Turner Syndrome and the Thyroid Function—A Systematic and Critical Review
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Considered Population
2.2. Thyroid Parameters
2.2.1. Thyroid Function
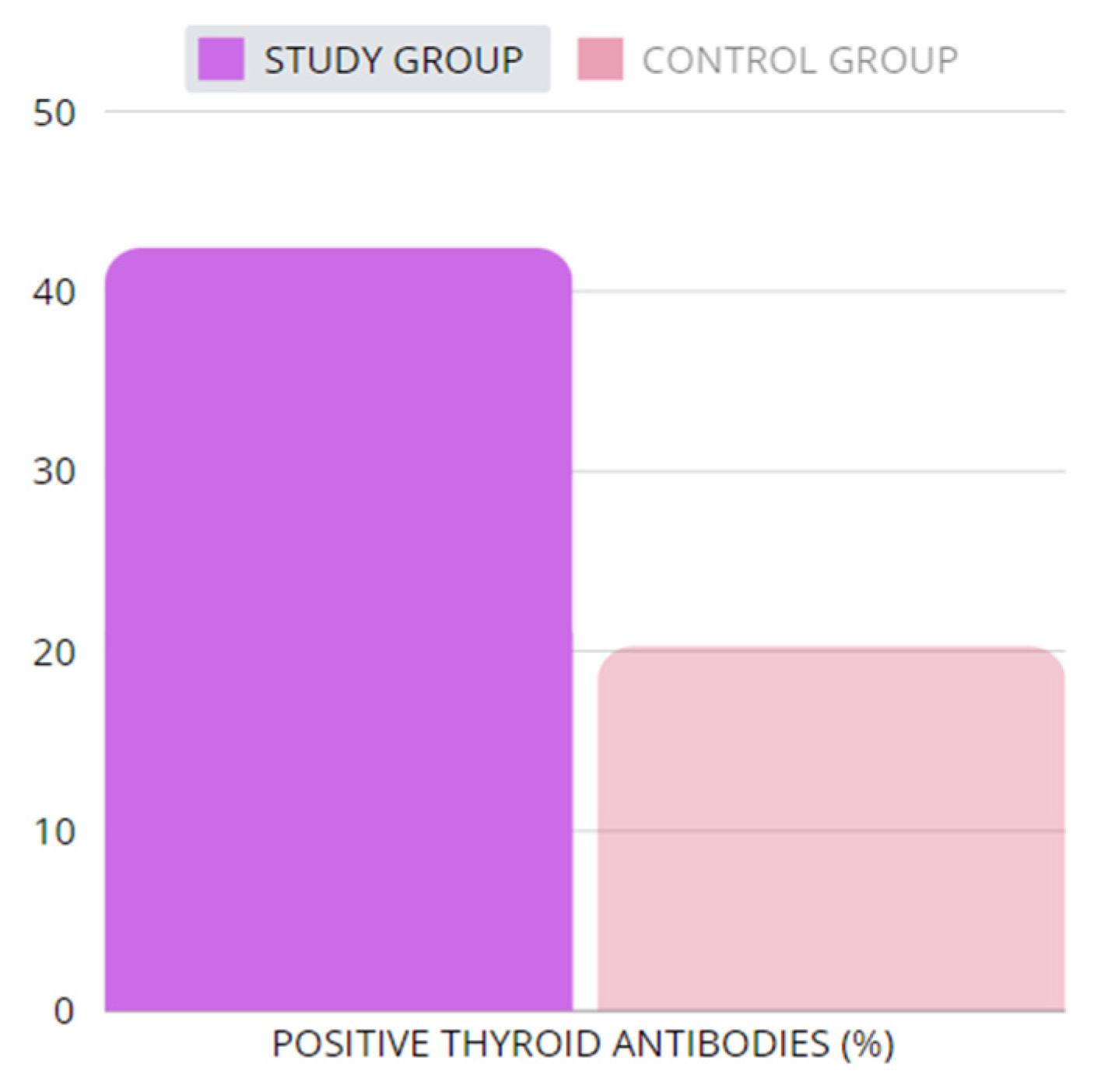
2.2.2. Thyroid Antibodies
2.2.3. Thyroid in Ultrasonography
2.3. Screening and Treatment
Thyroid Gland
2.4. Future Prospects
2.5. Limitations
3. Materials and Methods
3.1. Search Strategy
3.2. Inclusion and Exclusion Criteria
3.3. Data Extraction and Quality Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| anti-Tg | antithyroglobulin antibody |
| anti-TPO | thyroid peroxidase antibody |
| BDNF | brain-derived neurotrophic factor |
| BMI | body mass index |
| CLTRN | collectrin amino acid transport regulator |
| CSF2RA | colony-stimulating factor 2 receptor subunit alpha |
| FOXP3 | forkhead box P3 |
| fT3 | free triiodothyronine |
| fT4 | free thyroxine |
| GH | growth hormone |
| HDL | high density lipoproteins |
| IGF-I | insulin-like growth factor 1 |
| IL3RA | interleukin 3 receptor alpha |
| KDM6A | lysine demethylase 6A |
| L-thyroxine | levothyroxine |
| PTPN22 | protein tyrosine phosphatase non-receptor type 22 |
| SHOX | short stature homeobox-containing gene |
| TIMP 1 | metallopeptidase inhibitor 1 |
| TIMP 3 | metallopeptidase inhibitor 3 |
| TS | Turner syndrome |
| TSH | thyroid-stimulating hormone |
| TT3 | total triiodothyronine |
| TT4 | total thyroxine |
| USG | ultrasonography |
References
- Bondy, C.A. Care of girls and women with Turner syndrome: A guideline of the Turner Syndrome Study Group. J. Clin. Endocrinol. Metab. 2007, 92, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Gravholt, C.H.; Andersen, N.H.; Conway, G.S.; Dekkers, O.M.; Geffner, M.E.; Klein, K.O.; Lin, A.E.; Mauras, N.; Quigley, C.A.; Rubin, K.; et al. Clinical practice guidelines for the care of girls and women with Turner syndrome: Proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. Clin. Endocrinol. 2017, 55, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Ćatović, A.; Kendić, S. Cytogenetics findings at Turner Syndrome and their correlation with clinical findings. Bosn. J. Basic Med. Sci. 2005, 5, 54–58. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Czyzyk, A.; Lacka, K.; Krawczynski, M.; Andrzejewska, J.; Wojda, A.; Skolozdrzy, J.; Latos-Bielanska, A. Analysis of phenotype and karyotype of children born from mothers with 45, X/46, XX mosaicism. Endocrinologist 2010, 20, 283–285. [Google Scholar] [CrossRef]
