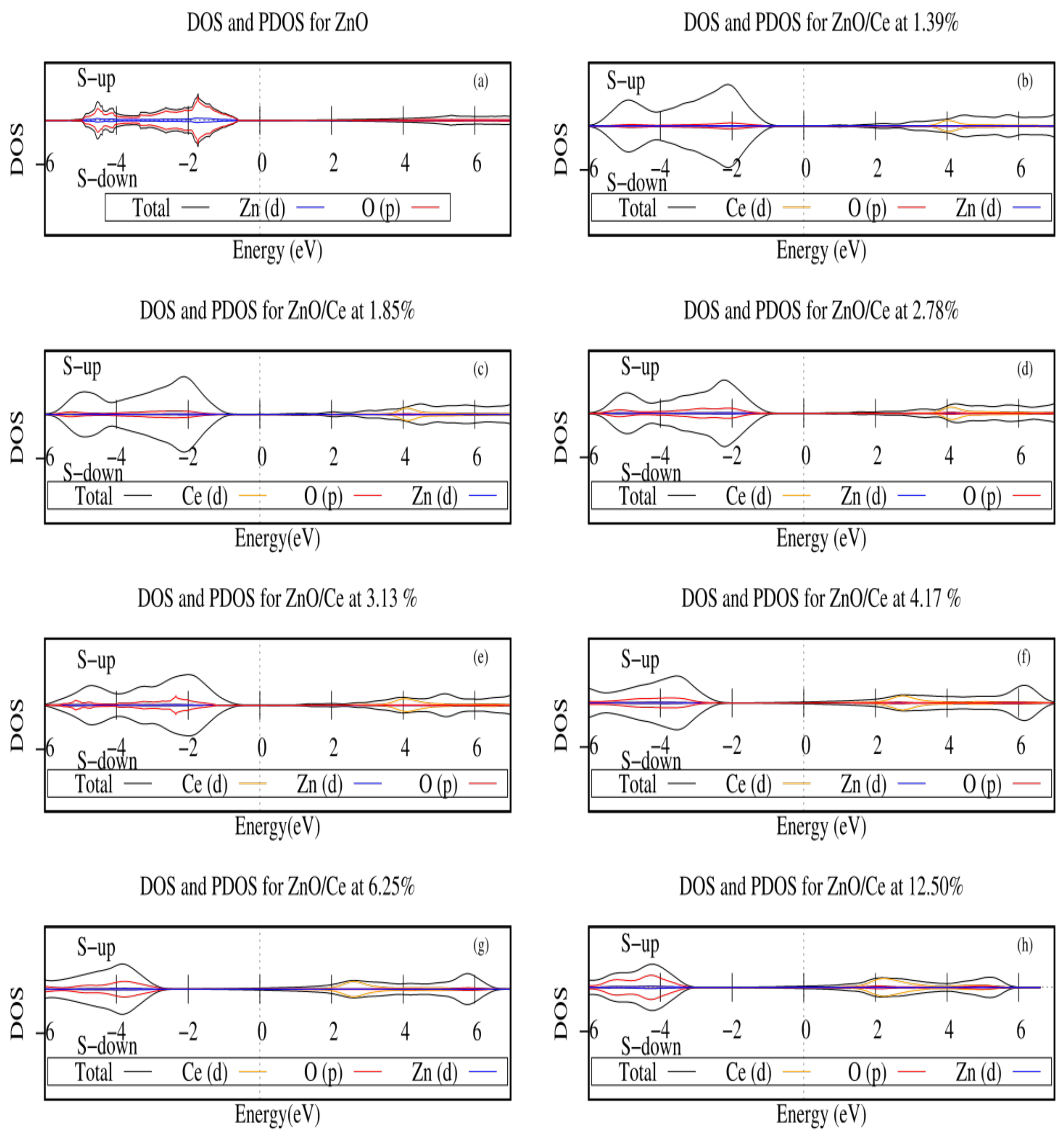
2.2. Electronic Structures
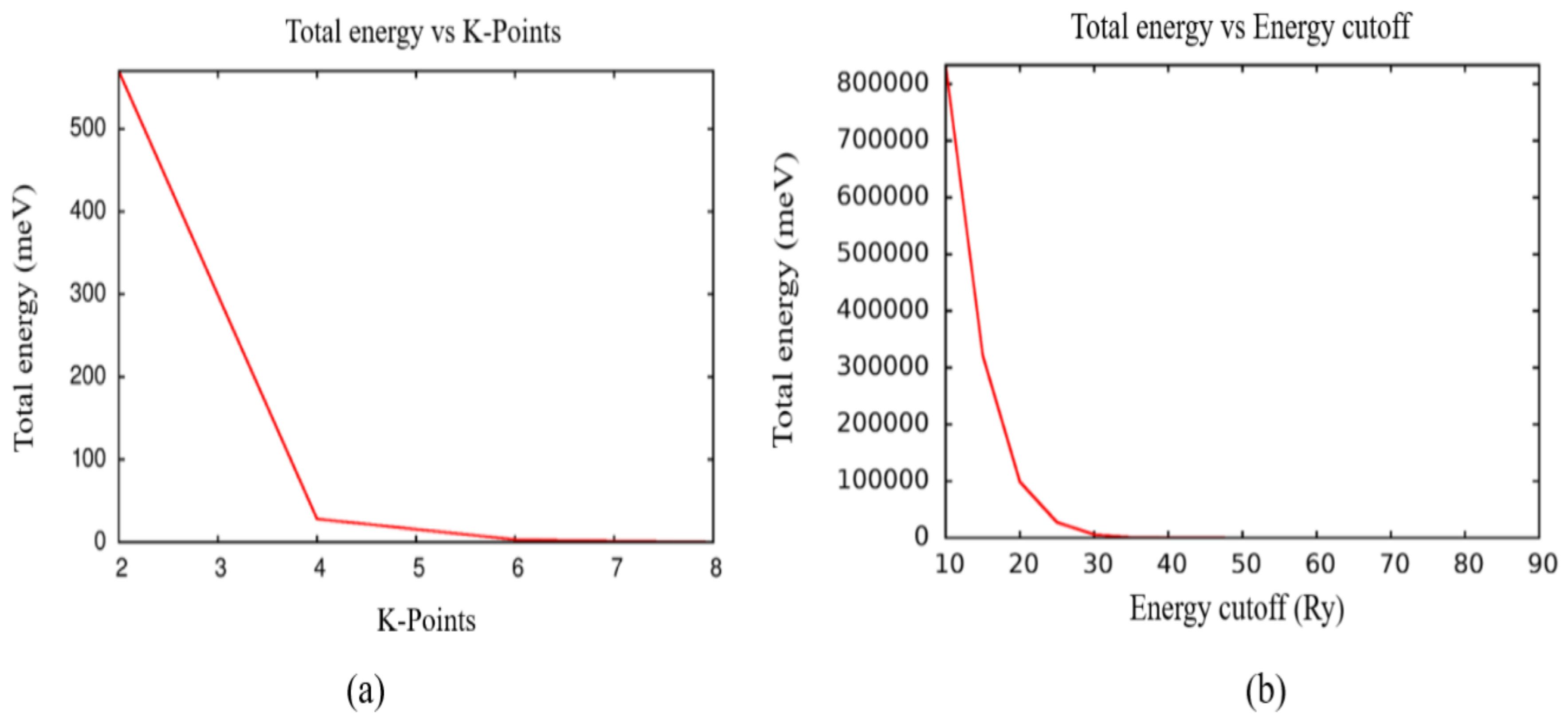
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mondragón-Suárez, J.H.; Sandoval-Villalbazo, A.; Breña-Ramos, F. Calentamiento global: Una secuencia didáctica. Revista Mexicana de Física E 2018, 65, 1. [Google Scholar] [CrossRef]
- Inumaru, J.; Hasegawa, T.; Shirai, H.; Nishida, H.; Noda, N.; Ohyama, S. Fossil fuels combustion and environmental issues. In Advances in Power Boilers; Elsevier: Amsterdam, The Netherlands, 2021; Volume 2, pp. 1–56. [Google Scholar] [CrossRef]
- Roy, S.C.; Varghese, O.K.; Paulose, M.; Grimes, C.A. Toward Solar Fuels: Photocatalytic Conversion of Carbon Dioxide to Hydrocarbons. ACS Nano 2010, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, Y. Understanding the Reaction Mechanism of Photocatalytic Reduction of CO2 with H2O on TiO2-Based Photocatalysts: A Review. Aerosol Air Qual. Res. 2014, 14, 453–469. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Tao, Y.; Shen, L.; Xu, Z.; Bian, Z.; Li, H. Challenges of photocatalysis and their coping strategies. Chem. Catal. 2022, 14, 1315–1345. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.-N.; Zhan, Z.; Woo, M.-H.; Wu, C.-Y.; Biswas, P. Photocatalytic Reduction of CO2 with H2O on Mesoporous Silica Supported Cu/TiO2 Catalysts. Appl. Catal. B Environ. 2010, 100, 386–392. [Google Scholar] [CrossRef]
- Karakurt, H.; Kartal, O.E. Investigation of photocatalytic activity of TiO2 nanotubes synthesized by hydrothermal method. Chem. Eng. Commun. 2022, 210, 1383–1403. [Google Scholar] [CrossRef]
- Ha, Y.; Kwon, J.; An, H.; Jung, D. Improvement of Carbon Dioxide Reduction Efficiency of Titanium Dioxide Photocatalyst Using 1-propanol. J. Sens. Sci. Technol. 2022, 31, 343–347. [Google Scholar] [CrossRef]
- Danyliuk, N.; Mironyuk, I.; Tatarchuk, T.; Shyichuk, A. Optimal H2O2 Concentration in Advanced Oxidation over Titanium Dioxide Photocatalyst. Phys. Chem. Solid State 2021, 22, 73–79. [Google Scholar] [CrossRef]
- Le, T.M.; Nguyen, T.D. Enhancement of visible-light photocatalysis of TiO2 via nanocomposite incorporating with Fe(III) species. Results Eng. 2024, 23, 102500. [Google Scholar] [CrossRef]
