Involvement of Melatonin, Oxidative Stress, and Inflammation in the Protective Mechanism of the Carotid Artery over the Torpor–Arousal Cycle of Ground Squirrels
Abstract
1. Introduction
2. Results
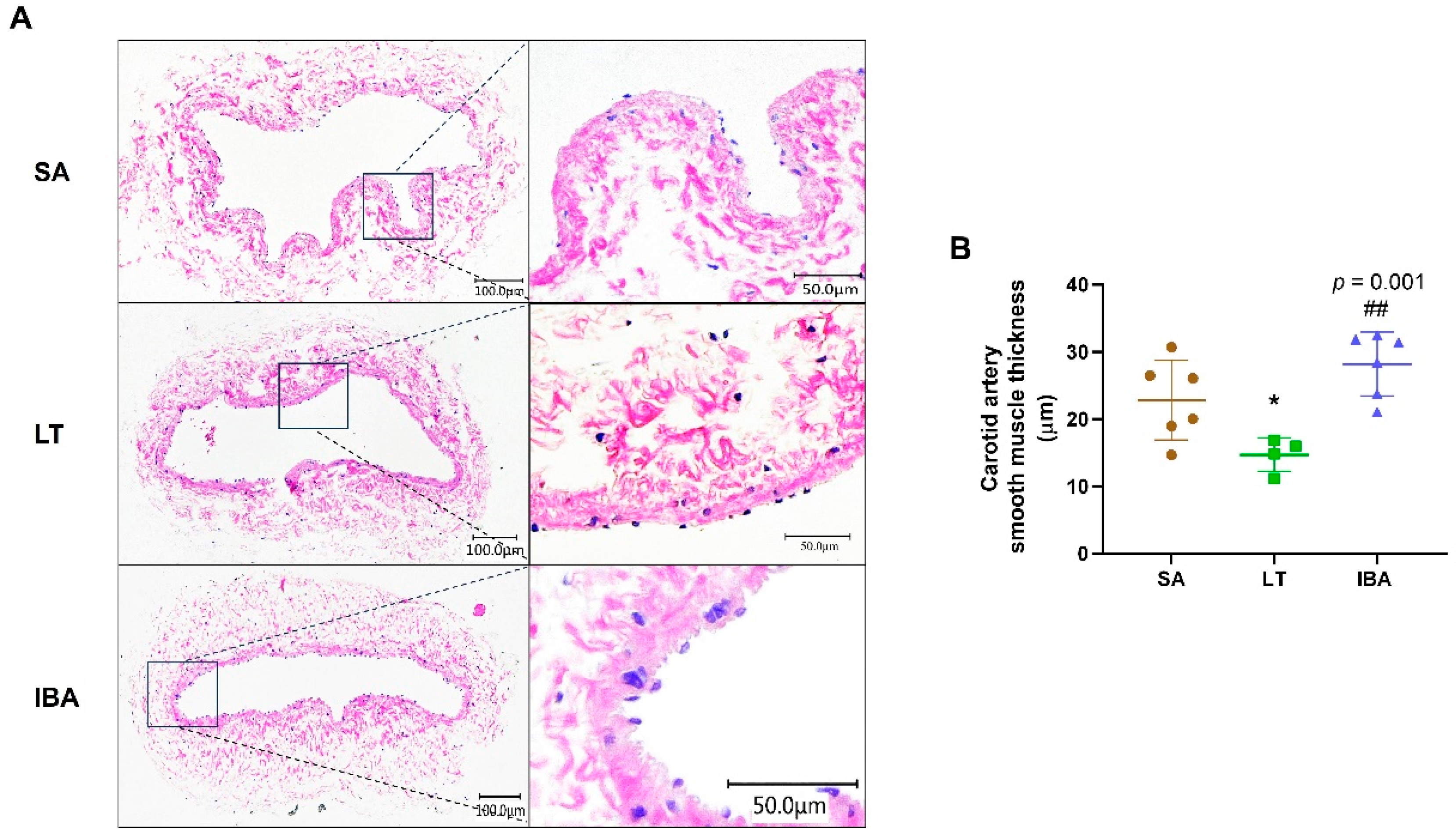
2.1. Changes in Carotid Artery Intima-Media Thickness of Ground Squirrels
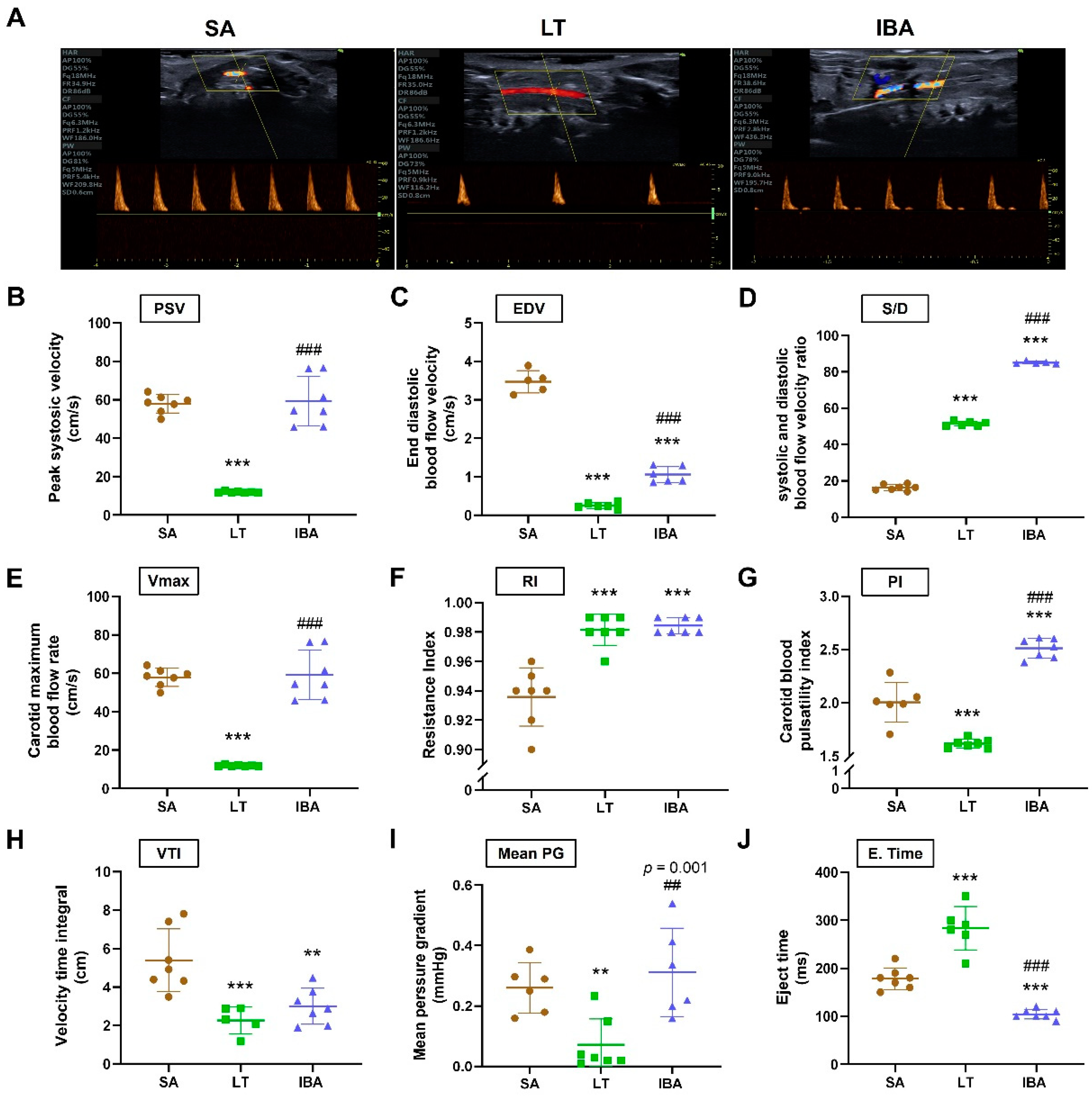
2.2. Changes in Carotid Artery Hemodynamics of Ground Squirrels
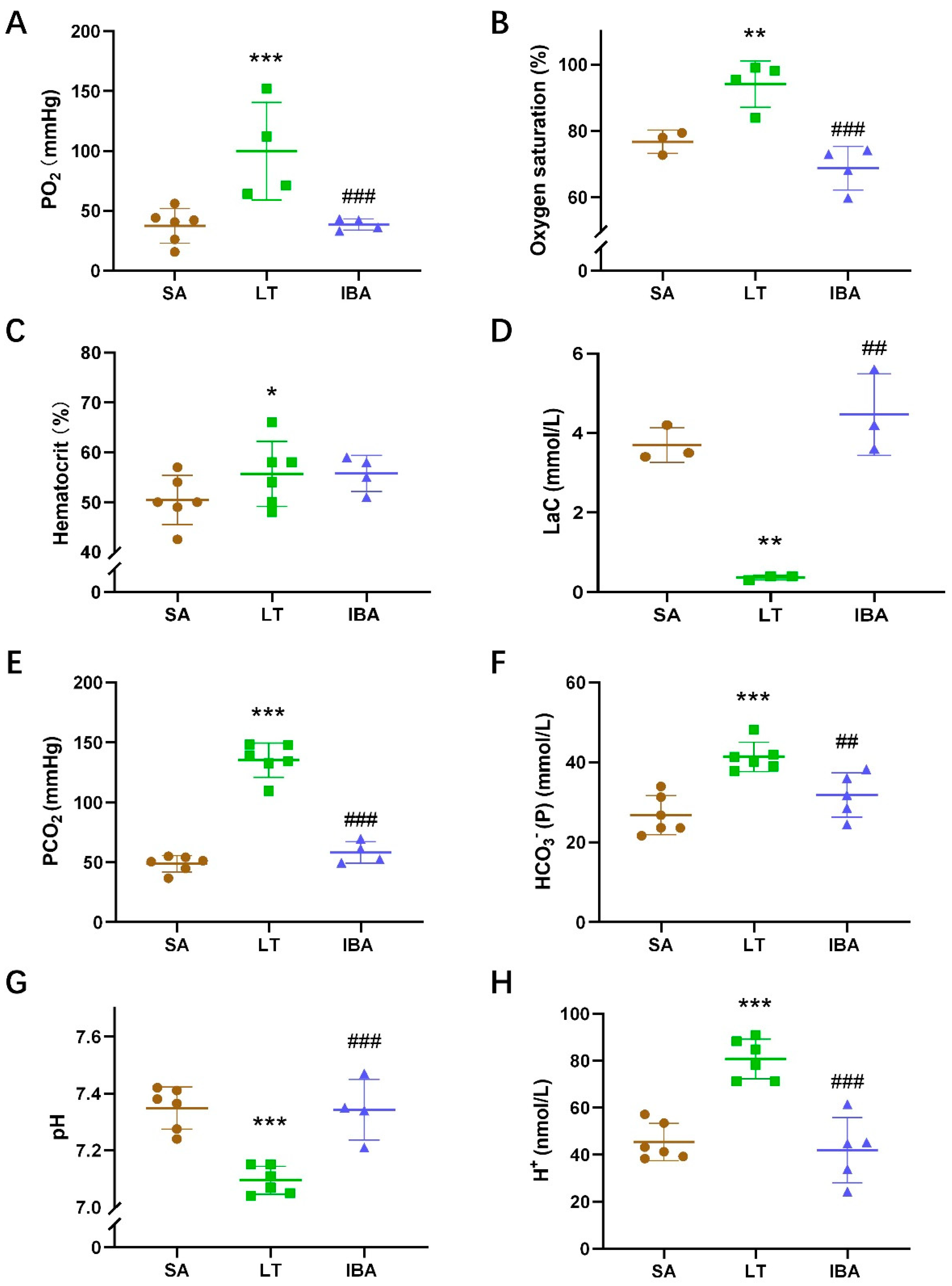
2.3. Arterial Blood Gas Value of Ground Squirrels
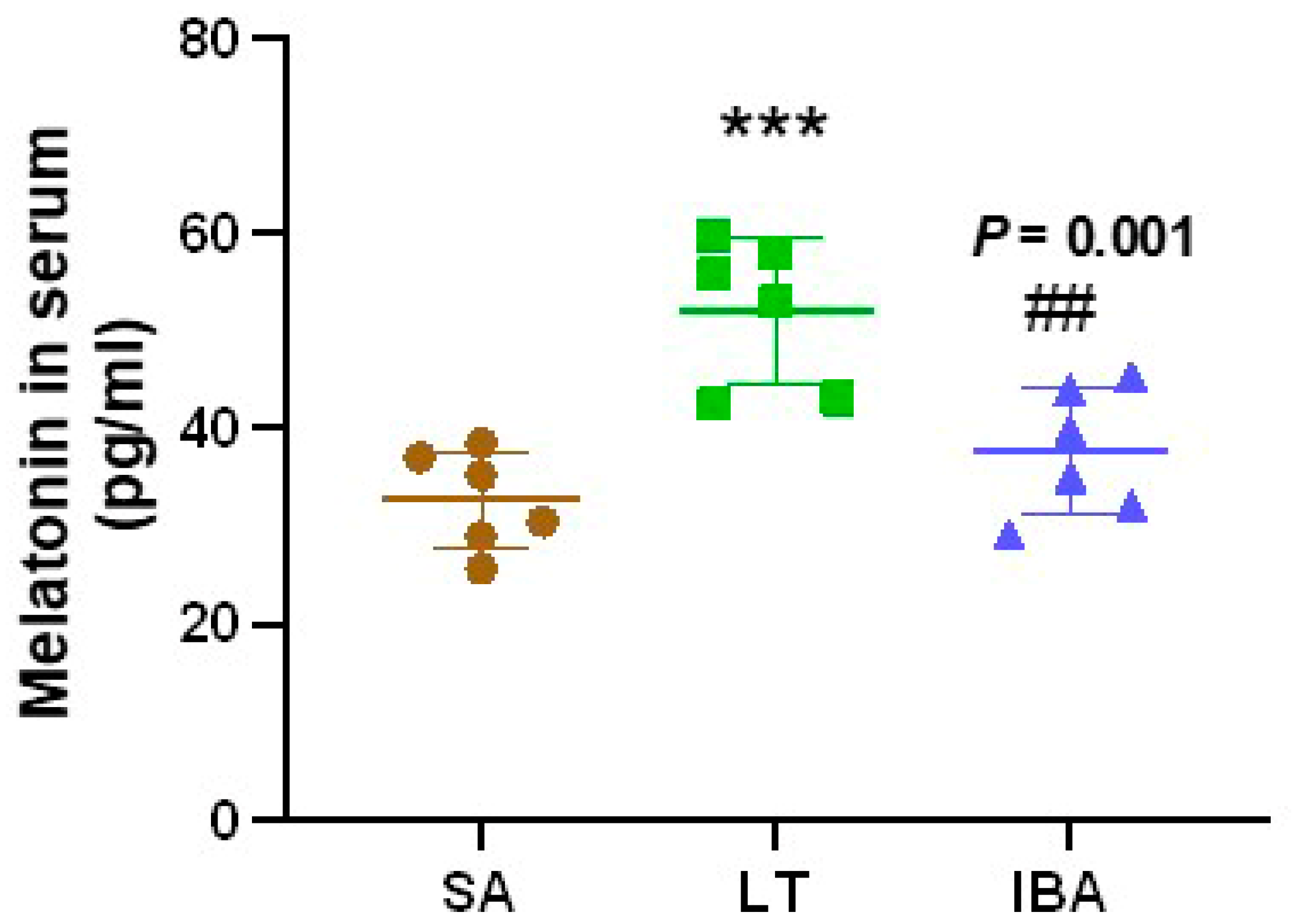
2.4. Melatonin Level in Serum of Ground Squirrels
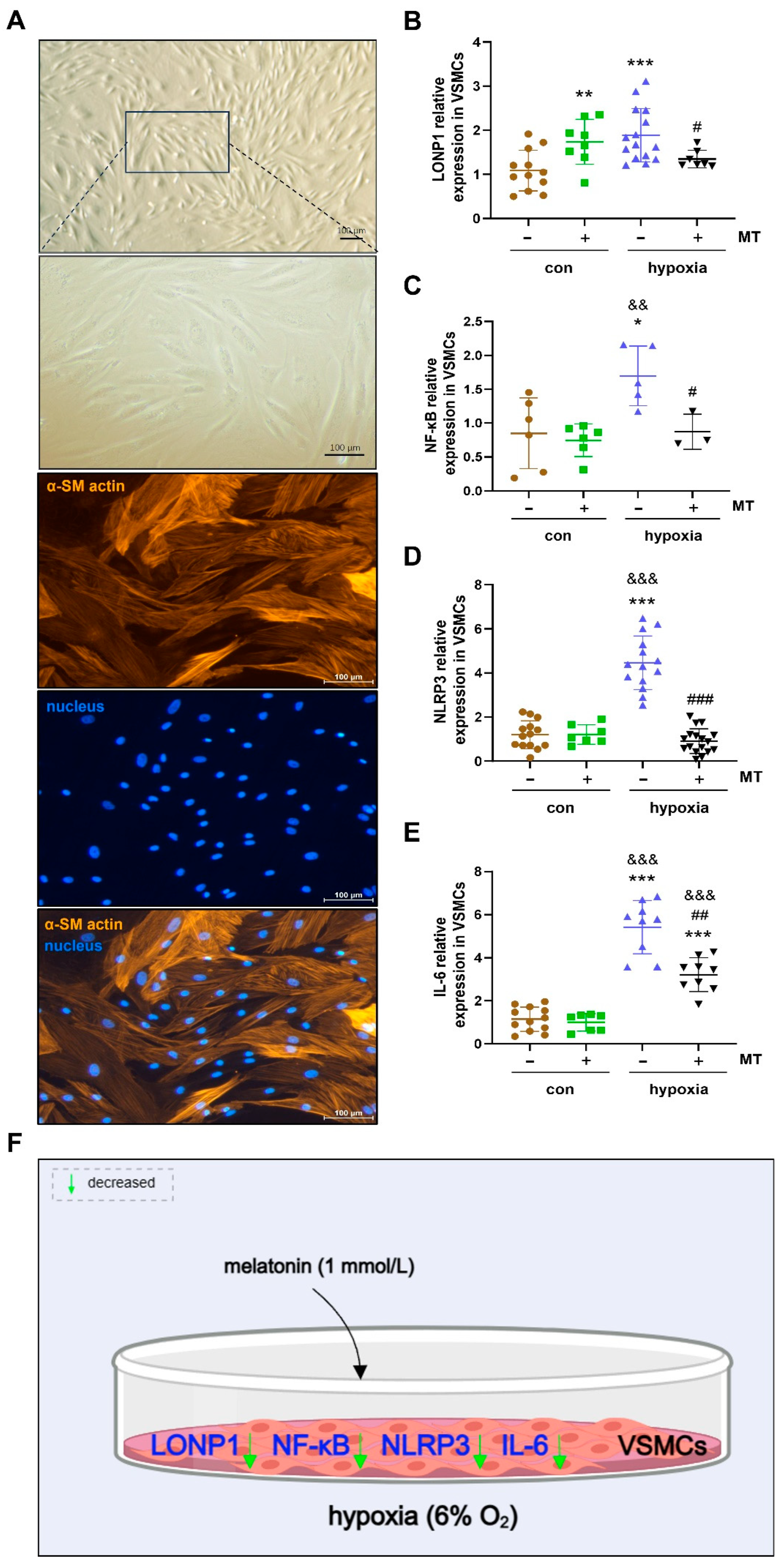
2.5. The Effect of MT on Oxidative Stress and Inflammatory Factor Expression in Hypoxia-Induced Primary Cultured VSMCs of Ground Squirrels
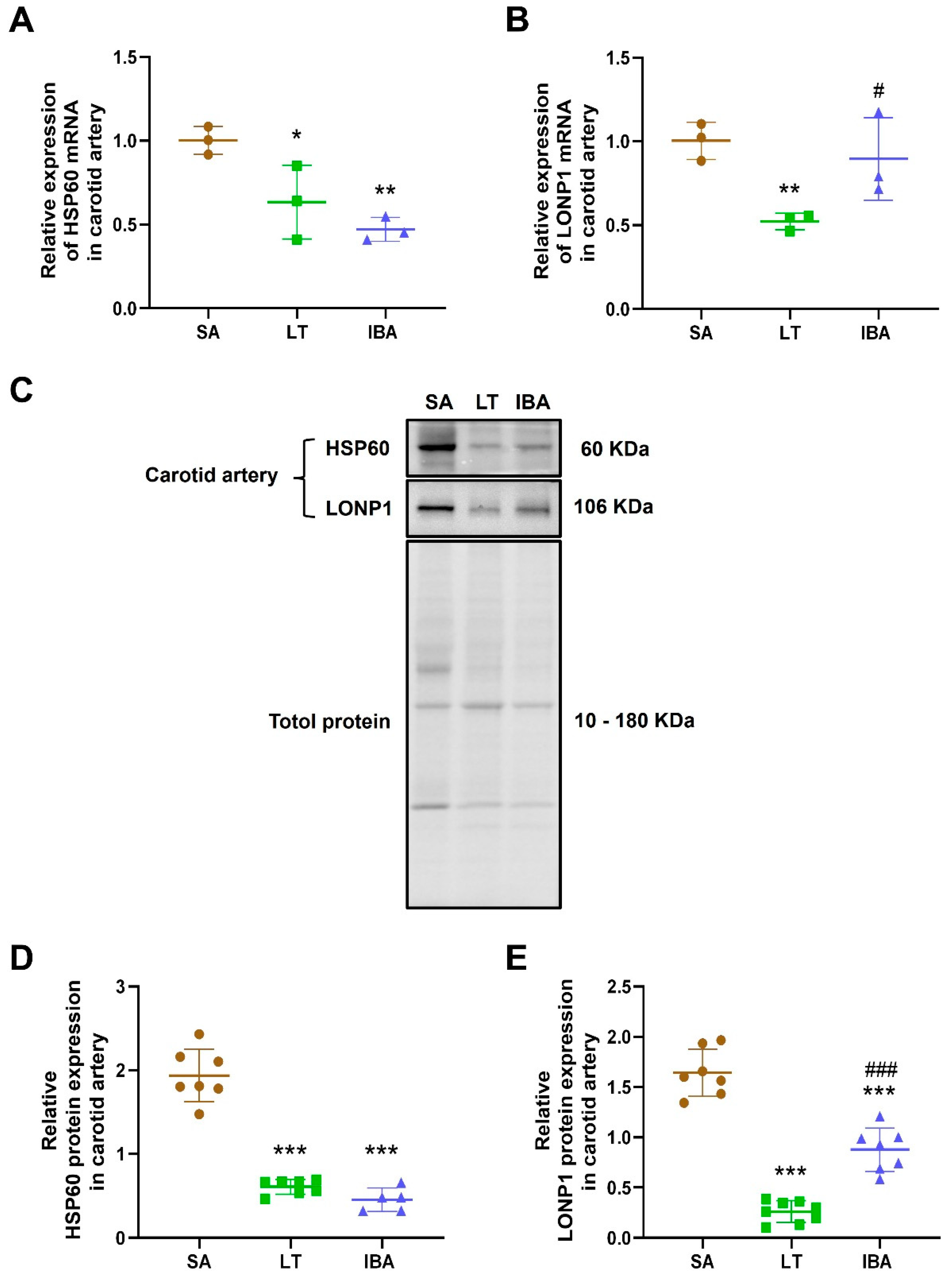
2.6. Oxidative Stress Levels in Carotid Arteries of Ground Squirrels
2.7. Inflammatory Factor Levels in Carotid Arteries of Ground Squirrels
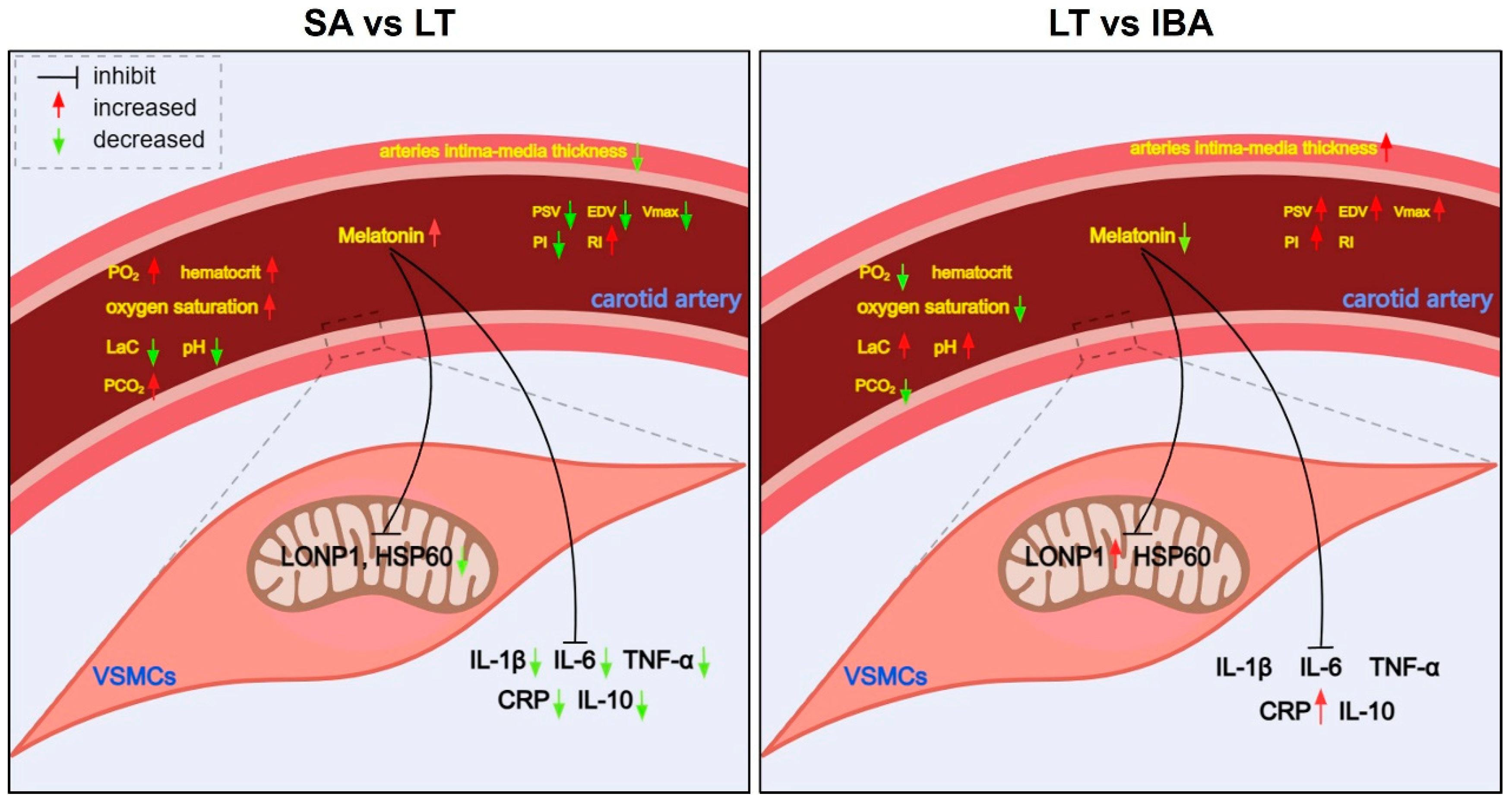
3. Discussion
4. Materials and Methods
4.1. Animal Collection and Grouping
4.2. Sample Collection
4.2.1. Carotid Artery Tissue Sample Collection
4.2.2. Serum Sample Collection
4.3. Protein Extraction and Concentration Determination
4.4. Western Blots
4.5. RNA Extraction and RT-qPCR (Real-Time Fluorescence Quantitative PCR)
4.5.1. RNA Extraction
4.5.2. Reverse Transcription and RT-qPCR
4.6. Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Hematoxylin and Eosin (HE) Staining
4.8. Color Doppler Ultrasonography
4.9. Arterial Blood Gas Measurement
4.10. Cell Culture, Identification, and Sample Collection
4.11. Statistical Analysis of Data
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Geiser, F. Hibernation. Curr. Biol. 2013, 23, R188–R193. [Google Scholar] [CrossRef]
- Carey, H.V.; Andrews, M.T.; Martin, S.L. Mammalian hibernation: Cellular and molecular responses to depressed metabolism and low temperature. Physiol. Rev. 2003, 83, 1153–1181. [Google Scholar] [CrossRef]
- Andrews, M.T. Molecular interactions underpinning the phenotype of hibernation in mammals. J. Exp. Biol. 2019, 222 Pt 2, jeb160606. [Google Scholar] [CrossRef]
- Han, Y.; Miao, W.; Hao, Z.; An, N.; Yang, Y.; Zhang, Z.; Chen, J.; Storey, K.B.; Lefai, E.; Chang, H. The Protective Effects on Ischemia–Reperfusion Injury Mechanisms of the Thoracic Aorta in Daurian Ground Squirrels (Spermophilus dauricus) over the Torpor–Arousal Cycle of Hibernation. Int. J. Mol. Sci. 2022, 23, 10248. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Han, Y.; Yang, Y.; Hao, Z.; An, N.; Chen, J.; Zhang, Z.; Gao, X.; Storey, K.B.; Chang, H.; et al. Dynamic Changes in Colonic Structure and Protein Expression Suggest Regulatory Mechanisms of Colonic Barrier Function in Torpor–Arousal Cycles of the Daurian Ground Squirrel. Int. J. Mol. Sci. 2022, 23, 9026. [Google Scholar] [CrossRef]
