2′-Hydroxycinnamaldehyde, a Natural Product from Cinnamon, Alleviates Ischemia/Reperfusion-Induced Microvascular Dysfunction and Oxidative Damage in Rats by Upregulating Cytosolic BAG3 and Nrf2/HO-1
Abstract
1. Introduction
2. Results
2.1. HCA Preconditioning Inhibited H2O2-Induced Cell Death and Upregulated BAG3 Expression in H9c2 Cells
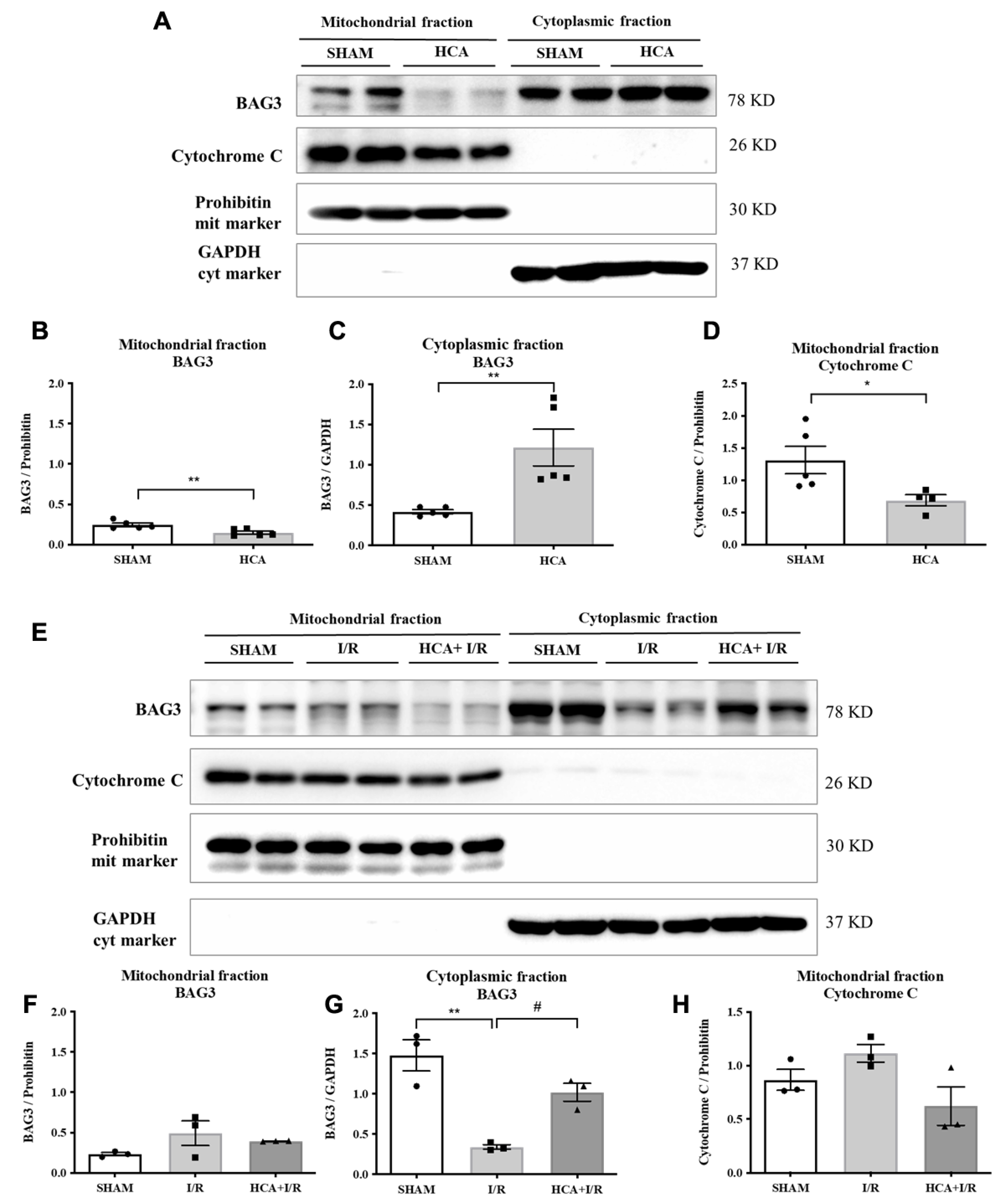
2.2. HCA Preconditioning Increased Cytosolic but Not Mitochondrial BAG3 Expression in HCA and HCA+I/R Hearts
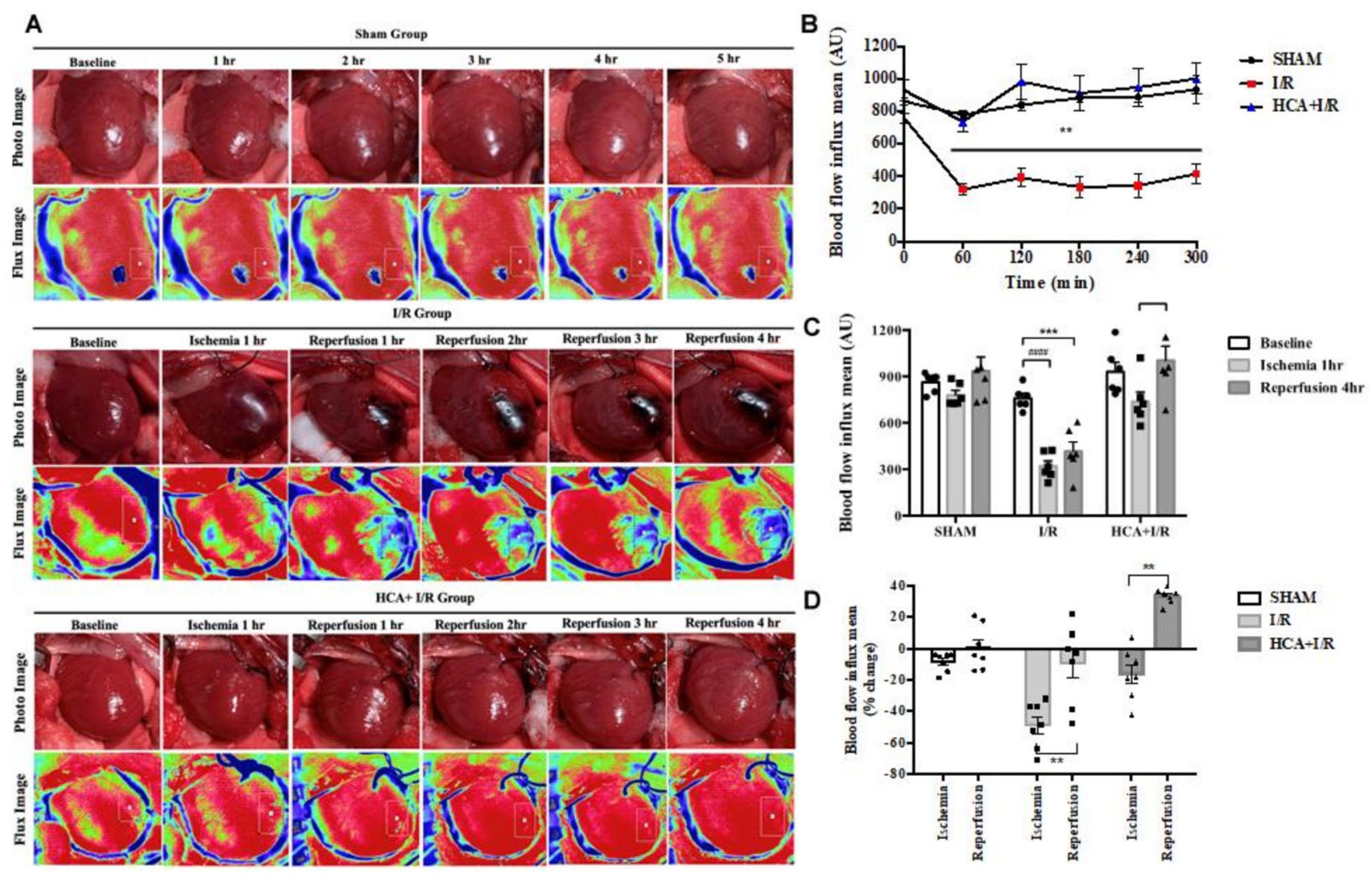
2.3. HCA Preconditioning Preserved Cardiac I/R-Induced Decrease in Cardiac Surface Microcirculation
2.4. HCA Preconditioning Restored Cardiac I/R-Induced Increase in LVEDP and Contractile Activity
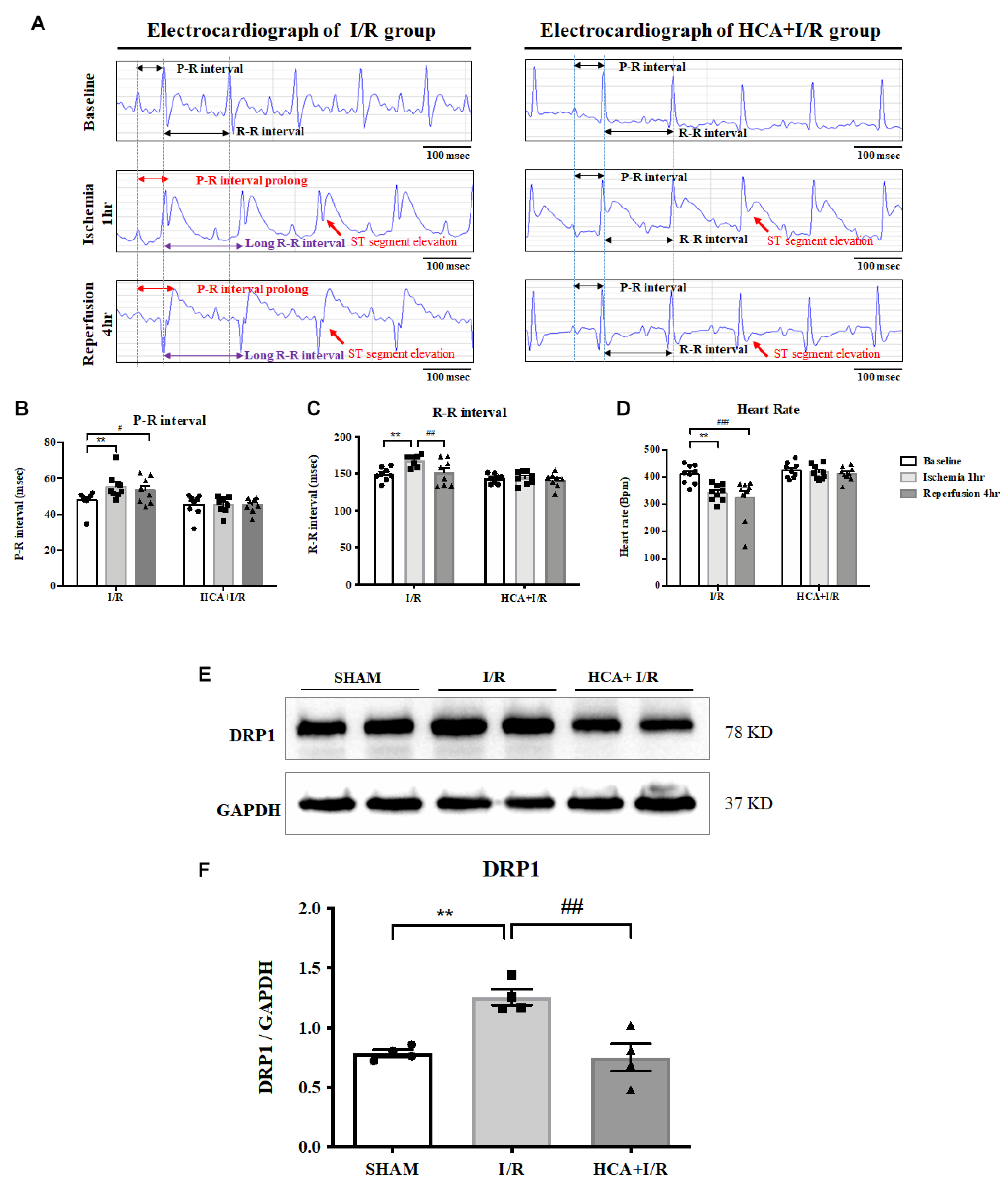
2.5. HCA Preconditioning Improved Cardiac I/R-Altered EKG Parameters
2.6. Effects of Bioactive Peptides from Tortoiseshell, Antler, and Their Combination on the Cell Viability of MC3T3-E1 Osteoblasts and HIG-82 Chondrocytes
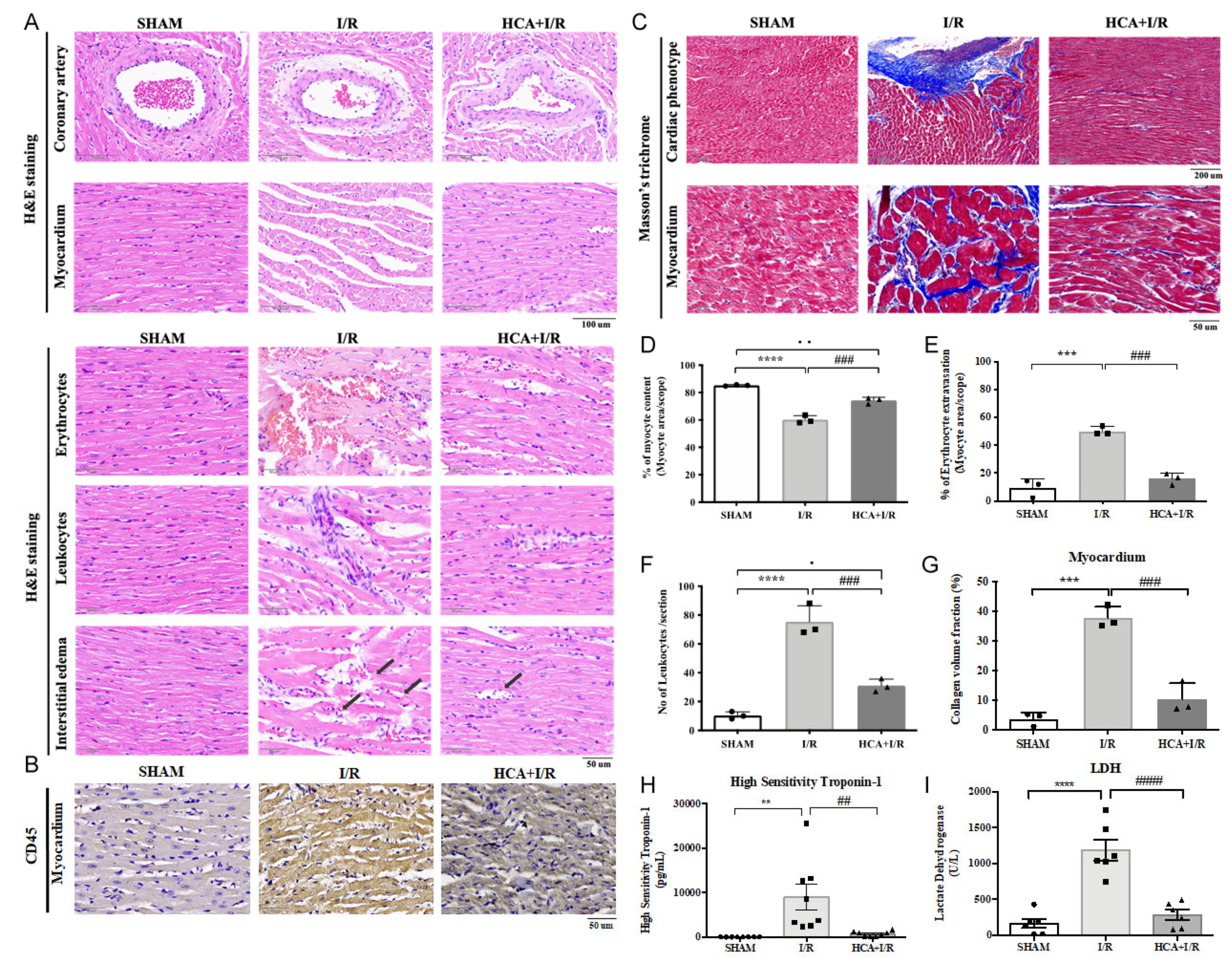
2.7. HCA Preconditioning Obviously Improved Cardiac I/R-Induced Pathologic Alterations
2.8. HCA Preconditioning Decreased Cardiac I/R-Induced Fibrosis
2.9. HCA Preconditioning Attenuated Cardiac I/R-Induced Increase in cTn I and LDH Levels
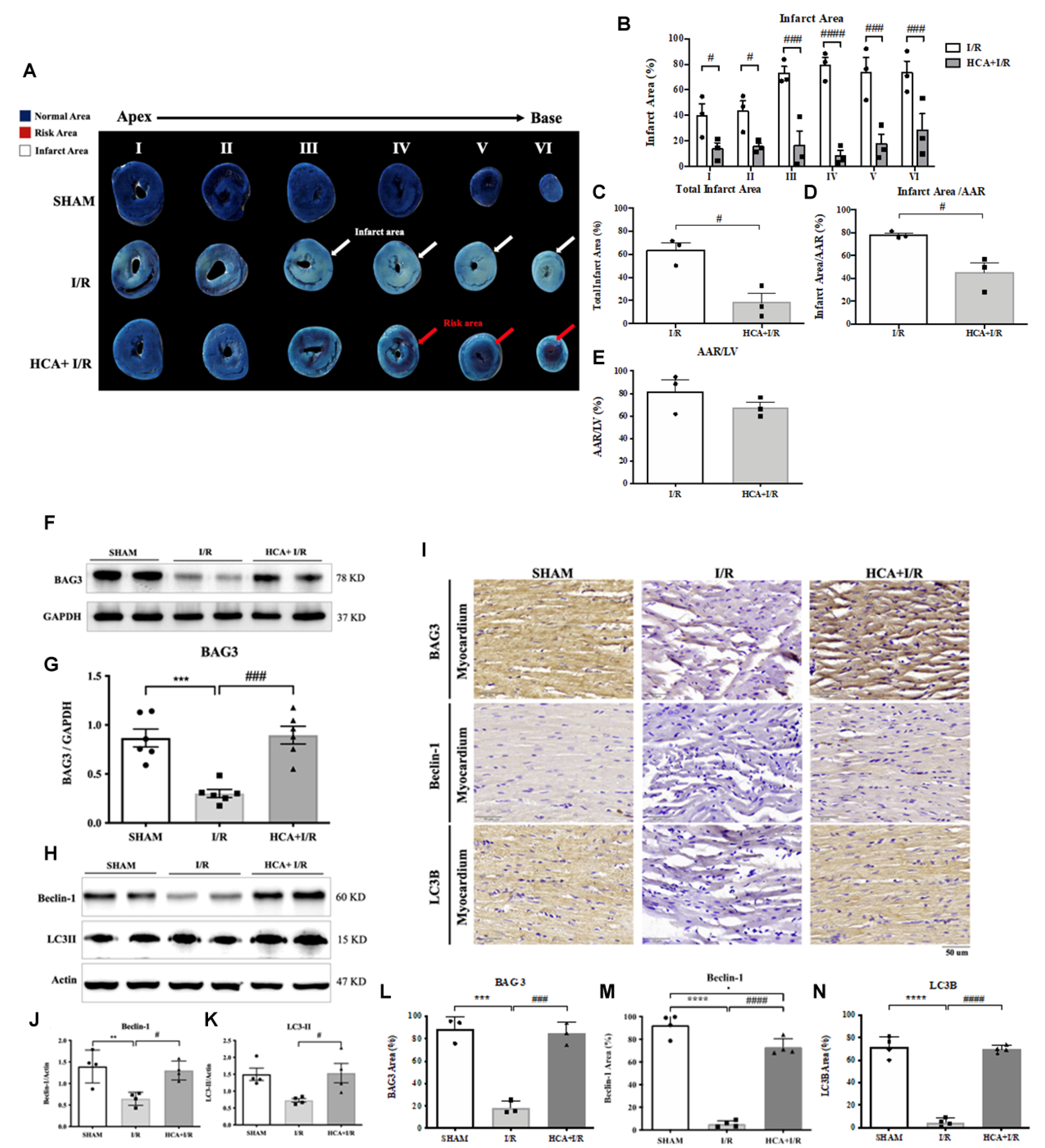
2.10. HCA Preconditioning Decreased Cardiac I/R-Induced Infarct Size
2.11. HCA Preconditioning Enhanced BAG3 Expression and Restored Autophagy-Related Proteins in I/R Hearts
2.12. HCA Preconditioning Decreased Cardiac I/R-Induced 4HNE/GPX4-Mediated Ferroptosis and Caspase 3-Mediated Apoptosis
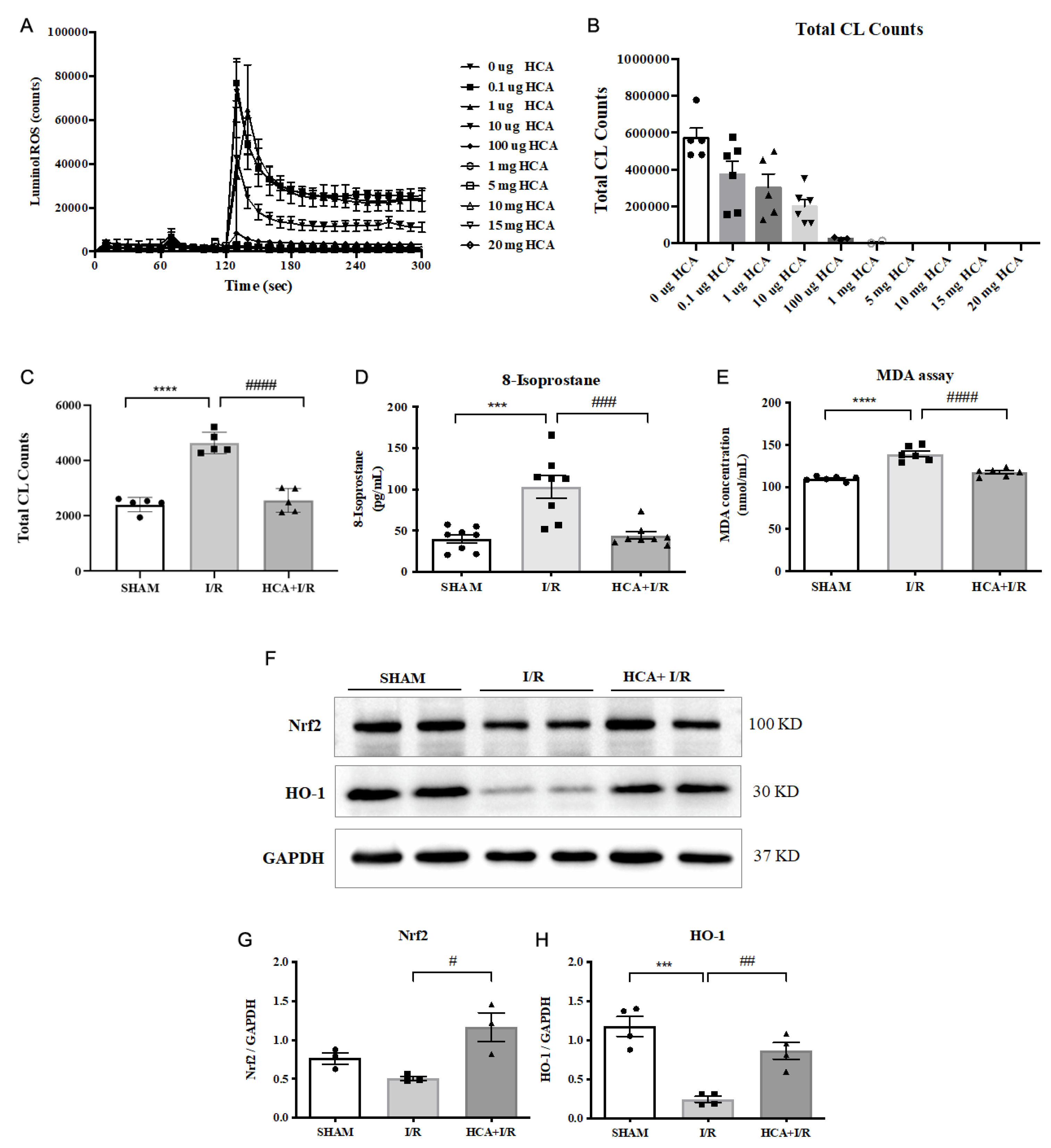
2.13. HCA Provided Antioxidant Activity In Vitro and In Vivo
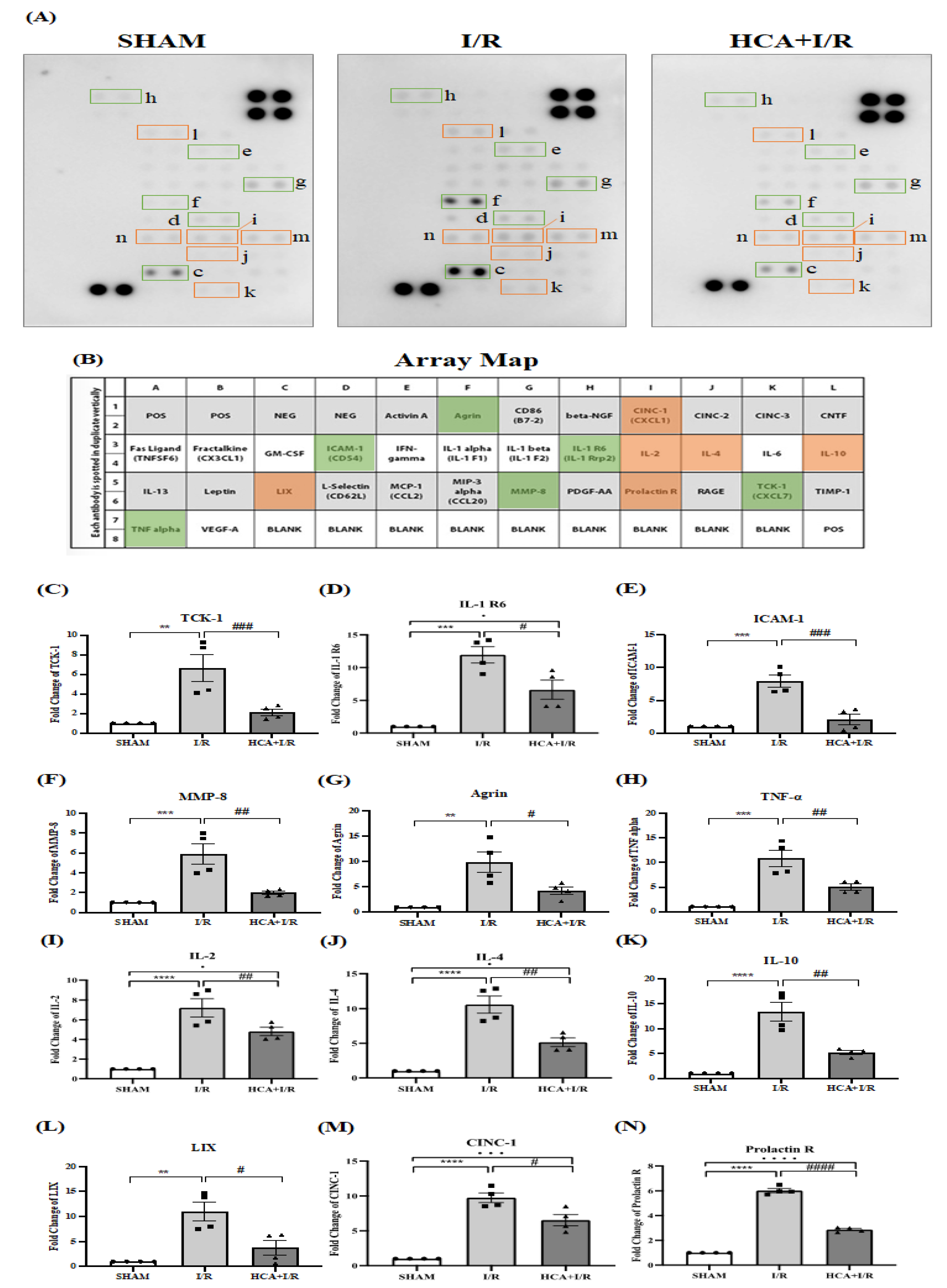
2.14. HCA Preconditioning Reduced Cardiac I/R Injury-Induced Expression of Multiple Cytokines
3. Discussion
4. Materials and Methods
4.1. Effect of HCA on In Vitro Model of Cardiac I/R in H9c2 Cells for Determining Viability
4.2. Animal Model
4.3. Cardiac I/R Injury Induction
4.4. EKG Measurement
4.5. Parameters of Left Ventricle Pressure
4.6. Cardiac Surface Microcirculation
4.7. Infarct Size Calculation