- Saenger, P. Turner’s syndrome. NEJM 1996, 335, 1749–1754. [Google Scholar] [CrossRef]
- Lacka, K. Turner’s Syndrome-correlation between kariotype and fenotype. Endokrynol. Pol. 2005, 6, 986–993. [Google Scholar]
- Turner, H.H. A syndrome of infantilism, congenital webbed neck and cubitus vulgaris. Endocrinologist 1938, 28, 566–574. [Google Scholar] [CrossRef]
- Morgagni, J.B. Desedibus et causis Morborum; Ex Typographia Remondiniana: Venice, Italy, 1761; Volume 6, p. 462. [Google Scholar]
- Gravholt, C.H. Epidemiological, endocrine and metabolic features in Turner syndrome. Arq. Bras. Endocrinol. Metabol. 2005, 49, 145–156. [Google Scholar] [CrossRef]
- Shankar, R.K.; Backeljauwa, P.F. Current best practice in the management of Turner syndrome. Ther. Adv. Endocrinol. Metab. 2018, 9, 33–40. [Google Scholar] [CrossRef]
- Chen, R.M.; Zhang, Y.; Yang, X.H.; Lin, X.Q.; Yuan, X. Thyroid disease in Chinese girls with Turner syndrome. J. Pediatr. Endocrinol. Metab. 2015, 28, 201–205. [Google Scholar] [CrossRef]
- Elsheikh, M.; Wass, J.A.; Conway, G.S. Autoimmune thyroid syndrome in women with Turner syndrome-the association with karyotype. Clin. Endocrinol. 2001, 55, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Grossi, A.; Palma, A.; Zanni, G.; Novelli, A.; Loddo, S.; Cappa, M.; Fierabracci, A. Multiorgan autoimmunity in a Turner syndrome patient with partial monosomy 2q and trisomy 10p. Gene 2013, 515, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Gawlik, A.; Gawlik, T.; Januszek-Trzciakowska, A.; Patel, H.; Małecka-Tendera, E. Incidence and dynamics of thyroid dysfunction and thyroid autoimmunity in girls with Turner’s syndrome: A long-term follow-up study. Horm. Res. Paediatr. 2011, 76, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Aversa, T.; Messina, M.F.; Mazzanti, L.; Salerno, M.; Mussa, A.; Faienza, M.F.; Scarano, E.; De Luca, F.; Wasniewska, M. The association with Turner syndrome significantly affects the course of Hashimoto’s thyroiditis in children, irrespective of karyotype. Endocrine 2015, 50, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Cui, Y.; Shi, L.; Luan, J.; Zhou, X.; Han, J. A basic understanding of Turner syndrome: Incidence, complications, diagnosis, and treatment. Intractable Rare Dis. Res. 2018, 7, 223–228. [Google Scholar] [CrossRef]
- Turner, S. NORD (National Organization for Rare Disorders). Available online: https://rarediseases.org/rarediseases/turner-syndrome/ (accessed on 20 September 2024).