- Saito, H.; Thapaliya, S.; Matsuyama, H.; Nishimura, M.; Unno, T.; Komori, S.; Takewaki, T. Reversible impairment of endothelium-dependent relaxation in golden hamster carotid arteries during hibernation. J. Physiol. 2002, 540 Pt 1, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Zatzman, M.L. Renal and cardiovascular effects of hibernation and hypothermia. Cryobiology 1984, 21, 593–614. [Google Scholar] [CrossRef] [PubMed]
- Henning, R.H.; Deelman, L.E.; Hut, R.A.; Van der Zee, E.A.; Buikema, H.; Nelemans, S.; Lip, H.; De Zeeuw, D.; Daan, S.; Epema, A.H. Normalization of aortic function during arousal episodes in the hibernating ground squirrel. Life Sci. 2002, 70, 2071–2083. [Google Scholar] [CrossRef] [PubMed]
- Hermes-Lima, M.; Zenteno-Savín, T. Animal response to drastic changes in oxygen availability and physiological oxidative stress. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2002, 133, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Vasconcelos, G.R.; Hermes-Lima, M. Hypometabolism, antioxidant defenses and free radical metabolism in the pulmonate land snail Helix aspersa. J. Exp. Biol. 2003, 206 Pt 4, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Tøien, Ø.; Drew, K.L.; Chao, M.L.; Rice, M.E. Ascorbate dynamics and oxygen consumption during arousal from hibernation in Arctic ground squirrels. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 281, R572–R583. [Google Scholar] [CrossRef]
- Drew, K.; Tøien, Ø.; Rivera, P.; Smith, M.; Perry, G.; Rice, M. Role of the antioxidant ascorbate in hibernation and warming from hibernation. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2002, 133, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Ballo, P.; Quatrini, I.; Giacomin, E.; Motto, A.; Mondillo, S. Circumferential versus longitudinal systolic function in patients with hypertension: A nonlinear relation. J. Am. Soc. Echocardiogr. 2007, 20, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Samaja, M. Blood gas transport at high altitude. Respiration 1997, 64, 422–428. [Google Scholar] [CrossRef]
- Sutton, J.R.; Reeves, J.T.; Wagner, P.D.; Groves, B.M.; Cymerman, A.; Malconian, M.K.; Rock, P.B.; Young, P.M.; Walter, S.D.; Houston, C.S. Operation Everest II: Oxygen transport during exercise at extreme simulated altitude. J. Appl. Physiol. 1988, 64, 1309–1321. [Google Scholar] [CrossRef]
- Jelicks, L.A.; Gupta, R. 31P-NMR of high-energy phosphates in perfused rat heart during metabolic acidosis. Am. J. Physiol. Circ. Physiol. 1992, 263 Pt 2, H903–H909. [Google Scholar] [CrossRef] [PubMed]
- da Silva, T.F.G.; de Bem, G.F.; da Costa, C.A.; Santos, I.B.; de Andrade Soares, R.; Ognibene, D.T.; Rito-Costa, F.; Cavalheira, M.A.; da Conceição, S.P.; Ferraz, M.R.; et al. Prenatal hypoxia predisposes vascular functional and structural changes associated with oxidative stress damage and depressive behavior in adult offspring male rats. Physiol. Behav. 2021, 230, 113293. [Google Scholar] [CrossRef]
