Abstract
The treatment of infections caused by Staphylococcus aureus is currently complicated by the increasing number of strains resistant to antimicrobial agents. One promising way to solve this problem is phage therapy. Due to the lack of data on the effectiveness and safety of phage preparations, STAFAL® is the only registered phage preparation for the treatment of infectious diseases in the Slovak Republic and the entire European Union. The aim of this work was to determine the effectiveness of the STAFAL® phage preparation against S. aureus strains of different origins with variable sensitivity to antimicrobial substances and with different genetic backgrounds. For this purpose, 111 carrier strains, 35 clinical isolates from bloodstream infections, and 46 strains from skin and soft tissue infections were analysed. The effectiveness of STAFAL® was determined by the plaque forming method. STAFAL® was effective against 74.0% of the strains tested. Susceptibility to this phage preparation was significantly higher in strains resistant to methicillin (MRSA), erythromycin and clindamycin (p < 0.05). The high efficiency of the STAFAL® preparation was confirmed against spa types t003, t024 and t032, typical of the hospital environment. The in vitro results indicate high therapeutic potential of the STAFAL® antistaphylococcal phage preparation, especially against MRSA strains.
1. Introduction
Staphylococcus aureus is an opportunistic pathogen that commonly colonises the nasal mucosa and human skin [1,2]. However, colonisation by S. aureus may be the basis for the development of infection [3]. This bacterium is capable of causing a wide range of infections, from skin and soft tissue infections to systemic infections, such as pneumonia, osteomyelitis, sepsis or endocarditis; thus, it is a leading cause of infection-related mortality worldwide [1,2,4]. The development of infection is facilitated by its set of virulence factors [2,5].
The treatment as well as decolonisation of S. aureus from body sites to prevent infectious events is complicated by the increasing number of strains resistant to beta-lactam antibiotics, designated as MRSA strains, that are circulating in the population. These strains are also often able to acquire resistance to other groups of antimicrobial agents (ATBs)—macrolides, tetracyclines, aminoglycosides, chloramphenicol and quinolones [3,6]. Moreover, the use of vancomycin as the final line in the treatment of MRSA infection has led to decreased susceptibility and treatment failure in some cases [7,8]. Other significant limitation in the treatment of S. aureus by ATBs include the risk of renal failure, allergy, drug–drug interactions and intolerable side effects, particularly in immunocompromised patients in the hospital environment [9,10,11]. For these reasons, it is necessary to search for new strategies that would be effective in the treatment of infections caused by resistant strains of microorganisms—in this particular case, by S. aureus [12,13].
Treatment by bacteriophages may be an alternative to classic antibiotic therapy, or its suitable supplement [14]. Bacteriophages (shortly phages) are viruses capable of specifically lysing bacterial cells. They are the most widespread form of organism in the world, present everywhere where bacteria are found [15].
Phage therapy has several advantages over conventional antibiotic therapy. Firstly, due to the specificity of the phage, it has a minimal impact on the physiological microbiota of the host. After the elimination of host bacteria, phages naturally diminish [12,13]. Phage therapy is considered safe for the human body, since phages are not toxic to or mutagenic in mammalian cells [13,16]. Another advantage is that resistance of bacteria to phages is developed 10 times slower than the resistance to antibiotics [17,18]. An indisputable advantage is the lower financial burden compared with the development and production of new antibiotics. The disadvantages of phage therapy include the possibility of neutralising antibody production by the patient’s body, which can reduce the effectiveness of bacteriophages [19]. Another disadvantage is the potential spread of genes encoding virulence or antibiotic resistance. The spread of these genes can be eliminated by whole-genome sequencing and the subsequent selection of phages that do not contain such genes [20]. Despite the limitations mentioned, treatment by bacteriophages may be the last possible therapeutic option in some cases [21,22,23,24,25]. In addition to the therapy of infections, phages can also be used to decolonise S. aureus carriers [19].
Bacteriophages were discovered as early as 1915 (and separately in 1917) and were used for the treatment of infectious diseases, such as staphylococcal skin infections, cholera or dysentery [26,27,28]. However, after the discovery and introduction of antibiotics into clinical practice, phage therapy was abandoned in most parts of the world. The production of phage preparations for the treatment of infectious diseases was continued in Eastern Europe in countries such as Georgia or Russia. However, these phage preparations are not recognised by Western regulatory agencies. Nowadays, due to the emergence of multidrug-resistant (MDR) strains, there is a restoration of efforts to produce safe and effective phage preparations also in other countries, such as France, Belgium, the United Kingdom, Australia, China, India or the United States [17,29,30,31,32,33]. Actually, phage therapy in Poland is administered to patients in the Phage Therapy Unit as a personalised experimental therapy [30]. In Slovakia, the antistaphylococcal phage preparation STAFAL® is currently the only phage preparation registered in a member state of the European Union [32,34].
The STAFAL® phage preparation includes lytic polyvalent bacteriophages of the family Herelleviridae, genus Kayvirus [35,36]. STAFAL® is indicated for the elimination of S. aureus from infectious foci and from possible reservoirs of infection (mainly from the upper respiratory tract—nose, nasopharynx and paranasal sinuses—and secondarily also from the gastrointestinal and uropoietic systems). It is also a drug for the treatment of purulent wounds, chronic skin and subcutaneous tissue infections and infections affecting deep-seated soft tissue and for the prevention of septic state development [34].
The aim of this work was to determine the in vitro efficacy of the phage preparation STAFAL® against clinical and even carrier S. aureus strains with variable susceptibility to antimicrobial compounds and variable genetic backgrounds in Slovakia, where the phage preparation is already registered.
2. Results
2.1. Antimicrobial Susceptibility of Isolated Staphylococcus aureus Strains
The summary antimicrobial susceptibility of S. aureus strains analysed in this study is shown in Table 1. Thirty-three MRSA strains belonging especially to the group of clinical strains (29 isolates—87.9%) were included. A total of 3 carrier strains and 24 clinical isolates were classified as MDR. One hundred and ten carrier strains (99.1%) were susceptible to local antimicrobial agents mupirocin, neomycin, bacitracin and fusidic acid.