- Muruganandham, M.; Chen, I.S.; Wu, J.J. Effect of temperature on the formation of macroporous ZnO bundles and its application in photocatalysis. J. Hazard. Mater. 2009, 172, 700–706. [Google Scholar] [CrossRef]
- Kansal, S.K.; Singh, M.; Sud, D. Studies on photodegradation of two commercial dyes in aqueous phase using different photocatalysts. J. Hazard. Mater. 2007, 141, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.; Bibi, S.; Jatoi, W.B.; Tuzen, M.; Jakhrani, M.A.; Feng, X.; Saleh, T.A. Green synthesized zinc oxide nanostructures and their applications in dye-sensitized solar cells and photocatalysis: A review. Mater. Today Commun. 2023, 141, 581–590. [Google Scholar] [CrossRef]
- Raha, S.; Ahmaruzzaman, M. ZnO nanostructured materials and their potential applications: Progress, challenges and perspectives. Nanoscale Adv. 2022, 4, 1868–1925. [Google Scholar] [CrossRef] [PubMed]
- Doria, A.; Ponnefz Durango, R.E.; Alcalá Varilla, L.A. Activation of carbon dioxide on zinc oxide surfaces doped with cerium. Emergent Mater. 2024, 7, 1997–2005. [Google Scholar] [CrossRef]
- Azizi, A.; Kazemi, M. Green synthesis of zinc oxide magnetic nanocomposite via zinc electroplating effluent: Its characterization and application as a photocatalyst. Results Opt. 2024, 16, 100698. [Google Scholar] [CrossRef]
- Stefan, M.; Popa, A.; Toloman, D.; Leostean, C.; Barbu-Tudoran, L.; Falamas, A. Enhanced Plasmonic Photocatalysis of Au-Decorated ZnO Nanocomposites. Inorganics 2023, 11, 157. [Google Scholar] [CrossRef]
- Bawazeer, T.M. Facile and Novel Route for the Preparation of ZnO Nanoparticles with Different Cr Loadings for Opto-Photocatalysis Applications. Catalysts 2022, 12, 100698. [Google Scholar] [CrossRef]
- Su, X.; Zhao, X.; Cui, C.; Xi, N.; Zhang, X.L.; Liu, H.; Yu, X.; Sang, Y. Influence of Wurtzite ZnO Morphology on Piezophototronic Effect in Photocatalysis. Catalysts 2022, 12, 946. [Google Scholar] [CrossRef]
- Vallejo, W.; Cantillo, A.; Díaz-Uribe, C. Improvement of the photocatalytic activity of ZnO thin films doped with manganese. EHeliyon 2023, 9, e20809. [Google Scholar] [CrossRef]
- Gebretsadik, A.; Kefale, B.; Sori, C.; Tsegaye, D.; Murthy, H.A.; Abebe, B. Cu-doped ZnO/Ag/CuO heterostructure: Superior photocatalysis and charge transfer. RSC Adv. 2024, 14, 41. [Google Scholar] [CrossRef]