- Chen, M.; Liu, Y.; Yi, D.; Wei, L.; Li, Y.; Zhang, L. Tanshinone IIA promotes pulmonary artery smooth muscle cell apoptosis in vitro by inhibiting the JAK2/STAT3 signaling pathway. Cell. Physiol. Biochem. 2014, 33, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, S.; Lee, J.; Singh, K.; Lee, I.; Suzuki, C.K. Multitasking in the mitochondrion by the ATP-dependent Lon protease. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2012, 1823, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Malkov, M.I.; Lee, C.T.; Taylor, C.T. Regulation of the Hypoxia-Inducible Factor (HIF) by Pro-Inflammatory Cytokines. Cells 2021, 10, 2340. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.L.; Zhu, X.; Rivera, P.M.; Tøien, Ø.; Barnes, B.M.; LaManna, J.C.; Smith, M.A.; Drew, K.L. Absence of cellular stress in brain after hypoxia induced by arousal from hibernation in Arctic ground squirrels. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R1297–R1306. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-L.; Hsiao, C.-J.; Hsu, C.-H.; Wang, S.-E.; Jen, P.H.-S.; Wu, C.-H. Hypothermic neuroprotections in the brain of an echolocation bat, Hipposideros terasensis. NeuroReport 2017, 28, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Heldmaier, G.; Ortmann, S.; Elvert, R. Natural hypometabolism during hibernation and daily torpor in mammals. Respir. Physiol. Neurobiol. 2004, 141, 317–329. [Google Scholar] [CrossRef]
- Revsbech, I.G.; Fago, A. Regulation of blood oxygen transport in hibernating mammals. J. Comp. Physiol. B 2017, 187, 847–856. [Google Scholar] [CrossRef]
- McArthur, M.D.; Jourdan, M.L.; Wang, L.C. Prolonged stable hypothermia: Effect on blood gases and pH in rats and ground squirrels. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1992, 262 Pt 2, R190–R197. [Google Scholar] [CrossRef] [PubMed]
- Stanton, T.L.; Daley, J.C., 3rd; Salzman, S.K. Prolongation of hibernation bout duration by continuous intracerebroventricular infusion of melatonin in hibernating ground squirrels. Brain Res. 1987, 413, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Perreau-Lenz, S.; Kalsbeek, A.; Garidou, M.; Wortel, J.; Van Der Vliet, J.; Van Heijningen, C.; Simonneaux, V.; Pévet, P.; Buijs, R.M. Suprachiasmatic control of melatonin synthesis in rats: Inhibitory and stimulatory mechanisms. Eur. J. Neurosci. 2003, 17, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Zeman, M.; Herichova, I. Melatonin and clock genes expression in the cardiovascular system. Front. Biosci. 2013, 5, 743–753. [Google Scholar] [CrossRef]
- Govender, J.; Loos, B.; Marais, E.; Engelbrecht, A. Mitochondrial catastrophe during doxorubicin-induced cardiotoxicity: A review of the protective role of melatonin. J. Pineal Res. 2014, 57, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Simko, F.; Paulis, L. Melatonin as a potential antihypertensive treatment. J. Pineal Res. 2007, 42, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Zawilska, J.B.; Skene, D.J.; Arendt, J. Physiology and pharmacology of melatonin in relation to biological rhythms. Pharmacol. Rep. 2009, 61, 383–410. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E.A.; Macchiavello, R.; Montt, C.; Ebensperger, G.; Díaz, M.; Ramírez, S.; Parer, J.T.; Serón-Ferré, M.; Reyes, R.V.; Llanos, A.J. Melatonin improves cerebrovascular function and decreases oxidative stress in chronically hypoxic lambs. J. Pineal Res. 2014, 57, 33–42. [Google Scholar] [CrossRef]