4.8. Effect of HCA Preconditioning on Cytosolic and Mitochondrial BAG3 Expression
4.9. Western Blotting
4.10. Antioxidant Activity Determination
4.11. Pathologic Findings
4.12. Immunohistochemistry (IHC)
4.13. 8-Isoprostane, MDA, and Biochemical Determination
4.14. Multiple Cytokine Antibody Array
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAR | area at risk |
| AMI | acute myocardial infarction |
| BAG3 | Bcl-2–associated athanogene |
| I/R | ischemia/reperfusion |
| HCA | 2′-Hydroxycinnamaldehyde |
| 4HNE | 4-hydroxynonenal |
| HR | heart rate |
| +dp/dt | maximal rate of rise in left ventricular pressure |
| -dp/dt | maximal rate of fall in left ventricular pressure |
| ECG | electrocardiogram |
| GPX4 | glutathione peroxidase 4 |
| HO-1 | heme oxygenase-1 |
| HRP | horseradish peroxidase |
| LAD | left anterior descending coronary artery |
| LV | left ventricle |
| LVEDP | left ventricular end-diastolic pressure |
| LVP | left ventricular pressure |
| LVSP | left ventricular systolic pressure |
| MDA | malondialdehyde |
| Nrf2 | nuclear factor erythroid 2–related factor 2 |
| ROS | reactive oxygen species |
| TTC | 2,3,5-triphenyltetrazolium chloride |
References
- Luan, F.; Lei, Z.; Peng, X.; Chen, L.; Peng, L.; Liu, Y.; Rao, Z.; Yang, R.; Zeng, N. Cardioprotective effect of cinnamaldehyde pretreatment on ischemia/reperfusion injury via inhibiting NLRP3 inflammasome activation and gasdermin D mediated cardiomyocyte pyroptosis. Chem. Interact. 2022, 368, 110245. [Google Scholar] [CrossRef]
- Heusch, G. Myocardial ischaemia–reperfusion injury and cardioprotection in perspective. Nat. Rev. Cardiol. 2020, 17, 773–789. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Yellon, D.M. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J. Clin. Investig. 2013, 123, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, X.; Zhang, J.; Han, X.; Wang, H.; Du, F.; Zeng, X.; Guo, C. Protective Effects of the Soluble Receptor for Advanced Glycation End-Products on Pyroptosis during Myocardial Ischemia-Reperfusion. Oxid. Med. Cell. Longev. 2021, 2021, 9570971. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-C.; Lee, W.-S.; Yang, K.