- Kikkeri, N.S.; Nagalli, S. Turner Syndrome. In StatPearls. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554621/ (accessed on 20 September 2024).
- Sävendahl, L.; Davenport, M.L. Delayed diagnoses of Turner’s syndrome: Proposed guidelines for change. J. Pediatr. 2000, 137, 455–459. [Google Scholar] [CrossRef]
- Barrenäs, M.L.; Nylen, O.; Hanson, C. The influence of karyotype on the auricle, otitis media and hearing in Turner syndrome. Hear. Res. 1999, 138, 163–170. [Google Scholar] [CrossRef]
- Bianchi, I.; Lleo, A.; Gershwin, M.E.; Invernizzi, P. The X chromosome and immune associated genes. J. Autoimmun. 2012, 38, 87–92. [Google Scholar] [CrossRef]
- Gawlik, A.; Sołtysik, K.; Berdej-Szczot, E. Autoimmune disease profile in Turner syndrome patients. Endokrynol. Pediatr. 2013, 12, 57–68. [Google Scholar] [CrossRef]
- Libert, C.; Dejager, L.; Pinheiro, I. The X chromosome in immune functions: When a chromosome makes the difference. Nat. Rev. Immunol. 2010, 10, 594–604. [Google Scholar] [CrossRef]
- Gawlik, A.M.; Berdej-Szczot, E.; Blat, D.; Klekotka, R.; Gawlik, T.; Blaszczyk, E.; Hankus, M.; Malecka-Tendera, E. Immunological profile and predisposition to autoimmunity in girls with Turner syndrome. Front. Endocrinol. 2018, 9, 307. [Google Scholar] [CrossRef] [PubMed]
- Chiovato, L.; Larizza, D.; Bendinelli, G.; Tonacchera, M.; Marinó, M.; Mammoli, C.; Lorini, R.; Severi, F.; Pinchera, A. Autoimmune hypothyroidism and hyperthyroidism in patients with Turner’s syndrome. Eur. J. Endocrinol. 1996, 134, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Naessén, S.; Eliasson, m.; Berntorp, K.; Kitlinski, M.; Trimpou, P.; Amundson, E.; Thunström, S.; Ekman, B.; Wahlberg, J.; Karlsson, A.; et al. Autoimmune Disease in Turner Syndrome in Sweden: An up to 25 Years’ Controlled Follow-up Study. J. Clin. Endocrinol. Metab. 2024, 109, e602–e612. [Google Scholar] [CrossRef] [PubMed]
- Sylvén, L.; Hagenfeldt, K.; Bröndrum-Nielsen, K.; von-Schoultz, B. Middle-aged women with Turner´s syndrome. Medical status, hormonal treatment, and social life. Acta Endocrinol. 1991, 125, 359–365. [Google Scholar]
- Chelikani, V.; Rao, D.N.; Balmuri, S.; Arida, A.K. A Rare Case of Hashimoto’s Encephalopathy With Mosaic Turner Syndrome. Cureus 2022, 20, e28215. [Google Scholar] [CrossRef]
- Pempera, N.; Miedziaszczyk, M.; Lacka, K. Difficulties in the Diagnostics and Treatment of Hashimoto’s Encephalopathy-A Systematic and Critical Review. Int. J. Mol. Sci. 2024, 25, 7101. [Google Scholar] [CrossRef]
- Mitsibounas, D.N.; Kosmaidou, Z.; Kontoleon, P.; Deligeoroglou, E.; Liberatou, E. Hyperlipidemia and presence of thyroid autoantibodies in girls with Turner’s syndrome or mosaic variance. J. Pediatr. Adolesc. Gynecol. 1997, 10, 133–139. [Google Scholar] [CrossRef]