- Lin, H.-W.; Lee, E.-J. Effects of melatonin in experimental stroke models in acute, sub-acute, and chronic stages. Neuropsychiatr. Dis. Treat. 2009, 5, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Pearce, W.J.; Williams, J.M.; White, C.R.; Lincoln, T.M.; Ducsay, C.A.; Goyal, R.; Wilson, S.; Hu, X.-Q.; Zhang, L.; Curran-Everett, D.; et al. Effects of chronic hypoxia on soluble guanylate cyclase activity in fetal and adult ovine cerebral arteries. J. Appl. Physiol. 2009, 107, 192–199. [Google Scholar] [CrossRef]
- Lee, F.; Sun, C.; Sung, P.; Chen, K.; Chua, S.; Sheu, J.; Chung, S.; Chai, H.; Chen, Y.; Huang, T.; et al. Daily melatonin protects the endothelial lineage and functional integrity against the aging process, oxidative stress, and toxic environment and restores blood flow in critical limb ischemia area in mice. J. Pineal Res. 2018, 65, e12489. [Google Scholar] [CrossRef] [PubMed]
- Ralph, C.L.; Harlow, H.J.; Phillips, J.A. Delayed effect of pinealectomy on hibernation of the golden-mantled ground squirrel. Int. J. Biometeorol. 1982, 26, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, C.; Ballinger, M.A.; Andrews, M.T. Melatonin receptor signaling contributes to neuroprotection upon arousal from torpor in thirteen-lined ground squirrels. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R1292–R1300. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weekley, B.; Harlow, H.J. Effects of pharmacological manipulation of the renin-angiotensin system on the hibernation cycle of the 13-lined ground squirrel (Spermophilus tridecemlineatus). Physiol. Behav. 1985, 34, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Baluk, P.; Hashizume, H.; McDonald, D.M. Cellular abnormalities of blood vessels as targets in cancer. Curr. Opin. Genet. Dev. 2005, 15, 102–111. [Google Scholar] [CrossRef]
- Barton, M.; Haudenschild, C.C. Endothelium and atherogenesis: Endothelial therapy revisited. J. Cardiovasc. Pharmacol. 2001, 38 (Suppl. 2), S23–S25. [Google Scholar] [CrossRef]
- Lin, X.; Zhan, J.-K.; Wang, Y.-J.; Tan, P.; Chen, Y.-Y.; Deng, H.-Q.; Liu, Y.-S. Function, Role, and Clinical Application of MicroRNAs in Vascular Aging. BioMed Res. Int. 2016, 2016, 6021394. [Google Scholar] [CrossRef] [PubMed]
- Arinell, K.; Sahdo, B.; Evans, A.L.; Arnemo, J.M.; Baandrup, U.; Fröbert, O. Brown bears (Ursus arctos) seem resistant to atherosclerosis despite highly elevated plasma lipids during hibernation and active state. Clin. Transl. Sci. 2012, 5, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ren, W.; Wang, X.; Yu, X.; Cui, L.; Li, X.; Zhang, X.; Shi, B. MicroRNA-150 relieves vascular remodeling and fibrosis in hypoxia-induced pulmonary hypertension. Biomed. Pharmacother. 2019, 109, 1740–1749. [Google Scholar] [CrossRef]