-T. Methyl palmitate protects heart against ischemia/reperfusion-induced injury through G-protein coupled receptor 40-mediated activation of the PI3K/AKT pathway. Eur. J. Pharmacol. 2021, 905, 174183. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.N.; Jiang, Y.; Yan, L.Y.; Yin, S.Y.; Wang, Y.H.; Wang, S.B.; Fang, L.H.; Du, G.H. Aesculin suppresses the NLRP3 inflam-masome-mediated pyroptosis via the Akt/GSK3β/NF-κB pathway to mitigate myocardial ischemia/reperfusion injury. Phytomedicine 2021, 92, 153687. [Google Scholar] [CrossRef]
- Liu, X.-M.; Zhang, Z.; Zhong, J.; Li, N.; Wang, T.; Wang, L.; Zhang, Q. Long non-coding RNA MALAT1 modulates myocardial ischemia-reperfusion injury through the PI3K/Akt/eNOS pathway by sponging miRNA-133a-3p to target IGF1R expression. Eur. J. Pharmacol. 2021, 916, 174719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-J.; Liu, X.; Hu, M.; Zhao, G.-J.; Sun, D.; Cheng, X.; Xiang, H.; Huang, Y.-P.; Tian, R.-F.; Shen, L.-J.; et al. Pharmacological inhibition of arachidonate 12-lipoxygenase ameliorates myocardial ischemia-reperfusion injury in multiple species. Cell Metab. 2021, 33, 2059–2075.e10. [Google Scholar] [CrossRef] [PubMed]
- Maslov, L.N.; Popov, S.V.; Naryzhnaya, N.V.; Mukhomedzyanov, A.V.; Kurbatov, B.K.; Derkachev, I.A.; Boshchenko, A.A.; Khaliulin, I.; Prasad, N.R.; Singh, N.; et al. The regulation of necroptosis and perspectives for the development of new drugs preventing ischemic/reperfusion of cardiac injury. Apoptosis 2022, 27, 697–719. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.T.; Miller, J.H.; Day, M.M.; Munger, J.C.; Brookes, P.S. Accumulation of Succinate in Cardiac Ischemia Primarily Occurs via Canonical Krebs Cycle Activity. Cell Rep. 2018, 23, 2617–2628. [Google Scholar] [CrossRef]
- Gottlieb, R.A.; Bernstein, D. Mitochondrial remodeling: Rearranging, recycling, and reprogramming. Cell Calcium 2016, 60, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Hahad, O.; Andreadou, I.; Steven, S.; Daub, S.; Münzel, T. Redox-related biomarkers in human cardiovascular disease-classical footprints and beyond. Redox Biol. 2021, 42, 101875. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Xiao, J.-H. The Keap1-Nrf2 System: A Mediator between Oxidative Stress and Aging. Oxidative Med. Cell. Longev. 2021, 2021, 6635460. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Myers, V.D.; Knezevic, T.; Wang, J.; Gao, E.; Madesh, M.; Tahrir, F.G.; Gupta, M.K.; Gordon, J.; Rabinowitz, J.; et al. Bcl-2-associated athanogene 3 protects the heart from ische-mia/reperfusion injury. JCI Insight 2016, 1, e90931. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, T.; Myers, V.D.; Gordon, J.; Tilley, D.G.; Sharp, T.E., 3rd; Wang, J.; Khalili, K.; Cheung, J.Y.; Feldman, A.M. BAG3: A new player in the heart failure paradigm. Heart Fail Rev. 2015, 20, 423–434. [Google Scholar] [CrossRef]
- Homma, S.; Iwasaki, M.; Shelton, G.D.; Engvall, E.; Reed, J.C.; Takayama, S. BAG3 Deficiency Results in Fulminant Myopathy and Early Lethality. Am. J. Pathol. 2006, 169, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.; Schaefer, J.; Meinhardt, M.; Reichmann, H. Mitochondrial abnormalities in the myofibrillar myopathies. Eur. J. Neurol. 2015, 22, 1429–1435. [Google Scholar] [CrossRef]
- Mizushima, W.; Sadoshima, J. BAG3 plays a central role in proteostasis in the heart. J. Clin. Investig. 2017, 127, 2900–2903. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carrizzo, A.; Damato, A.; Ambrosio, M.; Falco, A.; Rosati, A.; Capunzo, M.; Madonna, M.; Turco, M.C.; Januzzi, J.L.; De Laurenzi, V.; et al. The prosurvival protein BAG3: A new participant in vascular homeostasis. Cell Death Dis. 2016, 7, e2431. [Google Scholar] [CrossRef][Green Version]
- Tahrir, F.G.; Knezevic, T.; Gupta, M.K.; Gordon, J.; Cheung, J.Y.; Feldman, A.M.; Khalili, K. Evidence for the Role of BAG3 in Mitochondrial Quality Control in Cardiomyocytes. J. Cell. Physiol. 2016, 232, 797–805. [Google Scholar] [CrossRef]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Li, J.; Sun, Y.; Chen, Q.; Zhang, L.; Le, T. The antifungal effects of cinnamaldehyde against Aspergillus niger and its application in bread preservation. Food Chem. 2020, 317, 126405. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W. Cinnamaldehyde induces autophagy-mediated cell death through ER stress and epigenetic modification in gastric cancer cells. Acta Pharmacol. Sin. 2021, 43, 712–723. [Google Scholar] [CrossRef]