- Wasniewska, M.; Salerno, M.; Corrias, A.; Mazzanti, L.; Matarazzo, P.; Corica, D.; Aversa, T.; Messina, M.F.; De Luca, F.; Valenzise, M. The Evolution of Thyroid Function after Presenting with Hashimoto Thyroiditis Is Different between Initially Euthyroid Girls with and Those without Turner Syndrome. Horm. Res. Paediatr. 2016, 86, 403–409. [Google Scholar] [CrossRef]
- Susperreguy, S.; Miras, M.B.; Montesinos, M.M.; Mascanfroni, I.D.; Muñoz, L.; Sobrero, G.; Silvano, L.; Masini-Repiso, A.M.; Coleoni, A.H.; Targovnik, H.M.; et al. Growth hormone (GH) treatment reduces peripheral thyroid hormone action in girls with Turner syndrome. Clin. Endocrinol. 2007, 67, 629–636. [Google Scholar] [CrossRef]
- El-Mansoury, M.; Bryman, I.; Berntorp, K.; Hanson, C.; Wilhelmsen, L.; Landin-Wilhelmsen, K. Hypothyroidism is common in turner syndrome: Results of a five-year follow-up. J. Clin. Endocrinol. Metab. 2005, 90, 2131–2135. [Google Scholar] [CrossRef]
- Wilson, R.; Chu, C.E.; Donaldson, M.D.C.; Thomson, J.A.; McKillop, J.H.; Connor, J.M. An increased incidence of thyroid antibodies in patients with Turner’s syndrome and their first degree relatives. Autoimmun 1996, 25, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Łacka, K.; Wawrzyniak, M.; Andrzejewska, J.; Krysińska, I.; Ruchała, M.; Bauman-Antczak, A. Evaluation of thyroid structure and function in patients with Turner syndrome. Pol. Arch. Med. Wewn. 2000, 104, 583–589. [Google Scholar] [PubMed]
- Calcaterra, V.; Klersy, C.; Muratori, T.; Caramagna, C.; Brizzi, V.; Albertini, R.; Larizza, D. Thyroid ultra-sound in patients with Turner syndrome: Influence of clinical and auxological parameters. J. Endocrinol. Invest 2011, 34, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Cogni, G.; Chiovato, L. An overview of the pathogenesis of thyroid autoimmunity. Hormones 2013, 39, 19–29. [Google Scholar] [CrossRef]
- Atria, A.; Sanz, R.; Donoso, S. Necropsy study of a case of Turner’s syndrome, Case report. J. Clin. Endocrinol. 1948, 8, 397–405. [Google Scholar] [CrossRef]
- Gravholt, C.H.; Viuff, M.H.; Brun, S.; Stochholm, K.; Andersen, N.H. Turner syndrome: Mechanisms and management. Nat. Rev. Endocrinol. 2019, 15, 601–614. [Google Scholar] [CrossRef]
- Sharland, M.; Burch, M.; McKenna, W.M.; Paton, M.A. A clinical study of Noonan syndrome. Arch. Dis. Child. 1992, 67, 178–183. [Google Scholar] [CrossRef]
- Vesterhus, P.; Aarskog, D. Noonan’s syndrome and autoimmune thyroiditis. J. Pediatr. 1973, 83, 237–240. [Google Scholar] [CrossRef]
- Fukami, M.; Seki, A.; Ogata, T. SHOX Haploinsufficiency as a cause of syndromic and nonsyndromic short stature. Mol. Syndromol. 2016, 7, 3–11. [Google Scholar] [CrossRef]