- Kyotani, Y.; Ota, H.; Itaya-Hironaka, A.; Yamauchi, A.; Sakuramoto-Tsuchida, S.; Zhao, J.; Ozawa, K.; Nagayama, K.; Ito, S.; Takasawa, S.; et al. Intermittent hypoxia induces the proliferation of rat vascular smooth muscle cell with the increases in epidermal growth factor family and erbB2 receptor. Exp. Cell Res. 2013, 319, 3042–3050. [Google Scholar] [CrossRef] [PubMed]
- Bonis, A.; Anderson, L.; Talhouarne, G.; Schueller, E.; Unke, J.; Krus, C.; Stokka, J.; Koepke, A.; Lehrer, B.; Schuh, A.; et al. Cardiovascular resistance to thrombosis in 13-lined ground squirrels. J. Comp. Physiol. B 2019, 189, 167–177. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Collard, C.D. Vascular ischaemia and reperfusion injury. Br. Med. Bull. 2004, 70, 71–86. [Google Scholar] [CrossRef]
- Ozari, H.O.; Oktenli, C.; Celik, S.; Tangi, F.; Ipcioglu, O.; Terekeci, H.M.; Top, C.; Uzun, M.; Sanisoglu, Y.S.; Nalbant, S. Are increased carotid artery pulsatility and resistance indexes early signs of vascular abnormalities in young obese males? J. Clin. Ultrasound. 2012, 40, 335–340. [Google Scholar] [CrossRef]
- Yang, Y.; Hao, Z.; An, N.; Han, Y.; Miao, W.; Storey, K.B.; Lefai, E.; Liu, X.; Wang, J.; Liu, S.; et al. Integrated transcriptomics and metabolomics reveal protective effects on heart of hibernating Daurian ground squirrels. J. Cell. Physiol. 2023, 238, 2724–2748. [Google Scholar] [CrossRef] [PubMed]
- Milsom, W.K.; Jackson, D.C. Hibernation and gas exchange. Compr. Physiol. 2011, 1, 397–420. [Google Scholar] [PubMed]
- Revsbech, I.G.; Malte, H.; Fröbert, O.; Evans, A.; Blanc, S.; Josefsson, J.; Fago, A. Decrease in the red cell cofactor 2,3-diphosphoglycerate increases hemoglobin oxygen affinity in the hibernating brown bear Ursus arctos. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R43–R49. [Google Scholar] [CrossRef] [PubMed]
- Maginniss, L.A.; Milsom, W.K. Effects of hibernation on blood oxygen transport in the golden-mantled ground squirrel. Respir. Physiol. 1994, 95, 195–208. [Google Scholar] [CrossRef]
- Clausen, G.; Ersland, A. The respiratory properties of the blood of the hibernating hedgehog Erinaceus europaeus L. Respir. Physiol. 1968, 5, 221–233. [Google Scholar] [CrossRef]
- Græsli, A.R.; Evans, A.L.; Fahlman, Å.; Bertelsen, M.F.; Blanc, S.; Arnemo, J.M. Seasonal variation in haematological and biochemical variables in free-ranging subadult brown bears (Ursus arctos) in Sweden. BMC Vet. Res. 2015, 11, 301. [Google Scholar] [CrossRef] [PubMed]
- Christen, T.; Lemasson, B.; Pannetier, N.; Farion, R.; Segebarth, C.; Rémy, C.; Barbier, E.L. Evaluation of a quantitative blood oxygenation level-dependent (qBOLD) approach to map local blood oxygen saturation. NMR Biomed. 2011, 24, 393–403. [Google Scholar] [CrossRef]