- Liu, P.; Wang, J.; Wen, W.; Pan, T.; Chen, H.; Fu, Y.; Wang, F.; Huang, J.H.; Xu, S. Cinnamaldehyde suppresses NLRP3 derived IL-1β via activating succinate/HIF-1 in rheumatoid arthritis rats. Int. Immunopharmacol. 2020, 84, 106570. [Google Scholar] [CrossRef]
- Gao, Z.Y.; Chen, T.Y.; Yu, T.T.; Zhang, L.P.; Zhao, S.J.; Gu, X.Y.; Pan, Y.; Kong, L.D. Cinnamaldehyde prevents intergenera-tional effect of paternal depression in mice via regulating GR/miR-190b/BDNF pathway. Acta Pharmacol. Sin. 2022, 43, 1955–1969. [Google Scholar] [CrossRef] [PubMed]
- Pang, D.; Huang, Z.; Li, Q.; Wang, E.; Liao, S.; Li, E.; Zou, Y.; Wang, W. Antibacterial Mechanism of Cinnamaldehyde: Modu-lation of Biosynthesis of Phosphatidylethanolamine and Phosphatidylglycerol in Staphylococcus aureus and Escherichia coli. J. Agric. Food Chem. 2021, 69, 13628–13636. [Google Scholar] [CrossRef] [PubMed]
- Bektaşoğlu, P.K.; Koyuncuoğlu, T.; Demir, D.; Sucu, G.; Akakın, D.; Eyüboğlu, I.P.; Yüksel, M.; Çelikoğlu, E.; Yeğen, B.; Gürer, B. Neuroprotective Effect of Cinnamaldehyde on Secondary Brain Injury After Traumatic Brain Injury in a Rat Model. World Neurosurg. 2021, 153, e392–e402. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Shan, X.-L.; Wang, S.-N.; Chen, H.-H.; Zhao, P.; Qian, D.-D.; Xu, M.; Guo, W.; Zhang, C.; Lu, R. Trans-cinnamaldehyde suppresses microtubule detyrosination and alleviates cardiac hypertrophy. Eur. J. Pharmacol. 2021, 914, 174687. [Google Scholar] [CrossRef]
- Lan, H.; Zheng, Q.; Wang, K.; Li, C.; Xiong, T.; Shi, J.; Dong, N. Cinnamaldehyde protects donor heart from cold ische-mia-reperfusion injury via the PI3K/AKT/mTOR pathway. Biomed. Pharmacother. 2023, 165, 114867. [Google Scholar] [CrossRef]
- Lee, C.; Hong, D.; Han, S.; Park, S.; Kim, H.; Kwon, B.-M.; Kim, H. Inhibition of Human Tumor Growth by 2′-Hydroxy- and 2′-Benzoyl-oxycinnamaldehydes. Planta Medica 1999, 65, 263–266. [Google Scholar] [CrossRef]