Table 1.
Susceptibility of Staphylococcus aureus strains to antimicrobial compounds [37].
The susceptibility of MRSA strains to the other tested ATBs was statistically lower compared with the susceptibility of MSSA strains. These differences were detected in the susceptibility to erythromycin (36.6% vs. 67.3%), clindamycin (39.4% vs. 73.0%) and ciprofloxacin (21.2% vs. 91.2%) (Table 2).

Table 2.
Comparison of the susceptibility profiles of methicillin-resistant (MRSA) strains and methicillin-susceptible (MSSA) strains of Staphylococcus aureus to other antimicrobial compounds.
2.2. Susceptibility to STAFAL®
The overall efficacy of the STAFAL® phage preparation was confirmed in 142 S. aureus strains (74.0%). Slightly higher susceptibility to the phage preparation was demonstrated in clinical strains compared with carrier strains (73.9% vs. 74.1%), but these differences were not statistically significant (p = 0.985). The highest susceptibility was assessed in strains isolated from bloodstream infections (80.0%), followed by carrier strains (73.9%) and strains from skin and soft tissue infections (69.6%). No statistically significant differences were detected either (p = 0.571) (Figure 1).
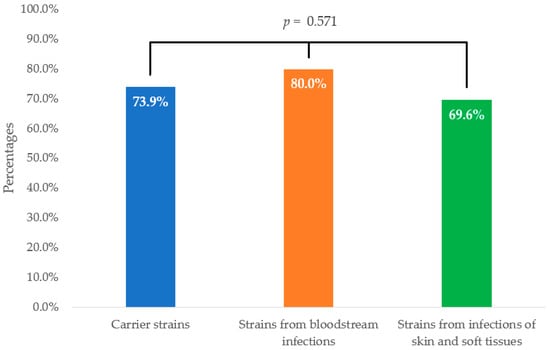
Figure 1.
Comparison of efficacy of STAFAL® against Staphylococcus aureus strains according to their origin. p—statistical significance among carrier strains, isolates from bloodstream infections and strains from skin and soft tissue infections according to Fisher–Freeman–Halton exact test.
The susceptibility of the tested bacterial strains to STAFAL® was different in the strains susceptible to ATBs and the strains resistant to ATBs. A statistically significant difference was found in the phage susceptibility of MRSA strains (31 phage-susceptible strains (93.9%)) and MSSA strains (111 phage-susceptible strains (69.8%)). Two other statistically significant difference were found in the phage susceptibility of erythromycin-resistant (60 phage-susceptible strains (82.2%)) and erythromycin-susceptible strains (82 phage-susceptible strains (68.9%)) and in the susceptibility of clindamycin-resistant (53 phage-susceptible strains (82.8%)) and clindamycin-susceptible strains (89 phage-susceptible strains (69.5%)) (Figure 2).
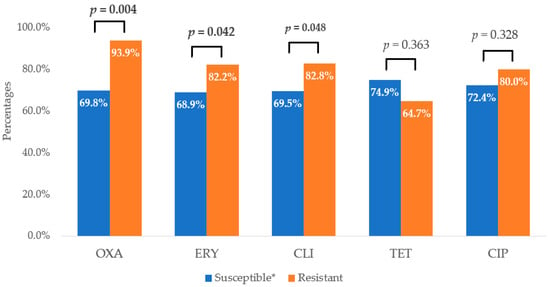
Figure 2.
Comparison of efficacy of STAFAL® against ATB-susceptible and ATB-resistant strains of Staphylococcus aureus. OXA—oxacillin; ERY—erythromycin; CLI—clindamycin; TET—tetracycline; CIP—ciprofloxacin; p—statistical significance between susceptible and resistant strains to various antimicrobial compounds according to χ2-test; in the case of statistical significance (p < 0.05), the values are expressed in bold; * in the case of ciprofloxacin—susceptible, increased exposure.
The efficacy of STAFAL® was confirmed in 23 MDR strains (85.2%), and the susceptibility of non-MDR strains was detected in 119 cases (72.1%). A non-significant difference was detected in the phage susceptibility of MDR and non-MDR strains to STAFAL® (p = 0.235).
The STAFAL® phage preparation was effective against strains belonging to 63 of the detected 97 spa types (64.9%). Five spa types included strains with variable susceptibility (5.2%). Strains belonging to the other 29 spa types were resistant to STAFAL® (29.9%) (Table 3).

Table 3.
Susceptibility of Staphylococcus aureus strains of various molecular genetic affiliations to STAFAL®.
3. Discussion
Nowadays, the complications associated with the treatment of infections caused by MDR strains are emerging. Phage therapy constitutes an alternative way of treating infectious diseases. However, only a few phage preparations are currently registered, since there is still a lack of studies confirming the effectiveness and safety of this type of products [32,38,39,40,41]. The only phage preparation registered within the European Union is STAFAL®. This phage preparation is indicated for topical application, especially for the treatment of purulent infections of the skin and soft tissues caused by S. aureus [32,34].
The basic prerequisite for the use of phage preparations in the therapy of infections in patients is their ability to lyse bacterial cells in vitro [42,43]. Therefore, 192 S. aureus strains of Slovak origin were selected to evaluate the therapeutic potential of the STAFAL® phage preparation. This collection included clinical strains isolated from infected patients, but also carrier strains from the nasal mucosa of healthy students of medicine, since they participate in healthcare facilities during their internships and nasal carriage contributes significantly to the spread of staphylococcal infections in vulnerable individuals [44,45]. The other aim of this study was to test the effect of STAFAL® on MRSA strains, since they are often associated with other types of resistance and these strains are often the reason for complicating therapy [46,47,48].
The antibacterial activity of the STAFAL® phage preparation has already been proven in studies by Dvořáčková et al. [49,50]. In the first study, the effectiveness of this preparation was assessed against clinical strains of MRSA and MSSA, and higher susceptibility of MRSA was detected (more than 99%). MSSA strains were susceptible in 87.9% of cases [49]. The high effectiveness of the antistaphylococcal preparation against clinical strains in planktonic form and in biofilm was determined in the second study. The strains analysed in this work were included in spa types t003, t024, t032 and t056 [50].