- Franchini, K.G.; Cestari, I.A.; Krieger, E.M. Restoration of arterial blood oxygen tension increases arterial pressure in sinoaortic-denervated rats. Am. J. Physiol. Circ. Physiol. 1994, 266 Pt 2, H1055–H1061. [Google Scholar] [CrossRef]
- Frerichs, K.U.; Dienel, G.A.; Cruz, N.F.; Sokoloff, L.; Hallenbeck, J.M. Rates of glucose utilization in brain of active and hibernating ground squirrels. Am. J. Physiol. Integr. Comp. Physiol. 1995, 268 Pt 2, R445–R453. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Choi, I.; Park, K. Activation of stress signaling molecules in bat brain during arousal from hibernation. J. Neurochem. 2002, 82, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Hanson, A.; Johansson, B.W. Myocardial lactate concentration in guinea-pigs, normothermic and hypothermic, and hedgehogs, in a hibernating and a non-hibernating state. Acta Physiol. Scand. 1961, 53, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, C. Acid-base balance in euthermic and hibernating marmots. Am. J. Physiol. 1973, 224, 1185–1189. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.B.; Milsom, W.K. pH regulation in hibernation: Implications for ventilatory and metabolic control. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2019, 237, 110536. [Google Scholar] [CrossRef] [PubMed]
- Kreienbühl, G.; Strittmatter, J.; Ayim, E. Blood gas analyses of hibernating hamsters and dormice. Pflug. Arch. 1976, 366, 167–172. [Google Scholar] [CrossRef]
- Snapp, B.D.; Heller, H.C. Suppression of Metabolism during Hibernation in Ground Squirrels (Citellus lateralis). Physiol. Zool. 1981, 54, 297–307. [Google Scholar] [CrossRef]
- Malan, A.; Rodeau, J.L.; Daull, F. Intracellular pH in hibernation and respiratory acidosis in the European hamster. J. Comp. Physiol. B 1985, 156, 251–258. [Google Scholar] [CrossRef]
- Lyman, C.P.; Hastings, A.B. Total CO2, plasma pH and pCO2 of hamsters and ground squirrels during hibernation. Am. J. Physiol. 1951, 167, 633–637. [Google Scholar] [CrossRef]
- Bharma, S.; Milsom, W.K. Acidosis and metabolic rate in golden mantled ground squirrels (Spermophilus lateralis). Respir. Physiol. 1993, 94, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Kuhnen, G.; Wloch, B.; Wünnenberg, W. Effects of acute hypoxia and/or hypercapnia on body temperatures and cold induced thermogenesis in the golden hamster. J. Therm. Biol. 1987, 12, 103–107. [Google Scholar] [CrossRef]
- Amaral, F.G.D.; Cipolla-Neto, J. A brief review about melatonin, a pineal hormone. Arch. Endocrinol. Metab. 2018, 62, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.D.; Iwamoto, A.; Kawai, M.; Goda, R.; Matsuo, H.; Otsuka, T.; Nagasawa, M.; Furuse, M.; Yasuo, S. Melatonin adjusts the expression pattern of clock genes in the suprachiasmatic nucleus and induces antidepressant-like effect in a mouse model of seasonal affective disorder. Chronobiol. Int. 2015, 32, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Acuna-Castroviejo, D.; Escames, G.; Rodriguez, M.I.; Lopez, L.C. Melatonin role in the mitochondrial function. Front. Biosci. 2007, 12, 947–963. [Google Scholar] [CrossRef]