- Mohamed, S.O.O.; Elkhidir, I.H.E.; Abuzied, A.I.H.; Noureddin, A.A.M.H.; Ibrahim, G.A.A.; Mahmoud, A.A.A. Prevalence of autoimmune thyroid diseases among the Turner Syndrome patients: Meta-analysis of cross sectional studies. BMC Res. Notes 2018, 11, 842. [Google Scholar] [CrossRef]
- Brix, T.; Hegedüs, L. Twin studies as a model for exploring the aetiology of autoimmune thyroid disease. Clin. Endocrinol. 2012, 76, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, I.; Hizuka, N.; Kurimoto, M.; Morita, J.; Tanaka, S.; Yamakado, Y.; Takano, K. Autoimmune thyroid diseases in 65 Japanese women with Turner syndrome. Endocr. J. 2009, 56, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Weeke, J.; Jens, S. Christiansen. Thyroid function during growth hormone therapy. Horm. Res. 1992, 38, 63–67. [Google Scholar]
- Jonklaas, J.; Bianco, A.C.; Bauer, A.J.; Burman, K.D.; Cappola, A.R.; Celi, F.S.; Cooper, D.S.; Kim, B.W.; Peeters, R.P.; Rosenthal, M.S.; et al. Guidelines for the treatment of hypothyroidism: Prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid 2014, 24, 1670–1751. [Google Scholar] [CrossRef] [PubMed]
- Somwaru, L.L.; Rariy, C.M.; Arnold, A.M.; Cappola, A.R. The natural history of subclinical hypothyroidism in the elderly: The cardiovascular health study. J. Clin. Endocrinol. Metab. 2012, 97, 1962–1969. [Google Scholar] [CrossRef]
- Vanderpump, M.P.; Tunbridge, W.M.; French, J.M.; Appleton, D.; Bates, D.; Clark, F.; Grimley Evans, J.; Hasan, D.M.; Rodgers, H.; Tunbridge, F.; et al. The incidence of thyroid disorders in the community: A twenty-year follow-up of the whickham survey. Clin. Endocrinol. 1995, 43, 55–68. [Google Scholar] [CrossRef]
- Jorde, R.; Waterloo, K.; Storhaug, H.; Nyrnes, A.; Sundsfjord, J.; Jenssen, T.G. Neuropsychological function and symptoms in subjects with subclinical hypothyroidism and the effect of thyroxine treatment. J. Clin. Endocrinol. Metab. 2006, 91, 145–153. [Google Scholar] [CrossRef]
- Almandoz, J.P.; Gharib, H. Hypothyroidism: Etiology, diagnosis, and management. Med. Clin. N. Am. 2012, 96, 203–221. [Google Scholar] [CrossRef]
- Pearce, S.H.; Brabant, G.; Duntas, L.H.; Monzani, F.; Peeters, R.P.; Razvi, S.; Wemeau, J.L. 2013 eta guideline: Management of subclinical hypothyroidism. Eur. Thyroid J. 2013, 2, 215–228. [Google Scholar] [CrossRef]
- Jonklaas, J.; Razvi, S. Reference intervals in the diagnosis of thyroid dysfunction: Treating patients not numbers. Lancet Diabetes Endocrinol. 2019, 7, 473–483. [Google Scholar] [CrossRef]
- Razvi, S.; Korevaar, T.I.M.; Taylor, P. Trends, determinants, and associations of treated hypothyroidism in the United Kingdom, 2005–2014. Thyroid 2019, 29, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Syamsunder, A.N.; Pal, G.K.; Pal, P.; Kamalanathan, C.S.; Parija, S.C.; Nanda, N. Association of sympathovagal imbalance with cardiovascular risks in overt hypothyroidism. N. Am. J. Med. Sci. 2013, 5, 554. [Google Scholar] [PubMed]