In this study, the analysis of these clinical and carrier strains of S. aureus revealed 74.0% efficacy of the STAFAL® phage preparation. Similar effectiveness of phage preparations from Russia, Bakteriophag Stafilokokovyj (74.5%) and Sextaphag® (73.4%), and Georgia, Staphylococcal bacteriophage (75.6%), was detected in this collection of strains in the previous study. The efficiency of the Georgian preparation Pyo-bacteriophage (80.2%) was slightly higher. These phage preparations and STAFAL® had similar spectra of action in our strain collection. Most of the strains were susceptible to all these five phage preparations, including STAFAL® (67.7%). On the other hand, 16.1% were resistant to all the preparations tested. Of all S. aureus strains, 83.9% were susceptible to at least one of the phage preparations tested [37].
The susceptibility of S. aureus strains to STAFAL® was compared in terms of the origin of the strains, their susceptibility to ATBs, and their molecular genetic affiliation. Slightly higher susceptibility of clinical strains compared with carrier strains to STAFAL® was detected. The highest efficacy of STAFAL® was observed in the group of strains of bloodstream infection isolated in severe-to-life-threatening infections. Similar results were also detected for the other four phage cocktails [37].
The susceptibility of the tested strains to STAFAL® varied in the strains with different susceptibility to ATBs. The tested phage preparation showed significantly higher activity against ATB-resistant strains (except tetracycline), especially against MRSA strains. Significantly higher phage susceptibility was obtained also for erythromycin and clindamycin-resistant strains. Similar results were also observed in this strain collection during the testing of the Russian and Georgian preparations [37]. Oechslin [51] stated that bacterial mutations that lead to resistance to phages often result in an increased survival cost for this bacterium. Additionally, the effect of two distinct inhibiting factors may reduce the risk of resistance development [52].
The efficacy of the STAFAL® preparation has been confirmed against a genetically diverse range of S. aureus strains, including typical nosocomial spa types t003, t024 and t032 [53,54,55,56,57]. Strains within the same spa type had similar patterns of susceptibility to the phage preparation, and only a few exceptions were found. Similarly, it was described also for the previously tested preparations. In the case of three strains (affiliated with t362, t701 and t1333), their susceptibility to STAFAL® was detected, while the other four phage preparations were ineffective. On the other hand, four different strains (affiliated with t008, t3884, t6943 and t12469) were susceptible to all the preparations tested, except for the STAFAL® preparation. Six other strains (affiliated with t091) and two strains (affiliated with t2716) were susceptible to Pyo-bacteriophage, but STAFAL® was not effective [37]. S. aureus strains with declared resistance to the STAFAL® preparation can be used as indicator strains to expand the spectrum of this preparation. The resistance of the bacterial strains to the phage preparation may be a result of mutation in receptors for phage adsorption, acquiring genes encoding restriction enzymes or CRISPR-Cas system-cleaving phage nucleic acid, the blocking of virus progeny release, etc. [58,59].
The American Antibiotic Resistance Leadership Group and Health Improvement Scotland have recommended the consideration of phage therapy for difficult-to-treat bacterial infections [9,60]. Our results confirm that the use of the STAFAL® phage preparation may be beneficial in the treatment of bacterial infections, primarily in patients infected or colonised by MRSA strains [17,38,61]. Moreover, it may be also a beneficial tool for the decolonisation of S. aureus from body sites [3] The successful application of the STAFAL® preparation in patients with infections caused by S. aureus took place in the 1960s and 1970s in the Czech Republic [62]. More recent experience with the treatment of ulcus cruris in chronic venous insufficiency, vasculitis, diabetic legs or severe carbuncles by STAFAL® in Slovakia was described in a study by Zelenková [63]. Here, the beneficial effect of STAFAL® was also confirmed.
The efforts to treat infections caused by S. aureus by other phages or phage preparations with antistaphylococcal activity have already been described. Pyo-bacteriophage was administered by intravesical titration in a double-blind study to patients with urinary tract infection. The effect of the phage cocktail was comparable with the efficacy of ATBs and placebo, but it was well tolerated [64]. Ferry et al. [65] used a phage cocktail (including phages PP1493, PP1815 and PP1957) followed by antibiotics for the treatment of three patients with prosthetic knee infection with significant clinical improvement in all of them. In another study, a patient with knee and hip prosthetic joint infection by MRSA underwent the exchange of a new spacer and was treated by phage Sa WIQ0488ø1 and daptomycin. Finally, all bacteriological cultures were negative, and no evidence of recurrence was detected [66]. The intravenous administration of phage cocktail AB-SA01 was described in patients with bacteremia by Petrovic Fabijan et al. [67]. Patients were also treated by ATBs. No adverse effects were reported, and probably, a synergic effect between phages and ATBs was achieved.
Despite the successful experience with phages in vitro or even in clinical use, STAFAL® is still the only phage preparation registered in a member state of the European Union and, in addition, is currently unavailable [32,34].
4. Materials and Methods
4.1. Characteristics of Bacterial Strains
In this study, 111 carrier strains and 81 clinical isolates of S. aureus characterised in a former study [37] were submitted to analysis. The carrier strains were obtained from nasal swabs of healthy volunteers—students of the Faculty of Medicine, Comenius University in Bratislava. The clinical isolates originated from the samples of patients of University Hospital Bratislava indicated for microbiological examination, where 35 isolates were from bloodstream and 46 from skin and soft tissue infections. Carrier strains were isolated from nasal swabs cultivated on BD Columbia Agar with 5% Sheep Blood (Becton Dickinson GmbH, Heidelberg, Germany). S. aureus strains were identified according to the cultivation properties and positive hyaluronidase test [68]. Clinical strains were identified within the routine microbiological diagnostics at University Hospital Bratislava [68]. Strains with uncertain identification were analysed by PCR according to Martineau et al. [69]. The antimicrobial susceptibility of carrier strains to erythromycin (15 µg), clindamycin (2 µg), tetracycline (30 µg), trimethoprim–sulfamethoxazole (co-trimoxazole) (2 µg + 23 µg) and ciprofloxacin (5 µg) (all Oxoid, Basingstoke, UK) was tested by the disc diffusion test according to EUCAST recommendations [70]. In this group of strains, the susceptibility to local ATBs, such as mupirocin (200 µg), bacitracin (200 µg), neomycin (200 µg) and fusidic acid (200 µg), was also assessed [70]. The screening of MRSA strains was conducted by the disc diffusion test by using cefoxitin (30 µg) [69], and confirmation in both carrier and clinical strains was conducted by the detection of the mecA gene by PCR according to Martineau et al. [71]. Strains resistant to at least one ATB from three or more ATB groups were classified as multidrug-resistant (MDR) [72].