- Acuña-Castroviejo, D.; Escames, G.; López, L.C.; Hitos, A.B.; León, J. Melatonin and nitric oxide: Two required antagonists for mitochondrial homeostasis. Endocrine 2005, 27, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, S.; Li, M.; Saito, T.; Tong, M.; Rashed, E.; Mareedu, S.; Zhai, P.; Bárcena, C.; López-Otín, C.; Yehia, G.; et al. Mitochondrial LonP1 protects cardiomyocytes from ischemia/reperfusion injury in vivo. J. Mol. Cell. Cardiol. 2019, 128, 38–50. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Volt, H.; García, J.A.; Doerrier, C.; Díaz-Casado, M.E.; Guerra-Librero, A.; López, L.C.; Escames, G.; Tresguerres, J.A.; Acuña-Castroviejo, D. Same molecule but different expression: Aging and sepsis trigger NLRP3 inflammasome activation, a target of melatonin. J. Pineal Res. 2016, 60, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Su, L.-Y.; Sun, C.; Jiao, L.; Miao, Y.; Xu, M.; Luo, R.; Zuo, X.; Zhou, R.; Zheng, P.; et al. Melatonin alleviates morphine analgesic tolerance in mice by decreasing NLRP3 inflammasome activation. Redox Biol. 2020, 34, 101560. [Google Scholar] [CrossRef]
- Qin, M.; Liu, Y.; Sun, M.; Li, X.; Xu, J.; Zhang, L.; Jiang, H. Protective effects of melatonin on the white matter damage of neonatal rats by regulating NLRP3 inflammasome activity. NeuroReport 2021, 32, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Sagrillo-Fagundes, L.; Salustiano, E.M.A.; Ruano, R.; Markus, R.P.; Vaillancourt, C. Melatonin modulates autophagy and inflammation protecting human placental trophoblast from hypoxia/reoxygenation. J. Pineal Res. 2018, 65, e12520. [Google Scholar] [CrossRef] [PubMed]
- Liyanagamage, D.S.N.K.; Martinus, R.D. Role of Mitochondrial Stress Protein HSP60 in Diabetes-Induced Neuroinflammation. Mediat. Inflamm. 2020, 2020, 8073516. [Google Scholar] [CrossRef]
- Bitting, L.; Watson, F.L.; O’Hara, B.F.; Kilduff, T.S.; Heller, H.C. HSP70 expression is increased during the day in a diurnal animal, the golden-mantled ground squirrel Spermophilus lateralis. Mol. Cell. Biochem. 1999, 199, 25–34. [Google Scholar] [CrossRef]
- Rouble, A.N.; Tessier, S.N.; Storey, K.B. Characterization of adipocyte stress response pathways during hibernation in thirteen-lined ground squirrels. Mol. Cell. Biochem. 2014, 393, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Shetty, R.; Noland, R.; Nandi, G.; Suzuki, C.K. Powering down the mitochondrial LonP1 protease: A novel strategy for anticancer therapeutics. Expert Opin. Ther. Targets 2023, 28, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Inflammaging: Disturbed interplay between autophagy and inflammasomes. Aging 2012, 4, 166–175. [Google Scholar] [CrossRef]
- Campbell, A.K.; Beaumont, A.J.; Hayes, L.; Herbert, P.; Gardner, D.; Ritchie, L.; Sculthorpe, N. Habitual exercise influences carotid artery strain and strain rate, but not cognitive function in healthy middle-aged females. Eur. J. Appl. Physiol. 2023, 123, 1051–1066. [Google Scholar] [CrossRef] [PubMed]