- Biondi, B.; Klein, I. Hypothyroidism as a risk factor for cardiovascular disease. Endocrine 2004, 24, 1–14. [Google Scholar] [CrossRef]
- Fiot, E.; Alauze, B.; Donadille, B.; Samara-Boustani, D.; Houang, M.; De Filippo, G.; Bachelot, A.; Delcour, C.; Beyler, C.; Bois, E.; et al. Turner syndrome: French National Diagnosis and Care Protocol (NDCP.; National Diagnosis and Care Protocol). Orphanet. J. Rare Dis. 2022, 17, 261. [Google Scholar] [CrossRef] [PubMed]
- Gravholt, C.H.; Niels, H.A.; Christin-Maitre, S.; Shanlee, M.D.; Duijnhouwer, A.; Gawlik, A.; Maciel-Guerra, A.T.; Gutmark-Little, I.; Fleischer, K.; Hong, K.; et al. Clinical practice guidelines for the care of girls and women with Turner syndrome: Proceedings from the 2023 Aarhus International Turner Syndrome Meeting. Eur. J. Endocrinol. 2023, 6, G53–G151. [Google Scholar]
- Corbitt, H.; Morris, S.A.; Gravholt, C.H.; Mortenson, K.H.; Tippner-Hedges, R.; Silberbach, M.; Maslen, C.L. TIMP3 and TIMP1 are risk genes for bicuspid aortic valve and aortopathy in Turner syndrome. PLoS Genet. 2018, 14, e1007692. [Google Scholar] [CrossRef]
- Khan, N.; Farooqui, A.; Ishrat, R. Turner Syndrome where are we? Orphanet J. Rare Dis. 2024, 19, 314. [Google Scholar] [CrossRef]
- Farooqui, A.; Tazyeen, S.; Ahmed, M.M.; Alam, A.; Ali, S.; Malik, M.Z.; Ali, S.; Ishrat, R. Assessment of the key regulatory genes and their Interologs for Turner Syndrome employing network approach. Sci. Rep. 2018, 8, 10091. [Google Scholar] [CrossRef]
- Pajenda, S.; Wagner, L.; Gerges, D.; Herkner, H.; Tevdoradze, T.; Mechtler, K.; Schmidt, A.; Winnicki, W. Urinary Collectrin (TMEM27) as Novel Marker for Acute Kidney Injury. Life 2022, 12, 1391. [Google Scholar] [CrossRef]
- Bonnard, Å.; Bark, R.; Hederstierna, C. Clinical update on sensorineural hearing loss in Turner syndrome and the X-chromosome. Am. J. Med. Genet. C Semin. Med. Genet. 2019, 181, 18–24. [Google Scholar] [CrossRef]
- Farooqui, A.; Anwer, A.; Alam, A.; Bagabir, S.A.; Haque, S.; Khadgawat, R.; Kazim, S.N.; Ali, S.; Ishrat, R. Brain-derived neurotrophic factor G196A (rs6265) gene polymorphism increases Turner syndrome susceptibility. Biotechnol. Genet. Eng. Rev. 2023, 39, 882–896. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]


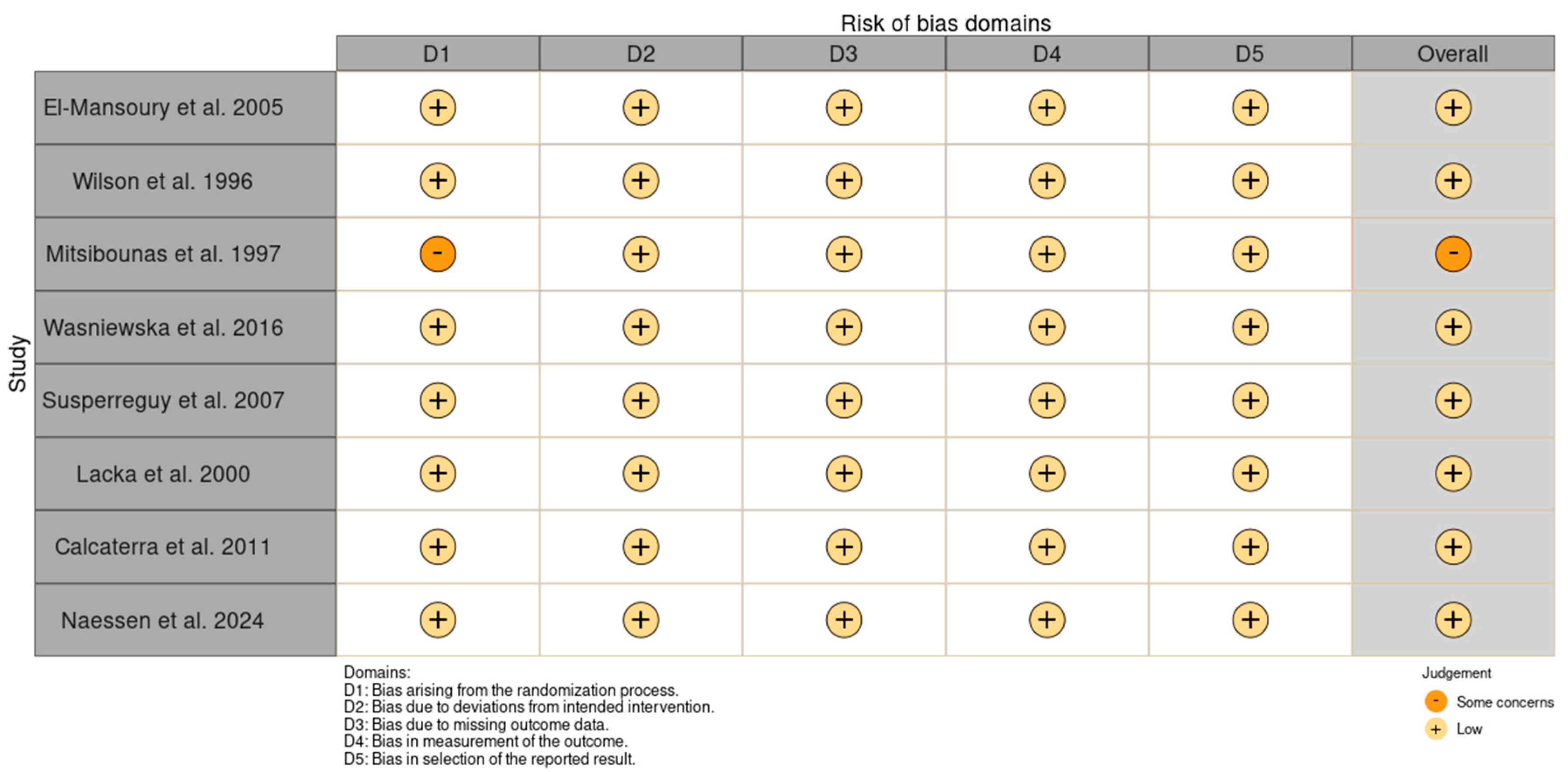
| Author and Year | El-Mansoury et al., 2005 [33] | Wilson et al., 1996 [34] | Mitsibounas et al., 1997 [30] | Wasniewska et al., 2016 [31] | Susperreguy et al., 2007 [32] | Lacka et al., 2000 [35] | Calcaterra et al., 2011 [36] | Naessen et al., 2024 [26] | |
|---|---|---|---|---|---|---|---|---|---|
| Group (n) | study | 91 | 60 | 29 | 66 | 20 (10 treated with GH) | 37 | 73 | 503 |