- Selvam, P.P.; Rathinam, V.; Arunraj, A. Synthesis effect of Mg-doped ZnO nanoparticles for visible light photocatalysis. Ionics 2023, 29, 3723–3729. [Google Scholar] [CrossRef]
- Jellal, I.; Daoudi, O.; Nouneh, K. Comparative study on the properties of Al- and Ni-doped ZnO nanostructured thin films grown by SILAR technique: Application to solar photocatalysis. Opt. Quantum Electron. 2023, 55, 620. [Google Scholar] [CrossRef]
- Sin, J.C.; Lam, S.M.; Lee, K.T.; Mohamed, A.R. Preparation of rare earth-doped ZnO hierarchical micro/nanospheres and their enhanced photocatalytic activity under visible light irradiation. Ceram. Int. 2014, 40, 4. [Google Scholar] [CrossRef]
- Bechambi, O.; Touati, A.; Sayadi, S.; Najjar, W. Effect of cerium doping on the textural, structural and optical properties of zinc oxide: Role of cerium and hydrogen peroxide to enhance the photocatalytic degradation of endocrine disrupting compounds. Mater. Sci. Semicond. Process. 2015, 39, 807–816. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmed, E.; Zafar, F.; Khalid, N.R.; Niaz, N.A.; Hafeez, A.; Ikram, M.; Khan, M.A.; Zhanglian, H.O. Enhanced photocatalytic activity of Ce-doped ZnO nanopowders synthesized by combustion method. J. Rare Earths 2015, 33, 3. [Google Scholar] [CrossRef]
- Pathak, T.K.; Coetsee-Hugo, E.; Swart, H.C.; Swart, C.W.; Kroon, R.E. Preparation and characterization of Ce doped ZnO nanomaterial for photocatalytic and biological applications. Mater. Sci. Eng. B 2020, 261, 114780. [Google Scholar] [CrossRef]
- Wen, J.Q.; Zhang, J.M.; Qiu, Z.G.; Yang, X.; Li, Z.Q. The investigation of Ce doped ZnO crystal: The electronic. optical and magnetic properties. Phys. B Condens. Matter 2018, 534, 44–50. [Google Scholar] [CrossRef]
- Vijayaprasath, G.; Soundarrajan, P.; Ravi, G. Synthesis of ZnO Nanosheets Morphology by Ce Doping for Photocatalytic Activity. J. Electron. Mater. 2019, 48, 684–695. [Google Scholar] [CrossRef]
- Parangusan, H.; Ponnamma, D.; Al-Maadeed, M.A.A. Effect of cerium doping on the optical and photocatalytic properties of ZnO nanoflowers. Bull. Mater. Sci. 2019, 42, 179. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, J.; Liu, C.; Wang, T.; Liu, H.; Shen, T. A first-principle study on photoelectric characteristics of Ce-doped ZnO. Ferroelectrics 2021, 573, 214–223. [Google Scholar] [CrossRef]