- Baylis, D.; Bartlett, D.B.; Patel, H.P.; Roberts, H.C. Understanding how we age: Insights into inflammaging. Longev. Heal. 2013, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Galluzzi, L.; Kroemer, G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science 2011, 333, 1109–1112. [Google Scholar] [CrossRef]
- Mominoki, K.; Morimatsu, M.; Karjalainen, M.; Hohtola, E.; Hissa, R.; Saito, M. Elevated plasma concentrations of haptoglobin in European brown bears during hibernation. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005, 142, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P.; Jani, A.H.; Johnson, R.J. Hibernating bears (Ursidae): Metabolic magicians of definite interest for the nephrologist. Kidney Int. 2013, 83, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Bogren, L.K.; Olson, J.M.; Carpluk, J.; Moore, J.M.; Drew, K.L. Resistance to systemic inflammation and multi organ damage after global ischemia/reperfusion in the arctic ground squirrel. PLoS ONE 2014, 9, e94225. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Hu, H.; Dang, K.; Chang, H.; Du, B.; Wu, X.; Gao, Y. Remarkable preservation of Ca2+ homeostasis and inhibition of apoptosis contribute to anti-muscle atrophy effect in hibernating Daurian ground squirrels. Sci. Rep. 2016, 6, 27020. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Z.; Han, Y.; Zhao, Q.; Zhu, M.; Liu, X.; Yang, Y.; An, N.; He, D.; Lefai, E.; Storey, K.B.; et al. Involvement of Melatonin, Oxidative Stress, and Inflammation in the Protective Mechanism of the Carotid Artery over the Torpor–Arousal Cycle of Ground Squirrels. Int. J. Mol. Sci. 2024, 25, 12888. https://doi.org/10.3390/ijms252312888
Hao Z, Han Y, Zhao Q, Zhu M, Liu X, Yang Y, An N, He D, Lefai E, Storey KB, et al. Involvement of Melatonin, Oxidative Stress, and Inflammation in the Protective Mechanism of the Carotid Artery over the Torpor–Arousal Cycle of Ground Squirrels. International Journal of Molecular Sciences. 2024; 25(23):12888. https://doi.org/10.3390/ijms252312888
Chicago/Turabian StyleHao, Ziwei, Yuting Han, Qi Zhao, Minghui Zhu, Xiaoxuan Liu, Yingyu Yang, Ning An, Dinglin He, Etienne Lefai, Kenneth B. Storey, and et al. 2024. "Involvement of Melatonin, Oxidative Stress, and Inflammation in the Protective Mechanism of the Carotid Artery over the Torpor–Arousal Cycle of Ground Squirrels" International Journal of Molecular Sciences 25, no. 23: 12888. https://doi.org/10.3390/ijms252312888
APA StyleHao, Z., Han, Y., Zhao, Q., Zhu, M., Liu, X., Yang, Y., An, N., He, D., Lefai, E., Storey, K. B., Chang, H., & Xie, M. (2024). Involvement of Melatonin, Oxidative Stress, and Inflammation in the Protective Mechanism of the Carotid Artery over the Torpor–Arousal Cycle of Ground Squirrels. International Journal of Molecular Sciences, 25(23), 12888. https://doi.org/10.3390/ijms252312888