| control | 228 | 57 | 22 | 132 | 10 | 37 | 93 | 401 | |
| Hashimoto diagnosis (n) | study | No data | 3 | No data | 66 | No data | No data | 32 | No data |
| control | No data | No data | No data | 132 | No data | No data | 0 | No data | |
| Age (y) | study | 37.7 ± 11 | No data | 22.55 ± 6.34 | 13.0 (4.5–17.9) | 9.6 ± 3.5 (TS) 10.9 ± 3.1(GH) | 13.8 ± 6 | 25.01 ± 10.43 | 27.6 ± 11.5 |
| control | 37.3 ± 9 | No data | 22.05 ± 2.299 | 10.6 (2.8–15.0) | 9.9 ± 2.8 | No data | 1.95 to 37.2 | 35.5 ± 5.7 | |
| Height (cm) | study | 149.4 ± 7 | No data | 148 ± 9 | No data | No data | No data | No data | 153.6 ± 7.2 |
| control | 165.7 ± 6.6 | No data | 158 ± 5.5 | No data | No data | No data | No data | 166.3 ± 6.6 | |
| Weight (kg) | study | 56.4 ± 12.4 | No data | 53.16 ± 10.12 | No data | No data | No data | No data | 58.9 ± 11.9 |
| control | 65.4 ± 10.3 | No data | 52.86 ± 4.663 | No data | No data | No data | No data | 65.2 ± 10.2 | |
| Body mass index (BMI) (kg/m2) | study | 25.3 ± 4.7 | No data | 23.75 ± 3.64 | No data | No data | No data | No data | 25.1 ± 5.7 |
| control | 23.8 ± 3.7 | No data | 21.90 ± 1.681 | No data | No data | No data | No data | 23.6 ± 3.4 | |
| Author and Year | El-Mansoury et al., 2005 [33] | Wilson et al., 1996 [34] | Mitsibounas et al., 1997 [30] | Wasniewska et al., 2016 [31] | Susperreguy et al., 2007 [32] | Lacka et al., 2000 [35] | Calcaterra et al., 2011 [36] | Naessen et al., 2024 [26] | |
|---|---|---|---|---|---|---|---|---|---|
| Group (n) | study | 91 | 60 | 29 | 66 | 20 (10 treated with GH) | 37 | 73 | 503 |
| control | 228 | 57 | 22 | 132 | 10 | 37 | 93 | 401 | |
| Serum TSH (U/mL) | study | 2.5 ± 2.3 | 2.5 ± 2.5 | 2.468 ± 1.2003 | 2.8 (0.6–4.8) | 2.5 ± 0.6 | 3.9 ± 6.61 | No data | 3.18 ± 7.78 |
| control | 1.2 ± 0.7 | 2.4 ± 1.7 | 1.95 ± 1.45 | 2.5 (0.47–4.9) | 2.8 ± 1.0 | No data | No data | 1.10 ± 0.63 | |
| Serum fT4 (ng/dL) | study | 1.07 ± 0.2 | No data | No data | 1.173 ± 0.225 | 1.38 ± 0.23 | 1.71 ± 2.1 | No data | 1.227 ± 0.326 |
| control | 1.16 ±0.2 | No data | No data | 1.196 ± 0.28 | 1.45 ± 0.28 | No data | No data | 1.158 ± 0.194 | |
| Serum TT3 (nmol/L) | study | No data | No data | 1.59 ± 0.35 | No data | 1.7 ± 0.3 | 2.6 ± 0.1 | No data | No data |
| control | No data | No data | 1.88 ± 284 | No data | 1.8 ± 0.3 | No data | No data | No data | |
| Serum TT4 (nmol/L) | study | No data | 104 ± 25 | 124.131 ± 24.3063 | No data | 128 ± 23 | 119.7 ± 46.3 | No data | No data |
| control | No data | 129 ± 44 | 119.37 ± 15.773 | No data | 128 ± 25 | No data | No data | No data | |
| Author and Year | El-Mansoury et al., 2005 [33] | Wilson et al., 1996 [34] | Mitsibounas et al., 1997 [30] | Wasniewska et al., 2016 [31] | Susperreguy et al., 2007 [32] | Lacka et al., 2000 [35] | Calcaterra et al., 2011 [36] | Naessen et al., 2024 [26] | |