- Hong, S.; Ismail, I.A.; Kang, S.; Han, D.C.; Kwon, B. Cinnamaldehydes in Cancer Chemotherapy. Phytother. Res. 2016, 30, 754–767. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.-A.; Kim, S.-A. 2′-Hydroxycinnamaldehyde induces apoptosis through HSF1-mediated BAG3 expression. Int. J. Oncol. 2016, 50, 283–289. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Song, F.; Li, H.; Sun, J.; Wang, S. Protective effects of cinnamic acid and cinnamic aldehyde on isoproterenol-induced acute myocardial ischemia in rats. J. Ethnopharmacol. 2013, 150, 125–130. [Google Scholar] [CrossRef]
- Kim, N.; Trinh, N.; Ahn, S.; Kim, S. Cinnamaldehyde protects against oxidative stress and inhibits the TNF-α-induced inflammatory response in human umbilical vein endothelial cells. Int. J. Mol. Med. 2020, 46, 449–457. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chiang, C.Y.; Chang, T.C.; Chien, C.T. Multiple Progressive Thermopreconditioning Improves Cardiac Ische-mia/Reperfusion-induced Left Ventricular Contractile Dysfunction and Structural Abnormality in Rat. Transplantation 2020, 104, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Murry, C.E.; Jennings, R.B.; Reimer, K.A. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation 1986, 74, 1124–1136. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, F.; Cuenca, S.; Bilińska, Z.; Toro, R.; Villard, E.; Barriales-Villa, R.; Ochoa, J.P.; Asselbergs, F.; Sammani, A.; Garcia-Pavia, P.; et al. Dilated Cardiomyopathy Due to BLC2-Associated Athanogene 3 (BAG3) Muta-tions. J. Am. Coll. Cardiol. 2018, 72, 2471–2481. [Google Scholar] [CrossRef]
- Martin, T.G.; Myers, V.D.; Dubey, P.; Dubey, S.; Perez, E.; Moravec, C.S.; Willis, M.S.; Feldman, A.M.; Kirk, J.A. Cardiomyo-cyte contractile impairment in heart failure results from reduced BAG3-mediated sarcomeric protein turnover. Nat. Commun. 2021, 12, 2942. [Google Scholar] [CrossRef]
- Liu, B.-Q.; Du, Z.-X.; Zong, Z.-H.; Li, C.; Li, N.; Zhang, Q.; Kong, D.-H.; Wang, H.-Q. BAG3-dependent noncanonical autophagy induced by proteasome inhibition in HepG2 cells. Autophagy 2013, 9, 905–916. [Google Scholar] [CrossRef]