The molecular characterisation of all S. aureus strains was performed by spa typing based on the sequence typing of the spa gene repeat region [73,74]. Bacterial genomic DNA was isolated from an overnight culture of the isolated strain in 10 mL of LB medium (BioLife, Milan, Italy) by using the Illustra TM bacteria genomicPrepMini Spin Kit according to the manufacturer’s instructions (GE Healthcare, Chicago, IL, USA). The amplification of the spa gene was carried out in 25 µL reactions by using primers spa-1113f (TAAAGACGATCCTTCGGTGAGC) and spa-1514r (CAGCAGTAGTGCCGTTTGCTT) (Merck KGaA, Darmstadt, Germany). The PCR reaction contained 2 µL of isolated DNA, 320 µM deoxynucleoside triphosphates (Solis bioDyne, Tartu, Estonia), 12 pmol of each primer, 10-fold concentrated DreamTaq green Buffer and 1.25 U of DreamTaq DNA polymerase (both ThermoFisher Scientific, Waltham, MA, USA). Thermal cycling reactions consisted of initial denaturation (2 min at 94 °C) followed by 35 cycles of denaturation (45 s at 94 °C), annealing (45 s at 66 °C) and extension (90 s at 72 °C), with final extension (10 min at 72 °C) in the thermocycler Biometra TAdvanced 96 (Analytik Jena, Jena, Germany). The presence of amplicons was detected by separation in 1% agarose gel. Subsequently, the PCR products were purified with the illustra TM ExoProStar TM kit (GE Healthcare, Chicago, IL, USA) according to the manufacturer’s instructions. Sequencing was performed by using the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Waltham, MA, USA) with an ABI PRISM 3130xl instrument (Applied Biosystems, Waltham, MA, USA). The obtained DNA sequences were analysed by using BIONUMERICS software version 7.5 (Applied Maths, Sint-Martens-Latem, Belgium). New spa types were uploaded to the Ridom SpaServer database [75].
4.2. Testing of Susceptibility of Staphylococcus aureus Strains to STAFAL® Phage Preparation
S. aureus strains were subjected to the testing of susceptibility to the STAFAL® phage preparation (Bohemia Pharmaceuticals, Brno, Czech Republic, currently AUMED, Praha, Czech Republic [76]). This preparation contains 107 phage particles per mL that are preserved by thiomersal.
The susceptibility of bacterial strains to this preparation was determined by the plaque formation method as described in a previous study [37]. After overnight cultivation of S. aureus strains at 37 °C on BD Columbia Agar with 5% Sheep Blood (Becton Dickinson GmbH, Heidelberg, Germany) a standardised inoculum of bacterial strains (McFarland 0.5, corresponding to 1–5 × 108 CFU/mL) was prepared turbidimetrically, using Densitometer DEN-1 (Biosan, Riga, Latvia). Luria–Bertani agar (BioLife, Milan, Italy) plates were overlaid with 2 mL of standardised bacterial inoculum. The inoculum excess was sucked out by a pipette. After the inoculum had soaked into the agar, the phage preparation STAFAL® was point-inoculated in 10 μL volumes of non-diluted phage preparation and preparation diluted in 1:10, 1:100 and 1:1000. Incubation lasted for 16 h at 37 °C. The activity of the phage preparations was determined by the detection of confluent bacterial lysis, semi-confluent plaques, or individual isolated plaques in the spot area of inoculated phage suspensions. Any of these reactions at any dilution were considered positive. The effect of thiomersal on bacteria was distinguished from the phage effect by testing different dilutions of the phage preparation and the preservative itself.
4.3. Statistical Analysis
The distribution of categorical variables between two groups was compared by using the Pearson χ2 test. Fisher’s exact test was used if the expected count was less than five in ≥20% of cells of the 2 × 2 contingency table. The Fisher–Freeman–Halton exact test was applied to compare the distribution of categorical variables among the three groups in 2 × 3 tables. All the statistical tests were performed at the level of statistical significance α = 0.05. The analyses were performed by using IBM SPSS statistical software for Windows, version 28 (IBM SPSS Inc., Armonk, NY, USA).
5. Conclusions
In the “post-antibiotic era”, phage therapy may constitute a helpful tool to manage the treatment of bacterial infections caused by MDR strains and thus decrease the rate of morbidity and mortality in patients. In this study, the high in vitro efficacy of the STAFAL® phage preparation against strains isolated in Slovakia with various genetic background indicates its therapeutical potential, especially in the infections caused by MRSA strains. Due to several advantages that phages have over ATBs, STAFAL® preparation may play an important role as an alternative or complementary treatment, especially in case of failure of ATB therapy. Moreover, there is a potential for use of this phage preparation as a decolonising agent for carriers.
Author Contributions
Conceptualisation, H.D. and L.S.; methodology, H.D. and L.S.; validation, H.D., L.S. and A.B.; formal analysis, A.B., M.S. and M.W.; investigation, Z.H., A.B., L.J., A.M., M.S. and L.S.; data curation, H.D., L.S., M.S., A.B. and M.W.; writing—original draft preparation, M.S., L.J. and Z.H.; writing—review and editing, L.S., H.D. and A.L.; supervision, L.S., H.D. and A.L.; project administration, L.S. and H.D.; funding acquisition, L.S., H.D. and A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research study was funded by the Slovak Research and Development Agency under contract No. APVV-23-0140.