- Jia, X.F.; Hou, Q.Y.; Xu, Z.C.; Qu, L.F. Effect of Ce doping on the magnetic and optical properties of ZnO by the first principle. J. Magn. Magn. Mater. 2018, 465, 128–135. [Google Scholar] [CrossRef]
- Ravbar, M.; Maver, K.; Knaflič, T.; Arčon, I.; Tušar, N.N.; Štangar, U.L.; Šuligoj, A. Nickel-decorated ZnO nanoparticles for effective solar reduction of hexavalent chromium and removal of selected pharmaceuticals. Appl. Surf. Sci. 2025, 681, 161463. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. Quantum Espresso:A modular and open-source software project for quantum simulations of material. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef]
- Scandolo, S.; Giannozzi, P.; Cavazzoni, C.; de Gironcoli, S.; Pasquarello, A.; Baroni, S. First-principles codes for computational crystallography in the Quantum-ESPRESSO package. Z. Krist. Cryst. Mater. 2005, 220, 574–579. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 1990, 41, 7892. [Google Scholar] [CrossRef]
- Head, J.D.; Zerner, M.C. A Broyden—Fletcher—Goldfarb—Shanno optimization procedure for molecular geometries. Chem. Phys. Lett. 1985, 122, 264–270. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez-Mena, E.Y.; Alcalá-Varilla, L.A.; Ortiz-Romero, J.D. First-Principles Study of the Consequences of Cerium Doping on the Photocatalytic Activity of Zinc Oxide. Int. J. Mol. Sci. 2024, 25, 13033. https://doi.org/10.3390/ijms252313033
Rodriguez-Mena EY, Alcalá-Varilla LA, Ortiz-Romero JD. First-Principles Study of the Consequences of Cerium Doping on the Photocatalytic Activity of Zinc Oxide. International Journal of Molecular Sciences. 2024; 25(23):13033. https://doi.org/10.3390/ijms252313033
Chicago/Turabian StyleRodriguez-Mena, Eimy Y., Luis A. Alcalá-Varilla, and José D. Ortiz-Romero. 2024. "First-Principles Study of the Consequences of Cerium Doping on the Photocatalytic Activity of Zinc Oxide" International Journal of Molecular Sciences 25, no. 23: 13033. https://doi.org/10.3390/ijms252313033
APA StyleRodriguez-Mena, E. Y., Alcalá-Varilla, L. A., & Ortiz-Romero, J. D. (2024). First-Principles Study of the Consequences of Cerium Doping on the Photocatalytic Activity of Zinc Oxide. International Journal of Molecular Sciences, 25(23), 13033. https://doi.org/10.3390/ijms252313033