|---|---|---|---|---|---|---|---|---|---|
| Group (n) | study | 91 | 60 | 29 | 66 | 20 (10 treated with GH) | 37 | 73 | 503 |
| control | 228 | 57 | 22 | 132 | 10 | 37 | 93 | 401 | |
| Euthyroidism (n) | study | No data | 60 | No data | 66 | 20 | 27 | 73 | No data |
| control | No data | 57 | No data | 132 | 10 | No data | 93 | No data | |
| Hyperthyroidism (n) | study | 3 | 0 | 0 | 0 | 0 | 1 | 0 | No data |
| control | 0 | 0 | 0 | 0 | 0 | No data | 0 | No data | |
| Hypothyroidism (n) | study | 23 | 0 | 0 | 0 | 0 | 8 | 0 | 37 |
| control | 5 | 0 | 0 | 0 | 0 | No data | 0 | 0 | |
| Author and Year | El-Mansoury et al., 2005 [33] | Wilson et al., 1996 [34] | Mitsibounas et al., 1997 [30] | Wasniewska et al., 2016 [31] | Susperreguy et al., 2007 [32] | Lacka et al., 2000 [35] | Calcaterra et al., 2011 [36] | Naessen et al., 2024 [26] | |
|---|---|---|---|---|---|---|---|---|---|
| Group (n) | study | 91 | 60 | 29 | 66 | 20 | 37 | 73 | 503 |
| control | 228 | 57 | 22 | 132 | 10 | 37 | 93 | 401 | |
| Positive thyroid antibodies (n) | study | 25 | 18 | 4 | 66 | 0 | 23 | No data | 206 |
| control | 0 | 1 | 0 | 132 | 0 | 6 | No data | 41 | |
| Serum antiTPO level (mIU/L) | study | 475 ± 960 | No data | No data | 119 (5–6.4) | No data | 10.9 ± 12.7 | No data | No data |
| control | <0.01 | No data | No data | 374 (31–29.95) | No data | No data | No data | No data | |
| Serum antiTg level (mIU/L) | study | No data | No data | No data | 230 (5–4.9) | No data | 3300 ± 9100 | No data | No data |
| control | No data | No data | No data | 227 (10–6.4) | No data | No data | No data | No data | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacka, K.; Pempera, N.; Główka, A.; Mariowska, A.; Miedziaszczyk, M. Turner Syndrome and the Thyroid Function—A Systematic and Critical Review. Int. J. Mol. Sci. 2024, 25, 12937. https://doi.org/10.3390/ijms252312937
Lacka K, Pempera N, Główka A, Mariowska A, Miedziaszczyk M. Turner Syndrome and the Thyroid Function—A Systematic and Critical Review. International Journal of Molecular Sciences. 2024; 25(23):12937. https://doi.org/10.3390/ijms252312937
Chicago/Turabian StyleLacka, Katarzyna, Nikola Pempera, Alicja Główka, Agnieszka Mariowska, and Miłosz Miedziaszczyk. 2024. "Turner Syndrome and the Thyroid Function—A Systematic and Critical Review" International Journal of Molecular Sciences 25, no. 23: 12937. https://doi.org/10.3390/ijms252312937
APA StyleLacka, K., Pempera, N., Główka, A., Mariowska, A., & Miedziaszczyk, M. (2024). Turner Syndrome and the Thyroid Function—A Systematic and Critical Review. International Journal of Molecular Sciences, 25(23), 12937. https://doi.org/10.3390/ijms252312937