Institutional Review Board Statement
The study was treated by the Ethics Committee of Faculty of Medicine of Comenius University and University Hospital Bratislava (protocol code 72/2024).
Informed Consent Statement
According to the statement of Ethics Committee.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Alves, D.R.; Gaudion, A.; Bean, J.E.; Perez Esteban, P.; Arnot, T.C.; Harper, D.R.; Kot, W.; Hansen, L.H.; Enright, M.C.; Jenkins, A.T. Combined use of bacteriophage K and a novel bacteriophage to reduce Staphylococcus aureus biofilm formation. Appl. Environ. Microbiol. 2014, 80, 6694–6703. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, P.; Mudgil, P. The Cell Wall, Cell Membrane and Virulence Factors of Staphylococcus aureus and Their Role in Antibiotic Resistance. Microorganisms 2023, 11, 259. [Google Scholar] [CrossRef] [PubMed]
- Piewngam, P.; Otto, M. Staphylococcus aureus colonisation and strategies for decolonisation. Lancet Microbe 2024, 5, e606–e618. [Google Scholar] [CrossRef] [PubMed]
- Agnello, S.; Wardlow, L.C.; Reed, E.; Smith, J.M.; Coe, K.; Day, S.R. Clinical Outcomes of Daptomycin Versus Anti-Staphylococcal Beta-Lactams in Definitive Treatment of Methicillin-susceptible Staphylococcus aureus Bloodstream Infections. Int. J. Antimicrob. Agents 2021, 58, 106363. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.; Wu, S.M.; Soni, I.; Wang-Crocker, C.; Matern, T.; Beck, J.P.; Loc-Carrillo, C. Phage and Antibiotic Combinations Reduce Staphylococcus aureus in Static and Dynamic Biofilms Grown on an Implant Material. Viruses 2023, 15, 460. [Google Scholar] [CrossRef]
- Łubowska, N.; Grygorcewicz, B.; Kosznik-Kwaśnicka, K.; Zauszkiewicz-Pawlak, A.; Węgrzyn, A.; Dołęgowska, B.; Piechowicz, L. Characterization of the Three New Kayviruses and Their Lytic Activity Against Multidrug-Resistant Staphylococcus aureus. Microorganisms 2019, 7, 471. [Google Scholar] [CrossRef]
- Tong, S.Y.C.; Lye, D.C.; Yahav, D.; Sud, A.; Robinson, J.O.; Nelson, J.; Archuleta, S.; Roberts, M.A.; Cass, A.; Paterson, D.L.; et al. Effect of vancomycin or daptomycin with vs without an antistaphylococcal β-lactam on mortality, bacteremia, relapse, or treatment failure in patients with MRSA bacteremia: A randomized clinical trial. JAMA 2020, 323, 527–537. [Google Scholar] [CrossRef]
- Liu, K.; Wang, C.; Zhou, X.; Guo, X.; Yang, Y.; Liu, W.; Zhao, R.; Song, H. Bacteriophage therapy for drug-resistant Staphylococcus aureus infections. Front. Cell Infect. Microbiol. 2024, 14, 1336821. [Google Scholar] [CrossRef]
- Scottish Health Technologies Group HIS. SHTG Recommendation. 2023. Available online: https://shtg.scot/our-advice/bacteriophage-therapy-for-patients-with-difficult-to-treat-bacterial-infections/ (accessed on 23 September 2024).
- Möllers, M.; von Wahlde, M.-K.; Schuler, F.; Mellmann, A.; Böing, C.; Schwierzeck, V.; Schneider, J.S.; Kampmeier, S. Outbreak of MRSA in a Gynecology/Obstetrics Department during the COVID-19 Pandemic: A Cautionary Tale. Microorganisms 2022, 10, 689. [Google Scholar] [CrossRef]
- Zohra, T.; Numan, M.; Ikram, A.; Salman, M.; Khan, T.; Din, M.; Salman, M.; Farooq, A.; Amir, A.; Ali, M. Cracking the Challenge of Antimicrobial Drug Resistance with CRISPR/Cas9, Nanotechnology and Other Strategies in ESKAPE Pathogens. Microorganisms 2021, 9, 954. [Google Scholar] [CrossRef]
- Freitas, A.R.; Werner, G. Nosocomial Pathogens and Antimicrobial Resistance: Modern Challenges and Future Opportunities. Microorganisms 2023, 11, 1685. [Google Scholar] [CrossRef] [PubMed]
- Bozidis, P.; Markou, E.; Gouni, A.; Gartzonika, K. Does Phage Therapy Need a Pan-Phage? Pathogens 2024, 13, 522. [Google Scholar] [CrossRef] [PubMed]
- D’Accolti, M.; Soffritti, I.; Mazzacane, S.; Caselli, E. Bacteriophages as a Potential 360-Degree Pathogen Control Strategy. Microorganisms 2021, 9, 261. [Google Scholar] [CrossRef] [PubMed]
- Baláž, A.; Kajsik, M.; Budiš, J.; Szemes, T.; Turňa, J. PHERI—Phage Host ExploRation Pipeline. Microorganisms 2023, 11, 1398. [Google Scholar] [CrossRef]
- Uyttebroek, S.; Chen, B.; Onsea, J.; Ruythooren, F.; Debaveye, Y.; Devolder, D.; Spriet, I.; Depypere, M.; Wagemans, J.; Lavigne, R.; et al. Safety and efficacy of phage therapy in difficult-to-treat infections: A systematic review. Lancet Infect. Dis. 2022, 22, e208–e220. [Google Scholar] [CrossRef]