- Tahrir, F.G.; Langford, D.; Amini, S.; Mohseni Ahooyi, T.; Khalili, K. Mitochondrial quality control in cardiac cells: Mecha-nisms and role in cardiac cell injury and disease. J. Cell. Physiol. 2019, 234, 8122–8133. [Google Scholar] [CrossRef]
- Große, L.; Wurm, C.A.; Brüser, C.; Neumann, D.; Jans, D.C.; Jakobs, S. Bax assembles into large ring-like structures remodel-ing the mitochondrial outer membrane in apoptosis. EMBO J. 2016, 35, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.G.; Sherer, L.A.; Kirk, J.A. BAG3 localizes to mitochondria in cardiac fibroblasts and regulates mitophagy. Am. J. Physiol. Circ. Physiol. 2024, 326, H1124–H1130. [Google Scholar] [CrossRef] [PubMed]
- Hwa, J.S.; Jin, Y.C.; Lee, Y.S.; Ko, Y.S.; Kim, Y.M.; Shi, L.Y.; Kim, H.J.; Lee, J.H.; Ngoc, T.M.; Bae, K.H.; et al. 2-methoxycinnamaldehyde from Cinnamomum cassia reduces rat myocardial ischemia and reperfusion injury in vivo due to HO-1 induction. J. Ethnopharmacol. 2012, 139, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Regitz-Zagrosek, V.; Gebhard, C. Gender medicine: Effects of sex and gender on cardiovascular disease manifestation and outcomes. Nat. Rev. Cardiol. 2023, 20, 236–247. [Google Scholar] [CrossRef]
- Kararigas, G.; Bito, V.; Tinel, H.; Becher, E.; Baczko, I.; Knosalla, C.; Albrecht-Küpper, B.; Sipido, K.R.; Regitz-Zagrosek, V. Transcriptome characterization of estrogen-treated human myocardium identifies myosin regulatory light chain interacting protein as a sex-specific element influencing contractile function. J. Am. Coll. Cardiol. 2012, 59, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Dworatzek, E.; Mahmoodzadeh, S.; Schriever, C.; Kusumoto, K.; Kramer, L.; Santos, G.; Fliegner, D.; Leung, Y.K.; Ho, S.M.; Zimmermann, W.H.; et al. Sex-specific regulation of collagen I and III expression by 17β-estradiol in cardiac fibroblasts: Role of estrogen receptors. Cardiovasc. Res. 2019, 115, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Regitz-Zagrosek, V.; Kararigas, G. Mechanistic pathways of sex differences in cardiovascular disease. Physiol. Rev. 2017, 97, 1–37. [Google Scholar] [CrossRef]