- Vázquez, R.; Díez-Martínez, R.; Domingo-Calap, P.; García, P.; Gutiérrez, D.; Muniesa, M.; Ruiz-Ruigómez, M.; Sanjuán, R.; Tomás, M.; Tormo-Mas, M.Á.; et al. Essential Topics for the Regulatory Consideration of Phages as Clinically Valuable Therapeutic Agents: A Perspective from Spain. Microorganisms 2022, 10, 717. [Google Scholar] [CrossRef]
- Doss, J.; Culbertson, K.; Hahn, D.; Camacho, J.; Barekzi, N. A Review of Phage Therapy against Bacterial Pathogens of Aquatic and Terrestrial Organisms. Viruses 2017, 9, 50. [Google Scholar] [CrossRef]
- Straka, M.; Dubinová, M.; Liptáková, A. Phascinating Phages. Microorganisms 2022, 10, 1365. [Google Scholar] [CrossRef]
- Weber-Dąbrowska, B.; Jończyk-Matysiak, E.; Żaczek, M.; Łobocka, M.; Łusiak-Szelachowska, M.; Górski, A. Bacteriophage Procurement for Therapeutic Purposes. Front. Microbiol. 2016, 7, 1177. [Google Scholar] [CrossRef]
- Weber-Dąbrowska, B.; Mulczyk, M.; Górski, A. Bacteriophages as an efficient therapy for antibiotic-resistant septicemia in man. Transplant. Proc. 2003, 35, 1385–1386. [Google Scholar] [CrossRef]
- Jennes, S.; Merabishvili, M.; Soentjens, P.; Pang, K.W.; Rose, T.; Keersebilck, E.; Soete, O.; Francois, P.M.; Teodorescu, S.; Verween, G.; et al. Use of bacteriophages in the treatment of colistin-only-sensitive Pseudomonas aeruginosa septicaemia in a patient with acute kidney injury-a case report. Crit. Care 2017, 21, 129. [Google Scholar] [CrossRef] [PubMed]
- Rogóż, P.; Amanatullah, D.F.; Międzybrodzki, R.; Manasherob, R.; Weber-Dąbrowska, B.; Fortuna, W.; Letkiewicz, S.; Górski, A. Phage therapy in orthopaedic implant-associated infections. In Phage Therapy: A Practical Approach; Górski, A., Międzybrodzki, R., Borysowski, J., Eds.; Springer Nature Switzerland AG: Berlin/Heidelberg, Germany, 2019; pp. 189–211. [Google Scholar]
- Rubalskii, E.; Ruemke, S.; Salmoukas, C.; Boyle, E.C.; Warnecke, G.; Tudorache, I.; Shrestha, M.; Schmitto, J.D.; Martens, A.; Rojas, S.V.; et al. Bacteriophage therapy for critical infections related to cardiothoracic surgery. Antibiotics 2020, 9, 232. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Dai, J.; Guo, M.; Li, J.; Zhou, X.; Li, F.; Gao, Y.; Qu, H.; Lu, H.; Jin, J.; et al. Pre-optimized phage therapy on secondary Acinetobacter baumannii infection in four critical COVID-19 patients. Emerg. Microbes Infect. 2021, 10, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Abedon, S.T.; Kuhl, S.J.; Blasdel, B.G.; Kutter, E.M. Phage treatment of human infections. Bacteriophage 2011, 1, 66–85. [Google Scholar] [CrossRef] [PubMed]
- Fruciano, D.E.; Bourne, S. Phage as an antimicrobial agent: D’Herelle’s heretical theories and their role in the decline of phage prophylaxis in the West. Can. J. Infect. Dis. Med. Microbiol. 2007, 18, 19–26. [Google Scholar] [CrossRef]
- Chanishvili, N. Bacteriophages as Therapeutic and Prophylactic Means: Summary of the Soviet and Post Soviet Experiences. Curr. Drug Deliv. 2016, 13, 309–323. [Google Scholar] [CrossRef]
- Kutateladze, M.; Adamia, R. Phage therapy experience at the Eliava Institute. Med. Mal. Infect. 2008, 38, 426–430. [Google Scholar] [CrossRef]
- Żaczek, M.; Górski, A.; Weber-Dąbrowska, B.; Letkiewicz, S.; Fortuna, W.; Rogóż, P.; Pasternak, E.; Międzybrodzki, R. A Thorough Synthesis of Phage Therapy Unit Activity in Poland—Its History, Milestones and International Recognition. Viruses 2022, 14, 1170. [Google Scholar] [CrossRef]
- Jault, P.; Leclerc, T.; Jennes, S.; Pirnay, J.P.; Que, Y.A.; Resch, G.; Rousseau, A.F.; Ravat, F.; Carsin, H.; Floch, R.; et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): A randomised, controlled, double-blind phase 1/2 trial. Lancet Infect. Dis. 2019, 19, 35–45. [Google Scholar] [CrossRef]
- Naureen, Z.; Malacarne, D.; Anpilogov, K.; Dautaj, A.; Camilleri, G.; Cecchin, S.; Bressan, S.; Casadei, A.; Albion, E.; Sorrentino, E.; et al. Comparison between American and European legislation in the therapeutical and alimentary bacteriophage usage. Acta Biomed 2020, 91, e2020023. [Google Scholar] [CrossRef]
- Yang, Q.; Le, S.; Zhu, T.; Wu, N. Regulations of phage therapy across the world. Front. Microbiol. 2023, 14, 1250848. [Google Scholar] [CrossRef] [PubMed]
- ŠÚKL. Available online: https://www.sukl.sk/hlavna-stranka/slovenska-verzia/pomocne-stranky/detail-lieku?page_id=386&lie_id=24546 (accessed on 19 July 2024).
- Pulverer, G.; Pillich, J.; Kocur, M. Zwei neue gegen pathogene Staphylokokken wirksame Bakteriophagen. Zentralbl Bakteriol. Parasit. Infekt. Hyg. I Orig. 1966, 201, 321–325. [Google Scholar]
- Barylski, J.; Enault, F.; Dutilh, B.E.; Schuller, M.B.P.; Edwards, R.A.; Gillis, A.; Klumpp, J.; Knezevic, P.; Krupovic, M.; Kuhn, J.H.; et al. Analysis of Spounaviruses as a Case Study for the Overdue Reclassification of Tailed Phages. Syst. Biol. 2020, 69, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Straka, M.; Hubenakova, Z.; Lichvarikova, A.; Janosikova, L.; Markuskova, B.; Minich, A.; Liptakova, A.; Drahovska, H.; Slobodnikova, L. Susceptibility of Staphylococcus aureus strains to commercial therapeutic phage preparations. Bratisl. Lek. Listy. 2022, 123, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Romero-Calle, D.; Guimarães Benevides, R.; Góes-Neto, A.; Billington, C. Bacteriophages as Alternatives to Antibiotics in Clinical Care. Antibiotics 2019, 8, 138. [Google Scholar] [CrossRef]
- Wang, B.; Du, L.; Dong, B.; Kou, E.; Wang, L.; Zhu, Y. Current Knowledge and Perspectives of Phage Therapy for Combating Refractory Wound Infections. Int. J. Mol. Sci. 2024, 25, 5465. [Google Scholar] [CrossRef]
- Pirnay, J.P. Phage Therapy in the Year 2035. Front. Microbiol. 2020, 11, 1171. [Google Scholar] [CrossRef]
- Verbeken, G.; Pirnay, J.P. European regulatory aspects of phage therapy: Magistral phage preparations. Curr. Opin. Virol. 2022, 52, 24–29. [Google Scholar] [CrossRef]
- Erol, H.B.; Kaskatepe, B.; Bakkaloglu, Z.; Suzuk Yildiz, S. The evaluation of five commercial bacteriophage cocktails against methicillin-resistant Staphylococcus aureus isolated from nasal swab samples. Arch. Microbiol. 2021, 203, 5735–5743. [Google Scholar] [CrossRef]
- Terwilliger, A.; Clark, J.; Karris, M.; Hernandez-Santos, H.; Green, S.; Aslam, S.; Maresso, A. Phage Therapy Related Microbial Succession Associated with Successful Clinical Outcome for a Recurrent Urinary Tract Infection. Viruses 2021, 13, 2049. [Google Scholar] [CrossRef]
- Price, J.R.; Cole, K.; Bexley, A.; Kostiou, V.; Eyre, D.W.; Golubchik, T.; Wilson, D.J.; Crook, D.W.; Walker, A.S.; Peto, T.E.A.; et al. Transmission of Staphylococcus aureus between health-care workers, the environment, and patients in an intensive care unit: A longitudinal cohort study based on whole-genome sequencing. Lancet Infect. Dis. 2017, 17, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Sakr, A.; Brégeon, F.; Mège, J.L.; Rolain, J.M.; Blin, O. Staphylococcus aureus Nasal Colonization: An Update on Mechanisms, Epidemiology, Risk Factors, and Subsequent Infections. Front. Microbiol. 2018, 9, 2419. [Google Scholar] [CrossRef] [PubMed]
- Attia, N.; Ghazal, A.; El Sherbini, E.; Shalaby, M. Have Methicillin Resistant Staphylococcus aureus clinical isolates to be also resistant to Streptogramins? Microbes Infect. Dis. 2021, 2, 286–294. [Google Scholar] [CrossRef]
- Jangale, N.P.; Joshi, P.A.; Gaigawale, A.S. Association of different phenotypes of MLSB and mupirocin resistance in clinical isolates of Staphylococcus aureus. J. Popul. Ther. Clin. Pharmacol. 2024, 31, 2173–2178. [Google Scholar] [CrossRef]
- Alseqely, M.; Newton-Foot, M.; Khalil, A.; El-Nakeeb, M.; Whitelaw, A.; Abouelfetouh, A. Association between fluoroquinolone resistance and MRSA genotype in Alexandria, Egypt. Sci. Rep. 2021, 11, 4253. [Google Scholar] [CrossRef] [PubMed]
- Dvořáčková, M.; Růžička, F.; Dvořáková-Heroldová, M.; Vacek, L.; Bezděková, D.; Benešík, M.; Petráš, P.; Pantůček, R. Možnosti terapeutického ovlivnění stafylokokových infekcí prostřednictvím bakteriofágů a vybrané metody testování citlivosti stafylokoků in vitro (Therapeutic potential of bacteriophages for staphylococcal infections and selected methods for in vitro susceptibility testing). Epidemiol. Mikrobiol. Imunol. 2020, 69, 10–18. [Google Scholar]
- Dvořáčková, M.; Růžička, F.; Benešík, M.; Pantůček, R.; Dvořáková-Heroldová, M. Antimicrobial effect of commercial phage preparation Stafal® on biofilm and planktonic forms of methicillin-resistant Staphylococcus aureus. Folia Microbiol. 2019, 64, 121–126. [Google Scholar] [CrossRef]
- Oechslin, F. Resistance Development to Bacteriophages Occurring during Bacteriophage Therapy. Viruses 2018, 10, 351. [Google Scholar] [CrossRef]
- Torres-Barceló, C.; Hochberg, M.E. Evolutionary rationale for phages as complements of antibiotics. Trends Microbiol. 2016, 24, 249–256. [Google Scholar] [CrossRef]
- Engelthaler, D.M.; Kelley, E.; Driebe, E.M.; Bowers, J.; Eberhard, C.F.; Trujillo, J.; Decruyenaere, F.; Schupp, J.M.; Mossong, J.; Keim, P.; et al. Rapid and robust phylotyping of spa t003, a dominant MRSA clone in Luxembourg and other European countries. BMC Infect. Dis. 2013, 13, 339. [Google Scholar] [CrossRef]