- Shi, W.; Sheng, X.; Dorr, K.M.; Hutton, J.E.; Emerson, J.I.; Davies, H.A.; Andrade, T.D.; Wasson, L.K.; Greco, T.M.; Hashimoto, Y.; et al. Cardiac proteomics reveals sex chromosome-dependent differences between males and females that arise prior to gonad formation. Dev. Cell 2021, 56, 3019–3034.e3017. [Google Scholar] [CrossRef]
- Guo, J.; Yan, S.; Jiang, X.; Su, Z.; Zhang, F.; Xie, J.; Hao, E.; Yao, C. Advances in pharmacological effects and mechanism of action of cinnamaldehyde. Front Pharmacol. 2024, 15, 1365949. [Google Scholar] [CrossRef]
- Chien, C.Y.; Chien, C.T.; Wang, S.S. Progressive thermopreconditioning attenuates rat cardiac ischemia/reperfusion injury by mitochondria-mediated antioxidant and antiapoptotic mechanisms. J. Thorac. Cardiovasc. Surg. 2014, 148, 705–713. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.-H.; Chiang, C.-Y.; Wu, C.-H.; Chien, C.-T. 2′-Hydroxycinnamaldehyde, a Natural Product from Cinnamon, Alleviates Ischemia/Reperfusion-Induced Microvascular Dysfunction and Oxidative Damage in Rats by Upregulating Cytosolic BAG3 and Nrf2/HO-1. Int. J. Mol. Sci. 2024, 25, 12962. https://doi.org/10.3390/ijms252312962
Cheng Y-H, Chiang C-Y, Wu C-H, Chien C-T. 2′-Hydroxycinnamaldehyde, a Natural Product from Cinnamon, Alleviates Ischemia/Reperfusion-Induced Microvascular Dysfunction and Oxidative Damage in Rats by Upregulating Cytosolic BAG3 and Nrf2/HO-1. International Journal of Molecular Sciences. 2024; 25(23):12962. https://doi.org/10.3390/ijms252312962
Chicago/Turabian StyleCheng, Yu-Hsuan, Chih-Yao Chiang, Chung-Hsin Wu, and Chiang-Ting Chien. 2024. "2′-Hydroxycinnamaldehyde, a Natural Product from Cinnamon, Alleviates Ischemia/Reperfusion-Induced Microvascular Dysfunction and Oxidative Damage in Rats by Upregulating Cytosolic BAG3 and Nrf2/HO-1" International Journal of Molecular Sciences 25, no. 23: 12962. https://doi.org/10.3390/ijms252312962
APA StyleCheng, Y.-H., Chiang, C.-Y., Wu, C.-H., & Chien, C.-T. (2024). 2′-Hydroxycinnamaldehyde, a Natural Product from Cinnamon, Alleviates Ischemia/Reperfusion-Induced Microvascular Dysfunction and Oxidative Damage in Rats by Upregulating Cytosolic BAG3 and Nrf2/HO-1. International Journal of Molecular Sciences, 25(23), 12962. https://doi.org/10.3390/ijms252312962