- Neradova, K.; Fridrichova, M.; Jakubu, V.; Pomorska, K.; Zemlickova, H. Epidemiological characteristics of methicillin-resistant Staphylococcus aureus isolates from bloodstream cultures at University Hospital in the Czech Republic. Folia Microbiol. 2020, 65, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Bartels, M.D.; Boye, K.; Rhod Larsen, A.; Skov, R.; Westh, H. Rapid increase of genetically diverse methicillin-resistant Staphylococcus aureus, Copenhagen, Denmark. Emerg. Infect. Dis. 2007, 13, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.U.; Chua, K.H.; Chew, C.H.; Yeo, C.C.; Abdullah, F.H.; Othman, N.; Kee, B.P.; Puah, S.M. spa diversity of methicillin-resistant and -susceptible Staphylococcus aureus in clinical strains from Malaysia: A high prevalence of invasive European spa-type t032. PeerJ 2021, 9, e11195. [Google Scholar] [CrossRef] [PubMed]
- Pomorska, K.; Jakubu, V.; Malisova, L.; Fridrichova, M.; Musilek, M.; Zemlickova, H. Antibiotic Resistance, spa Typing and Clonal Analysis of Methicillin-Resistant Staphylococcus aureus (MRSA) Isolates from Blood of Patients Hospitalized in the Czech Republic. Antibiotics 2021, 10, 395. [Google Scholar] [CrossRef]
- Sutton, T.D.S.; Hill, C. Gut Bacteriophage: Current Understanding and Challenges. Front. Endocrinol. 2019, 10, 784. [Google Scholar] [CrossRef]
- Barathan, M.; Ng, S.L.; Lokanathan, Y.; Ng, M.H.; Law, J.X. The Profound Influence of Gut Microbiome and Extracellular Vesicles on Animal Health and Disease. Int. J. Mol. Sci. 2024, 25, 4024. [Google Scholar] [CrossRef]
- Suh, G.A.; Lodise, T.P.; Tamma, P.D.; Knisely, J.M.; Alexander, J.; Aslam, S.; Barton, K.D.; Bizzell, E.; Totten, K.M.; Campbell, J.L.; et al. Considerations for the Use of Phage Therapy in Clinical Practice. Antimicrob. Agents Chemother. 2022, 66, e0207121. [Google Scholar] [CrossRef]
- Abedon, S.T.; Danis-Wlodarczyk, K.M.; Alves, D.R. Phage Therapy in the 21st Century: Is There Modern, Clinical Evidence of Phage-Mediated Efficacy? Pharmaceuticals 2021, 14, 1157. [Google Scholar] [CrossRef]
- Pillich, J.; Výmola, F.; Buda, J. Voraussetzungen für eine erfolgreiche Therapie durch Staphylokokken-Phagenlysate [Assumptions for successful therapy using staphylococcal phage lysates]. Zentralbl. Bakteriol. Orig. 1969, 210, 377–381. [Google Scholar]
- Zelenková, H. Antistafylokokový fágový lyzát v liečbe chronických rán predkolenia na podklade chronickej venóznej insuficiencie a diabetes mellitus [Antistaphylococcal phage lysate in the treatment of chronic lower leg wounds based on chronic venous insufficiency and diabetes mellitus]. Kazuistiky Diabetol. 2014, 12, 15–19. [Google Scholar]
- Leitner, L.; Ujmajuridze, A.; Chanishvili, N.; Goderdzishvili, M.; Chkonia, I.; Rigvava, S.; Chkhotua, A.; Changashvili, G.; McCallin, S.; Schneider, M.P.; et al. Intravesical bacteriophages for treating urinary tract infections in patients undergoing transurethral resection of the prostate: A randomised, placebo-controlled, double-blind clinical trial. Lancet Infect. Dis. 2021, 21, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Ferry, T.; Kolenda, C.; Batailler, C.; Gustave, C.A.; Lustig, S.; Malatray, M.; Fevre, C.; Josse, J.; Petitjean, C.; Chidiac, C.; et al. Phage therapy as adjuvant to conservative surgery and antibiotics to salvage patients with relapsing S. aureus prosthetic knee infection. Front. Med. 2020, 7, 570572. [Google Scholar] [CrossRef] [PubMed]
- Schoeffel, J.; Wang, E.W.; Gill, D.; Frackler, J.; Horne, B.A.; Manson, T.; Doub, J.B. Successful use of salvage bacteriophage therapy for a recalcitrant MRSA knee and hip prosthetic joint infection. Pharmaceuticals 2022, 15, 177. [Google Scholar] [CrossRef] [PubMed]
- Fabijan, A.P.; Lin, R.C.; Ho, J.; Maddocks, S.; Zakour, N.L.; Iredell, J.R.; Team, W.B. Publisher correction: Safety of bacteriophage therapy in severe Staphylococcus aureus infection. Nat. Microbiol. 2020, 5, 652. [Google Scholar] [CrossRef]
- Jorgensen, J.H.; Pfaller, M.A. (Eds.) Manual of Clinical Microbiology; ASM Press: Washington, DC, USA, 2015. [Google Scholar]
- Martineau, F.; Picard, F.J.; Paradis, D.K.S.; Roy, P.H.; Ouellette, M.; Bergeron, M.G. Development of a PCR Assay for Identifi cation of Staphylococci at Genus and Species Levels. J. Clin. Microbiol. 2001, 39, 2541–2547. [Google Scholar] [CrossRef]
- EUCAST. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_14.0_Breakpoint_Tables.pdf (accessed on 22 November 2024).
- Martineau, F.; Picard, F.J.; Roy, P.H.; Ouellette, M.; Bergeron, M.G. Species-specific and ubiquitous-DNA-based assays for rapid identification of Staphylococcus aureus. J. Clin. Microbiol. 1998, 36, 618–623. [Google Scholar] [CrossRef]
- CDC. Available online: https://www.cdc.gov/narms/resources/glossary.html (accessed on 22 May 2024).
- Croes, S.; Deurenberg, R.H.; Boumans, M.L.; Beisser, P.S.; Neef, C.; Stobberingh, E.E. Staphylococcus aureus biofilm formation at the physiologic glucose concentration depends on the S. aureus lineage. BMC Microbiol. 2009, 9, 229. [Google Scholar] [CrossRef]
- Schwierzeck, V.; Effner, R.; Abel, F.; Reiger, M.; Notheis, G.; Held, J.; Simon, V.; Dintner, S.; Hoffmann, R.; Hagl, B.; et al. Molecular Assessment of Staphylococcus aureus Strains in STAT3 Hyper-IgE Syndrome Patients. J. Clin. Immunol. 2022, 42, 1301–1309. [Google Scholar] [CrossRef]
- Ridom Spa Server. Available online: http://spaserver.ridom.de/ (accessed on 22 November 2024).
- AUMED. Available online: https://aumed.cz/stafal/ (accessed on 22 November 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).


